Abstract
The significance of lipid droplets (LD) in lipid metabolism, cell signaling, and membrane trafficking is increasingly recognized, yet the role of the LD phospholipid monolayer in LD protein targeting and function remains unknown. To begin to address this issue, two populations of LD were isolated by ConA sepharose affinity chromatography: 1) functionally active LD enriched in perilipin, caveolin-1, and several lipolytic proteins, including ATGL and HSL; and 2) LD enriched in ADRP and TIP47 that contained little to no lipase activity. Coimmunoprecipitation experiments confirmed the close association of caveolin and perilipin and lack of interaction between caveolin and ADRP, in keeping with the separation observed with the ConA procedure. The phospholipid monolayer structure was evaluated to reveal that the perilipin-enriched LD exhibited increased rigidity (less fluidity), as shown by increased cholesterol/phospholipid, Sat/Unsat, and Sat/MUFA ratios. These results were confirmed by DPH-TMA, NBD-cholesterol, and NBD-sphingomyelin fluorescence polarization studies. By structure and organization, the perilipin-enriched LD most closely resembled the adipocyte PM. In contrast, the ADRP/TIP47-enriched LD contained a more fluid monolayer membrane, reflecting decreased polarizations and lipid order based on phospholipid fatty acid analysis. Taken together, results indicate that perilipin and associated lipolytic enzymes target areas in the phospholipid monolayer that are highly organized and rigid, similar in structure to localized areas of the PM where cholesterol and fatty acid uptake and efflux occur.
Keywords: lipolysis, caveolae, caveolin, concanavalin-A, adipose differentiation-related protein
it is increasingly clear that lipid droplets (LD) are dynamic organelles with regulatory roles in many cellular processes besides lipid metabolism, including cell signaling, immune function, membrane trafficking, and regulation of longevity (42, 70). Whereas the mechanism of these processes is only partially understood, much less is known about how the structure of the LD phospholipid monolayer may affect LD protein targeting and function. Embedded in the monolayer that surrounds the LD neutral lipid (NL) core are proteins, such as perilipins (65) and adipose differentiation-related protein (ADRP) (16, 41), that coat the LD surface and lipids such as cholesterol (4, 80) that help to define the LD structure. Perilipin and ADRP are two members of the PAT [perilipin, ADRP, tail-interacting protein 47 (TIP47)] family from the Plin group of genes (56) that were initially thought to act simply as barriers to protect the NL core against lipolytic action (63, 65). Current evidence suggests a more complex mechanism, one that requires coordination of perilipin with several lipases, including adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and the activator molecule comparative gene identification-58 (CGI-58), a protein with no lipolytic activity that increases ATGL activity to promote triacylglyerol hydrolysis (11, 14, 44). Perilipin and HSL upon hormonal stimulation are phosphorylated, causing HSL to translocate to the LD surface and perilipin to release CGI-58, which can then activate ATGL (105). Both lipases then work in concert to hydrolyze triacylglyerols stored in the LD NL core. Although it not known how lipases on the LD surface gain access to core lipids, studies with mice deficient in AGTL and HSL revealed that the primary lipase responsible for triacylglyerol metabolism was AGTL, whereas HSL was key to diacylglycerol hydrolysis (91). In the final step, monoacylglycerol lipase (MAGL) releases the last of the three fatty acid molecules and glycerol (3, 61). Despite a greater understanding of the molecular details regarding triacylglyerol hydrolysis, the influence of the LD phospholipid monolayer in regulating lipolysis remains largely unknown.
Apart from the surface coat proteins (perilipins, ADRP) and lipolytic enzymes (HSL, AGTL), other proteins associate with LD, including those involved in lipid synthesis, signaling, and membrane trafficking (13, 35, 64). Surprisingly, caveolins and flotillin, proteins commonly found in plasma membrane caveolae, were also detected (13, 34, 35, 64, 75, 76, 81). The presence of these proteins, along with cholesterol (9, 80) and sphingomyelin (48, 53, 57, 68, 101) in the LD phospholipid monolayer, gives rise to the hypothesis that localized areas may exist within the LD monolayer that on the basis of membrane structure attract proteins involved in lipolysis, much like the highly organized areas within the plasma membrane where lipid uptake/efflux occurs (18, 30, 62, 79). The affinity of cholesterol for sphingolipids represents a driving force for selective recruitment and sequestering of proteins of similar function (18). In the plasma membrane, cellular processes such as fatty acid transport protein-mediated fatty acid uptake/efflux and scavenger receptor class B1 (SR-B1)-mediated cholesterol ester uptake/efflux to HDL are organized within highly rigid cholesterol- and sphingomyelin-enriched domains in caveolin-1 containing caveolae and/or lipid rafts (30, 62, 79). Likewise, the presence of LD-organized domains represents a potential mechanism to