Abstract
Hyaluronan (HA), a glycosaminoglycan critical to the lung extracellular matrix, has been shown to dissociate into low-molecular-weight (LMW) HA fragments following exposure to injurious stimuli. In the present study we questioned whether lung HA changed during ischemia and whether changes had an effect on subsequent angiogenesis. After left pulmonary artery ligation (LPAL) in mice, we analyzed left lung homogenates immediately after the onset of ischemia (0 h) and intermittently for 14 days. The relative expression of HA synthase (HAS)1, HAS2, and HAS3 was determined by real-time RT-PCR, total HA in the lung was measured by an ELISA-like assay, gel electrophoresis was performed to determine changes in HA size distribution, and the activity of hyaluronidases was determined by zymography. A 50% increase in total HA was measured 16 h after the onset of ischemia and remained elevated for up to 7 days. Furthermore, a fourfold increase in LMW HA fragments (495–30 kDa) was observed by 4 h after LPAL. Both HAS1 and HAS2 showed increased expression 4–16 h after LPAL, yet no changes were seen in hyaluronidase activity. These results suggest that both HA fragmentation and activation of HA synthesis contribute to increased HA levels during lung ischemia. Delivery of LMW HA fragments in an in vitro tube formation assay or directly to the ischemic mouse lung in vivo both resulted in increased angiogenesis. We conclude that ischemic injury results in matrix fragmentation, which leads to stimulation of neovascularization.
Keywords: N-acetyl-l-cysteine, hyaluronan synthase
an extensive literature documents that tissue-specific matrix conditions are required to support organ structure, modulate fluid dynamics, and regulate the behavior of resident cells within a tissue. Specifically, hyaluronan (HA), a hydrophilic glycosaminoglycan found in the extracellular matrix, has been shown to possess a range of biological activities based on its size. HA plays an important role maintaining the structural integrity of the lung where normal single HA chains range in size from 1,000 to 4,000 kDa. Each HA chain is made up of repeating polymeric disaccharides of d-glucuronic acid (1-β-3) and N-acetyl-d-glucosamine (1-β-4). An increase in matrix turnover, the breakdown of HA chains, and an overall change in the size distribution of HA have been associated with tissue injury (13). Gao and colleagues (3) have confirmed that increased levels of oxidants in the lung lead to an increase in low-molecular-weight (LMW) HA. Furthermore, LMW HA fragments (<500 kDa) have been shown to activate inflammatory cells, leading to cytokine release likely through binding Toll-like receptors (2). Interestingly, high-molecular-weight (HMW) HA has been shown to provide protection in some injury models such as after ozone exposure (4), LPS-induced vascular permeability (17), and smoke-induced lung injury (6), competitively inhibiting the effects of LMW HA. Since each of these lung injury models includes changes in the redox balance of the lung, one might predict that LMW HA initiates an inflammatory response in the lung whenever reactive oxygen species (ROS) are released. We have shown recently that ischemia-induced angiogenesis in the lung is initiated by the release of ROS (12). Complete obstruction of the left pulmonary artery in mice caused an early and transient increase in ROS release, which appeared to play a central role in promoting subsequent systemic neovascularization of the lung. However, whether HA fragmentation occurs during lung ischemia is unknown.
Within the field of angiogenesis, HA has been studied both as an endothelial cell proliferative agonist as well as an essential biomaterial for therapeutic neovascularization for peripheral artery occlusive disease (20). LMW HA has been shown to have direct stimulatory effects on endothelial cells leading to increased cell proliferation and vessel formation (22, 23).
Given these reported associations of LMW HA, ROS, and angiogenesis, we tested whether HA fragmentation occurred during ischemia caused by complete cessation of pulmonary perfusion in the left lung of mice. In the present study we hypothesized that acute pulmonary ischemia causes HA fragmentation, which contributes to the cascade of events culminating in angiogenesis. Our results are consistent with this hypothesis and suggest an important role for lung matrix components in recovery from ischemic injury.
METHODS
LPAL.
