Abstract
Intermittent hypoxia (IH) resulting from sleep apnea causes both systemic and pulmonary hypertension. Enhanced endothelin-1 (ET-1)-induced vasoconstrictor reactivity is thought to play a central role in the systemic hypertensive response to IH. However, whether IH similarly increases pulmonary vasoreactivity and the signaling mechanisms involved are unknown. The objective of the present study was to test the hypothesis that IH augments ET-1-induced pulmonary vasoconstrictor reactivity through a PKCβ-dependent signaling pathway. Responses to ET-1 were assessed in endothelium-disrupted, pressurized pulmonary arteries (∼150 μm inner diameter) from eucapnic-IH [(E-IH) 3 min cycles, 5% O2-5% CO2/air flush, 7 h/day; 4 wk] and sham (air-cycled) rats. Arteries were loaded with fura-2 AM to monitor vascular smooth muscle (VSM) intracellular Ca2+ concentration ([Ca2+]i). E-IH increased vasoconstrictor reactivity without altering Ca2+ responses, suggestive of myofilament Ca2+ sensitization. Consistent with our hypothesis, inhibitors of both PKCα/β (myr-PKC) and PKCβ (LY-333-531) selectively decreased vasoconstriction to ET-1 in arteries from E-IH rats and normalized responses between groups, whereas Rho kinase (fasudil) and PKCδ (rottlerin) inhibition were without effect. Although E-IH did not alter arterial PKCα/β mRNA or protein expression, E-IH increased basal PKCβI/II membrane localization and caused ET-1-induced translocation of these isoforms away from the membrane fraction. We conclude that E-IH augments pulmonary vasoconstrictor reactivity to ET-1 through a novel PKCβ-dependent mechanism that is independent of altered PKC expression. These findings provide new insights into signaling mechanisms that contribute to vasoconstriction in the hypertensive pulmonary circulation.
Keywords: sleep apnea, pulmonary hypertension, Ca2+ sensitization, Rho kinase
sleep apnea (SA) is a common disorder affecting an estimated one in five adults in the United States that is characterized by frequent nocturnal episodes of apnea, leading to nighttime intermittent hypoxia (IH) (48). SA increases the incidence of cardiovascular complications such as heart failure, stroke, and systemic hypertension (13, 39). In addition, SA is a risk factor for pulmonary hypertension (PH) and can exacerbate PH in patients with chronic obstructive pulmonary disease (11, 15). Thus understanding the etiology of PH associated with SA is crucial to identifying effective therapeutic strategies to treat this condition.
Although mechanisms leading to SA-induced PH remain poorly characterized, enhanced vasoconstrictor sensitivity to endogenous agonists such as ET-1 may provide a contributing influence. Indeed, several studies have identified an association between SA and increased circulating ET-1, suggesting that ET-1 contributes to cardiovascular disease in this setting (34, 38). Consistent with this possibility, we have previously reported that rats exposed to eucapnic-IH (E-IH) as a model of SA display increased circulating ET-1 levels and ET-1-dependent systemic hypertension (2, 25). In addition, E-IH increases ET-1-induced constriction of systemic arteries via PKCδ-dependent vascular smooth muscle (VSM) Ca2+ sensitization, which may account for elevated vascular resistance and resultant hypertension in this model (3).
Whereas effects of E-IH on pulmonary vasoreactivity to ET-1 are unknown, recent studies from our laboratory have demonstrated greater vasoconstrictor sensitivity to the thromboxane analog U-46619 in isolated lungs from E-IH rats compared with controls (41). Interestingly, PH resulting from chronic sustained hypoxia has been attributed in part to enhanced vasoconstrictor responsiveness to receptor-mediated agonists, including ET-1 (17, 23). However, in contrast to effects of E-IH to augment PKCδ-dependent vasoconstriction in the systemic circulation (3), greater ET-1-mediated vasoconstriction in the pulmonary circulation of chronically hypoxic rats is mediated by a distinct myofilament Ca2+ sensitization pathway involving RhoA/Rho kinase (ROCK) signaling (8, 23, 47).
The goal of the present study was to identify VSM signaling mechanisms responsible for E-IH-mediated increases in pulmonary vasoconstrictor reactivity by using an isolated, pressurized small pulmonary artery preparation. Our findings demonstrate a novel effect of IH to augment ET-1-induced pulmonary vasoreactivity through a mechanism dependent on PKCβ but independent of either ROCK or PKCδ. Furthermore, this response to IH appears to be a function of increased PKCβ activity rather than altered PKC expression.
MATERIALS AND METHODS
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center (Albuquerque, NM).
Experimental Groups
Age-matched male Wistar rats (∼250–350 g; Harlan Industries) were housed in Plexiglas cages with free access to food and water and exposed to either IH with CO2 supplementation (3 min cycles of 5% O2-5% CO2/air flush) or sham conditions for 7 h/day for 4 wk as described previously (41). This method of CO2 supplementation maintains normal arterial Pco2 and pH during hypoxic episodes (41) and is therefore termed E-IH. We have previously demonstrated evidence for both PH (41) and systemic hypertension characteristic of SA in this model of E-IH (4, 25).
Measurement of Arterial Blood Gases
To assess whether chronic acid-base adjustments occur as a result of E-IH exposure, we measured arterial blood gases at the end of 4 wk of E-IH treatment during cycling conditions in conscious, chronically instrumented rats as described previously (41). Three arterial blood samples were drawn to measure arterial blood gases via an ABL5 blood-gas analyzer (Radiometer): the first before cycling began for the day, the second during the nadir or most hypoxic portion of the cycling period (∼5% O2-5% CO2), and the third at the peak of the air flush (∼20% O2-0% CO2).
Assessment of RV Weight and Hematocrit
Blood samples were obtained by direct cardiac puncture at the time of lung isolation for measurement of hematocrit. Right ventricular (RV) hypertrophy was assessed as an index of pulmonary hypertension, as previously described (36, 37). The degree of RV hypertrophy is expressed as the ratio of RV to total ventricle weight (T) and as the ratio of RV to body weight (BW).
