Abstract
Prostaglandin E2 (PGE2) is a lipid mediator that is produced via the metabolism of arachidonic acid by cyclooxygenase enzymes. In the lung, PGE2 acts as an anti-inflammatory factor and plays an important role in tissue repair processes. Although several studies have examined the role of PGE2 in the pathogenesis of pulmonary fibrosis in rodents, results have generally been conflicting, and few studies have examined the therapeutic effects of PGE2 on the accompanying lung dysfunction. In this study, an established model of pulmonary fibrosis was used in which 10–12-wk-old male C57BL/6 mice were administered a single dose (1.0 mg/kg) of bleomycin via oropharyngeal aspiration. To test the role of prostaglandins in this model, mice were dosed, via surgically implanted minipumps, with either vehicle, PGE2 (1.32 μg/h), or the prostacyclin analog iloprost (0.33 μg/h) beginning 7 days before or 14 days after bleomycin administration. Endpoints assessed at 7 days after bleomycin administration included proinflammatory cytokine levels and measurement of cellular infiltration into the lung. Endpoints assessed at 21 days after bleomycin administration included lung function assessment via invasive (FlexiVent) analysis, cellular infiltration, lung collagen content, and semiquantitative histological analysis of the degree of lung fibrosis (Ashcroft method). Seven days after bleomycin administration, lymphocyte numbers and chemokine C-C motif ligand 2 expression were significantly lower in PGE2- and iloprost-treated animals compared with vehicle-treated controls (P < 0.05). When administered 7 days before bleomycin challenge, PGE2 also protected against the decline in lung static compliance, lung fibrosis, and collagen production that is associated with 3 wk of bleomycin exposure. However, PGE2 had no therapeutic effect on these parameters when administered 14 days after bleomycin challenge. In summary, PGE2 prevented the decline in lung static compliance and protected against lung fibrosis when it was administered before bleomycin challenge but had no therapeutic effect when administered after bleomycin challenge.
Keywords: mouse, cyclooxygenase, FlexiVent
idiopathic pulmonary fibrosis (IPF) is a serious and usually fatal disease that is characterized by progressive scarring of lung tissue and impaired pulmonary function (7, 21, 59). The outlook for patients suffering from this debilitating condition is poor, with a median survival of ∼2–5 yr following diagnosis (1, 2, 32–34). Unfortunately, present therapeutic strategies to treat IPF are less than satisfactory, and lung transplant is often the only viable treatment option (12, 52). Although the exact cause of IPF is unknown, several risk factors, including cigarette smoking, exposure to commonly prescribed drugs, environmental factors, and genetic predispositions are likely culprits (1). IPF is primarily an epithelial-fibroblast disorder in which disease progression is initiated by injury to pulmonary tissue followed by an inflammatory response that is characterized by an influx of eosinophils, neutrophils, lymphocytes, and macrophages that produce profibrotic cytokines such as TGF-β, IL-4, and IL-13. Such cytokines are known to stimulate epithelial-to-myofibroblast differentiation and/or act directly on fibroblasts to enhance proliferation and myofibroblast differentiation. The resulting surplus of myofibroblasts ultimately leads to increased extracellular matrix deposition, collagen production, and pathological fibrosis (7, 21, 59).
Cyclooxygenase (COX)-derived prostanoids are important biological mediators that are produced in the lung and have been shown to regulate pulmonary function under normal and pathological conditions (6, 8, 9, 15, 16). Two COX isoforms, COX-1 and COX-2, have been identified (22, 23). Whereas COX-1 is constitutively expressed, COX-2 is normally expressed at low levels but can be induced by a variety of stimuli such as bacterial endotoxin, cytokines, growth factors, and pharmacological agents (19, 28, 37, 40, 41, 43, 48). Both COX isoforms catalyze the conversion of arachidonic acid to the endoperoxide intermediate prostaglandin H2 (PGH2), which is further metabolized by prostanoid-specific synthases to produce biologically active prostaglandins (PGI2, PGF2α, PGD2, and PGE2) and thromboxane (50).
Several studies have examined the role of COX isoforms, as well as their prostanoid products, in the development and/or progression of pulmonary fibrosis (7). Oga et al. (42) recently showed that loss of PGF2α signaling through its cognate receptor (prostaglandin F receptor) attenuates bleomycin-induced pulmonary fibrosis in mice, thereby demonstrating the profibrotic effects of this prostaglandin (42). On the other hand, the stable prostacyclin (PGI2) analog, iloprost, was shown to protect against TGF-β2-induced fibrosis via PKA-dependent inhibition of the Ras/MEK/ERK pathway (51). Earlier reports showed that PGE2 decreases fibroblast proliferation and inhibits collagen synthesis while promoting its degradation (4, 5, 13, 20, 36). Whereas most in vitro studies agree that COX-2 and PGE2 are antifibrotic, in vivo data have been inconsistent. In a model of vanadium pentoxide-induced pulmonary fibrosis, mice lacking COX-2 experienced a more extreme inflammatory response and were more susceptible to developing fibrosis than wild-type controls or mice lacking COX-1 (6). Additional work from our laboratory showed that COX-2−/− mice had exacerbated lung dysfunction, but not fibrosis, when exposed to bleomycin by oropharyngeal aspiration (9). In contrast, Lovgren et al. (39) argue that COX-2−/− mice do indeed develop more severe fibrosis in addition to exacerbated loss of pulmonary function in response to bleomycin. Several factors may contribute to the discrepancies between the results of these studies, including differences in sex and genetic makeup of the mice, as well as the amount of bleomycin that was used to induce fibrosis.
There have also been discrepancies as to which prostanoid is primarily responsible for conferring the protective effects of COX-2 in pulmonary fibrosis. The above mentioned study by Lovgren and coworkers used several in vivo genetic models to elegantly show that prostacyclin, rather than PGE2, protects against the development of fibrosis and the decline in lung function in response to bleomycin (39). However, a separate study showed that administration of the PGE2 analog, 16,16-dimethyl PGE2 (dmPGE2), protected against bleomycin-induced lung inflammation and fibrosis in mice (17). Unfortunately, this study did not examine the effect of dmPGE2 on pulmonary function in this model.
The current study was designed to clarify the effect of exogenously administered PGE2 or iloprost on lung function, fibrosis, and inflammation in a mouse model of bleomycin-induced pulmonary fibrosis. In addition to examining the protective effects of these two compounds, we also investigated their potential therapeutic effects by administering them 2 wk after bleomycin challenge was initiated. This is an important aspect of our study, as it has not yet been examined by other groups. Our results show that mice receiving PGE2 before the initiation of bleomycin challenge were significantly protected from the decline in pulmonary static compliance and had attenuated inflammatory and fibrotic responses to bleomycin. However, when administered 14 days after bleomycin, PGE2 failed to provide significant therapeutic effects on lung function or fibrosis but did attenuate the inflammatory response that is normally observed during the fibrotic stage. Although iloprost prevented excessive collagen production and inflammation in the lung, it failed to protect against the decline in pulmonary static compliance induced by bleomycin. Like PGE2, when iloprost was administered after bleomycin, it attenuated inflammation but had no effect on lung function or fibrosis.