describe protein-mediated lipid uptake/efflux from the NL interior. Consistent with this, kinetic analysis of HDL-mediated {22-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-23,24-bisnor-5-cholen-3b-ol} (NBD-cholesterol) efflux from LD in living cells detected multiple sterol domains (9). Efflux curves were best fit to a biexponential decay equation describing at least two cholesterol pools, the half-times of which were consistent with protein- (t1/2 ∼ 1 min) and vesicular-mediated (t1/2 = 10–20 min) sterol transfer. These results were similar to plasma membrane cholesterol distribution into multiple domains. The transbilayer distribution of cholesterol in the plasma membrane is asymmetric, with cholesterol enriched in the cytofacial leaflet (400%) vs. the exofacial leaflet (15, 52, 55, 88, 90), and transbilayer movement of cholesterol across the plasma membrane appears fast (t1/2 = 1–6 min). However, most of the plasma membrane cholesterol is localized in lateral domains that are relatively inert in terms of transfer kinetics (t1/2 = hours to days). Although movement between such domains is slow, small pools of cholesterol associated with caveolae and enriched in caveolins and the HDL receptor SR-B1 were found to be highly dynamic (93). Furthermore, sterol efflux from LD revealed multiple dynamic cholesterol domains, one of which was similar to the small, rapid pools found in the plasma membrane. In addition, kinetic analysis of HDL-mediated 6-{[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-hexanoyl}sphingosyl-phosphocholine (NBD-sphingomyelin) efflux from LD in living cells allowed resolution of multiple sphingomyelin pools, revealing a small, dynamic pool with a half-time of 11.8 min and another larger, relatively inert pool with a half-time on the order of hours to days (68). A comparison of LD and plasma membrane sphingomyelin dynamic pools revealed that the LD pool was smaller with faster efflux kinetics. Taken together, these studies indicate that, like the plasma membrane, LD contain resolvable lipid pools that are available to facilitate protein-mediated lipid uptake/efflux from the NL interior.
To extend the earlier discovery of dynamic sterol and sphingomyelin LD pools (9, 68), the investigation herein examined how the membrane characteristics of the LD phospholipid monolayer affected LD protein targeting and function. The present work demonstrates for the first time the following novel insights. Adipocyte LD proteins involved with lipolysis, including perilipins, HSL, and AGTL, target highly organized, rigid phospholipid membrane structures as observed by the higher cholesterol, phospholipid, and saturated phospholipid fatty acyl content in perilipin-enriched LD. Increased membrane rigidity in the phospholipid monolayer of perilipin-enriched LD was further indicated by significantly higher fluorescence polarizations in the presence of DPH-TMA, NBD-labeled cholesterol, and sphingomyelin. Moreover, radiolabeled lipolysis assays confirmed function. In all, these results indicate that perilipin and lipolytic LD proteins associate with highly organized, rigid membrane structures similar to those found in caveolin/cholesterol-rich regions within the plasma membrane wherein cholesterol and fatty acid uptake/efflux occur. These results point to an active role for the LD phospholipid monolayer in regulating lipid storage through protein and lipid membrane interactions.
MATERIALS AND METHODS
Materials.
Lipid standards were purchased from Nu-Chek Prep, (Elysian, MN) and Avanti (Alabasta, AL). Silica Gel G and Silica Gel 60 thin-layer chromatography (TLC) plates were from Analtech (Newark, DE) and EM Industries (Darmstadt, Germany), respectively. Concanavalin-A (ConA) sepharose resin was purchased from Pharmacia (Piscataway, NJ). Rabbit polyclonal antiserum to ADRP and TIP47 was prepared in house, as described (8). Rabbit anti-perilipin, anti-CD-14, and anti-Gsα were purchased from Abcam (Cambridge, MA). Rabbit anti-cytochrome c oxidase was purchased from Sigma (St. Louis, MO). Rabbit polyclonal anti-sera to caveolins and flotillin were purchased from BD Transduction Laboratories (Palo Alto, CA). Rabbit anti-H-Ras and anti-Smad7 were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA). Rabbit anti-Na+,K+-ATPase was purchased from Novus Biologicals (Littleton, CO). Rabbit anti-transferrin receptor (CD71) was purchased from Zymed Laboratory (San Francisco, CA). The following were obtained from Molecular Probes (Eugene, OR): 1,6-diphyenyl-1,3,5-hexatrienyl-trimethylammonium (DPH-TMA), NBD-sphingomyelin, and NBD-cholesterol. All reagents and solvents used were of the highest grade available and were cell culture tested.
Animals.
Male (8–12 wk old) inbred C57BL/6NCr mice used for adipocyte isolations were obtained from the National Cancer Institute (Frederick Cancer Research and Developmental Center). Mice were maintained on a standard rodent chow mix (5% calories from fat) and kept under a 12:12-h light-dark cycle in a temperature-controlled facility (25°C) with access to food and water ad libitum. Animal protocols were approved by the Institutional Animal Care and Use Committees at Texas A & M University and Michigan State University.
Isolation and resolution of adipocyte LD.