Isolated Small Pulmonary Artery Experiments
To characterize effects of E-IH on agonist-induced pulmonary vasoreactivity and VSM Ca2+ mobilization, small pulmonary arteries (4th to 5th order) were isolated from the left lungs of sham and E-IH rats, cannulated, and pressurized as previously described (9, 21, 40). Inner diameter (ID) and 340/380 emission ratios (F340/F380) were measured simultaneously to assess vasoconstriction and changes in VSM intracellular Ca2+ concentration ([Ca2+]i). All arteries in these and subsequent protocols were studied at a transmural pressure of 12 Torr and were endothelium disrupted, by rubbing the lumen with a strand of moose mane, to eliminate influences of endothelium-derived factors on E-IH-dependent changes in VSM function. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to ACh (1 μmol/l) in UTP (5 μmol/l)-constricted vessels as we have previously reported (9, 19, 21, 22, 40).
Effect of E-IH on endothelin-1- and KCl-induced vasoconstrictor and VSM [Ca2+]i responses: roles of Rho kinase and PKC.
Both ID and VSM [Ca2+]i responses to increasing concentrations of ET-1 (10−11 to 10−7 M) were assessed in endothelium-disrupted pulmonary arteries from sham and E-IH rats in the presence of either the ROCK inhibitor fasudil (10 μmol/l, LC Laboratories), the general PKC inhibitor Ro 31-8220 (5 μmol/l, Biomol), the PKCδ inhibitor rottlerin (3 μmol/l, Biomol), the cell-permeable myristoylated PKCα/β pseudosubstrate inhibitor [20–28] (myr-PKC; 10 μmol/l, Biomol), the selective PKCβ inhibitor LY-333-531 (10 nmol/l, Enzo, also known as ruboxistaurin), or their respective vehicles. We have previously demonstrated that this concentration of fasudil inhibits ROCK-dependent constriction in this preparation (9, 23). To determine the efficacy of Ro 31-8220, rottlerin, and myr-PKC to inhibit PKC, we additionally assessed vasoconstrictor responses to the PKC activator 12-myristate 13-acetate (PMA) in the presence and absence of each inhibitor. In parallel protocols, we performed concentration-response curves to KCl (18–100 mmol/l) in isolated arteries from each group of rats to evaluate effects of E-IH on vasoconstrictor responsiveness to a depolarizing stimulus.
In situ calibrations of VSM [Ca2+]i.
In situ calibrations for VSM Ca2+ were performed in pressurized arteries from sham and E-IH rats by methods similar to those previously described (27, 40). Following each experiment, as described above, the VSM was permeabilized to Ca2+ with 10 μmol/l ionomycin, and the arteries were incubated with a bath solution containing 140 mmol/l KCl, 5 mmol/l NaCl, 5 mmol/l HEPES, 1 mmol/l MgCl2, 5 μmol/l nigericin, and either 5 mmol/l EGTA (low-Ca2+ solution) or 2 mmol/l CaCl2 (high-Ca2+ solution), with a pH of 7.15 at 37°C until nadir and peak ratios were achieved. Calibrations were performed in the presence of each drug used in the study.
Effects of E-IH on Pulmonary Arterial PKC Expression
Real-time PCR.
Intrapulmonary arteries (∼2nd to 5th order; endothelium intact) from the left lungs of Wistar rats were dissected from accompanying airways and surrounding lung tissue in PSS then stored in RNA Later (Ambion). Total RNA was isolated by using the RNEasy Fibrous Mini Kit (Qiagen) according to the manufacturer's protocol. The High Capacity Reverse Transcription kit (Applied Biosystems) was used for reverse transcription of total RNA to cDNA. For real-time PCR detection, Taqman gene expression assay kits were used for rat PKCα and β. β-Actin was used as endogenous control and detection was performed by using an Applied Biosystems 7500 Real-Time PCR System. The normalized gene expression method (2−ΔΔCT) for relative quantification of gene expression was used.
Western blotting.
Expression of classical PKC isoforms was measured by Western blot using antibodies specific for PKCα, PKCβI and PKCβII, using a protocol similar to previously published work (23). To obtain sufficient tissue for analysis, intrapulmonary arteries (∼2nd to 5th order; endothelium intact) were isolated from the right and left lungs of sham and E-IH rats in HEPES-based PSS (in mmol/l, 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 1.8 CaCl2, 10 HEPES, 1.18 KH2PO4, 6 glucose, and 0.03 EDTA, pH adjusted to 7.4 with NaOH, all from Sigma) as described previously (20, 21, 41).
Pulmonary artery lysates (25 μg/lane) were separated by SDS-PAGE (7.5% Tris·HCl gels, Bio-Rad) and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h at room temperature with 2.5% BSA and 2.5% milk in 0.05% Tween 20 (Bio-Rad) Tris-buffered saline (T-TBS). Blots were then incubated overnight at 4°C with monoclonal antibodies to PKCα or PKCβI, or a polyclonal antibody raised against PKCβII (1:200, Santa Cruz). For immunochemical labeling, blots were incubated 1 h at room temperature with goat anti-mouse (PKCα/βI) or goat anti-rabbit (PKCβII) IgG-horseradish peroxidase (1:3,000, Bio-Rad). After chemiluminescence labeling (ECL, Amersham), PKCα/βI/βII bands were detected by exposing the blots to chemiluminescence-sensitive film (Kodak). Quantification of the bands was achieved by densitometric analysis of scanned images (SigmaGel software, SPSS). Control experiments for each antibody were conducted using increasing concentrations of protein to ensure linearity of the densitometry curve. Bands for PKC α/βI/βII were normalized to total protein (Coomassie staining). Preliminary experiments revealed a linear relationship between protein loading and Coomassie staining band density (Supplemental Fig. S1; the online version of this article contains supplemental data), thus demonstrating the utility of this stain for protein normalization.
Effects of E-IH on PKC Membrane Localization
Because PKC activity is generally reflected by changes in membrane-association of the enzyme (31, 35, 45), the effect of ET-1 on levels of membrane-localized PKCα, βI, or βII were assessed in intrapulmonary arteries from sham and E-IH rats by Western blotting (3, 43). Intrapulmonary arteries were isolated as above and placed in 1 ml of HEPES buffer with or without 10−8 mol/l ET-1 present for 8 min at 37°C, then snap frozen and later homogenized as described above. Lysates were then centrifuged at 800 g for 10 min at 4°C to remove the nuclear fraction and cellular debris. The supernatant was then spun in an ultracentrifuge at 100,000 g for 1 h at 4°C to separate the microsomal (pellet) and cytosolic (supernatant) fractions. A Pierce BCA protein assay was then performed by use of a plate reader. Microsomal (membrane-enriched) and cytosolic samples were loaded (10 μg/lane) onto 7.5% Tris·HCl gels, and the Western blotting procedure was performed as above, with each blot containing paired membrane and cytosol samples from each group-treatment combination. For PKCβI/βII an Odyssey Imaging System from Li-cor Biosciences and corresponding secondary antibodies were used.