MATERIALS AND METHODS
Animal care and treatments.
All studies were conducted in accordance with principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Wild-type male C57BL/6 mice (10–12 wk old) were purchased from Taconic Farms (Rockville, MD).
PGE2 and iloprost (Cayman, Ann Arbor, MI) were dissolved in vehicle containing 15% ethanol/sterile saline and were individually delivered 1 wk before or 2 wk after bleomycin treatment via subcutaneously implanted osmotic minipumps (model 1004; Alzet, Cupertino, CA). Minipumps were filled with 100 μl of 12 mg/ml of PGE2 or 3 mg/ml of iloprost. Considering a release rate of 0.11 μl/h, this provided a final dose of 1.32 μg/h for PGE2, a dose that resulted in significantly elevated serum PGE2 levels and no obvious adverse side effects. For iloprost, this provided a dose of 0.33 μg/h and also showed no harmful side effects.
For bleomycin treatments, mice were anesthetized with isoflurane/oxygen and were administered bleomycin-sulfate (Sigma-Aldrich, St. Louis, MO) dissolved in sterile, endotoxin-free saline, or an equivalent volume of sterile saline (up to 75 μl) as a vehicle control, via an oropharyngeal aspiration method previously described (8). Bleomycin was administered at a dose of 1.0 mg/kg body wt. Mice were observed and weighed at regular intervals after aspiration of bleomycin or saline until assessment of inflammation or fibrosis/lung function at 7 or 21 days postdosing, respectively.
Invasive assessment of lung function.
Lung function was measured invasively as previously described (9) using the FlexiVent system (SCIREQ, Montreal, PQ, Canada). Briefly, mice were anesthetized with urethane and paralyzed with pancuronium bromide, and a portable heart rate monitor (CardioMonitor; BAS Vetronics, West Lafayette, IN) was used to ensure that appropriate anesthesia was maintained throughout the duration of ventilation. Ventilation was maintained at a rate of 150 breaths/min, a tidal volume of 7.5 ml/kg, and a positive end expiratory pressure of 3 cm of water. Mice were allowed to acclimate to the ventilator for 2–3 min before initiation of readings, and three to four total lung-capacity functions were performed during this acclimation period to prevent atelectasis and to ensure maximum airway and alveolar recruitment.
Static compliance (Cst), a measurement of the elasticity of the lungs, was calculated from pressure-volume curves by the FlexiVent software (version 5.1) using the Salazar-Knowles equation (46). Thirty seconds after each pressure-volume curve, a 2-s perturbation at a frequency of 2.5 Hz was applied to generate data using the single-compartment model of respiratory mechanics. Only measurements with a coefficient of determination of 0.95 or greater were used, and measurements were repeated until a total of three pressure-volume curves and three single-compartment perturbations, each with acceptable coefficients of determination, were obtained. The averages of these three measurements were determined for each mouse and averaged for each experimental group.
Analysis of cellular infiltration.
Bronchoalveolar lavage fluid (BALF) was collected from mice immediately following lung-function analysis. BALF collection was performed with two 1.0-ml aliquots of Hank's Balanced Salt Solution, and recovery was ≥80% for each animal. Recovered BALF was processed and analyzed for total cell counts using a Coulter Counter (Coulter Electronics, Hialeah, FL). Cell differential counts were determined by light microscopic assessment of Romanowsky-stained cytospin (Shandon, Pittsburg, PA) preparations of BALF.
Collagen assay.
Total collagen was measured in the right caudal lobe by homogenization in RIPA buffer containing protease inhibitors. Collagen content was determined on lysates using the colorimetric Sircol collagen assay kit (Biocolor, Carrickfergus, UK) according to the manufacturer's instructions.
Histopathological analysis.
The left lung was inflated and fixed with 4% paraformaldehyde, processed, and embedded in paraffin wax. Five-micron sections were cut and stained with hematoxylin and eosin or Masson's Trichrome. Histological assessment of the extent and severity of pulmonary fibrosis was determined using the Ashcroft method (3) by a pathologist who was blinded to the treatment groups.
Real-time PCR analysis.
Total RNA was purified from lung lysates using Qiagen RNeasy Mini kits according to the manufacturer's instructions (Qiagen, Valencia, CA), which included the optional on-column treatment with Qiagen RNase-free DNase (Qiagen cat. no. 79254). RNA was then reverse-transcribed into first-strand cDNA using the ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real-time PCR reactions were performed in duplicate as follows: 2 min at 50°C, 10 min at 95°C, then 40 cycles each at 95°C for 15 s followed by 60°C for 60 s in the ABI Prism 7900 HT Sequence Detector System. GAPDH was used as an endogenous control for all samples. The ΔΔCt method was used to determine the relative levels of gene expression (38). All TaqMan probes were purchased from Applied Biosystems with the following catalogue numbers: Gapdh (Mm99999915_g1), Tnf-α (Mm00443258_m1), intercellular adhesion molecule-1 (Icam) (Mm00516023_m1), and chemokine C-C motif ligand 2 (Ccl2) (Mm00441242).
BALF cytokine analysis.
Levels of CCL2, TNF-α, and macrophage inflammatory protein (MIP)-1α in BALF were determined with a Bio-Plex mouse cytokine kit (Bio-Rad, Hercules, CA), using fluorescently labeled microsphere beads and a Bio-Plex suspension array system (Bio-Rad) according to the manufacturer's instructions.
Analysis of serum and BALF prostaglandin levels.
Serum and BALF prostaglandin levels were analyzed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). Briefly, 200 μl of serum or 500 μl of BALF was applied to Amprep Octadecyl C18 columns (Amersham Biosciences, Piscataway, NJ), followed by washes with 0.1% acetic acid: 5% methanol. Samples were eluted from columns with methanol into a 2-ml collection tube, dried by SpeedVac (Thermo Scientific, Waltham, MA), flooded with argon and stored at −80°C until analysis. Details of the analytical methods have been previously described (8).
cAMP ELISA.
Serum cAMP levels were measured with the R&D Systems Parameter cAMP assay per manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis.
Statistical comparisons were performed by one-way analysis of variance (31) followed by Newman-Keuls post hoc tests, using GraphPad Prism software (GraphPad Software, San Diego, CA). In all instances, statistical significance was denoted when the P value was <0.05.
RESULTS
Implantation of PGE2 osmotic minipumps leads to significantly elevated serum PGE2 levels.
