Abstract
Estrogens can either relax or contract arteries via rapid, nongenomic mechanisms involving classic estrogen receptors (ER). In addition to ERα and ERβ, estrogen may also stimulate G protein-coupled estrogen receptor 1 (GPER) in nonvascular tissue; however, a potential role for GPER in coronary arteries is unclear. The purpose of this study was to determine how GPER activity influenced coronary artery reactivity. In vitro isometric force recordings were performed on endothelium-denuded porcine arteries. These studies were augmented by RT-PCR and single-cell patch-clamp experiments. RT-PCR and immunoblot studies confirmed expression of GPER mRNA and protein, respectively, in smooth muscle from either porcine or human coronary arteries. G-1, a selective GPER agonist, produced a concentration-dependent relaxation of endothelium-denuded porcine coronary arteries in vitro. This response was attenuated by G15, a GPER-selective antagonist, or by inhibiting large-conductance calcium-activated potassium (BKCa) channels with iberiotoxin, but not by inhibiting NO signaling. Last, single-channel patch-clamp studies demonstrated that G-1 stimulates BKCa channel activity in intact smooth muscle cells from either porcine or human coronary arteries but had no effect on channels isolated in excised membrane patches. In summary, GPER activation relaxes coronary artery smooth muscle by increasing potassium efflux via BKCa channels and requires an intact cellular signaling mechanism. This novel action of estrogen-like compounds may help clarify some of the controversy surrounding the vascular effects of estrogens.
Keywords: selective estrogen receptor modulator, calcium-activated potassium channels, coronary, G-1, G15
estrogen induces a rapid, nongenomic relaxation of coronary arteries in vitro (2, 16) and can also enhance blood flow in intact hearts (17, 21). On the other hand, more recent studies indicate that estrogen can contract coronary arteries by increasing oxidative stress under certain conditions (28). Clinical trials indicate that estrogen replacement therapy (ERT) may increase the risk of coronary heart disease in postmenopausal women (22), thus limiting the utility of ERT. As an alternative, selective estrogen receptor modulators (SERMs) are increasingly being employed (24). However, the mechanism(s) of SERM action, especially in cardiovascular tissues, is largely unknown. SERMs and other estrogen-like compounds may function as either agonists, antagonists, or partial agonists of classic estrogen receptors (ERα and ERβ), and increasing evidence indicates that some of these compounds may also activate a more novel transmembrane estrogen-binding protein, G protein-coupled estrogen receptor 1 (GPER) (7, 20).
Although studies have implicated GPER in mediating effects of estrogen on protein transcription and cell proliferation, the potential role of GPER in mediating nongenomic effects of estrogen in the vasculature remains to be established. Infusion of a selective GPER agonist (G-1) reduced mean arterial pressure acutely in rats and relaxed human mammary arteries in vitro (8); however, a molecular effector mechanism underlying GPER-mediated vasodilation was not identified. Moreover, there is virtually nothing known about GPER signaling in coronary arteries. We now provide tissue and cellular evidence that GPER can mediate estrogen signaling in coronary arteries: endothelium-independent relaxation, which involves stimulation of large-conductance, calcium- (BKCa) and voltage-activated potassium channels expressed in coronary artery smooth muscle (CASM). These findings provide direct evidence that signaling via GPER activation can contribute to the vascular effects of estrogens and suggest a potential rationale for why some SERMs may offer benefits of traditional ERT with fewer potential side effects.
MATERIALS AND METHODS
Cell culture.
Human coronary artery smooth muscle cells were purchased from Cambrex and grown in phenol red free smooth muscle growth medium with 5% fetal bovine serum (FBS), as described previously (29). Steroid hormones and growth factors were removed from FBS by charcoal stripping. MCF-7 cells were purchased from American Type Culture Collection. Porcine coronary arteries were isolated from fresh porcine hearts obtained from a local abattoir. Single CASM myocytes were isolated as described previously (27). Briefly, the media layer from fresh coronary arteries was dissected free from adventitia and gently shaken (37°C) for 30 min in a dissociation medium containing 45 μM papain, 4 mM dithiothreitol, and 0.2% bovine serum albumin. Cells were then dissociated by gentle trituration. Experiments were performed 6–8 h after isolation.
Tension studies.