Effects of E-IH on ET-1-Induced MLC Phosphorylation
ET-1 may signal via PKC to cause VSM contraction by increasing myosin light chain (MLC) monophosphorylation (p-MLC) at Ser19 (42). Additionally, kinases such as zipper interacting protein kinase and integrin-linked kinase can diphosphorylate the MLC (pp-MLC) at Ser19 and Thr18, and this may occur downstream of PKC to mediate contraction (12). Therefore, we assessed effects of ET-1 on levels of p-MLC and pp-MLC in arteries from each group by Western blotting using a protocol adapted from Walsh et al. (46). Briefly, intrapulmonary arteries were isolated as described above, subjected to treatment with either 10−8 mol/l ET-1 or saline for 8 min at 37°C, and then immersed in an ice-cold solution of trichloroacetate (10%) and dithiothreitol (10 mmol/l) in acetone. Samples were lyophilized for 24–48 h, and proteins were separated by SDS-PAGE (12% Mini-Protean TGX gels, Bio-Rad) and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% milk in T-TBS and incubated with primary antibodies for p-MLC (1:5,000, Abcam) or pp-MLC (1:1,000, Cell Signaling) overnight at 4°C. Blots were incubated with anti-rabbit secondary antibodies (1:5,000) conjugated to IR800 dyes (Licor) and imaged on an Odyssey Infrared Imaging System. Blots were then stripped of primary and secondary antibodies, reblocked, and reprobed for total MLC (rabbit polyclonal, 1:200, Santa Cruz).
Calculations and Statistical Analysis
Vasoconstrictor responses were calculated as a percent of baseline ID (following pharmacological inhibitor administration) for all experiments. VSM [Ca2+]i is expressed as the background-subtracted ratios of fluorescence (F) emitted following excitation at 340 and 380 nm (F340/F380). All data are expressed as means ± SE, and values of n refer to the number of animals in each group. A t-test, one-way ANOVA, or two-way ANOVA was used to make comparisons when appropriate. A general linear model was used to make comparisons between vasoconstriction vs. [Ca2+]i. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. A probability of P < 0.05 was accepted as statistically significant for all comparisons.
RESULTS
Effects of E-IH on Arterial Blood Gases, RV Weight, and Hematocrit
Prior to the first hypoxic stimulus on the final day of treatment, arterial Po2, Pco2, and pH values (Table 1) reflected normal values observed before E-IH exposure (41), indicating that E-IH does not chronically alter blood gases. As previously reported, inhaled CO2 prevented the development of hypocapnia during hypoxic exposure, resulting in the maintenance of normal pH during the complete cycle (41).
Table 1.
Arterial blood gas data in E-IH rats during cycling
| Parameter | Pre | Nadir | Post | n |
|---|---|---|---|---|
| Po2, Torr | 69.7 ± 0.9 | 38.0 ± 7.6* | 71.0 ± 1.7 | 4 |
| Pco2, Torr | 31.3 ± 1.8 | 26.3 ± 1.5 | 27.7 ± 0.7 | 3 |
| pH | 7.47 ± 0.040 | 7.47 ± 0.020 | 7.48 ± 0.009 | 3 |
Arterial Po2, Pco2, and pH following 4 wk eucapnic intermittent hypoxia (E-IH) exposure. Measurements were obtained in conscious rats before (Pre), during (Nadir), and after (Post) 1 E-IH cycle. Values are means ± SE; n= number of rats.
P < 0.05 vs. respective precycling value.
E-IH-treated rats developed RV hypertrophy as indicated by greater ratios of RV/BW and RV/T compared with sham animals (Table 2). However, ratios of left ventricle + septum to BW and hematocrit values were similar between sham and E-IH rats (Table 2),demonstrating that E-IH does not induce significant left ventricular hypertrophy or polycythemia, as previously reported (41). In addition, age-matched E-IH rats weighed significantly less than sham animals after 4 wk of exposure (Table 2), suggesting that E-IH decreases growth rate.
Table 2.
Heart and body weights in sham and E-IH rats
| Group | PreBW, g | PostBW, g | RV/T, g/g | RV/BW, g/kg | LV+S/BW, g/kg | Hct, % | n |
|---|---|---|---|---|---|---|---|
| Sham | 253 ± 11 | 392 ± 10* | 0.220 ± 0.007 | 0.531 ± 0.014 | 1.91 ± 0.04 | 42 ± 1 | 7 |
| E-IH | 244 ± 12 | 324 ± 12*† | 0.244 ± 0.008‡ | 0.662 ± 0.048‡ | 2.03 ± 0.07 | 45 ± 1 | 8 |
Values are means ± SE; n = number of rats. PreBW and PostBW, body weight (BW) values before and after, respectively, exposure to sham or E-IH; RV, right ventricular weight; T, total ventricular weight; LV+S, left ventricle + septum weight.
P < 0.05 vs. preexposure value.
P < 0.05 vs. sham postexposure BW.
P < 0.05 vs. sham.