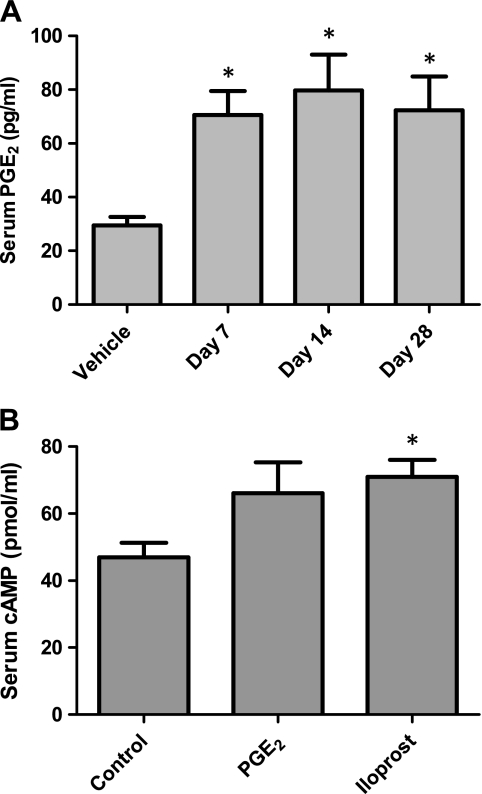
We first wanted to confirm that implantation of osmotic minipumps containing PGE2 led to elevated circulating PGE2 levels throughout the duration of our study. Vehicle- or PGE2-containing minipumps were implanted subcutaneously, and serum was obtained after 7, 14, and 28 days of treatment. Serum analysis by LC/MS/MS showed that mice receiving PGE2-containing minipumps had significantly higher (∼2.5-fold) PGE2 levels than mice receiving vehicle containing minipumps at all time points examined (Fig. 1A). In contrast, there were no differences between the two groups in serum levels of 6-keto-PGF1α (the stable PGI2 metabolite), PGF2α, PGD2, thromboxane B2 (the stable thromboxane A2 metabolite), or other eicosanoids (data not shown). There were no differences in BALF PGE2 levels following minipump implantation (data not shown).
Fig. 1.
Surgical implantation of subcutaneous prostaglandin E2 (PGE2) osmotic minipumps results in elevated serum PGE2 and cAMP levels. A: mice were given vehicle osmotic minipumps for 7 days or minipumps containing PGE2 for the designated time points. Serum PGE2 levels were determined by liquid chromatography-tandem mass spectrometry (LC/MS/MS). B: serum was collected 1 wk after implantation of osmotic minipumps containing either PGE2 or iloprost. ELISA showed a similar induction in cAMP levels in both PGE2- and iloprost-treated mice. *P < 0.05 vs. saline-treated group; n = 3–5 per group for serum PGE2 analysis and 9–11 per group for serum cAMP analysis.
Serum cAMP levels in PGE2- and iloprost-treated mice.
To assess the downstream signaling effect of PGE2 and iloprost, we measured serum cAMP levels as a biomarker of prostanoid signaling. As shown in Fig. 1B, both PGE2 and iloprost induced a similar increase in serum cAMP levels following 1 wk of dosing. On the basis of these data, we conclude that the PGE2 and iloprost doses chosen for our study are physiologically effective and comparable, at least from a cell-signaling standpoint.
Bleomycin treatment leads to increased BALF PGE2 levels.
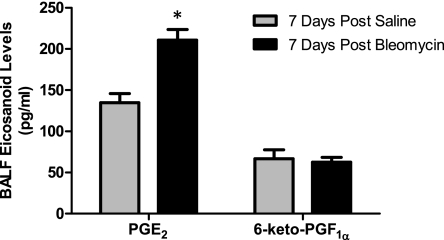
To assess the effect of bleomycin exposure on endogenous prostaglandin levels, we collected BALF from mice 7 days after saline or bleomycin challenge and measured prostaglandin levels by LC/MS/MS. As shown in Fig. 2, bleomycin treatment led to an ∼1.6-fold elevation in BALF PGE2 levels. In contrast, no changes in 6-keto-PGF1α levels were observed following bleomycin challenge. Similar analysis of other prostaglandins also showed no differences between saline- and bleomycin-treated animals (data not shown). These results suggest that, at least at this time point, PGE2 (rather than PGI2 or other prostaglandins) may mediate or be involved in mitigating the inflammatory, fibrotic, and/or physiological responses to bleomycin exposure in vivo.
Fig. 2.
Bronchoalveolar lavage fluid (BALF) PGE2, but not 6-keto-PGF1α, is elevated in response to bleomycin. Mice were treated once with either saline or 1 mg/kg bleomycin, and BALF PGE2 and 6-keto-PGF1α levels were measured 7 days later by LC/MS/MS. *P < 0.05 vs. saline-treated group; n = 6–7 per group.
Both PGE2 and iloprost attenuate bleomycin-induced weight loss.
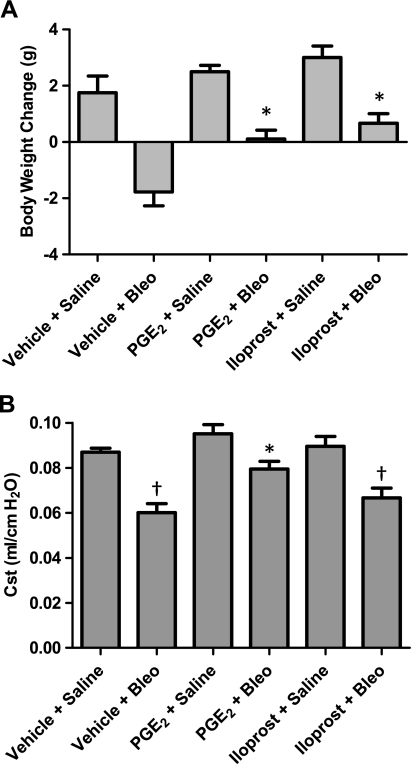
Over the course of 7 days, we observed an increase in body weight in animals that were administered saline, an effect that was similar in vehicle-, PGE2- and iloprost-treated animals (Fig. 3A). The increased body weight is likely due in part to the fact that mice in this study were fed ground NIH-31 food mixed with reverse osmosis deionized (RODI) water. Essentially, this is a standard rodent chow that has been “mashed” in RODI water to make it easily digestible for animals that may experience a loss of appetite or difficulty eating, which often is the case following bleomycin treatment. It is thus provided on the cage floor in an effort to prevent excessive weight loss and mortality following bleomycin administration. Nevertheless, mice that were administered bleomycin experienced a loss of body weight in the first week. This weight loss was significantly attenuated in PGE2- and iloprost-treated mice compared with vehicle-treated controls (Fig. 3A). After 21 days of treatment, all saline- and bleomycin-treated mice had similar body weights that were ∼10% above predosing levels (data not shown). None of the animals in this study died as a result of bleomycin treatment.
Fig. 3.
PGE2 and iloprost attenuate bleomycin-induced weight loss and decline in lung function. Mice were given vehicle, PGE2, or iloprost via minipumps and treated with bleomycin 1 wk later. A: body weights were recorded after 1 wk of bleomycin (Bleo) administration. B: static compliance (Cst) was measured via Flexivent 21 days after bleomycin administration. Bleomycin-induced decrease in Cst observed in vehicle-treated animals was significantly attenuated in PGE2-treated mice. *P < 0.05 vs. vehicle + bleomycin group, †P < 0.05 vs. vehicle + saline group; for body weight analysis, n = 4–8 mice for saline groups, n = 9 for bleomycin groups. For lung function analysis, n ≥ 6 for saline groups, n ≥ 11 for bleomycin groups.