Porcine coronary arteries were obtained as described previously (28, 29). Briefly, left anterior descending (LAD) coronary arteries were dissected and cleaned of excess fat and connective tissue. Two to four 5-mm rings were obtained from each LAD and prepared for isometric contractile force recordings. To control for possible indirect effects of endothelium-derived vasoactive factors, the endothelium was removed physically by rubbing the intimal surface and tested by observing the absence of acetylcholine-induced relaxation of the initial stabilizing contraction. Rings were mounted on two triangular tissue supports, with one support fixed to a stationary glass rod and the other attached to a force displacement transducer. Isometric contractions or relaxations were recorded on a PC computer using MacLab software. The tissue bathing solution was the modified Krebs-Henseleit buffer (in mM): 122 NaCl, 4.7 KCl, 15.5 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, and 11.5 glucose, pH 7.2. The solution was oxygenated continuously (95% O2-5% CO2) and maintained at 37°C. Coronary artery ring preparations were equilibrated for 90 min under an optimal resting tension of 2 g, and fresh bath solution was added to the tissue chamber every 30 min to prevent accumulation of metabolic end products. After the initial equilibration, preparations were exposed to effective concentrations of a contractile agonist, i.e., 1 μM prostaglandin (PG)F2α, to insure stabilization of the muscles. Vasodilatory responses were calculated as the percent reduction in tension (i.e., relaxation) from the precontracted state. Pharmacological inhibitors were allowed to equilibrate with the arteries for ≥30 min prior to measurement of a complete G-1 concentration-response relationship (1–3,000 nM). One vessel was exposed only to the constrictor agent to control for potential fading of the contractile response. In addition, control responses to DMSO vehicle alone were also measured, and the slight relaxation response to higher concentrations of DMSO was substracted from responses to G-1.
Patch-clamp studies.
Cell-attached and excised patch-clamp recordings on isolated coronary myocytes were performed as described previously (27, 29). Whole cell and cell-attached patch-clamp recordings on single cells were performed as described previously (27). For perforated-patch, whole cell recordings, cells were placed in a recording solution of the following composition (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 20 HEPES, and 20 glucose (pH 7.2). Patch pipettes (≤3 MΩ) were fabricated from capillaries by a P-2000 laser pipette puller (Sutter Instruments). The tip of patch pipette was filled with a solution containing (in mM) 90 KCH3SO3, 40 KCl, 5 MgCl2, and 20 HEPES to approximate normal cellular [K+] and [Cl−] (pH 7.2 with KOH). The remainder of the pipette was back-filled with a similar solution to which 200 μg/ml amphotericin B (diluted by sonication from a 50 mg/ml stock in dimethylsulfoxide) was added. Voltage clamp and voltage pulse generation was controlled with an Axopatch 200B patch-clamp amplifier (Molecular Devices). Data were acquired and analyzed with pCLAMP 10.0 (Molecular Devices). Voltage-activated currents were filtered at 2 kHz and digitized at 10 kHz. Capacitative and leakage currents were subtracted digitally. For cell-attached patch studies, the recording chamber contained the following solution (in mM): 140 KCl, 10 MgCl2, 0.1 CaCl2, 10 HEPES, and 30 glucose (pH 7.4, 22–25°C). Patch pipettes (2–5 MΩ) were filled with Ringer's solution (in mM): 110 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, and 10 HEPES. Voltage across the patch was controlled by clamping the cell at 0 mV with the high-concentration extracellular potassium solution. In experiments recording the potassium channel activity of inside-out patches, the bathing solution exposed to the cytoplasmic surface of the membrane consisted of the following (in mM): 60 K2SO4, 30 KCl, 2 MgCl2, 0.16 CaCl2, 1 BAPTA (pCa 7), 10 HEPES, 5 ATP, and 10 glucose (pH 7.4, 22–25°C). The pipette solution was the same Ringer's solution described above. Average channel activity (expressed as number of channels × single-channel open probability; NPo) in patches with multiple BKCa channels was determined as described previously (27). NPo calculations were based on 10–15 s of continuous recording during periods of stable channel activity. Although channel activity was observed at a variety of membrane potentials, most single-channel data were analyzed at a potential of +40 mV, where BKCa channel openings are easily distinguished from other channel species to permit more accurate statistical analysis (10).