Mouse adipocytes were isolated from the epididymal fat pads of C57BL/6NCr mice, as described (21, 85). Briefly, mice (3–4 per isolation) were euthanized by CO2 asphyxiation, and the fat pads were removed and minced in HBSS buffer, followed by collagenase (type II; Sigma) digestion for 1 h at 37°C in a 400-rpm shaker. Floating fat cells, liberated from the tissue by gentle shaking, were filtered twice through sterile surgical gauze and washed two times with buffer. The cells were allowed to rest at 37°C for 30 min before the infranatant was removed from under the floating adipocyte layer with a sterile syringe. The remaining adipocytes were then prepared for organelle (LD, plasma membrane, and mitochondria) isolation. Adipocytes were placed in a N2 Bomb Cell Disrupter (Parr Instrument, Moline, IL) under 40 psi of N2 for 13 min, followed by separation on 30% Percoll in sucrose/Tris buffer by centrifuging at 70,000 g for 1 h. The adipocyte LD formed a distinct, white band on the surface of the Percoll preparation and was identified by Western blotting with known LD protein markers (ADRP, perilipin, TIP47). The plasma membrane fraction (located at the interphase between the Percoll and sample layer) and the mitochondrial fraction (located below the plasma membrane fraction) were identified by screening all of the visible protein bands separated by Percoll for presence of plasma membrane (transferrin receptor, Na+,K+-ATPase) and mitochondrial (cytochrome c oxidase) protein markers. Next, isolated LD were applied to a ConA sepharose affinity chromatography column. The ConA method, used previously to isolate caveolin-rich plasma membrane fractions (7, 38, 87, 98), allowed separation of two pools of LD, one enriched in perilipin and lipolytic proteins and the other enriched in ADRP and TIP47 based on the high affinity of caveolin-1/caveolae for the ConA protein (5, 7, 38, 87, 97, 98) and the strong interaction between caveolin-1 and perilipin (23, 83). In brief, isolated LD were applied to a ConA sepharose column equilibrated in buffer X (10 mM HEPES, pH 7.4, 140 mM KCL, 1 mM MgCl2, and 1 mM MnCl2) after a brief pulse with a Fisher 550 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA) to limit LD aggregation and increase binding. Prior to loading, intact LD were sized visually by light microscopy to confirm membrane integrity. The sample and resin were bubbled under N2 for 5 min at 4°C to allow thorough mixing and maximum interaction. The column was washed under bubbling N2 and fractions collected until the absorbance280 was close to zero. LD fractions designated as “nonbinding” were enriched in ADRP and TIP47. The column was then washed under bubbling N2 with buffer Y (buffer X plus 0.75 M α-methylmannoside) to elute bound proteins and lipids. Fractions were collected at 4°C until the absorbance280 leveled off close to zero. ConA-resolved LD fractions enriched in perilipin, caveolins, and LD lipolytic proteins were designated as “perilipin-enriched” LD. The two fractions were pooled separately and concentrated with Amicon Ultra-15 centrifuge filters (Ultracel 10K; Millipore, Billerica, MA) as indicated by the manufacturer.
Western blot analysis.
The purity and degree of contamination of adipocyte LD, plasma membrane, and mitochondrial fractions was assessed by the presence or absence of protein markers. Since a subset of plasma membrane proteins (caveolins, flotillin) is typically found in LD, as reported previously (34, 64, 75), the process of assessing enrichment was somewhat complicated. However, the absence of other plasma membrane-associated proteins, including transferrin receptor, Na+,K+-ATPase, CD-14, H-Ras, Gsα, and Smad7, showed that LD fractions were free from plasma membrane proteins that do not normally associate with LD. Expression of proteins found in adipocyte LD (ADRP, perilipins, TIP47), mitochondria (cytochrome c oxidase), plasma membrane (caveolins 1 and 2, flotillin, CD-14, H-Ras, Na+,K+-ATPase, Gsα, Smad7, transferrin), and ConA-resolved LD fractions was determined by Western blot analysis, as described (5). Briefly, samples (5–10 μg of protein) were loaded onto tricine gels (12%). Gels were run on a Mini-Protean II cell (Bio-Rad Laboratories, Hercules, CA) system at 100 V of constant voltage for ∼1.5–2 h (30 mA/gel initially). Proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad Laboratories) by applying a 100-V constant voltage for 2 h. After transfer, the blots were stained with Ponceau (46), a reversible protein stain that allowed estimation of protein loading (86). Next, the blots were blocked in 3% gelatin in Tris-buffered saline-Tween 20 (TBST; 10 mM Tris·HCl, pH 8, 100 mM NaCl, and 0.05% Tween-20) for 1 h at room temperature and then washed twice with TBST, followed by overnight incubation with primary antibodies in 1% gelatin in TBST at the following dilutions: 1:250 (anti-flotillin), 1:500 (anti-caveolins), or 1:1,000 (anti-ADRP, anti-perilipin, anti-TIP47, anti-cytochrome c oxidase, anti-CD-14, anti-H-Ras, anti-Na+,K+-ATPase, anti-Gαs, anti-Smad7, anti-transferrin receptor). The blot was washed three times with TBST and incubated for 2 h at room temperature with the appropriate secondary antibody (alkaline phosphatase conjugates of goat anti-rabbit IgG) diluted 1:4,500 in 1% gelatin TBST. Three more TBST washes were performed, and the bands of interest were visualized by development with Sigma Fast 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolim tablets (Sigma) according to the manufacturer's protocol. Images of each blot were acquired using a single-chip charge-coupled device video camera and a computer work station (IS-500 system from Alpha Innotech, San Leandro, CA). Densitometric analysis of image files was performed (mean 8-bit grayscale density) using National Institutes of Health Image J software, available by anonymous FTP from zippy (http://rsbweb.nih.gov/ij/index.html), to obtain relative levels of the respective proteins detected in each fraction. Relative protein levels were expressed as integrated density values or as fold differences between samples.