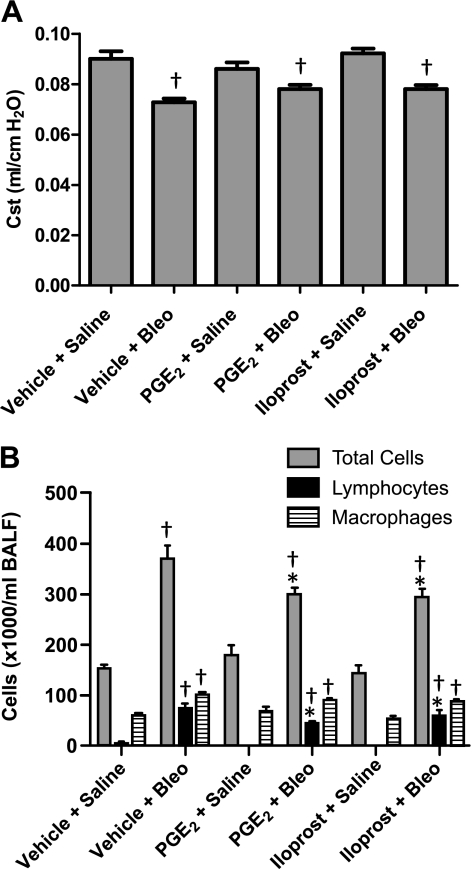
PGE2 protects against bleomycin-induced decline in lung function.
Studies from our group and others have consistently shown that bleomycin administration leads to a significant reduction in pulmonary static compliance (Cst) in mice (9, 39, 57). To test the effect of PGE2 and iloprost on bleomycin-induced pulmonary dysfunction, we challenged mice via oropharyngeal administration with 1 mg/kg of bleomycin and then conducted invasive assessment of lung function 21 days later. No differences in static compliance were observed among vehicle-, PGE2-, and iloprost-treated mice that received saline, indicating that neither PGE2 nor iloprost influenced lung static compliance under basal conditions (Fig. 3B). Relative to saline-treated controls, bleomycin administration led to reduced static compliance in all three experimental groups; however, PGE2-treated mice maintained significantly higher static compliance following bleomycin administration than vehicle-treated mice (Fig. 3B).
These functional changes in response to bleomycin may seem small. Nonetheless, small changes can have profound effects on an animal's overall health. Importantly, when given slightly higher doses of bleomycin that induce greater reductions in Cst, animal mortality became an issue. The dose we chose for our study provided a modest level of pulmonary dysfunction but was not associated with mortality.
Cellular infiltration is attenuated in airways of PGE2- and iloprost-treated mice.
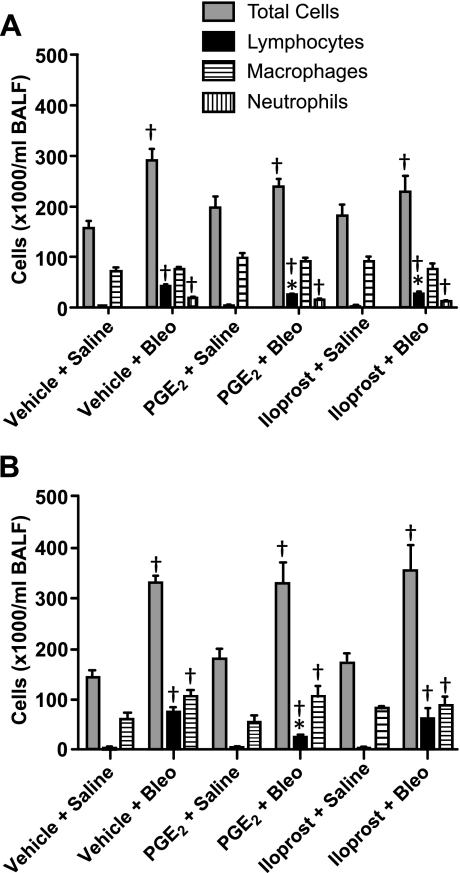
Bleomycin initiates an inflammatory response that includes infiltration of cells into the airways, an event that has been implicated as a causative factor in the development of pulmonary fibrosis (59). We therefore examined the effects of treatment with PGE2 or iloprost on this process during both the early inflammatory stage (day 7) and the late fibrotic stage (day 21) of bleomycin-induced pulmonary fibrosis. Relative to saline-administered controls, vehicle-, PGE2-, and iloprost-treated mice that were administered bleomycin had elevated total, lymphocyte, and neutrophil cell numbers in BALF 7 days after dosing. Whereas PGE2 and iloprost had modest effects on total cell numbers, both significantly attenuated lymphocyte infiltration at 7 days after bleomycin dosing. Macrophage cell numbers did not differ between saline- and bleomycin-treated animals in any of the treatment groups (Fig. 4A). We also observed a marked increase in total, lymphocyte, and macrophage cell numbers 21 days following bleomycin administration relative to control mice that received saline (Fig. 4B). Similar to our results at the 7-day time point, PGE2 significantly attenuated lymphocyte infiltration at 21 days. In contrast, iloprost had no significant effect on lymphocyte infiltration at 21 days. Although macrophage numbers were higher in bleomycin-treated animals, no differences were observed between the three different treatment groups (Fig. 4B). Furthermore, almost no neutrophils were observed in BALF from saline- or bleomycin-treated animals in any of the treatment groups and were thus not included in Fig. 4B. Importantly, neither PGE2 nor iloprost had significant effects on total, lymphocyte, neutrophil, or macrophage cell numbers at either of the examined time points in saline-treated animals (Fig. 4, A and B).
Fig. 4.
Cellular infiltration in PGE2- and iloprost-treated mice. A: although no differences were observed in total cell numbers among any of the bleomycin-treated groups, PGE2 and iloprost both inhibited lymphocyte influx into BALF 7 days after bleomycin challenge. B: no differences in total cell numbers were observed among any of the bleomycin-treated groups 21 days after challenge. In contrast, PGE2, but not iloprost, inhibited lymphocyte infiltration into BALF at this later time point. *P < 0.05 vs. vehicle + bleomycin group, †P < 0.05 vs. vehicle + saline group; n ≥ 4 for saline groups, n ≥ 9 for bleomycin groups.
Our results showing similar levels of cellular infiltration at 7 and 21 days after bleomycin challenge may seem a bit unusual. However, these results are consistent with previous studies from our laboratory and were reproducible in multiple independent experiments. Discrepancies between the current findings and previous reports from other groups may be attributable to a variety of factors including bleomycin dosing levels, age, sex, and strain of mice, or differences in other experimental conditions.
Cell adhesion molecule and inflammatory cytokine levels following bleomycin administration.