RT-PCR.
Total RNA was extracted from human CASM cells and MCF-7 cells and porcine coronary arteries using the TRI reagent (Sigma-Aldrich). Deoxyribonuclease 1 was used in the extracts to remove any genomic DNA contamination. Qiagen OneStep RT-PCR kit was used in this study. The sequences of the primers for GPER were as follows: porcine coronary arteries, sense 5′-GTGGCCGACTCCCTGATCG-3′ and antisense 5′-CGGGCATGGTGCTTGGTGC-3′ (product size is 200 bp) (1); human CASMC, sense 5′-GGCTTTGTGGGCAACATA-3′ and antisense 5′-CGGAAAGACTGCTTGCAGG-3′ (product size is 679 bp). PCR was performed for 40 cycles in an Eppendorf Mastercycler Gradient (Eppendorf, Hamburg, Germany): 94 for 1 min, 55 for 1 min, and 72 for 1 min. β-Actin primers (sense 5′-AGTCCTGTGGCATCCACGAAACTA and antisense ACTGTGTTGGCGTACAGGTCTTTG) were used as internal standard (13). The PCR products were analyzed on 1.5% agarose gel and stained with ethidium bromide.
Western blot.
Western blotting analysis was used to detect GPER. After being cleaned of excess fat and connective tissue and having endothelium removed by rubbing the intimal surface, LAD coronary arteries were snap-frozen in liquid nitrogen, pulverized (Fisher Scientific), and then lysed in homogenization buffer of the following composition: 50 mM Tris·HCl, 0.1 mM EGTA, 0.1 mM EDTA, 0.1% SDS, 1% NP-40, and 0.1% deoxycholic acid. Human CASM cells and MCF-7 cells were harvested and homogenized in the same solution. Protein concentrations were determined by Bio-Rad DC protein assay and separated on SDS-polyacrylamide gels with a Mini Protean II (Bio-Rad) gel kit according to the manufacturer's instructions. Proteins were then transferred to Hybond enhanced chemiluminescence (ECL) membrane (Amersham Pharmacia Biotech) with a Mini-Trans-Blot Electrophoretic Transfer Cell (Bio-Rad) at 100 V for 1 h. Membranes were blocked with 5% nonfat milk for 1 h at room temperature and then rinsed with Tris-buffered saline-Tween (TBST) three times for 15 min. Membranes were then probed with GPER primary antibody (Santa Cruz Biotechnology) in TBST containing 5% nonfat milk protein for overnight at 4°C. After washing, the membranes were then incubated with anti-rabbit IgG conjugated to horseradish peroxidase and visualized with an ECL system (Amersham). The membranes were stripped and then immunoblotted with β-actin antibody (Sigma-Aldrich) for protein loading control.
Statistical analysis.
Data were expressed as means ± SE. Significance between two groups was evaluated by Student's t-test for paired data. Comparison between multiple groups was made by analysis of variance. Probability of <0.05 indicated a significant difference.
Materials.
Antibodies were purchased from Santa Cruz Biotechnology, and G-1 was purchased from Calbiochem. All other chemicals were purchased from Sigma-Aldrich. Primers were purchased from Integrated DNA Technologies.
RESULTS
GPER is activated by 17β-estradiol and other estrogen-like compounds; however, the cardiovascular effects of these agents are poorly understood. To better target and identify the effects of GPER stimulation in CASM, we measured relaxation of endothelium-denuded porcine coronary arteries in response to G-1, which exhibits nanomolar affinity for GPER but does not bind ERα or ERβ (19). Endothelium-denuded coronary arteries were precontracted with 1 μM PGF2α, and cumulative addition of G-1 produced a concentration-dependent relaxation in coronary arteries. On average, the maximal relaxation effect of G-1 was 44.5 ± 3.2% (3 μM, n = 23; (Fig. 1A) with an average EC50 of 6.28 × 10−8 M. We then verified the specificity of action on GPER by employing a selective GPER inhibitor, G15 (4). Pretreating arteries with 3 μM G15 significantly attenuated G-1-induced relaxation at all G-1 concentrations above 1 nM (Fig. 1C). The average maximal G-1-induced (3 μM) relaxation was reduced nearly one-half (10.5 ± 2.0%, n = 21, P < 0.003) by G15 treatment; however, there was no significant change in EC50 value (5.2 × 10−8) in the presence of G15. However, parallel studies indicated a minor relaxation effect in the presence of solvent (DMSO) alone (Fig. 1A, top curve). Care was taken to assure that the total concentration of DMSO never exceeded 0.1% at any time. Therefore, to obtain a more accurate response to G-1, the G-1 concentration-response relationship was normalized by subtracting out this control component, and the resulting G-1 concentration-response relationship is illustrated in Fig. 1B.