E-IH Augments ET-1-Induced Vasoconstriction But Not the Global VSM [Ca2+]i Response
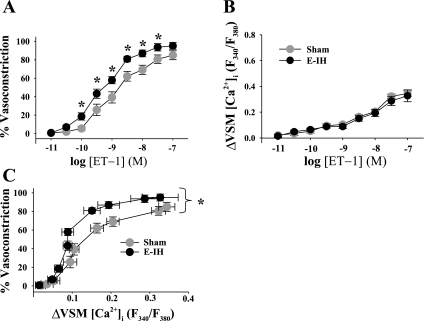
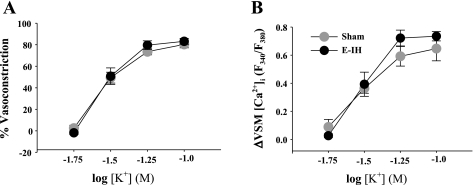
Basal ID and VSM [Ca2+]i were similar between groups (Supplemental Table S1). E-IH augmented vasoconstrictor reactivity to ET-1 (Fig. 1A) without altering the VSM [Ca2+]i response (Fig. 1B). A plot of vasoconstriction to ET-1 as a function of the VSM [Ca2+]i response reveals a left-shifted curve in the E-IH vs. the sham group (Fig. 1C), indicating greater vasoconstriction for a given change in VSM [Ca2+]i following E-IH. In contrast, vasoconstrictor and VSM [Ca2+]i responses to depolarizing concentrations of KCl were similar between arteries from sham and E-IH rats (Fig. 2, A and B). These findings suggest that E-IH selectively enhances receptor-mediated vasoconstrictor reactivity through myofilament Ca2+ sensitization.
Fig. 1.
Eucapnic intermittent hypoxia (E-IH) increases endothelin-1 (ET-1) concentration ([ET-1])-dependent vasoconstriction without a change in the global vascular smooth muscle (VSM) intracellular Ca2+ concentration ([Ca2+]i) response. Vasoconstrictor responses (percent baseline inner diameter) (A) and changes in VSM [Ca2+]i [ratio of fluorescence (F) (B) emitted following excitation at 340 and 380 nm (F340/F380)] to ET-1 in endothelium-disrupted, pressurized pulmonary arteries from sham (n = 6) and E-IH (n = 7) rats. C: vasoconstrictor responses to ET-1 plotted as a function of the change (Δ) in VSM [Ca2+]i. Each data point corresponds to 1 concentration of ET-1, as represented in A and B. Data are means ± SE. *P < 0.05 E-IH vs. sham.
Fig. 2.
E-IH does not alter vasoconstrictor (A) or VSM [Ca2+]i (B) responses to depolarizing concentrations of KCl in pulmonary arteries from sham and E-IH rats. Data are means ± SE of n = 5/group. There are no significant differences.
Greater ET-1-Mediated Vasoconstrictor Responsiveness Following E-IH Is Independent of ROCK
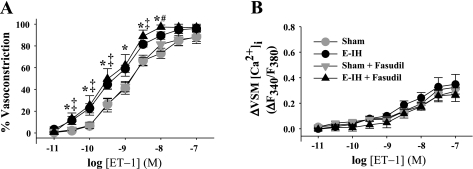
Vasoconstrictor (Fig. 3A) and VSM [Ca2+]i responses (Fig. 3B) to ET-1 were unaltered by ROCK inhibition with fasudil (10 μmol/l) in arteries from either sham or E-IH rats. Basal ID and VSM [Ca2+]i were not significantly different between arteries pretreated with fasudil and vehicle (Supplemental Table S1). Therefore, ROCK does not appear to contribute to augmented vasoconstrictor reactivity to ET-1 following E-IH.
Fig. 3.
Rho kinase (ROCK) does not mediate enhanced ET-1-induced vasoconstriction following E-IH. Vasoconstrictor (A) and VSM [Ca2+]i (B) responses to ET-1 in pulmonary arteries from sham and E-IH rats in the presence and absence of the ROCK inhibitor fasudil (10 μmol/l). Data are means ± SE of n = 6–9/group. *P < 0.05 E-IH vs. sham; #P < 0.05 E-IH vs. E-IH + fasudil; ‡P < 0.05 sham + fasudil vs. E-IH + fasudil.
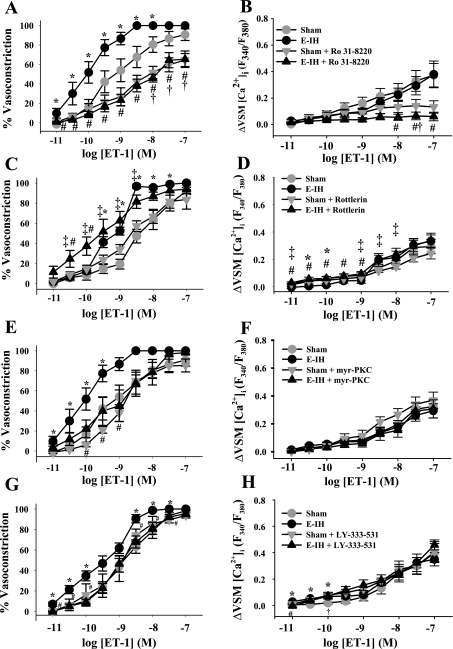
Enhanced Vasoreactivity to ET-1 After E-IH Is Mediated by PKCβ
The general PKC inhibitor Ro 31-8220 (5 μmol/l) attenuated constriction of pulmonary arteries to the PKC activator PMA (Supplemental Fig. S2A); therefore, this concentration was used for subsequent experiments. Ro 31-8220 attenuated constriction to ET-1 in vessels from both sham and E-IH rats and normalized responses between groups (Fig. 4A). In addition, Ro 31-8220 blunted Ca2+ responses to ET-1 in both sham and E-IH arteries (Fig. 4B).
Fig. 4.
PKCβ mediates enhanced vasoconstrictor reactivity to ET-1 following E-IH. Vasoconstrictor (A, C, E, G) and VSM [Ca2+]i (B, D, F, H) responses to ET-1 in the presence and absence of the general PKC antagonist Ro 31-8220 (5 μmol/l) (A and B), the PKCδ antagonist rottlerin (3 μmol/l) (C and D), the selective PKCα/β inhibitor myr-PKC (10 μmol/l) (E and F), or the selective PKCβ inhibitor LY-333-531 (10 nmol/l) (G and H). Data are means ± SE of n = 4–9/group. *P < 0.05 E-IH vs. sham; #P < 0.05 E-IH vs. E-IH + drug; †P < 0.05 sham vs. sham + drug, ‡P < 0.05 E-IH + drug vs. sham + drug.