Our experimental protocol was approved by the Johns Hopkins Animal Care and Use Committee. Six-week-old C57Bl/6 mice were anesthetized (2% isoflurane in room air), intubated, and ventilated (120 breaths/min, 200 μl/breath). A left lateral thoracotomy was performed between the third and fourth rib, exposing the left lung. The left pulmonary artery was located, separated from the left bronchus, and ligated (12, 15). The left lung was harvested and snap frozen immediately after ligation for the 0-h control time point. We used the 0-h lung immediately after left pulmonary artery ligation (LPAL) since it most closely matched the state of the vascular bed and lung components at subsequent time points and prior to significant ischemic injury. For 4-h to 14-day mice, the thorax was closed after LPAL and the mice were ventilated briefly and allowed to recover. Some animals were treated with the antioxidant N-acetyl-l-cysteine (NAC; reconstituted in 1 M NaOH and pH adjusted) 24 h, 12 h, and immediately before surgery (ip, 1 mg/g body wt; 0.3 ml).
Total HA.
Lungs were homogenized (PBS) weight by volume, and supernatants were measured by an ELISA-like assay according to manufacturer's protocol (Echelon Biosciences, Salt Lake City, UT). This commercially available ELISA-like assay has been shown to be effective at measuring all sizes of HA tested (2,000–6.4 kDa; Ref. 5).
HA fragmentation.
To ensure accurate loading, total HA was first determined (ELISA-like assay) before lung homogenates were treated with Pronase (13 mg/ml; Calbiochem, San Diego, CA) and incubated overnight (55°C). Samples were boiled (10 min) and concentrated (20K Icon Concentrators, Thermo Scientific, Rockford, IL; 12 min at 3,900 rpm). To avoid residual protein contamination in the gel analysis and to focus specifically on HA and not all glycosaminoglycans, paired samples were studied in parallel with and without lyase degradation of HA. One of the paired aliquots of each sample was treated with hyaluronate lyase (HYAL) from Streptomyces hyalurolyticus (1 unit per 10 μl; Sigma-Aldrich, St. Louis, MO), incubated (1 h at 37°C), and boiled. In pilot experiments using a known amount of HA in excess of the amount found in the lung, this HYAL treatment removed all HA. The paired lung homogenate samples were loaded into a 0.5% agarose gel (50 V for 5 h), subsequently stained with Stains-All (Sigma-Aldrich) overnight in the dark and then destained for 1–2 days in the dark. Images of gels were taken with a Kodak Gel Logic 112 Imaging System, and analyzed with Molecular Imaging Software, version 5.0 (Carestream Health, Rochester, NY). Using a HA ladder (Hyalose, Austin, TX) to set size limits, we determined HA fragments in the ranges 495–310 kDa, 310–110 kDa, and 110–30 kDa by measurement of pixel intensity of the original sample less the lyase-treated paired sample.
Hyaluronidase activity.
The activity of all hyaluronidases was determined by zymography using standard techniques. Zymograph images showing hyaluronidase activity were evaluated by use of the Kodak Gel Logic 112 imaging system (Carestream Health).
HA synthase expression.
A section of left lung was used to isolate mRNA for each sample. With use of standard techniques, real-time RT PCR was performed using a TaqMan PCR Core Reagent Kit (Applied Biosystems, Foster City, CA) on a Bio-Rad CFX96 Real-Time System (Hercules, CA).
Tube formation assay.
To evaluate effectiveness of LMW HA in promoting endothelial tube formation in lung pleural tissue, we adapted methods previously employed (9). Growth factor reduced Matrigel matrix (BD, Franklin Lakes, NJ) was mixed with endotoxin-free LMW HA (30 μg, 130 kDa HA, Lifecore Biomedical, Chaska, MN) or HMW HA (30 μg, 4,000 kDa, Healon, Abbott Medical Optics, Santa Ana, CA). Small pieces of the pleural surface from fresh lung tissue (1 mm3) were harvested from naive C57Bl/6 mice and plated on the Matrigel with different-sized HA (96-well plates). Each group was plated in triplicate, and medium (DMEM, 10% FBS, heparin) with LMW HA or HMW HA (60 μg/ml) was changed every other day. After 6 days of incubation, images of each well were obtained (Olympus IX51 microscope, Olympus, Center Valley, PA and High Performance SensiCam, Cooke, Auburn Hills, MI) by an individual blinded to the treatment protocol. Tube numbers per well and total tube lengths of connecting cells were measured by use of Image Pro Plus 5.1 software (Media Cybernetics, Bethesda, MD). Confirmation of endothelial cell phenotype was performed in parallel experiments by staining with FITC-labeled anti-mouse CD31 antibody (BD).