Fig. 1.
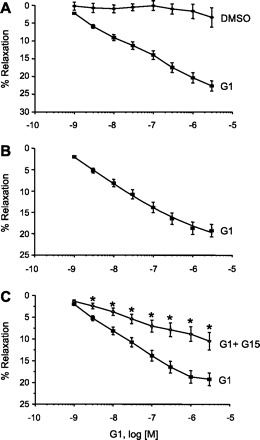
Stimulation of G protein-coupled estrogen receptor 1 (GPER) relaxes endothelium-denuded, precontracted coronary arteries. A: complete concentration-response relationship for G-1-induced relaxation. Each point represents the mean relaxation effect ± SE (n = 29). Top curve is a control concentration-response relationship in the presence of solvent (DMSO) alone (n = 6). B: actual G-1-induced relaxation response after normalization by subtracting the control component. C: complete concentration-response relationship for G-1-induced relaxation in the presence (○) or absence (■) of G15, a GPER antagonist. Each point represents the mean relaxation effect ± SE (n = 21). *P < 0.05.
Molecular experiments were done to confirm GPER expression in CASM. RT-PCR studies employed primers for both porcine and human GPER. RT-PCR results demonstrated that high levels of GPR30 mRNA expression were detected successfully in both human CASM cells and porcine coronary arteries (n = 3; Fig. 2) together with β-actin (data not shown). These findings are the first to report GPER mRNA expression coronary artery. Previous studies demonstrated that MCF-7 breast cancer tumor cells express GPER (7), and these cells were employed as a positive control for GPER mRNA in each study. In addition to these RT-PCR studies, immunoblotting experiments detected expression of an ∼38-kDa GPER protein in CASM from either porcine coronary arteries or human CASM cells (n = 3; Fig. 2B). GPER expression in MCF-7 cells was again employed as a positive control.
Fig. 2.
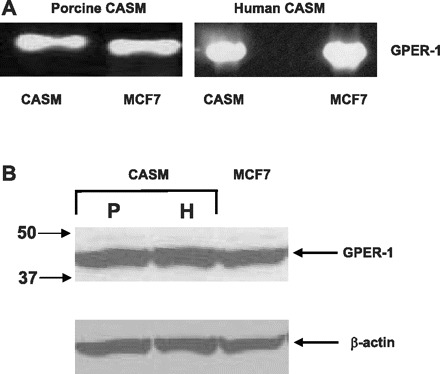
Coronary artery smooth muscle (CASM) cells express GPER. A: GPER mRNA is expressed in porcine and human CASM. RT-PCR products from MCF-7 cells were employed as positive controls. B: immunoblot detection of GPER protein expression in coronary myocytes from either porcine (P) or human (H) arteries (n = 3). MCF-7 cells were employed as positive controls. β-Actin was employed as a control for protein loading.
We had demonstrated previously that estrogen-induced relaxation of CASM (via ERα) involves nitrix oxide (NO) production and activation of the large-conductance BKCa channel (12). In contrast, inhibiting NO signaling had no effect on G-1-induced coronary artery relaxation. Inhibition of NO synthase (NOS) activity with Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) did not attenuate G-1-induced coronary artery relaxation significantly at any concentration (P > 0.05, n = 6; Fig. 3A). In addition, G-1-induced relaxation was unaffected by pretreating arteries with 10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, a selective inhibitor of guanylyl cyclase activity (n = 5; data not shown). However, similar to 17β-estradiol, the relaxation effect of G-1 was dependent upon potassium efflux from CASM cells. G-1-induced coronary artery relaxation was inhibited by 100 nM iberiotoxin, a highly selective inhibitor of BKCa channels (23). After the data were normalized by controlling for vehicle effects, iberiotoxin inhibited G-1-induced relaxation (1 μM) by an average of ∼65% [control 18.7 ± 1.4, iberiotoxin (IBTx) 6.8 ± 1.3%, n = 5, P = 0.001; Fig. 3B]. A completely normalized concentration-response relationship for G-1-induced relaxation in the absence and presence of 100 nM IBTx is illustrated in Fig. 3C and indicates a significant inhibition of G-1-induced relaxation at all concentrations >10 nM (n = 5).