Coimmunoprecipitation experiments.
Binding LD fractions were immunoprecipitated with antibodies specific for either perilipin (Abcam, Cambridge, MA) or caveolin (BD Transduction Laboratories) using the Catch and Release version 2.0 system from Millipore and then immunoblotted for caveolin or perilipin, respectively. The unfractionated LD were similarly immunoprecipitated with antibodies specific for either caveolin or ADRP (prepared in house as described in Ref. 8) and immunoblotted for ADRP or caveolin, respectively. A negative control (rabbit IgG) was used to assess nonspecific binding.
Lipid mass determination.
Lipids from adipocyte LD and ConA-resolved LD fractions were extracted and resolved into individual lipid classes [cholesterol, phospholipid (PL), fatty acids (FA), cholesteryl esters (CE), triacylglycerols (TG)], as described (6). In brief, samples were extracted with n-hexane-2-propanol 3:2 (vol/vol), spotted onto Silica gel G TLC plates, and developed in petroleum ether-diethyl ether-methanol-acetic acid (90:7:2:0.5, vol/vol/vol/vol). NL content, including total cholesterol, TG, free FAs, and CE, was determined by the method of Marzo et al. (66). Total PL, including sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS), were scraped from the Silica gel G TLC plates and eluted using chloroform-methanol-HCl (100:50:0.375, vol/vol/vol). PL samples were dried under N2 and resuspended in chloroform. One-half of each sample was used for PL FA mass analysis and one-half was applied to Silica gel 60 TLC plates to resolve individual phospholipids using chloroform-methanol-water-acetic acid (150:112.5:6:10.5, vol/vol/vol/vol). PL spots were visualized (iodine vapor), identified by comparison to known standards, and quantitated densitometrically as described previously (5, 29). Protein concentration was determined by the method of Bradford from the dried protein extract residue digested overnight in 0.2 M KOH (12). Lipids were stored under an atmosphere of N2 to limit oxidation, and all glassware was washed with sulfuric acid-chromate before use.
PL and TG FA distribution.
One-half of the PL extract described above was used for PL FA mass analysis by converting the lipid acyl chains to FA methyl esters (FAME) in an acid-catalyzed transesterification reaction. For TG FA mass analysis, the lipid acyl chains were converted to FAME using a base-catalyzed saponification with 1 M KOH in methanol, as described (4, 71). Individual FAME species were resolved according to chain length and unsaturation by gas chromatography linked to mass spectrometry (GC-MS) using a RTX-2330 capillary column (0.25 mm id × 30 m; Restek, West Chester, PA) on a Thermo-Finnigan Trace DSQ single quadrupole mass spectrometer (Thermo Electron, Austin, TX) with electron impact and chemical ionization sources. Injector and detector temperatures were set at 240°C with the temperature program of 100°C for 1 min, 10°C/min to 140°C, and then 2°C/min to 220°C, hold 1 min, then ramp at 20°C/min to 240°C. Individual peaks were identified by comparison to known FAME standards (NuChek, Elysian, MN) and referenced against a set concentration of C15:0. Sample identity was confirmed by GC-MS using the Trace DSQ single quadrupole in chemical ionization mode.
TG metabolism.
To determine whether LD resolved by ConA chromatography were functionally active, lipase activity was measured. The extent of TG hydrolysis was determined following a modified protocol (49, 50) by measuring the disappearance of [9,10-3H(N)]triolein (PerkinElmer, Waltham, MA). In brief, aliquots (4 μg) of each fraction were dissolved separately in PBS (pH 7.2, 250 μl) before addition to the reaction mix {250 μl of 1.1 pmol of 91.0 Ci/mmol [9,10-3H(N)]triolein and 0.120 mmol EDTA} to start the reaction. After 30 min at room temperature, the reaction was stopped by adding 10 volumes of hexane-isopropanol (3:2, vol/vol). This step was repeated once, followed by centrifugation at 1,000 rpm for 10 min to remove proteins. The extracted lipids were pooled and dried under nitrogen, and TG were resolved by TLC using silica gel G plates (Analtech) developed with a petroleum ether-diethyl ether-methanol-glacial acetic acid (180:14:4:1, vol/vol/vol/vol) solvent system. The radiolabeled TG spots from each reaction were scrapped, dissolved in ScintiSafe Gel (Thermo Fisher Scientific), and counted for radioactivity using a Tri-carb 1600 TR liquid scintillation analyzer (Packard Instrument, Meriden, CT). Postextraction protein samples were dried overnight and dissolved in 0.2 M KOH. The protein content in the samples was quantified by the method of Bradford (12). To determine the lipase activity in each LD fraction, values were determined as disintegrations per minute per milligram total protein ± SE based on average values derived from three to five replicate reactions and were reported as percent triacylglyerol remaining after 30 min of incubation.