Angiogenesis assessment.
After mice were anesthetized (2% isoflurane) and intubated for ventilation, the extent of neovascularization was determined by measuring systemic blood flow to the left lung 14 days after LPAL with use of labeled microspheres (12, 15). The carotid artery was cannulated and 360,000 microspheres (10 μm) were infused. Mice were euthanized by exsanguination and the left lung was removed. Fluorescent microspheres (Invitrogen, Eugene, OR) lodged in the left lung were quantified after tissue digestion and fluorescent dye extraction, and the number was calculated from a standard curve. Data are presented as the percent total microspheres delivered, i.e., % cardiac output. Angiogenesis was assessed in two treatment groups 14 days after LPAL. Mice received either PBS (40 μl) or endotoxin-free LMW HA (495–30 kDa; 40 μl of 2.3 mg/ml), delivered 4, 24, 48, and 72 h after LPAL by intratracheal aspiration. In preliminary studies, this treatment caused a substantial increase in total HA in bronchoalveolar lavage fluid (1,481.5 ± 237.1 ng/ml; n = 3 mice) 20 h after intratracheal delivery compared with no treatment (25.8 ± 8.4 ng/ml; n = 3 mice).
Statistics.
Average data are presented as means ± standard errors. A P value <0.05 was accepted as statistically significant. Fold-change data were analyzed by Wilcoxon's signed-rank test (total HA). Student's t-test was used to compare changes in blood flow. For multiple-group comparisons, Kruskal-Wallis followed by Dunn's multiple-comparison test were applied (HAS expression, HA fragment pixel intensities, tube lengths and numbers).
RESULTS
Total HA increases early after the onset of ischemia.
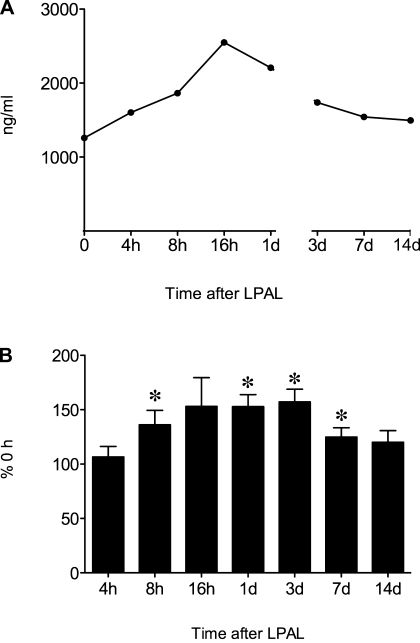
To determine whether HA increased after the onset of complete left lung ischemia, the time course of changes in total HA (ng/ml) in left lung homogenates was measured immediately (0 h); 4, 8, 16, and 24 h; and 3 and 14 days after LPAL. A representative time course obtained from one assay is shown in Fig. 1A (n = 1–2 mice/time point) demonstrating an increase in total HA followed by subsequent recovery at a late time point. Average group data (n = 6–9 mice/time point) are presented in Fig. 1B with each assay normalized to control (0 h). A significant increase in total HA was observed by 8 h, with a maximum average increase of 50% (P < 0.05). Total HA 14 days after LPAL was not different from control level (0 h).
Fig. 1.
A: representative time course of changes in total hyaluronan (HA) (ELISA-like assay) in the left lung after left pulmonary artery ligation (LPAL). An early increase is observed within the first 24 h that gradually recovers by 14 days (n = 1–2 mice/time point). d, Days. B: total HA in the left lung after LPAL (% 0 h left lung; n = 6–9 mice/time point). A significant increase in total HA was observed by 8 h after LPAL and remained elevated until the 14-day time point. (*P < 0.05).