Fig. 3.
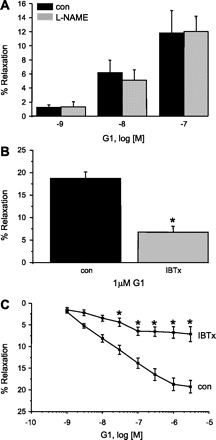
GPER-mediated coronary artery relaxation is nitric oxide independent and involves large-conductance, calcium-activated potassium (BKCa) channel activity. A: pretreating (30 min) coronary arteries with 100 μM Nω-nitro-l-arginine methyl ester (l-NAME) to inhibit nitric oxide synthase had no effect on G-1-induced relaxation. Each bar represents the mean ± SE (n = 6) B: pretreating vessels (30 min) with 100 nM iberiotoxin (IBTx; 30 min; n = 5) attenuated 1 μM G-1-induced relaxation significantly (*P = 0.001). C: complete concentration-response relationship for G-1-induced relaxation in the absence (■) or presence (○) of 100 nM IBTx (n = 5). *P < 0.05 or lower. Con, control.
To more directly identify a stimulatory effect of G-1 on BKCa channels, single-channel patch clamp experiments were performed. In cell-attached patches on intact porcine CASM myocytes, we observed that membrane electrical activity was dominated by a large-conductance, outwardly conducting channel that we have identified previously as the BKCa channel in these same cells (27, 29). As illustrated in Fig. 4A, we observed rather minimal activity of BKCa channels under control conditions (25–28°C, +40 mV). In contrast, BKCa channel activity increased dramatically after cells were treated with 100 nM G-1. On average, G-1 increased BKCa channel activity from an NPo of 0.006 ± 0.002 to 0.313 ± 0.092 (n = 4, P < 0.03). A time course plot of BKCa channel activity is illustrated in Fig. 4. We confirmed the identity of this molecule as the BKCa channel by measuring activity of excised inside-out membrane patches from porcine CASM myocytes (Fig. 4B). Under physiological concentrations of calcium (100 nM) at the cytoplasmic surface of the membrane, channel activity was minimal. In contrast, when [Ca2+]i was raised to 100 μM, there was a dramatic increase in channel opening (Fig. 4B, middle). On average, channel NPo was increased from 0.001 ± 0.001 to 0.684 ± 0.178 (n = 3, P < 0.05) by increasing [Ca2+]i. Furthermore, subsequent addition of 1 mM tetraethylammonium (TEA) abolished calcium-stimulated channel activity (Fig. 4B, right). At concentrations of 1 mM or less, TEA exhibits selectivity for blockade of BKCa channels.
Fig. 4.
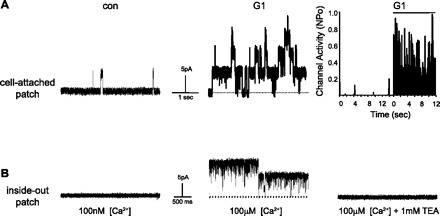
G-1 stimulates BKCa channel activity in porcine CASM cells. A: typical recordings from the same cell-attached patch (+40 mV) before (con) and 20 minutes after application of 100 nM G-1. Upward deflections indicate channel opening from the closed state (dashed line). Right: activity histogram of BKCa channel open probability (NPo) before and 20 minutes after application of 100 nM G-1. Total recording time under each condition was 12–14 s. Break in the time axis represents drug incubation. B: typical recordings from the same excised inside-out patch from a porcine CASM cell with either 100 nM (left) or 100 μM (middle) [Ca2+] exposed to the cytoplasmic face of the membrane (+40 mV). Right: recording after subsequent addition of 1 mM tetraethylammonium (TEA) to the cytoplasmic surface of the membrane (100 μM [Ca2+]).