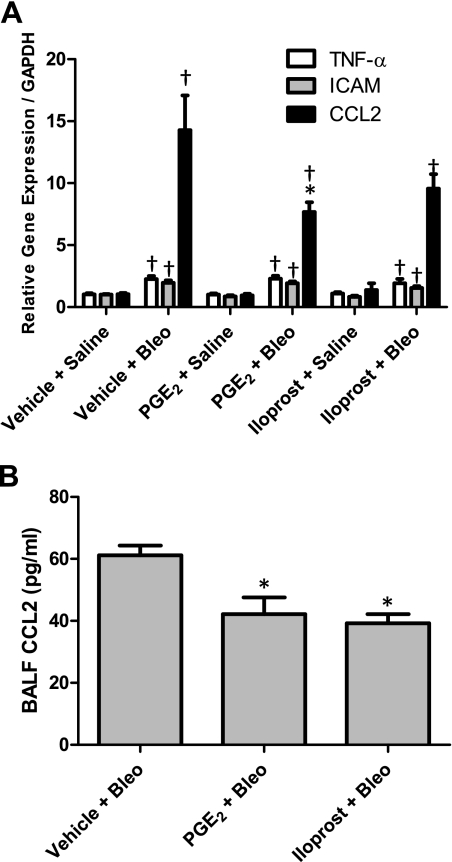
It is well established that the early inflammatory response to bleomycin includes increased expression of several proinflammatory cytokine, chemokine, and cell adhesion molecule genes in the lung (29, 30). Therefore, we used real-time RT-PCR to quantitatively assess Tnf-α, Icam, and Ccl2 (also known as monocyte chemoattractant protein 1) gene expression changes in the lung 7 days following bleomycin administration. As shown in Fig. 5A, bleomycin administration led to significantly elevated levels of TNF-α, ICAM, and CCL2 mRNAs in vehicle-, PGE2-, and iloprost-treated mice compared with saline-treated controls. Whereas PGE2 and iloprost had no significant effect on bleomycin-induced TNF-α or ICAM mRNA levels, PGE2 treatment led to significantly reduced CCL2 mRNA levels following bleomycin administration. We also observed a considerable, albeit statistically insignificant, reduction of bleomycin-induced CCL2 mRNA levels in iloprost-treated mice. It should be noted that neither PGE2 nor iloprost had a significant effect on TNF-α, ICAM or CCL2 mRNA levels in saline-treated groups (Fig. 5A).
Fig. 5.
Cell adhesion molecule and inflammatory cytokine levels in bleomycin-treated mice. A: bleomycin challenge resulted in elevated lung TNF-α, intercellular adhesion molecule (ICAM), and chemokine C-C motif ligand 2 (CCL2) mRNA levels after 7 days (determined by real-time RT-PCR). TNF-α and ICAM mRNA levels were not affected by PGE2 or iloprost treatment. In contrast, PGE2 significantly reduced CCL2 mRNA levels. B: BALF CCL2 protein levels were significantly reduced in PGE2- and iloprost-treated mice that received bleomycin (determined by Bio-Plex). *P < 0.05 vs. vehicle + bleomycin group, †P < 0.05 vs. vehicle + saline group; n ≥ 4 for saline groups, n ≥ 8 for bleomycin groups.
In addition to analysis of the above-mentioned markers at the mRNA level, we also examined CCL2, TNF-α, and MIP-1α protein levels by Bio-Plex analysis in BALF 7 days following treatment with saline or bleomycin. TNF-α and MIP-1α were undetectable in BALF from saline- and bleomycin-treated animals (data not shown). Whereas CCL2 protein levels were also undetectable in BALF from saline-treated mice, bleomycin exposure led to significantly increased CCL2 levels (Fig. 5B). Consistent with our observations at the mRNA level, treatment with either PGE2 or iloprost significantly attenuated the increase in BALF CCL2 protein levels following bleomycin challenge (Fig. 5B). Together, these findings demonstrate an anti-inflammatory role for PGE2 7 days following bleomycin administration. The anti-inflammatory properties of iloprost appear to be less consistent.
Effect of PGE2 and iloprost on bleomycin-induced collagen deposition and development of lung fibrosis.
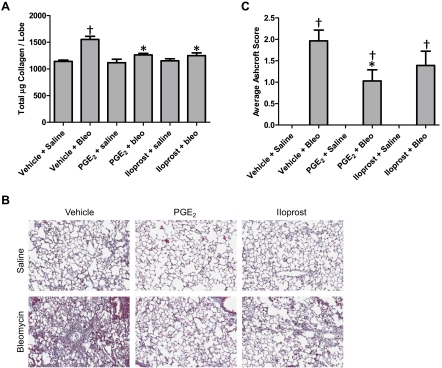
Twenty-one days following bleomycin administration, mice typically experience a robust fibrotic response in the lung characterized by a surplus of myofibroblasts that produce and release excessive amounts of collagen, resulting in histological changes similar to those observed in patients with pulmonary fibrosis (12, 59). Biochemical assessment of lung tissue showed that vehicle-, PGE2-, and iloprost-treated mice that received bleomycin had elevated lung collagen content relative to control mice that received saline (Fig. 6A). However, among mice dosed with bleomycin, collagen content in PGE2- and iloprost-treated mice was significantly lower than in vehicle-treated mice. Furthermore, bleomycin exposure led to a robust inflammatory and fibrotic response characterized by collagen deposition and cellular infiltration within the alveolar walls and alveolar spaces as observed on Masson's trichrome-stained histological sections (Fig. 6B). Histopathological quantification for fibrosis (Ashcroft scores) revealed a significant protective effect of PGE2 in bleomycin-treated animals (Fig. 6C). Although we also observed a modest reduction in Ashcroft scores in iloprost-treated animals, this group was not significantly different from vehicle-treated animals that received bleomycin (Fig. 6C). Importantly, PGE2 and iloprost had no significant effect on lung collagen content or histopathological evidence of lung fibrosis in animals that received saline instead of bleomycin (Fig. 6, A–C).
Fig. 6.
Effect of PGE2 and iloprost on bleomycin-induced collagen production and development of lung fibrosis. A: lung collagen content 21 days after bleomycin challenge was lower in mice treated with PGE2 or iloprost. B: representative histology of Masson's trichrome-stained slides. C: calculated fibrosis scores based on histopathological assessment of Masson's trichrome-stained sections revealed a protective effect of PGE2 in bleomycin-treated lungs. *P < 0.05 vs. vehicle + bleomycin group, †P < 0.05 vs. vehicle + saline group; n ≥ 6 for saline groups, n ≥ 15 for bleomycin groups.
Therapeutic effect of PGE2 and iloprost on lung function.
Although several studies have examined the protective effects of PGE2 and iloprost in pulmonary fibrosis, none have investigated the therapeutic effects of these compounds in a fibrosis model. We therefore administered PGE2 or iloprost 14 days after the initiation of fibrosis with bleomycin to test for potential therapeutic effects.
As shown previously, no differences in static compliance were observed among vehicle-, PGE2-, and iloprost-treated mice that received saline. Relative to saline-treated controls, bleomycin administration led to reduced static compliance in all three experimental groups. Interestingly, there were no significant differences between any of the bleomycin-treated groups (Fig. 7A). Thus PGE2 and iloprost lacked a significant therapeutic effect on lung function in our model.
Fig. 7.