Increased HA fragmentation.
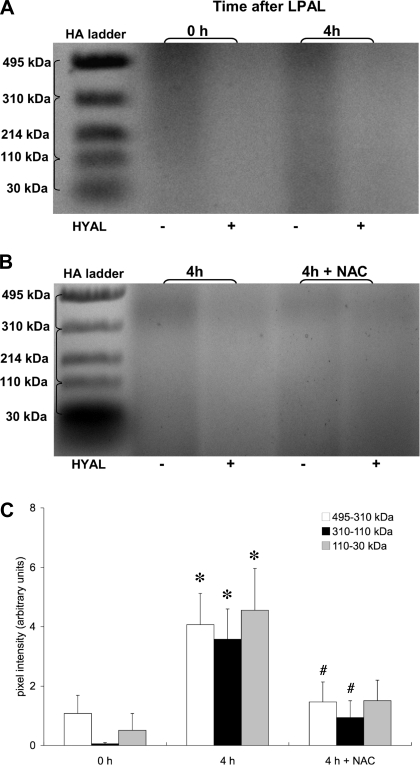
To determine whether HA fragmentation occurred after ischemia, changes in HA size in lung homogenates were evaluated by agarose gel electrophoresis. Figure 2A shows a representative experiment. Each sample (0 h, 4 h) was run without (left lane) and with (right lane) HYAL. The right-sided pair of each sample shows diminished intensity demonstrating removal of HA. On the basis of preliminary time course data in which smears associated with the 4-h sample showed the greatest intensity, we focused on comparing lungs from mice 0 and 4 h after LPAL (n = 7–10 mice/group). Pixel intensities were determined using the HA ladder as limits for each size range. Significant increases in each of the three fragment size ranges were observed when comparing 0-h left lungs with 4-h left lungs (Fig. 2C; P < 0.05). On the basis of these results and previous work from our laboratory demonstrating an increase in ROS by 4 h after LPAL (12), we selected the 4-h time point to evaluate changes in fragmentation in animals undergoing antioxidant treatment with NAC (n = 10 mice). Figure 2B shows a gel from lungs of mice 4 h after LPAL; the left two lanes are of lungs without NAC treatment and the right two lanes are of lungs with NAC pretreatment. As seen in Fig. 2C, fragmentation in lungs from NAC-treated mice was substantially reduced compared with untreated mice 4 h after LPAL within the ranges 495–310 kDa and 310–110 kDa (P < 0.05). Although the smallest fragment size also trended downward, the average value failed to reach statistical significance.
Fig. 2.
A: agarose gel showing HA fragmentation. Duplicate left lung samples were run without or with hyaluronan lyase (HYAL) to quantify the amount of HA fragments present. Left lungs were harvested immediately after LPAL (0 h) and 4 h after LPAL. Brackets next to the HA ladder indicate the size range where pixel intensities were evaluated. B: agarose gel showing HA fragmentation as in A. Left lungs were harvested 4 h after LPAL from mice without or with N-acetyl-l-cysteine (NAC) pretreatment. C: comparison of average changes in HA fragments in 3 size ranges (495–310 kDa, 310–110 kDa, and 110–30 kDa), 0 h and 4 h after LPAL (n = 7–10 mice/group). A significant increase in fragmentation occurred by 4 h after LPAL (*P < 0. 5 from 0 h). Fragmentation in lungs from NAC-treated mice was substantially reduced compared with untreated mice 4 h after LPAL within the ranges 495–310 kDa and 310–110 kDa (#P < 0.05 from 4 h).
Hyaluronidase activity remains constant.
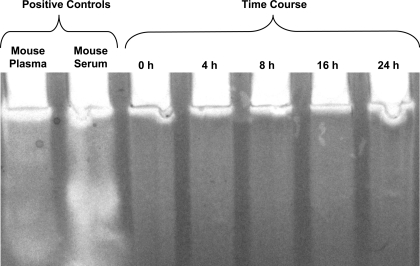
To determine whether changes in fragmentation were related to increased hyaluronidase activity, HA zymography was used to measure the activity of naturally occurring hyaluronidases in lung homogenate after LPAL. Figure 3 shows the results of a representative example from three separate experiments (n = 3 mice/time point). The activity of hyaluronidases remained unchanged over the time course from 0 h through 24 h.