We further confirmed the ability of G-1 to open a BKCa channel in CASM by observing a stimulatory effect of G-1 in myocytes from human coronary arteries (human CASM). As illustrated in Fig. 5A, addition of 100 nM G-1 to a cell-attached patch on a human CASM cell increased channel activity substantially. On average, channel activity increased from an NPo of 0.012 ± 0.004 to 0.027 ± 0.003 (n = 3, P < 0.05; Fig. 5, right). In contrast to these experiments on intact myocytes, G-1 was unable to stimulate BKCa channels isolated in a cell-free, inside-out patch (Fig. 5B). When we excised the membrane patch into the microscope chamber, the cytoplasmic surface of the patch was immediately exposed to a supraphysiological level of calcium (100 μM). As expected, this resulted in a dramatic increase channel activity, confirming that this protein is indeed the BKCa channel that we have characterized extensively in these same cells previously (29, 30). When [Ca2+] was subsequently lowered to a more physiological 100 nM, channel activity immediately decreased as expected. However, in contrast to what we observed in cell-attached patches, addition of 100 nM G-1 to this excised patch had no effect on BKCa channel activity (Fig. 5B, right). These experiments provide direct evidence that excludes potential effects of G-1 directly on the BKCa channel protein and demonstrate the necessity of an intact cellular transduction mechanism.
Fig. 5.
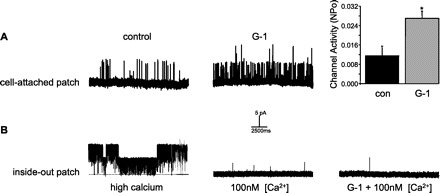
G-1 stimulates BKCa channel activity in human CASM cells. A: recordings (+40 mV) from the same cell-attached patch on human CASM cells before (control) and 25 min after addition of 100 nM G-1. Upward deflections indicate channel opening from the closed state (dashed line). Right: average increase in BKCa channel activity (NPo) stimulated by G-1. Bars represent the average of 3 membrane patches ± SE. B: recordings from the same excised inside-out patch from human CASM cells. Upward deflections indicate channel opening from the closed state (dashed line). Left: BKCa channel activity in the presence of high calcium (100 μM) at the cytoplasmic surface of the patch. Middle: channel activity recorded after calcium levels were lowered to 100 nM. Right: G-1 (100 nM) had no effect on channel activity in excised patch. *P < 0.05.
DISCUSSION
Recent studies suggest that GPER, a nonclassic, membrane-associated, estrogen-binding protein, may mediate rapid estrogen signaling in certain cell types (20); however, the role of GPER in mediating estrogen responses is controversial. For example, GPER-deficient mice exhibit a normal reproductive phenotype, indicating a nonessential role for GPER in mediating hypothalamic-pituitary-gonadal function (18). In contrast, other studies indicate an important if not essential role for GPER in reproductive function. Inhibition of GPER mRNA and protein expression markedly suppresses estrogen-stimulated follicle formation in hamster ovary (26), and estrogen-stimulated proliferation of mouse sperm cells is mediated via GPER (25). Thus, the physiological role of GPER remains unclear, and even less is known of a potential role for GPER in cardiovascular function. Although GPER mRNA has been detected in human blood vessels (9), we know little of its function and cellular/molecular transduction mechanisms in the cardiovascular system.
G-1 is reported to be a GPER agonist that exhibits nanomolar affinity for GPER but does not bind ERα or ERβ (19). We had demonstrated previously that ICI 182,780, a nonselective ER antagonist, relaxes porcine coronary arteries in vitro by an endothelium-independent mechanism (11). Meyer et al. (14) have also reported that G-1 and ICI 182,780 relaxed porcine epicardial coronary artery rings, but the mechanism of G-1 action was not investigated in detail in this study. The present results now demonstrate that G-1 induces endothelium-independent relaxation of coronary arteries and that this response is attenuated by G15 (selective GPER antagonist). These studies strongly suggest that activation of GPER in CASM causes relaxation. Furthermore, our functional studies are supported by RT-PCR and immunoblot experiments verifying expression of GPER in both porcine and human CASM. Taken together, these findings implicate a role for GPER in mediating acute estrogen and/or SERM action on CASM and hence, cardiac blood flow. Support for this mechanism is gained from prior studies indicating GPER mRNA expression in smooth muscle from human internal mammary arteries and saphenous veins (9) and findings that G-1 administration lowers blood pressure in vivo (8). Therefore, accumulating evidence indicates a novel, nongenomic mechanism mediating the effect of estrogens on coronary arteries, i.e., signaling via a G protein-coupled receptor that does not involve activation of either ERα or ERβ. It is unknown whether our findings on GPER signaling in CASM can be extended to coronary endothelium, which is also a target tissue of estrogen action.