PL membrane structure of adipocyte fractions.
The PL membrane surface fluidity of adipocyte LD, plasma membrane, and ConA-resolved LD fractions was examined by studying the incorporation of select fluorescent probes (DPH-TMA, NBD-cholesterol, and NBD-sphingomyelin), as described (38–40). Stock solutions of the probes were prepared in anhydrous ethanol, with 2% wt/vol butylated hydroxytoluene added as an antioxidant. In brief, an aliquot (5 μg/ml protein of 10 nM PIPES buffer, pH 7.4) of each adipocyte fraction was incubated at 37°C with a small amount of fluorescent probe (protein/fluorophore ratio = 1,000 μg of protein-1 μg of fluorophore). The final ethanol concentration was kept <25 μM, well below that which would perturb membrane structure, lipid distribution, and fluidity, induce artifacts (sterol self-aggregation/crystallization) in membrane fractions, or disrupt protein/lipid interactions (38). Maximal probe incorporation was assured by incubation for 15 min at 37°C. Steady-state fluorescence polarization was acquired with a Varian Carey Eclipse spectrofluorometer (Palo Alto, CA) in the L format (38–40). Polarization data were corrected for residual light scatter by converting polarization to anisotropy [r = 2P/(3-P)] and subtracting residual fluorescence anisotropy from all measurements. To avoid inner filter effects, the absorbance of each sample (fluorescent probe + sample) at the wavelength of excitation for each probe was maintained <0.15.
Statistics.
All values were expressed as means ± SE. Statistical analysis was performed using analysis of variance combined with the Newman-Keuls multiple comparisons test or the Student t-test where appropriate (GraphPad Prism, San Diego, CA). Values with P < 0.05 were considered statistically significant.
RESULTS
Adipocyte LD and plasma membrane isolation.
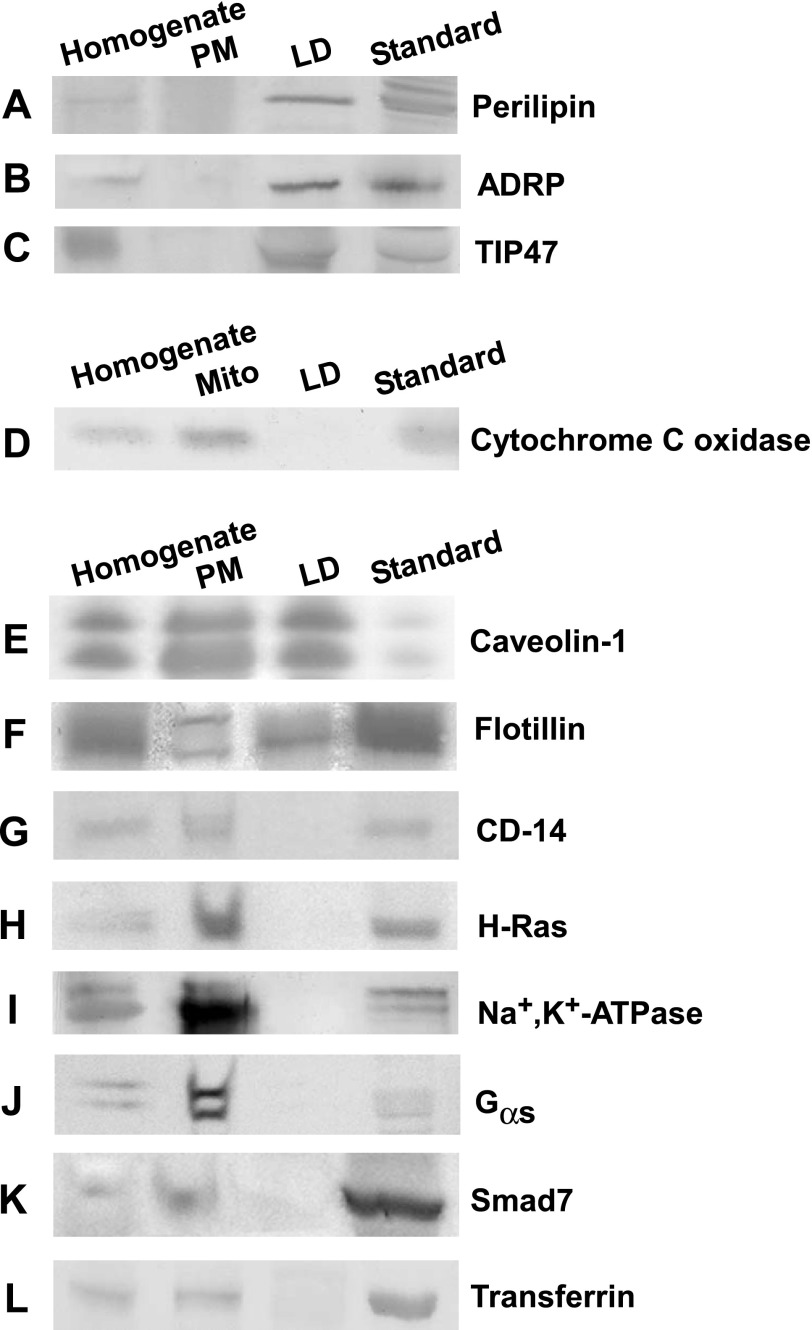
LD and plasma membrane fractions were isolated as indicated in materials and methods from adipocytes derived from the epididymal fat pads of C57BL/6NCr mice, using a previously established protocol (21, 85). Approximately 18 mg of adipocyte LD and 1.2 mg of plasma membrane proteins were obtained per 67 mg of adipocyte homogenate protein (Table 1). LD and plasma membrane fractions accounted for 27 and 1.8%, respectively, of total protein in the adipocyte homogenate, in keeping with the large commitment of adipocytes to LD proteins and lipids. Western blot analysis was performed to determine the distribution of proteins in LD, plasma membrane, and mitochondrial fractions by examining the expression of protein markers for LD (perilipin, ADRP, TIP47), mitochondria (cytochrome c oxidase), and plasma membrane (CD-14, H-Ras, Na+,K+-ATPase, Gsα, Smad7, transferrin, caveolin-1, flotillin) in each fraction (Fig. 1). As expected, the LD fraction was enriched severalfold in all LD markers (Fig. 1, A–C, lane 3) where expression of perilipin, ADRP, and TIP47 was observed in adipocyte homogenate and LD fractions but not the plasma membrane. The relative purity of LD fractions was further determined based on the absence of the mitochondrial protein marker cytochrome c oxidase (Fig. 1D, lane 3) and the lack of the following plasma membrane-associated proteins: 1) caveolae/lipid domain proteins such as CD-14 (Fig. 1G, lane 3) (73) and H-Ras (Fig. 1H, lane 3) (77); 2) nonlipid domain plasma membrane proteins such as Na+,K+-ATPase (Fig. 1I, lane 3) (29); 3) proteins found in lipid vesicles associated with the plasma membrane such as Gsα (Fig. 1J, lane 3) (2) and Smad7 (Fig. 1K, lane 3) (28); and 4) clathrin-dependent proteins such as transferrin receptor (Fig. 1L) (45). Thus, isolated LD fractions were shown by Western blot analysis to be enriched severalfold in perilipin, ADRP, TIP47, and caveolin-1 yet free from mitochondrial and plasma membrane protein markers.
Table 1.
Total protein distribution in mouse adipocyte LD, PM, and ConA-resolved LD fractions
| Fraction | Protein, mg | %Homogenate | %LD |
|---|---|---|---|
| Homogenate | 67 ± 8 | 100 | |
| PM | 1.2 ± 0.1 | 1.8 ± 0.1 | |
| LD | 18 ± 3 | 27 ± 2 | 100 |
| Nonbinding LD | 14 ± 3 | 23 ± 3 | 88 ± 2 |
| Perilipin-enriched LD | 1.9 ± 0.5 | 2.8 ± 0.7 | 12 ± 3 |
Values represent means ± SE; n = 3 preparations from pooled adipocytes derived from 3-4 mice/preparation. LD, lipid droplet; PM, plasma membrane; ConA, concanavalin-A. Adipocyte homogenate, PM, LD, and ConA-resolved LD fractions were isolated as described in materials and methods.
Fig. 1.
Western blot analysis of adipocyte homogenate, plasma membrane (PM), and lipid droplet (LD) fractions. The relative enrichment of LD, mitochondrial, and PM proteins in isolated adipocyte fractions was determined from Western blots loaded as follows: adipocyte homogenate (lane 1), PM (lane 2), LD (lane 3), and protein standards (lane 4). Proteins present in LD but not PM fractions included perilipin (A), adipose differentiation-related protein (ADRP; B), and tail-interacting protein 47 (TIP47; C). D: proteins in mitochondrial fractions but not LD included cytochrome c oxidase. Proteins in both LD and plasma membrane fractions included caveolin-1 (E) and flotillin (F). Proteins in plasma membrane but not LD fractions included CD-14 (G), H-ras (H), Na+,K+-ATPase (I), Gsα (J), Smad7 (K), and transferrin (L). The protein standard in A–C was detected from homogenates of mouse adipose tissue. Standard in D–L was detected in homogenates of mouse liver.