Fig. 3.
Representative time course of hyaluronidase (HAdase) activity as measured by hyaluronan zymography. The activity of HAdase remained constant through the first 24 h after LPAL. Positive controls (mouse plasma and serum) show high levels of HAdase activity.
HA synthase expression increases after the onset of ischemia.
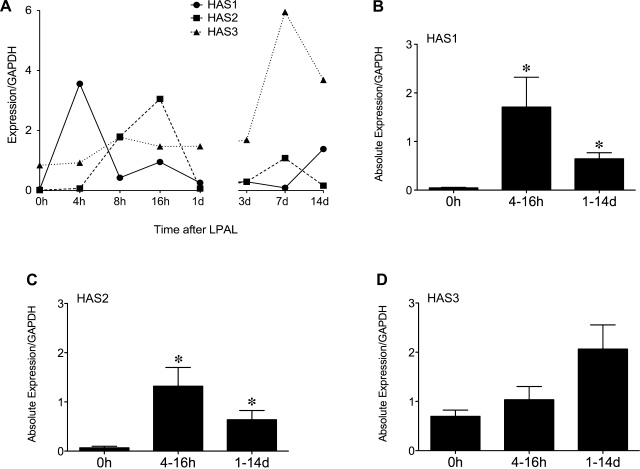
To determine whether synthesis of new HA also contributed to the increase in total HA, we examined the time course of changes in expression of the three HA synthases, HAS1, HAS2, and HAS3 using real-time RT PCR. Figure 4A shows the time course of changes in expression of HAS1, HAS2, and HAS3 normalized to GAPDH in a representative experiment (n = 2 mice/time point). HAS1 and HAS2 appear to show a maximum expression by 16 h after LPAL followed by relatively low expression levels at subsequent times. HAS3 showed a higher absolute baseline expression than HAS1 and HAS2 and an increased level of expression at later time points. On the basis of these observations and the variation in the exact time course profile in replicate experiments, we grouped expression data into 0 h (control), early (4–16 h), and late (1–14 days) expression. Group average data of absolute gene expression normalized to GAPDH are presented in Fig. 4, B–D. When grouped into early and late time responses after LPAL, both HAS1 and HAS2 showed significant increases compared with control (0 h; P < 0.01). because of very low control levels, these changes represented very large fold changes. Despite overall higher baseline expression levels, no changes in HAS3 were seen (Fig. 4D).
Fig. 4.
A: time course of HA synthase (HAS) expression by real-time RT-PCR after LPAL. A representative experiment showing the time course of HAS1, HAS2, and HAS3 expression, normalized to GAPDH (n = 2 mice/time point). HAS1 and HAS2 expression increased within the first 24 h after LPAL. HAS3 showed a greater initial expression; however, it did not change until late after LPAL (7 days). B: average HAS1 expression relative to GAPDH immediately after LPAL (0 h), early (4 h-16 h), and late (1 day-14 days) after LPAL (*P < 0.01 from 0 h; n = 8–14 mice/group). C: average HAS2 relative to GAPDH expression immediately after LPAL (0 h), early (4 h-16 h), and late (1 day-14 days) after LPAL (*P < 0.05 from 0 h; n = 9–15 mice/group). D: average HAS3 relative to GAPDH expression immediately after LPAL (0 h), early (4 h-16 h), and late (1 day-14 days) after LPAL (n = 5–16 mice/group).
LMW HA increases tube formation.
To confirm the angiogenic activity of LMW HA, pleural tissue was studied in vitro. Representative images from tube formation assays are presented in Fig. 5A. Cultures treated with LMW HA showed cells forming tubes radiating from tissue (right) whereas cultures treated with HMW HA showed only dispersed cells associated with tissue (left). Figure 5B shows average results from tube formation assays from pleural tissue cultures treated with vehicle, HMW HA, and LMW HA (n = 5). The sum of tube lengths in LMW HA-treated cultures was significantly greater than when HMW HA was delivered (P < 0.01). Vehicle-treated cultures resulted in an intermediate level of tube formation that was not statistically different from LMW HA or HMW HA. Additionally, the number of tubes after LMW HA treatment (34 ± 5 tubes) was significantly greater than in tissue treated with HMW HA (15 ± 1; P = 0.01). Staining cultures with an endothelial cell antibody showed both CD31+ and CD31− cells contributed to measured tubes.