Signaling mechanisms downstream to GPER activation in CASM remain to be elucidated. We have reported that estrogen-induced coronary artery relaxation involves generation of NO in both porcine and human CASM (3, 27, 29). In contrast, we found that inhibiting NOS activity in coronary arteries with l-NAME had no effect on G-1-induced relaxation. Moreover, inhibiting the activity of guanylyl cyclase (a primary target for NO signaling) also had no effect on responses to G-1. Taken together, these findings indicate a NO-independent mechanism of action for G-1/GPER in CASM. These findings are consistent with our previous report that ICI 182,780 does not stimulate NO production in CASM (12). Therefore, we conclude that GPER-mediated relaxation of CASM, unlike 17β-estradiol-stimulated coronary artery relaxation via ERα, is not dependent upon NO production. The fact that GPER-induced relaxation was endothelium independent further supports the idea that NO was not involved in this response. However, similar to 17β-estradiol, we found evidence that GPER activation leads to stimulation of BKCa channel activity in CASM.
Our tension studies demonstrated that coronary artery relaxation induced by G-1 involves potassium efflux. These findings implicate potassium channels in the vascular response to GPER activation. The fact that G-1-mediated relaxation was inhibited by iberiotoxin, which is a highly selective antagonist of BKCa channels, provides strong pharmacological evidence for involvement of this protein in GPER-mediated relaxation. Definitive evidence that GPER activation stimulates BKCa channel activity in CASM was then provided by single-cell, patch-clamp recordings on myocytes isolated from porcine and human coronary arteries. As we have reported in a number of previous studies (3, 11, 27), ion channel activity in CASM is dominated by a large-conductance (∼200 pS) potassium channel that is stimulated by cytoplasmic calcium and inhibited by iberiotoxin. This BKCa channel is a known target of estrogen action and is an important effector molecule that induces membrane repolarization and CASM relaxation.
We found G-1 to be a powerful stimulator of BKCa channel activity in intact CASM myocytes from either porcine or human arteries. Interestingly, however, G-1 was ineffective at stimulating channels isolated in excised membrane patches. These findings provide direct evidence that the BKCa channel protein complex is not a functional substrate for G-1 binding. Rather, G-1 action on CASM requires an intact cellular signaling mechanism involving diffusible cytosolic and/or membrane-associated second messenger compounds. Although we can find no previous patch-clamp studies that have employed G-1, there is a previous report that ICI 182,780 can stimulate BKCa channels in colonic smooth muscle cells (6). Therefore, we speculate that the effect of ICI 182,780 in these nonvascular smooth muscle cells is also mediated via GPER. In light of these findings, it is becoming apparent that estrogen and estrogen-like compounds may stimulate distinct receptor proteins in CASM (e.g., ERα, ERβ, and/or GPER), but ultimately these transduction pathways appear to converge on a common effector protein, the BKCa channel. Thus, the present findings from intact arteries and isolated myocytes are entirely consistent with a novel, nongenomic mechanism of estrogen action in the cardiovascular system, i.e., activation of GPER, which relaxes CASM via stimulation of BKCa channel activity. We certainly have not excluded other potential signaling mechanisms for GPER but propose BKCa channel activity to be a likely downstream effector mediating GPER-induced coronary artery relaxation.