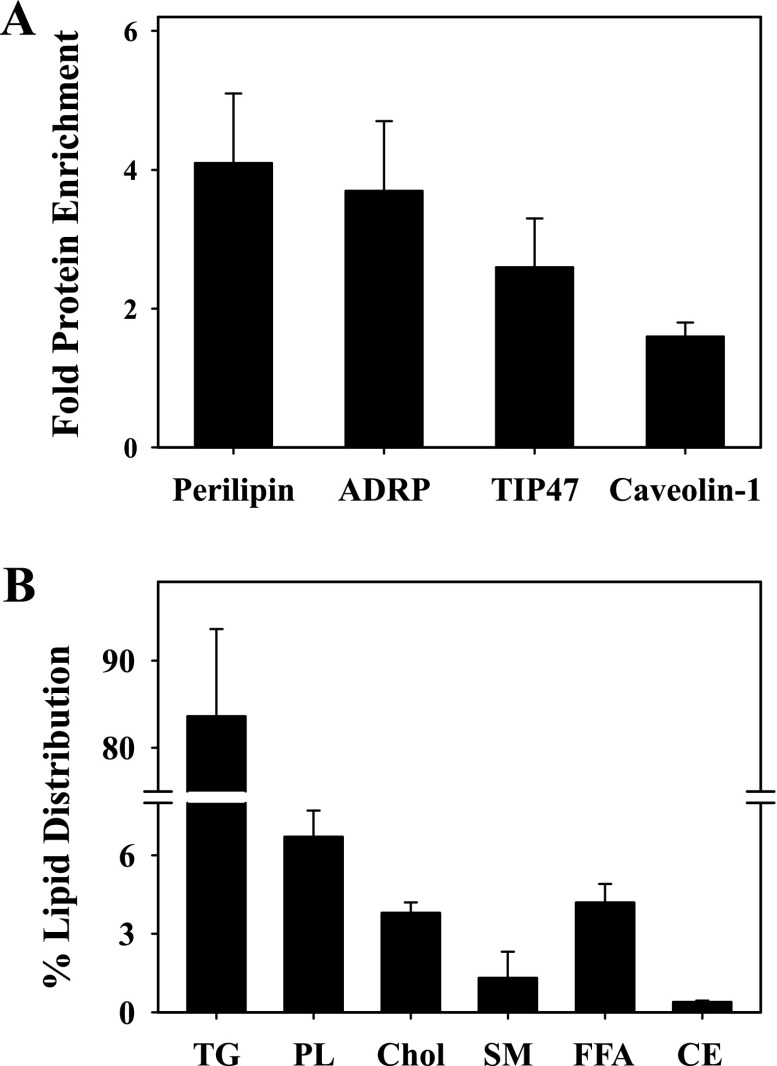
In addition, quantitative analysis of multiple Western blots was performed to reveal that levels of perilipin, ADRP, and TIP47 were increased 3.7-, 4.1-, and 2.6-fold, respectively, compared with the adipocyte homogenate (Fig. 2A). Since a small subset of plasma membrane proteins (caveolin-1, flotillin) has been found in LD (13, 34, 35, 64, 75, 76, 81), enrichment of caveolin-1 and flotillin was also determined to show that caveolin-1 was enriched 1.6-fold compared with the homogenate (Fig. 2A), with no fold enrichment observed for flotillin (data not shown). Isolated LD were then analyzed for lipid content, as described in materials and methods. The mole percent composition of cholesterol and sphingomyelin represented 3.8 ± 0.5 and 1.3 ± 0.3% of total lipids, respectively (Fig. 2B). In keeping with the lipidic nature of adipocyte LD, NLs such as TGs represented the majority lipid (84 ± 8%), with only small amounts of CEs (0.4% ± 0.1) observed. In addition, total PLs represented 8 ± 1% of adipocyte LD, whereas free FAs were 4.2 ± 0.8% of the total (Fig. 2B). Since isolated plasma membrane and other organelles are composed of mostly cholesterol and PLs to ∼75–90% of total lipid content (36, 37), these results further confirmed that the LD preparations were highly enriched and relatively free from contaminants from other organelles.
Fig. 2.
Fold protein enrichment and %lipid distribution in isolated adipocyte LD. The protein fold enrichment (A) of several LD-associated proteins, including perilipin, ADRP, TIP47, and caveolin-1, compared with the homogenate and the %distribution of lipids (B) in adipocyte LD was determined as described in materials and methods. TG, triacylglyerols; PL, total phospholipids; Chol, cholesterol; SM, sphingomyelin; FFA, free fatty acids; CE, cholesteryl esters.
ConA sepharose affinity chromatography resolution of adipocyte LD fractions.