Fig. 5.
A: representative images from tube formation assay showing the effects of high-molecular-weight (HMW) HA (4,000 kDa, left) with only a few isolated cells compared with cultures treated with low-molecular-weight (LMW) HA (130 kDa) showing tubes radiating from tissue (right). Small pieces (1 mm3) of the pleural surface from fresh lung tissue were harvested from naive lungs and plated on Matrigel with different-sized HA. After 6 days of incubation, images were obtained (Olympus IX51 microscope/SensiCam) and tube numbers per well were quantified. B: quantification of the length of tubes present in in vitro cultures of pleural tissue summed per well (each point indicates a separate experiment). LMW HA treatment showed significantly greater tube formation than HMW HA treatment (**P < 0.01).
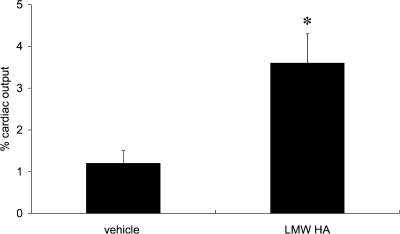
Increased angiogenesis after intratracheal LMW HA.
To determine the effects of additional LMW HA fragments on in vivo angiogenesis after LPAL, systemic blood flow to the left lung was determined. As seen in Fig. 6, blood flow to the left lung 14 days after LPAL was significantly greater in mice given additional intratracheal LMW HA fragments compared with vehicle-treated mice (P < 0.05).
Fig. 6.
Functional angiogenesis as assessed by blood flow (% cardiac output) to the left lung 14 days after LPAL. Mice received either PBS (40 μl) or endotoxin-free LMW HA (495–30 kDa; 40 μl of 2.3 mg/ml), delivered 4, 24, 48, and 72 h after LPAL by intratracheal aspiration. Angiogenesis was significantly greater in mice given additional LMW HA fragments compared with PBS-treated mice (*P < 0.05).
DISCUSSION
Changes in the HA size and content have been recognized as indexes of injury in the lung in models of pulmonary hypertension (Ref. 14, no. 1484), chronic obstructive pulmonary disease (Ref. 1, no. 1485), asthma (4), acute lung injury (8), and pulmonary fibrosis (11). The overall goal of the present study was to determine whether acute ischemia in the lung caused detectable changes in HA, a glycosaminoglycan critical to the structural integrity of the lung. Several other laboratories have demonstrated that ROS-induced injury in the lung results in fragmentation of HA (3, 4). Since our laboratory recently demonstrated that acute pulmonary ischemia in the mouse lung resulted in early increases in ROS (12), we questioned whether this stimulus was sufficient to result in the breakdown of HA. Our results demonstrated an early increase in LMW HA including a size range of fragments previously shown to elicit a range of inflammatory responses (2). Furthermore, antioxidant treatment of mice prior to the onset of ischemia resulted in a substantial reduction in the level of HA fragmentation. Our laboratory previously reported that antioxidant treatment lead to a decrease in ROS in the lung and a decrease in subsequent systemic angiogenesis (12). Therefore, we also questioned whether LMW HA fragments contributed to the process of neovascularization in the lung. Using two different angiogenesis assays, our results are consistent with the hypothesis that LMW HA contributes to the neovascularization that is part of the repair process after pulmonary ischemia.