Therapeutic effect of PGE2 and iloprost on lung dysfunction and cellular infiltration following bleomycin. A: mice were challenged with bleomycin and then given vehicle, PGE2, or iloprost via minipumps 14 days later. Static compliance was measured via FlexiVent 21 days after bleomycin administration. Bleomycin-induced decrease in Cst observed in vehicle-treated animals was not affected by PGE2 or iloprost. B: PGE2 and iloprost both inhibited total cell and lymphocyte influx into BALF when administered 14 days after bleomycin challenge. *P < 0.05 vs. vehicle + bleomycin group, †P < 0.05 vs. vehicle + saline group; n ≥ 8 for saline groups, n ≥ 21 for bleomycin groups.
Therapeutic effect of PGE2 and iloprost on cellular infiltration.
As shown previously, relative to saline-administered controls, vehicle-, PGE2-, and iloprost-treated mice that were administered bleomycin had elevated total, lymphocyte, and macrophage cell numbers in BALF 21 days after dosing. Mice treated with PGE2 or iloprost 14 days after the initiation of bleomycin challenge had significantly fewer total cells and lymphocytes, but macrophage numbers were similar in all three treatment groups (Fig. 7B). On the basis of these data, we conclude that PGE2 and iloprost act therapeutically to attenuate inflammation associated with the later stages of bleomycin-induced pulmonary fibrosis.
We would also like to note here that, although macrophages and lymphocytes make up a majority of the total cell population in BALF following bleomycin challenge, there are several other cell types, including neutrophils, eosinophils, and basophils, that contribute to the total population. Thus the large differences observed in total cell counts following bleomycin challenge are also impacted by these cell types. Although we did assess these cell types, no differences were observed between any of the treatment groups, and thus, in an effort to make the relevant data more presentable, these data were not included in our results.
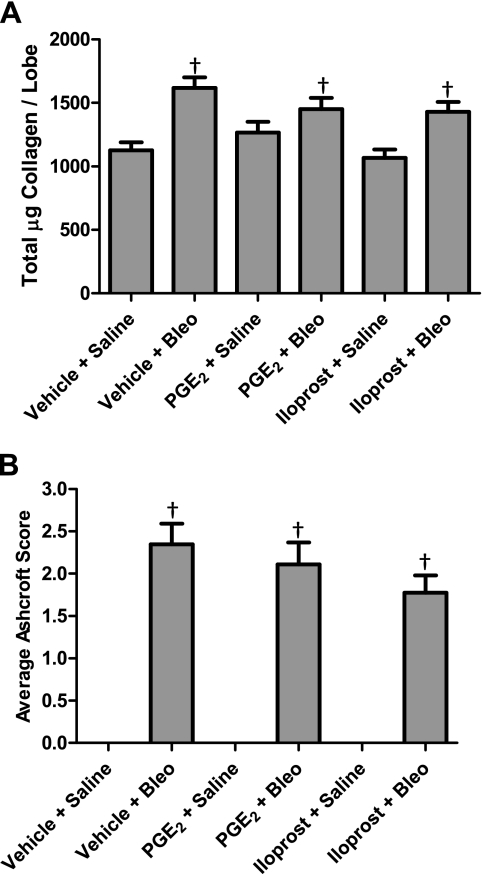
Therapeutic effect of PGE2 and iloprost on bleomycin-induced collagen deposition and development of lung fibrosis.
Consistent with previous results, biochemical assessment of lung tissue showed that vehicle-, PGE2-, and iloprost-treated mice that received bleomycin had elevated lung collagen content relative to control mice that received saline. There were no significant differences in collagen content between any of the bleomycin-treated groups (Fig. 8A). Furthermore, histopathological quantification for fibrosis showed no significant differences between any of the bleomycin-treated groups (Fig. 8B). Thus PGE2 and iloprost had no therapeutic effect on collagen content or fibrotic lung injury in our model.
Fig. 8.
Therapeutic effect of PGE2 and iloprost on bleomycin-induced collagen production and development of lung fibrosis. Lung collagen content (A) and calculated fibrosis scores (B) were not altered in mice receiving PGE2 or iloprost 14 days after bleomycin challenge. †P < 0.05 vs. vehicle + saline group; n ≥ 8 for saline groups, n ≥ 21 for bleomycin groups.
DISCUSSION
This was the first study designed to compare the protective vs. therapeutic effects of exogenously administered PGE2 or iloprost in a mouse model of pulmonary fibrosis. We used a well established model of bleomycin-induced pulmonary fibrosis and examined inflammatory, fibrotic, and lung functional endpoints in mice receiving osmotic minipumps containing vehicle, PGE2, or iloprost either before or after bleomycin administration. Our results demonstrate that PGE2 protects against the airway inflammation, lung fibrosis, and decline in lung function observed in this model. In contrast, iloprost did not protect against pulmonary dysfunction and had less pronounced effects on the inflammatory and fibrotic responses to bleomycin. The therapeutic effects of both PGE2 and iloprost were minimal, with significant beneficial effects only observed on cellular infiltration associated with later stages of fibrosis in this model. Thus the major finding from this study is that PGE2 modulates the early injury phase but not the later fibrotic phase in the bleomycin-induced model of pulmonary fibrosis.
A number of other reports have observed anti-inflammatory and antifibrotic effects of PGE2 in the lung; however, our report is the first to demonstrate PGE2-mediated protection from the decline in lung function associated with bleomycin administration. Although it seems logical that reduced inflammation and fibrosis would translate to improved pulmonary function, the association between histological/biochemical and functional defects is not always apparent. For example, a recent report from our laboratory showed that mice lacking COX-2 had exacerbated lung dysfunction, but not increased fibrosis, in response to bleomycin (9). Several possibilities for this phenomenon exist, including regional differences in collagen deposition, differences in collagen maturity, and/or variable effects of prostanoids on airway smooth muscle cells. In comparison, several studies have shown increased lung fibrosis in COX-2−/− mice in response to various stimuli (6, 30, 39). Possible explanations for these discrepancies include experimental design differences among studies such as differences in the doses of profibrotic agents used and in genetic background, age, and sex of the animals used. Our present study differs from the previously published reports in that we specifically tested the effects of PGE2, whereas other studies that used COX-2 knockout mice likely tested effects of several prostaglandins simultaneously, possibly confounding and/or masking the effects of PGE2.