Given that enhanced ET-1-induced Ca2+ sensitization following E-IH in systemic arteries is mediated by PKCδ (3), we next sought to determine the contribution of this novel PKC isoform to the observed increase in ET-1-induced pulmonary vasoconstriction following E-IH. However, PKCδ inhibition with rottlerin (3 μmol/l) did not decrease vasoconstrictor reactivity to ET-1 in arteries from E-IH rats (Fig. 4C). Rather, constriction was significantly greater in E-IH vessels in the presence of rottlerin (at 10−10.5 to 10−10 mol/l ET-1), and this was associated with a slightly but significantly greater Ca2+ response at lower concentrations of ET-1 (Fig. 4D), indicating a possible role for PKCδ to antagonize agonist-induced VSM Ca2+ mobilization after E-IH. This same concentration of rottlerin attenuated PMA-induced constriction in control arteries (Supplemental Fig. S2B), demonstrating the efficacy of this compound to inhibit PKC in this preparation. These results suggest that PKCδ does not mediate enhanced ET-1-induced vasoconstriction following E-IH.
To determine whether the observed increase in ET-1-induced, PKC-dependent vasoconstriction following E-IH is mediated by classical PKC isoforms, we measured ET-1-induced vasoconstrictor and Ca2+ responses in the presence of myr-PKC, which is selective for both PKCα and β isoforms. To validate the concentration of myr-PKC used in the present study, we assessed the effect of myr-PKC (10 μmol/l) on vasoconstrictor responsiveness to PMA in control arteries. PMA-induced vasoconstriction was significantly attenuated by this concentration of myr-PKC (Supplemental Fig. S2C). PKCα/β inhibition decreased ET-1-induced vasoconstriction in vessels from E-IH rats but was without effect on responses in arteries from sham rats (Fig. 4E). In addition, PKCα/β inhibition normalized vasoreactivity between sham and E-IH groups. Ca2+ responses to ET-1 were unaltered by myr-PKC in either group (Fig. 4F), suggesting that PKCα and/or β isoforms contribute to elevated ET-1-induced Ca2+ sensitization after E-IH.
To further investigate a role for PKCβ in enhanced ET-1-induced constriction following E-IH, we assessed responses to ET-1 in the presence of the selective PKCβ inhibitor LY-333-531 (10 nmol/l) or its vehicle. Similar to effects of myr-PKC, LY-333-531 blunted vasoconstrictor responses to ET-1 only in vessels from E-IH rats and normalized constriction between sham and E-IH groups (Fig. 4G), while having little effect on ET-1-induced Ca2+ mobilization. In the presence of the vehicle for LY-333-531, Ca2+ responses were slightly but significantly elevated in arteries from E-IH rats compared with those from sham rats at the lower concentrations of ET-1 (Fig. 4H). These results indicate a primary role for PKCβ in mediating enhanced ET-1 induced vasoconstriction following E-IH.
There were no differences in basal ID between groups, with the exception that E-IH vessels treated with DMSO (vehicle for LY-333-531) had a greater ID than those of the corresponding sham group (Supplemental Table S1). Basal VSM [Ca2+]i (F340/F380) was also similar between sham and E-IH for all drugs and vehicles, except in the presence of the vehicle for rottlerin (ethanol), in which sham arteries exhibited greater basal VSM [Ca2+]i.
In situ fura-2 calibrations were additionally performed to evaluate potential autofluorescence of drugs or effects of drugs on the affinity of fura-2 for Ca2+. Neither Ro 31-8220, myr-PKC, LY-333-531, nor rottlerin altered in situ calibration values (Supplemental Table S2). However, in the presence of DMSO, E-IH arteries displayed low calibration values that were greater than those of sham vessels. Furthermore, in the presence of LY-333-531, low calibration values in arteries from E-IH rats were significantly less than those of sham controls.
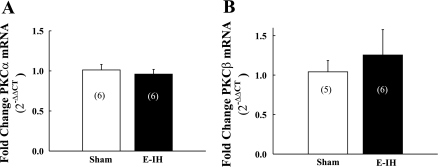
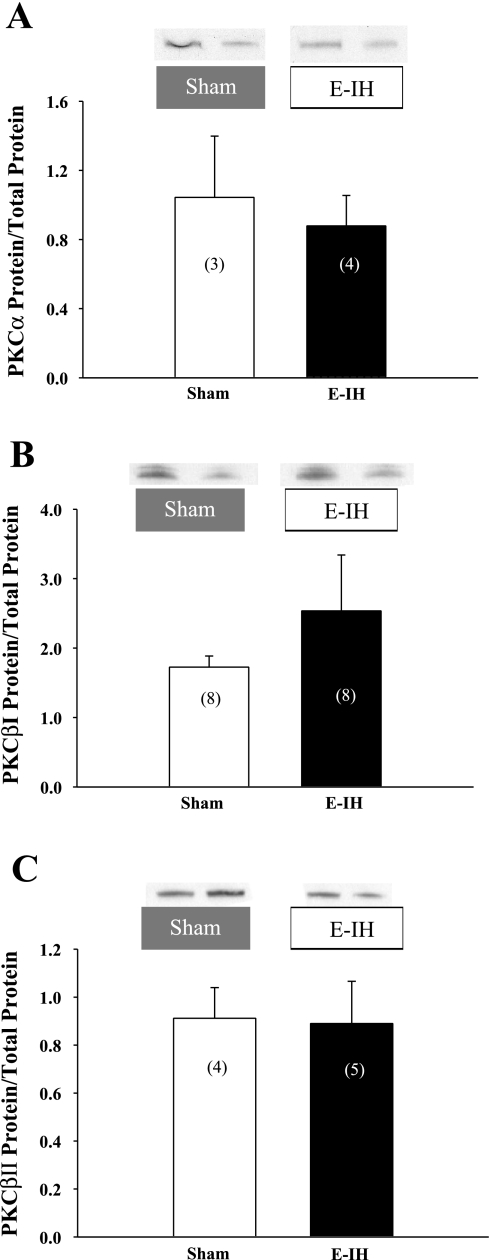
E-IH Does Not Alter Pulmonary Arterial PKC mRNA and Protein Expression
On the basis of our findings that E-IH augments vasoconstrictor reactivity to ET-1 through stimulation of a classical PKC isoform, we sought to determine whether E-IH increases PKC expression to mediate this response. However, E-IH was without effect on either PKCα or PKCβ mRNA levels (Fig. 5, A and B) as assessed by real-time PCR. Consistent with these results, E-IH did not significantly alter pulmonary arterial PKCα, βI, or βII protein expression (Fig. 6, A–C), as determined by Western blot analysis. Therefore, greater ET-1-induced vasoconstrictor responsiveness following E-IH exposure is likely mediated by an increase in PKC activity independent of changes in gene or protein expression.