In conclusion, because of increased cardiovascular risk associated with ERT, other estrogen-like compounds (e.g., SERMs) have been recommended as an alternative therapy (15). These compounds have a diversity of chemical structure and may have a unique and unpredictable array of clinical responses (24). The present study has demonstrated that, like estrogen, a nonsteroidal GPER agonist (G-1) relaxes endothelium-denuded coronary arteries. These studies indicate a novel, nongenomic estrogen-signaling mechanism in CASM, which we found to express GPER mRNA and protein. More conclusive proof of GPER involvement in these responses will require use of GPER-knockout mice, and future studies will certainly include such additional measures. Nonetheless, the present studies suggest a common signaling mechanism for nongenomic estrogen action in CASM, i.e., stimulation of GPER leading ultimately to increased activity of BKCa channels and coronary artery relaxation. Unlike 17β-estradiol, this response does not appear to involve NO production, nor does it require activation of classic ERs. We propose that GPER activation can enhance coronary blood flow and thus may help protect against myocardial ischemia. This hypothesis is supported by a recent finding that G-1 is cardioprotective when administered to rat hearts in vitro (5), although this protection also involved GPER expressed in cardiac myocytes. In summary, these findings strongly support the caveat that estrogen-like compounds used for treating specific disease states (e.g., cancer) may also affect other organs and systems.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-073890, HL-080402, and HL-068026. G. Han was supported in part by a Scientist Development Grant (0430226N) and a Beginning Grant-In-Aid (11BGIA7370061) from the American Heart Association.
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Louise Meadows for expert technical assistance.
REFERENCES
- 1. Canonaco M, Giusi G, Madeo A, Facciolo RM, Lappano R, Canonaco A, Maggiolini M. A sexually dimorphic distribution pattern of the novel estrogen receptor G-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol 196: 131–138, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Chester AH, Jiang C, Borland JA, Yacoub MH, Collins P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron Artery Dis 6: 417–422, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol Heart Circ Physiol 272: H2765–H2773, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol 5: 421–427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297: H1806–H1813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dick GM. The pure anti-oestrogen ICI 182,780 (Faslodex) activates large conductance Ca2+-activated K+ channels in smooth muscle. Br J Pharmacol 136: 961–964, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Haas E, Bhattacharya I, Brailoiu E, DamjanoviĆ M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49: 1358–1363, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Han G, Kryman JP, McMillin PJ, White RE, Carrier GO. A novel transduction mechanism mediating dopamine-induced vascular relaxation: opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J Cardiovasc Pharmacol 34: 619–627, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Han G, Ma H, Chintala R, Fulton DJ, Barman SA, White RE. Essential role of the 90-kilodalton heat shock protein in mediating nongenomic estrogen signaling in coronary artery smooth muscle. J Pharmacol Exp Ther 329: 850–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han G, Yu X, Lu L, Li S, Ma H, Zhu S, Cui X, White RE. Estrogen receptor alpha mediates acute potassium channel stimulation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther 316: 1025–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Li S, Kuhne WW, Kulharya A, Hudson FZ, Ha K, Cao Z, Dynan WS. Involvement of p54(nrb), a PSF partner protein, in DNA double-strand break repair and radioresistance. Nucleic Acids Res 37: 6746–6753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the g protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86: 58–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohandas B, Mehta JL. Lessons from hormone replacement therapy trials for primary prevention of cardiovascular disease. Curr Opin Cardiol 22: 434–442, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res 27: 1939–1942, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Node K, Kitakaze M, Kosaka H, Minamino T, Sato H, Kuzuya T, Hori M. Roles of NO and Ca2+-activated K+ channels in coronary vasodilation induced by 17beta-estradiol in ischemic heart failure. FASEB J 11: 793–799, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80: 34–41, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol 109: 350–353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci 29: 116–123, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Raddino R, Manca C, Poli E, Bolognesi R, Visioli O. Effects of 17 beta-estradiol on the isolated rabbit heart. Arch Int Pharmacodyn Ther 281: 57–65, 1986 [PubMed] [Google Scholar]
- 22. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Schroeder N, Mullmann TJ, Schmalhofer WA, Gao YD, Garcia ML, Giangiacomo KM. Glycine 30 in iberiotoxin is a critical determinant of its specificity for maxi-K versus KV channels. FEBS Lett 527: 298–302, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 63: 163–181, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Ando S, Maggiolini M, Pezzi V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 149: 5043–5051, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology 149: 4452–4461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res 77: 936–942, 1995 [DOI] [PubMed] [Google Scholar]
- 28. White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol 289: H1468–H1475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res 53: 650–661, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Zhu S, Han G, White RE. PGE2 action in human coronary artery smooth muscle: role of potassium channels and signaling cross-talk. J Vasc Res 39: 477–488, 2002 [DOI] [PubMed] [Google Scholar]