Three comparable studies are worth considering in more detail. Lovgren et al. (39) used several genetic models to show that prostacyclin, rather than PGE2, is responsible for both the histological and functional protective effects of COX-2 in bleomycin-induced pulmonary fibrosis. Differences between genetic models, which chronically alter gene expression levels, and administration of a drug for a relatively short duration cannot be underestimated. Furthermore, the amount of bleomycin used in our study (1 mg/kg) differs from the 0.025 and 0.05 units of bleomycin used by Lovgren et al. (39), regardless of body weight. In the present study, BALF prostacyclin levels were not altered by bleomycin treatment, but PGE2 in BALF was highly elevated in bleomycin-challenged mice, suggesting a key role for PGE2, but not prostacyclin, in this model. This observation could indeed be due to the fact that prostacyclin is primarily produced by endothelial cells, whereas PGE2 is produced by many cell types, including epithelial cells, macrophages, and many other cell types that are either in or contiguous to the airway. It is thus possible that PGE2 is more likely to be detected in BALF, and prostacyclin would more likely be detected in other compartments, such as urine. Another relevant study that should be noted is the one by Failla and coworkers (17), which showed that treatment with the PGE2 analog, dmPGE2, protects mice from bleomycin-induced pulmonary inflammation and fibrosis (17). Although this study utilized a similar experimental protocol, there are key differences between their report and ours. First, we used PGE2 rather than an analog and showed that its stability allows it to be effectively administered via osmotic minipumps for up to 4 wk. Second, our study utilized mice on a C57BL/6 genetic background, whereas the Failla study used CD mice. This is important because many other comparable studies, including the one by Lovgren et al. (39), also used C57BL/6 mice. Considering the variability in bleomycin response in different mouse strains, this is an important experimental variable that should be taken into consideration. Finally, the most important difference between our study and the one by Failla et al. (17) is that, in addition to examining the role of PGE2 in the inflammatory, histological, and biochemical response to bleomycin, we also studied the effect of PGE2 on lung function. Importantly, our data demonstrate significant protective effects of PGE2 on this endpoint. We believe that it is important to measure lung function in any model of pulmonary fibrosis because patients suffering from this condition often have profound changes in pulmonary function, and this significantly influences their quality of life. Indeed, by the time pulmonary function is compromised, the lungs have already undergone significant histological and biochemical deterioration. The prognosis for patients with reduced lung function attributable to fibrosis is generally poor, suggesting that functional assessment may be a more accurate predictor of disease status and survival than traditional pathological analysis of fibrotic lung disease. Finally, a very recent study by Zhu and coworkers (61) showed that one intraperitoneal injection of iloprost protected mice from bleomycin-induced pulmonary fibrosis and lung dysfunction. The most obvious difference between this study and ours is that we systemically treated mice with iloprost for the duration of the study, whereas Zhu et al. only gave a single intraperitoneal injection of iloprost at the time that bleomycin challenge was initiated. Another consideration is that our study dosed mice with 1 mg/kg of bleomycin, whereas Zhu et al. used 2 to 3 mg/kg of bleomycin, a dose that we have generally found to be lethal.
The beneficial role of PGE2 in the lung is also supported by data obtained using other experimental models of pulmonary fibrosis and inflammation. For example, PGE2 has been shown to inhibit fibroblast proliferation and collagen synthesis, regulate wound closure in airway epithelium, and inhibit TGF-β-induced fibroblast to myofibroblast transition (35, 47). PGE2 also prevents LPS-induced NF-κB activation in monocytes, thereby inhibiting TNF-α production while stimulating increased levels of the anti-inflammatory cytokine, IL-10 (11, 55, 56). Whereas we observed a significant increase in TNF-α and ICAM gene expression in bleomycin-treated animals, neither PGE2 nor iloprost had a significant effect on this response. Interestingly, we were unable to detect TNF-α or MIP-1α in BALF from saline- or bleomycin-treated animals. However, in support of our findings showing reduced lymphocyte infiltration in PGE2-treated animals, multiple studies have shown that PGE2 is involved in the regulation of lymphocyte trafficking and differentiation in a variety of models (44, 53). This is also consistent with our observation of reduced lung CCL2 mRNA levels and BALF CCL2 protein levels in PGE2-treated animals. Of note, CCL2 is known to recruit T lymphocytes to sites of injury and infection (10).
Although the role of inflammation and lymphocyte infiltration in the development of pulmonary fibrosis is debatable, several studies have demonstrated potent profibrotic effects of cytokines such as IL-4 and IL-13 that are produced by lymphocytes (14, 18, 49, 60). Examination of various inflammatory cells has also shown that increased eosinophils, neutrophils, and T lymphocytes are associated with poorer prognosis in patients suffering from IPF. In fact, a 2007 study by Parra et al. (45) showed that the most important predictor for survival in patients with IPF/usual interstitial fibrosis was the number of CD3+ T lymphocytes present in lung tissue. Thus, whether lymphocytes significantly contribute to the development of fibrosis or simply serve as a marker of overall pulmonary health, our results demonstrating reduced lymphocytes in PGE2-treated mice support the concept that targeting this eicosanoid pathway may benefit patients afflicted with IPF or other forms of pulmonary fibrosis.
The fact that PGE2 is such an effective protective agent against pulmonary fibrosis but has little therapeutic effect when administered during the fibrotic stage is interesting and deserves discussion. Our results show that the only endpoint in which PGE2 or iloprost acted therapeutically was on infiltration of total cells and lymphocytes. This observation, along with the results from our 7- and 21-day protective studies, suggests that both PGE2 and iloprost act potently to reduce early inflammation and injury associated with fibrosis but have little effect when administered at the later fibrotic phase. If we are to believe that inflammation contributes to the development of fibrosis and subsequent decline in lung dysfunction, then it is possible that PGE2 or iloprost treatment must be initiated earlier to be effective therapeutic agents. Dosing in our therapeutic experiments did not occur until animals were well into the fibrotic stages of disease, and, although inflammation was reduced, it may have been too late to have had an effect on fibrosis and lung function. It is also possible that, if we extended our study for another 1–2 wk, PGE2 and iloprost would have had more time to impact fibrosis and thus would have been more effective from a therapeutic standpoint.
Finally, it is clear that COX-derived eicosanoids, especially PGE2, have a profound effect in attenuating the inflammatory, fibrotic, and functional changes observed in pulmonary fibrosis. Previous studies have reported an induction of COX-2 expression in response to bleomycin exposure, which could result in significant alterations in the overall prostanoid profile (24). In our hands, the only COX-2 metabolite that is significantly altered in response to bleomycin challenge is PGE2, suggesting that it is likely the main COX metabolite involved in mediating the response to bleomycin. Although COX-2 induction leads to elevated PGE2 levels in a variety of disease states, the lung is unique in that PGE2 serves beneficial actions as an anti-inflammatory mediator, whereas it is mostly a proinflammatory factor in other tissues (54).
In summary, our data show that exogenously administered PGE2 via osmotic minipumps rescues mice from the lung functional decline, pulmonary inflammation, and lung fibrosis that are normally observed after bleomycin challenge. Furthermore, we show that iloprost selectively protects against some inflammatory and fibrotic endpoints but not against the associated decline in lung function. Interestingly, both PGE2 and iloprost had little therapeutic effect when administered 2 wk after initiation of bleomycin challenge. Further investigation comparing the protective and therapeutic properties of these two compounds is warranted.
GRANTS
This work was supported with funds from the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025043 and ES050167). J. Card was the recipient of a Senior Research Training Fellowship from the American Lung Association of North Carolina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Fessler and Dr. Stavros Garantziotis for providing helpful comments during preparation of this manuscript.