Fig. 5.
E-IH does not alter pulmonary arterial PKCα or β mRNA levels. PKCα (A) and PKCβ (B) mRNA levels determined by real-time PCR in intrapulmonary arteries from sham and E-IH rats. Data are means ± SE. Numbers of animals are indicated in bars. There are no significant differences.
Fig. 6.
E-IH does not alter PKCα, βI, or βII protein levels in intrapulmonary arteries. Western blot data for PKCα (A), PKCβI (B), and PKCβII (C) in intrapulmonary arteries from sham and E-IH rats. Blots were normalized to Coomassie-stained protein (total protein). Data are means ± SE. Numbers of animals are indicated in bars. There are no significant differences.
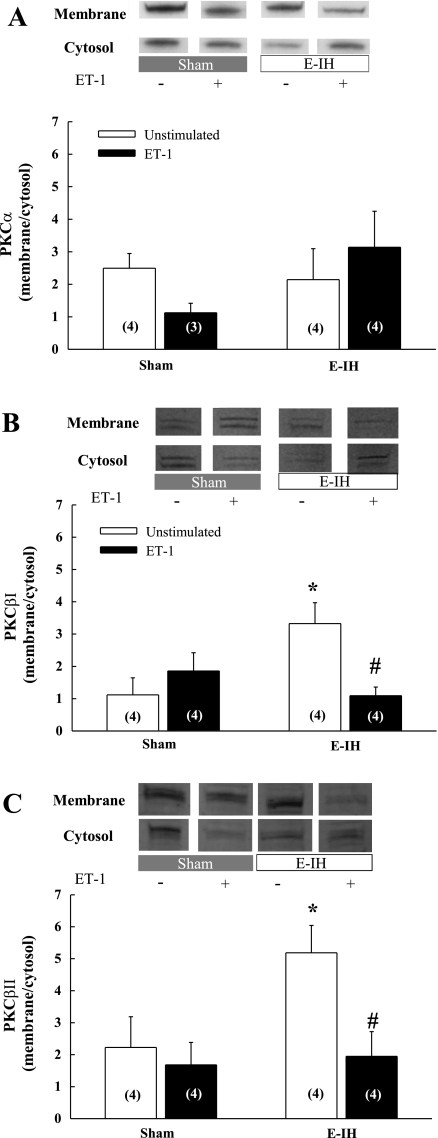
E-IH Increases Basal Levels of Membrane-Associated PKCβ and Promotes ET-1-Mediated Translocation of PKCβ from the Membrane Fraction
There were no differences in the ratio of basal membrane to cytosol PKCα levels between sham and E-IH arteries and there was no significant effect of ET-1 on PKCα membrane localization in either group (Fig. 7A). In contrast, E-IH increased membrane association of PKCβI and βII under basal conditions (Fig. 7, B and C). Furthermore, ET-1 selectively decreased membrane-localized PKCβI and βII in arteries from E-IH rats, while having no effect in the sham group.
Fig. 7.
E-IH increases membrane-localized PKCβI and βII in intrapulmonary arteries under basal conditions, and ET-1 stimulates translocation of PKCβI and βII from the membrane only in arteries from E-IH rats. Membrane/cytosol expression of PKCα (A), PKCβI (B), and PKCβII (C) in intrapulmonary arteries from sham and E-IH rats determined by Western blot. Bands were normalized to Coomassie-stained protein. Numbers of animals are indicated in bars. Data are means ± SE. *P < 0.05 vs. sham unstimulated. # P < 0.05 vs. E-IH unstimulated.
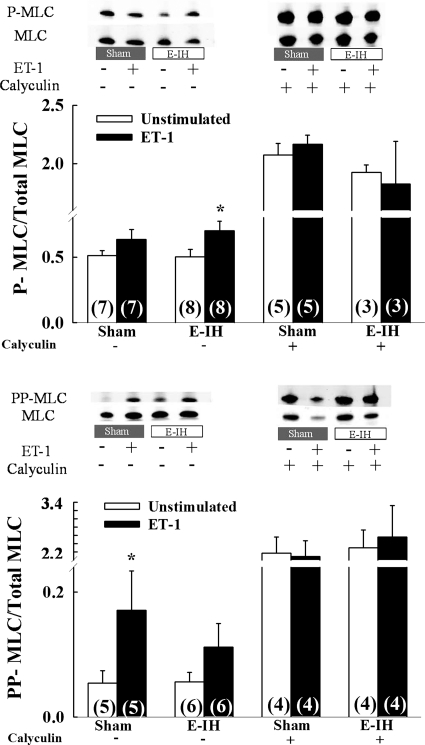
ET-1 Elicits MLC Phosphorylation
ET-1 tended to increase P-MLC in both groups, although a significant difference was observed only in E-IH arteries (Fig. 8A). Furthermore, we detected no differences in P-MLC levels between groups, either in the presence or absence of ET-1. ET-1 also increased PP-MLC in sham arteries, whereas no significant effect of ET-1 was observed in vessels from E-IH rats (Fig. 8B).
Fig. 8.
E-IH does not augment ET-1-induced myosin light chain (MLC) monophosphorylation (S20) or diphosphorylation (T18/S19) in intrapulmonary arteries. Ratios of phospho-MLC (P-MLC) to total MLC (A) and diphospho-MLC (PP-MLC) to total MLC (B) in intrapulmonary arteries from sham and E-IH rats as determined by Western blot. Numbers of animals are indicated in bars. Data are means ± SE. *P < 0.05 vs. corresponding unstimulated group.
DISCUSSION
The purpose of the present study was to identify VSM signaling mechanisms underlying augmented pulmonary vasoconstrictor reactivity in a rat model of sleep apnea. The major findings from this study are that 1) E-IH enhanced vasoconstrictor responsiveness to ET-1 in pressurized small pulmonary arteries independent of changes in global VSM [Ca2+]i but did not alter reactivity to a depolarizing stimulus; 2) greater ET-1-induced vasoconstriction following E-IH is mediated by PKCβ but not PKCδ or ROCK; 3) this response to E-IH was independent of an increase in arterial expression of classical PKC isoforms but was associated with greater basal membrane localization of PKCβI and II, as well as ET-1-induced translocation of these isoforms away from the membrane fraction; and 4) augmented vasoreactivity to ET-1 after E-IH was not associated with greater MLC phosphorylation. These results demonstrate a novel effect of E-IH to augment vasoconstrictor sensitivity to ET-1 through a PKCβ-dependent signaling pathway not present in control arteries.