Since measurements of HA content of the lung do not discriminate among different sizes of HA chains (5), specific evaluation of fragmentation was needed. We focused on fragment sizes shown previously to be increased after injury (495–30 kDa). On the basis of the initial time course (Fig. 2A) and previous estimates of maximum ROS release (12), and in advance of the significant change in total HA observed by the ELISA-like assay, we evaluated the lung 4 h after LPAL. Our results demonstrated a significant increase in each of the three size ranges, 495–310, 310–110, and 110–30 kDa of HA. Furthermore, since we have shown previously that antioxidant treatment limits ROS in the lung in this model, we evaluated fragmentation at the same time point in NAC-treated mice. Consistent with our hypothesis, results demonstrated a substantial attenuation in fragmentation. Additionally, these results are consistent with previous reports that ROS contribute to HA fragmentation in the lung (3, 4).
We also examined gene expression profiles of the HA synthases expecting that synthesis contributed to the increase in total HA content. These enzymes are membrane bound and, as the disaccharide chains are formed, they extend into the extracellular space. De novo synthesis of HA is through the activity of HA synthases (HAS1, HAS2, and HAS3), which differ in their production capacity. Itano and colleagues (7) have shown that HAS1 is the least active of the synthases and produces a wide range of sizes from 200 to 2,000 kDa. In contrast, HAS3 is the most active and produces HA of lower molecular weight (<1,000 kDa) whereas HAS2 produces the largest HA size chain with an average greater than 2,000 kDa. Our results showed an early (4–16 h) increase in HAS1 and HAS2 gene expression that may contribute to the overall increase in total HA content. Although HAS3 activity may contribute to the measured increase in HA fragments, it appears not to be induced by early events within the ischemic lung. Changes in the gene expression of HAS1 and HAS2 early after the onset of ischemia is suggestive of these synthases playing a role in the subsequent increase in total HA within the lung. Additional studies are required to confirm the precise role these enzymes play in the recovery of the lung from ischemic injury.
Another mechanism whereby fragmentation of lung HA might occur is through activation of any of the several hyaluronidases known to exist in the lung. Monzon and colleagues (10) showed that ROS can increase hyaluronidase 2 expression and activity in bronchial epithelial cells. However, we saw no change in activity in ischemic lung tissue when evaluating all hyaluronidases by zymography over a range of time points after the onset of ischemia. We concluded that the hyaluronidases are not activated by the ischemic status of the lung and that these enzymes appear unlikely to contribute to the large increase in fragmentation observed 4 h after LPAL.
Within the field of angiogenesis, HA has been studied both as an endothelial cell proliferative agonist as well as an essential biomaterial for therapeutic neovascularization for peripheral artery occlusive disease (20). LMW HA has been shown to have direct stimulatory effects on endothelial cells leading to increased cell proliferation and vessel formation (22, 23). However, others have shown the most effective are very short chains (3–10 disaccharide units; <6.5 kDa) referred to as oligosaccharides of HA (18). Using a tube formation assay, we demonstrated clear differences in pleural tissue cultures exposed to LMW HA (130 kDa) and HMW HA. Our laboratory previously showed this assay system to be useful to assess growth factors important to the process of endothelial cell tube formation (9). Furthermore, histological assessment of the ischemic lung after LPAL demonstrated vascular structures emanating to and from the pleural surface (21). In the present study we evaluated tube formation of endothelial cells originating in pleural tissue in culture medium with LMW HA and HMW HA. LMW HA (130 kDa) promoted significantly greater tube formation than HMW HA. This size (130 kDa), although found to increase significantly 4 h after LPAL, is considerably larger than what others have shown demonstrates angiogenic properties (18). In this experiment, we compared HMW HA with LMW HA, although HMW HA could also have been providing an angiostatic stimulus. HMW HA has been shown to be angiostatic, suppressing endothelial cell proliferation and disrupting endothelial cell monolayers (23). Consequently, the differences observed could be the result of the angiostatic properties of HMW HA as opposed to the proangiogenic properties of LMW HA. The vehicle control showed an intermediate level of tube formation.
We also tested our in vivo model after LPAL to determine whether intratracheal delivery of LMW HA fragments would stimulate neovascularization of the left lung. Delivery of HMW HA was technically difficult because of its high viscosity. Consequently, a PBS vehicle control was used for comparison. LMW HA increased the level of functional angiogenesis as assessed by blood flow to the left lung 14 days after LPAL. Thus both models were consistent in showing that LMW HA fragments increased properties of angiogenesis in lung tissue.