REFERENCES
- 1. American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society/European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165: 277–304, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41: 467–470, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baum BJ, Moss J, Breul SD, Berg RA, Crystal RG. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem 255: 2843–2847, 1980 [PubMed] [Google Scholar]
- 5. Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest 77: 700–708, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol 161: 459–470, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 445–452, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Card JW, Voltz JW, Carey MA, Bradbury JA, Degraff LM, Lih FB, Bonner JC, Morgan DL, Flake GP, Zeldin DC. Cyclooxygenase-2 deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol 37: 300–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 91: 3652–3656, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conte E, Bonaiuto C, Nesci C, Crimi N, Vancheri C, Messina A. Nuclear factor-kappaB activation in human monocytes stimulated with lipopolysaccharide is inhibited by fibroblast conditioned medium and exogenous PGE2. FEBS Lett 400: 315–318, 1997 [DOI] [PubMed] [Google Scholar]
- 12. du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov 9: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Elias JA. Tumor necrosis factor interacts with interleukin-1 and interferons to inhibit fibroblast proliferation via fibroblast prostaglandin-dependent and -independent mechanisms. Am Rev Respir Dis 138: 652–658, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Emura M, Nagai S, Takeuchi M, Kitaichi M, Izumi T. In vitro production of B cell growth factor and B cell differentiation factor by peripheral blood mononuclear cells and bronchoalveolar lavage T lymphocytes from patients with idiopathic pulmonary fibrosis. Clin Exp Immunol 82: 133–139, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ermert L, Dierkes C, Ermert M. Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clin Cancer Res 9: 1604–1610, 2003 [PubMed] [Google Scholar]
- 16. Ermert M, Kuttner D, Eisenhardt N, Dierkes C, Seeger W, Ermert L. Cyclooxygenase-2-dependent and thromboxane-dependent vascular and bronchial responses are regulated via p38 mitogen-activated protein kinase in control and endotoxin-primed rat lungs. Lab Invest 83: 333–347, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Failla M, Genovese T, Mazzon E, Fruciano M, Fagone E, Gili E, Barera A, La Rosa C, Conte E, Crimi N, Cuzzocrea S, Vancheri C. 16,16-Dimethyl prostaglandin E2 efficacy on prevention and protection from bleomycin-induced lung injury and fibrosis. Am J Respir Cell Mol Biol 41: 50–58, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol 37: 823–829, 1991 [PubMed] [Google Scholar]
- 19. Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 265: 16737–16740, 1990 [PubMed] [Google Scholar]
- 20. Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem 257: 8630–8633, 1982 [PubMed] [Google Scholar]
- 21. Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Hemler M, Lands WE. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem 251: 5575–5579, 1976 [PubMed] [Google Scholar]
- 23. Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA 89: 7384–7388, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol 165: 1663–1676, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E2 inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 292: L405–L413, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol 39: 482–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, Horowitz JC, Peters-Golden M. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J 23: 4317–4326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 268: 9049–9054, 1993 [PubMed] [Google Scholar]
- 29. Katsuma S, Nishi K, Tanigawara K, Ikawa H, Shiojima S, Takagaki K, Kaminishi Y, Suzuki Y, Hirasawa A, Ohgi T, Yano J, Murakami Y, Tsujimoto G. Molecular monitoring of bleomycin-induced pulmonary fibrosis by cDNA microarray-based gene expression profiling. Biochem Biophys Res Commun 288: 747–751, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158: 1411–1422, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenanova VE, Olafsen T, Salazar FB, Williams LE, Knowles S, Wu AM. Tuning the serum persistence of human serum albumin domain III:diabody fusion proteins. Protein Eng Des Sel 23: 789–798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, du Bois RM. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet 374: 222–228, 2009 [DOI] [PubMed] [Google Scholar]
- 33. King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 164: 1025–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 34. King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 164: 1171–1181, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 29: 537–544, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Korn JH, Halushka PV, LeRoy EC. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest 65: 543–554, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kujubu DA, Reddy ST, Fletcher BS, Herschman HR. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen-stimulated Swiss 3T3 cells. J Biol Chem 268: 5425–5430, 1993 [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 291: L144–L156, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci USA 89: 3917–3921, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett 330: 156–160, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Oga T, Matsuoka T, Yao C, Nonomura K, Kitaoka S, Sakata D, Kita Y, Tanizawa K, Taguchi Y, Chin K, Mishima M, Shimizu T, Narumiya S. Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat Med 15: 1426–1430, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Okamoto T, Hino O. Expression of cyclooxygenase-1 and -2 mRNA in rat tissues: tissue-specific difference in the expression of the basal level of mRNA. Int J Mol Med 6: 455–457, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Oppenheimer-Marks N, Kavanaugh AF, Lipsky PE. Inhibition of the transendothelial migration of human T lymphocytes by prostaglandin E2. J Immunol 152: 5703–5713, 1994 [PubMed] [Google Scholar]
- 45. Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E, Capelozzi VL. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration 74: 159–169, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964 [DOI] [PubMed] [Google Scholar]
- 47. Saltzman LE, Moss J, Berg RA, Hom B, Crystal RG. Modulation of collagen production by fibroblasts. Effects of chronic exposure to agonists that increase intracellular cyclic AMP. Biochem J 204: 25–30, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seibert K, Masferrer JL, Fu JY, Honda A, Raz A, Needleman P. The biochemical and pharmacological manipulation of cellular cyclooxygenase (COX) activity. Adv Prostaglandin Thromboxane Leukot Res 21A: 45–51, 1991 [PubMed] [Google Scholar]
- 49. Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol 152: 3606–3614, 1994 [PubMed] [Google Scholar]
- 50. Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62: 167–215, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Stratton R, Rajkumar V, Ponticos M, Nichols B, Shiwen X, Black CM, Abraham DJ, Leask A. Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J 16: 1949–1951, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Swigris JJ, Kuschner WG, Kelsey JL, Gould MK. Idiopathic pulmonary fibrosis: challenges and opportunities for the clinician and investigator. Chest 127: 275–283, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Takahashi HK, Liu K, Wake H, Mori S, Zhang J, Liu R, Yoshino T, Nishibori M. Prostaglandin E2 inhibits advanced glycation end product-induced adhesion molecule expression, cytokine production, and lymphocyte proliferation in human peripheral blood mononuclear cells. J Pharmacol Exp Ther 331: 656–670, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol 25: 40–46, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Vancheri C, Mastruzzo C, Tomaselli V, Sortino MA, D'Amico L, Bellistri G, Pistorio MP, Salinaro ET, Palermo F, Mistretta A, Crimi N. Normal human lung fibroblasts differently modulate interleukin-10 and interleukin-12 production by monocytes: implications for an altered immune response in pulmonary chronic inflammation. Am J Respir Cell Mol Biol 25: 592–599, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Vancheri C, Sortino MA, Tomaselli V, Mastruzzo C, Condorelli F, Bellistri G, Pistorio MP, Canonico PL, Crimi N. Different expression of TNF-alpha receptors and prostaglandin E(2)Production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am J Respir Cell Mol Biol 22: 628–634, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 39: 45–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wynn TA. IL13 effector functions. Annu Rev Immunol 21: 425–456, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, Xiao Y, Guo Z, Gao J. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice (Abstract). Respir Res 11: 34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]