The incidence of PH in SA patients has been reported to be between 17% (11, 49) and 43% (5, 44). Furthermore, 21% of SA patients without underlying cardiac or lung disease are reportedly pulmonary hypertensive, suggesting that SA is an independent risk factor for the development of PH (1). However, despite the growing recognition of the prevalence of this disorder, the pathogenesis of SA-induced PH remains ill defined. Rodent models of SA involving long-term exposure to episodic hypoxia reproduce not only the systemic hypertension characteristic of SA, but provide evidence for PH as well. For example, IH exposure (4–8 wk) in mice increases RV systolic pressure and causes RV hypertrophy, pulmonary arterial remodeling, and polycythemia (10, 14, 32). We have previously reported similar indexes of PH in rats exposed to IH for 2 wk (41). Although arterial remodeling and polycythemia may be contributing factors to the development of PH, effects of vasodilators to acutely reduce pulmonary vascular resistance in animal models of PH additionally support a major role for vasoconstriction in the PH response (29, 33). Although increases in basal arterial tone (9, 30) and reactivity to receptor-mediated agonists (23, 30, 41) have been demonstrated in chronic hypoxia-induced PH, little is known regarding effects of IH on pulmonary vasoreactivity. However, our present findings that E-IH augments vasoconstrictor sensitivity to ET-1 in small pulmonary arteries, along with previous evidence that vasoconstriction to the thromboxane mimetic U-46619 is greater in isolated lungs from E-IH compared with control rats (41), suggest that E-IH mediates a similar effect to enhance pulmonary vasoreactivity.
Consistent with effects of E-IH in the systemic circulation (3), the present results suggest that E-IH augments pulmonary vasoreactivity to ET-1 by increasing myofilament Ca2+ sensitivity. However, distinct differences exist between effects of E-IH on vasoreactivity in the systemic and pulmonary circulations. For example, whereas increased vasoconstrictor responsiveness following E-IH in systemic arteries appears to be specific to ET-1 (4), E-IH appears to mediate a generalized increase in reactivity to receptor-mediated vasoconstrictor agonists in the pulmonary circulation, since vasoconstriction to U-46619 (41) is similarly increased in isolated lungs from E-IH rats compared with controls. Furthermore, our finding that E-IH does not alter vasoconstrictor responses to depolarizing concentrations of KCl suggests that effects of E-IH to augment ET-1-induced vasoconstriction are not due to an increase in basal sensitivity to Ca2+ but rather are mediated by upregulation of a receptor-dependent signaling pathway that enhances VSM Ca2+ sensitivity or Ca2+ independent contractile mechanisms. However, because only global Ca2+ was measured in the present study, we cannot rule out a possible contribution of small localized Ca2+ signaling events to the observed responses.
The RhoA-ROCK signaling pathway plays a prominent role in myofilament Ca2+ sensitization in VSM (23, 42) and is thus a reasonable candidate for mediating altered pulmonary vasoreactivity following E-IH. This mechanism is largely responsible for enhanced pulmonary vasoconstrictor reactivity to both ET-1 and depolarizing stimuli in the hypertensive pulmonary circulation of chronically hypoxic rats (8, 23). However, we observed no effects of ROCK inhibition to attenuate ET-1-induced constriction of arteries from either E-IH or sham rats. These results suggest that an alternate Ca2+ sensitization pathway is responsible for increased pulmonary vasoconstrictor responsiveness after E-IH and reveal fundamental differences in ET-1 signaling between chronic hypoxia and E-IH models of PH.
Another well-established Ca2+ sensitization pathway is mediated by PKC, a key regulatory enzyme in the pulmonary circulation that contributes to both VSM cell contractility and proliferation (6, 7, 18). The PKC family of serine/threonine kinases consists of at least 13 isoforms in three subclasses: classical PKC isoforms (α, βI, βII, and γ) regulated by both Ca2+ and diacylglycerol (DAG); novel PKC isoforms (δ, ε, θ, η, μ) requiring only DAG for activation; and atypical PKC isoforms (ι, λ, ζ) activated by neither Ca2+ nor DAG but rather by phosphatidyl serine and phosphorylation. Although PKC can elicit VSM contraction through Ca2+-dependent mechanisms, PKC additionally inhibits the catalytic subunit of MLC phosphatase via phosphorylation of CPI-17 to increase myofilament Ca2+ sensitivity (26). On the basis of our previous findings that PKCδ is responsible for enhanced ET-1-induced Ca2+ sensitization in the systemic circulation of E-IH rats (3), we next examined the contribution of PKC to altered pulmonary vasoreactivity in this model. Although general PKC inhibition with Ro 31-8220 normalized vasoconstrictor responsiveness between arteries from sham and E-IH rats, this inhibitor significantly attenuated ET-1-induced vasoconstriction and associated Ca2+ mobilization in each group. Therefore, PKC contributes to both Ca2+ sensitization and Ca2+ influx in response to ET-1, perhaps resulting from stimulation of multiple PKC isoforms. However, in contrast to the role of PKCδ in the systemic circulation of E-IH rats (3) and in the hypertensive pulmonary circulation of fawn hooded rats (6), we found that inhibition of PKCδ with rottlerin did not prevent augmented pulmonary vasoreactivity to ET-1 following E-IH. Rather, PKCβ inhibitors with distinct mechanisms of action selectively attenuated vasoconstriction to ET-1 in E-IH arteries without inhibiting VSM Ca2+ responses, supporting a role for E-IH to elicit a PKCβ-dependent myofilament Ca2+ sensitization pathway that is absent in control arteries.