The mechanisms responsible for the effects of LMW HA in promoting angiogenesis in this model can only be speculated upon. Specific endothelial cell mechanisms are thought to be related to the binding of very-low-molecular-weight HA (<6.5 kDa) to CD44 and RHAMM and activating proliferation, migration, and tube formation (16, 18). Since we used larger size fragments shown to be present in the lung 4 h after LPAL, it is unclear whether these directly stimulated endothelial cells in the lung to form tubular structures that connect with the intercostal arteries that eventually invade the lung. However, HA fragments of the same size we used have been shown to induce cytokine release from inflammatory cells (2). Since this model of systemic angiogenesis has been shown to be dependent on chemokine growth factors (15, 19), the effects of HA fragments may be predominantly indirect, stimulating the release of growth factors that promote neovascularization. Additional studies are required to determine the precise mechanism by which LMW HA (130 kDa) exerts a proangiogenic outcome.
In summary, we have shown that left lung ischemia leads to an increase in total HA early after LPAL that persists for several days. This increase was due to both an early increase in HA fragmentation as well as an increase in HA synthase expression, but not due to a change in hyaluronidase activity. Furthermore, antioxidant pretreatment of mice before LPAL resulted in a decrease in fragmentation, suggesting that ROS released during acute ischemia contribute to HA fragmentation. LMW HA fragments delivered to the lung after LPAL as well as within an in vitro tube formation assay both showed enhanced angiogenesis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL071605.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, Cleutjens J, van Suylen RJ, Joos GF, Brusselle GG, Wouters EF. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am J Respir Cell Mol Biol 42: 753–761, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Eberlein M, Scheibner KA, Black KE, Collins SL, Chan-Li Y, Powell JD, Horton MR. Anti-oxidant inhibition of hyaluronan fragment-induced inflammatory gene expression. J Inflamm (Lond) 5: 20–30, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, Oury TD. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem 283: 6058–6066, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 5. Haserodt S, Aytekin M, Dweik RA. A comparison of the sensitivity, specificity, and molecular weight accuracy of three different commercially available Hyaluronan ELISA-like assays. Glycobiology 21: 175–183, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology 15: 1131–1139, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274: 25085–25092, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Moldobaeva A, Baek A, Eldridge L, Wagner EM. Differential activity of pro-angiogenic CXC chemokines. Microvasc Res 80: 18–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J Biol Chem 285: 26126–26134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nettelbladt O, Tengblad A, Hallgren R. High-dose corticosteroids during bleomycin-induced alveolitis in the rat do not suppress the accumulation of hyaluronan (hyaluronic acid) in lung tissue. Eur Respir J 3: 421–428, 1990 [PubMed] [Google Scholar]
- 12. Nijmeh J, Moldobaeva A, Wagner EM. Role of ROS in ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol 299: L535–L541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc 3: 401–404, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ormiston ML, Slaughter GR, Deng Y, Stewart DJ, Courtman DW. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 298: L148–L157, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Sánchez J, Moldobaeva A, McClintock J, Jenkins J, Wagner E. The role of CXCR2 in systemic neovascularization of the mouse lung. J Appl Physiol 103: 594–599, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 276: 36770–36778, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 299: L639–L651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 26: 58–68, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Srisuma S, Biswal SS, Mitzner WA, Gallagher SJ, Mai KH, Wagner EM. Identification of genes promoting angiogenesis in mouse lung by transcriptional profiling. Am J Respir Cell Mol Biol 29: 172–179, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Tang ZC, Liao WY, Tang AC, Tsai SJ, Hsieh PC. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials 32: 75–86, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Wagner EM, Petrache I, Schofield B, Mitzner W. Pulmonary ischemia induces lung remodeling and angiogenesis. J Appl Physiol 100: 587–593, 2006 [DOI] [PubMed] [Google Scholar]
- 22. West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 228: 1324–1326, 1985 [DOI] [PubMed] [Google Scholar]
- 23. West DC, Kumar S. Endothelial cell proliferation and diabetic retinopathy. Lancet 1: 715–716, 1988 [DOI] [PubMed] [Google Scholar]