Although upregulation of PKCβ signaling in response to E-IH was not associated with an increase in arterial PKCβI or PKCβII gene/protein expression, we did observe greater membrane association of these isoforms following E-IH, which may improve accessibility of the enzyme for stimulation by upstream mediators. Consistent with this possibility, cell fractionation experiments revealed an effect of ET-1 to stimulate translocation of both PKCβI and PKCβII away from the membrane fraction only in E-IH arteries. Recent studies have shown that PKCδ translocates to the nucleus of HeLa cells in response to agonist stimulation (24) and that ischemia reperfusion causes mitochondrial translocation of PKCβ, γ, δ in neurons. However, the subcellular target of PKCβ and the distal signaling pathway responsible for vasoconstriction following E-IH remain to be established. Indeed, whereas PKC can activate CPI-17 to inhibit MLC phosphatase and increase levels of phosphorylated MLC, we observed no significant effect of E-IH to augment ET-1 mediated mono- or diphosphorylation of the MLC. Whether PKCβ signals through MLC phosphorylation-independent mechanisms, including stimulation of actin polymerization (reviewed in Ref. 16), to augment ET-1-dependent vasoconstriction following E-IH is an important topic for future investigation. A potential caveat in interpretation of our studies, however, arises from technical limitations in the amount of mRNA/protein that could be derived from isolated small pulmonary arteries (4th to 5th order) used for vasoreactivity experiments. Consequently, arteries used for molecular assays were of varying size (2nd to 5th order) and could be neither pressurized nor endothelium disrupted. Therefore, we cannot rule out the possibility that vessels used for vasoreactivity and molecular studies may not exhibit similar PKC expression, localization, or MLC phosphorylation.
In summary, the present study demonstrates a novel effect of E-IH to augment ET-1-induced vasoconstriction through a PKCβ-dependent signaling mechanism in pulmonary VSM. This response is distinct from the role of PKCδ to mediate enhanced reactivity to ET-1 in mesenteric arteries in this same model. These results are also in marked contrast to effects of chronic sustained hypoxia to augment pulmonary vasoreactivity through a RhoA-ROCK signaling axis, thus emphasizing the divergent mechanisms mediating enhanced ET-1-dependent vasoconstriction between E-IH and chronic hypoxia-induced PH. Future studies are needed to address mechanisms by which E-IH couples ET receptor stimulation to PKCβ signaling in pulmonary VSM and the contribution of this pathway to E-IH-induced PH.
GRANTS
This work was supported by National Institutes of Health grants HL88151 (L. V. Gonzalez Bosc), HL82799 (N. L. Kanagy), HL58124 (B. R. Walker), HL88192 (T. C. Resta), an American Heart Association (AHA) Grant-In-Aid No. 0755775Z (T. C. Resta), and AHA Fellowships Nos. 0715682Z and 11POST5260026 (J. B. Snow).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Michael P. Walsh (University of Calgary) for assistance with MLC phosphorylation protocols and Minerva Murphy and Charles Norton for technical assistance.
REFERENCES
- 1. Alchanatis M, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB. Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration 68: 566–572, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol 295: H434–H440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol 294: H920–H927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45: 705–709, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 27: 1106–1113, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Barman SA. Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol 293: L472–L479, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Barman SA, Pauly JR. Mechanism of action of endothelin-1 in the canine pulmonary circulation. J Appl Physiol 79: 2014–2020, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 109: 380–386, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Cho YE, Ahn DS, Morgan KG, Lee YH. Enhanced contractility and myosin phosphorylation induced by Ca2+-independent MLCK activity in hypertensive rats. Cardiovasc Res 91: 162–170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension 35: 144–147, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Fagan KA. Selected Contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher EC. Chronic lung disease in the sleep apnea syndrome. Lung 168Suppl: 751–761, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hu J, Xu YJ, Zhang ZX, Tian F. Effect of cigarette smoke extract on proliferation of rat pulmonary artery smooth muscle cells and the relevant roles of protein kinase C. Chin Med J (Engl) 120: 1523–1528, 2007 [PubMed] [Google Scholar]
- 19. Jernigan NL, Broughton BR, Walker BR, Resta TC. Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle after chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 290: L517–L525, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 297: L271–L285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kajimoto T, Sawamura S, Tohyama Y, Mori Y, Newton AC. Protein kinase C δ-specific activity reporter reveals agonist-evoked nuclear activity controlled by Src family of kinases. J Biol Chem 285: 41896–41910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37: 511–515, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in Triton X-100-demembranated rabbit arterial smooth muscle. J Physiol 520: 139–152, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kowalczyk JE, Beresewicz M, Gajkowska B, Zablocka B. Association of protein kinase C delta and phospholipid scramblase 3 in hippocampal mitochondria correlates with neuronal vulnerability to brain ischemia. Neurochem Int 55: 157–163, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Nelson CP, Willets JM, Davies NW, Challiss RA, Standen NB. Visualizing the temporal effects of vasoconstrictors on PKC translocation and Ca2+ signaling in single resistance arterial smooth muscle cells. Am J Physiol Cell Physiol 295: C1590–C1601, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 17: 61–66, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Quittau-Prevostel C, Delaunay N, Collazos A, Vallentin A, Joubert D. Targeting of PKCalpha and epsilon in the pituitary: a highly regulated mechanism involving a GD(E)E motif of the V3 region. J Cell Sci 117: 63–72, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Resta TC, Walker BR. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol Heart Circ Physiol 270: H888–H896, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium 5: 115–118, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier NF, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Snow JB, Kanagy NL, Walker BR, Resta TC. Rat strain differences in pulmonary artery smooth muscle Ca2+ entry following chronic hypoxia. Microcirculation 16: 603–614, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Thamilselvan V, Menon M, Thamilselvan S. Oxalate-induced activation of PKC-α and -δ regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am J Physiol Renal Physiol 297: F1399–F1410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 85: 714–719, 1976 [DOI] [PubMed] [Google Scholar]
- 45. Vallentin A, Prevostel C, Fauquier T, Bonnefont X, Joubert D. Membrane targeting and cytoplasmic sequestration in the spatiotemporal localization of human protein kinase C alpha. J Biol Chem 275: 6014–6021, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Walsh MP, Thornbury K, Cole WC, Sergeant G, Hollywood M, McHale N. Rho-associated kinase plays a role in rabbit urethral smooth muscle contraction, but not via enhanced myosin light chain phosphorylation. Am J Physiol Renal Physiol 300: F73–F85, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Zielinski J. [Effects of intermittent hypoxia on pulmonary haemodynamics]. Pneumonol Alergol Pol 73: 86–93, 2005 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.