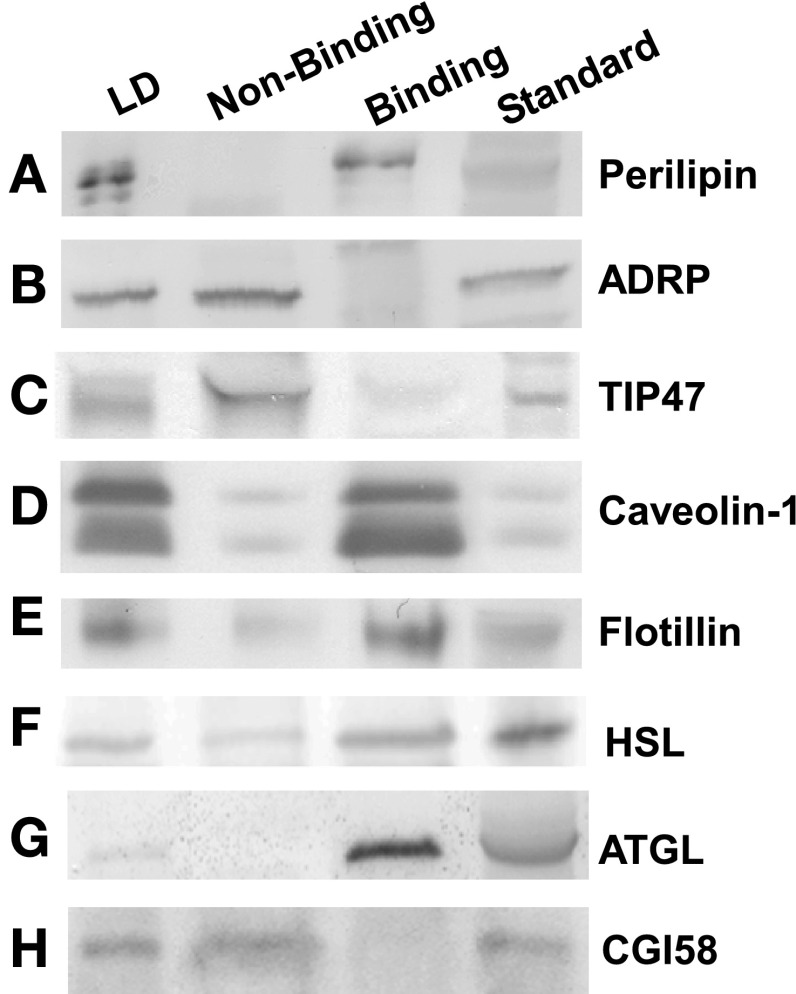
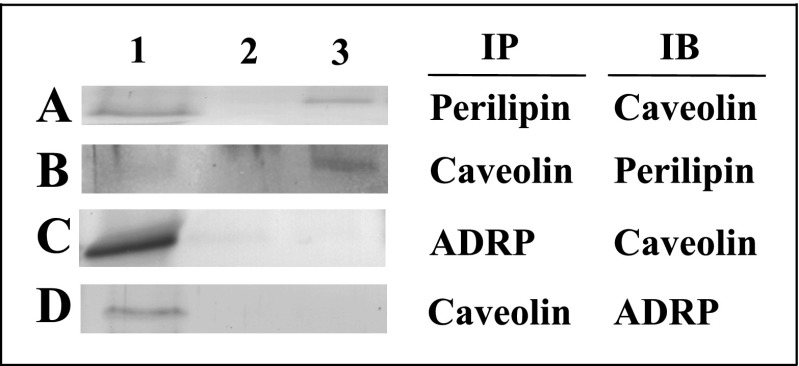
Isolated LD were passed over ConA sepharose affinity resin to yield two LD fractions (Supplemental Fig. S1; Supplemental Material for this article can be found online at the AJP-Endocrinology and Metabolism web site). The nonbinding LD fraction, enriched in ADRP and TIP47, was collected as flow through, whereas the perilipin-enriched LD fraction was eluted with buffer containing the displacing sugar α-methylmannoside. Approximately 18 mg of adipocyte LD protein was applied to the column. The nonbinding LD fraction, washed from the ConA resin within the first 100 ml of buffer, contained ∼14 mg protein, representing 88 ± 2% of LD and 23 ± 3% of adipocyte homogenate protein (Table 1). The perilipin-enriched LD fraction contained ∼1.9 mg protein, representing ∼12 ± 3% of LD and 2.8 ± 0.7% of adipocyte homogenate protein (Table 1). Western blot analysis performed on LD resolved by ConA revealed that, among the PAT family of proteins, perilipin was primarily in the fraction bound to the resin (Fig. 3A), whereas ADRP (Fig. 3B) and TIP47 (Fig. 3C) partitioned mostly to the nonbinding LD fraction. Based on these results, LD retained on the ConA resin and later eluted were designated “perilipin-enriched LD.” As expected from previous work (6, 7, 38, 97), caveolin-1 (Fig. 3D, lane 3) and flotillin (Fig. 3E, lane 3) were found primarily in the perilipin-enriched LD fraction. Next, coimmunoprecipitation experiments were performed to confirm the close association of bound proteins such as caveolin and perilipin and lack of interaction between bound (caveolin) and nonbound (ADRP) proteins, in keeping with the separation observed with the ConA procedure. Immunoprecipitation of the perilipin-enriched LD fraction with an antibody for perilipin pulled down a 20-kDa protein that was immunoreactive with a caveolin antibody in an immunoblot assay (Fig. 4A). These results verified the presence of perilipin and caveolin in the perilipin-enriched LD fraction and indicated a close association between the two proteins. Reverse immunoprecipitation experiments (Fig. 4B), where caveolin was immunoprecipitated from the perilipin-enriched LD fraction and immunoblotted for perilipin, confirmed the interaction. These results suggested that select proteins in the bound LD fraction were in close association and potentially colocalized on the same LD. It was also important to assess whether the nonbound LD fraction represented a physiologically discrete component of the LD population. Therefore, coimmunoprecipitated experiments were performed on unfractionated LD to determine whether ConA-bound and nonbound proteins associated with each other prior to the ConA procedure. Immunoprecipitation of unfractionated LD with an antibody for ADRP was not able to pull down a 20-kDa protein representing caveolin (Fig. 4C). A similar lack of results was found in the reverse experiment where caveolin was not able to pull down ADRP (Fig. 4D). These results indicated that little to no interaction occurred between the ConA-bound protein caveolin and nonbound ADRP and further validated the separation process.
Fig. 3.
Representative Western blots of proteins in adipocyte LD and concanavalin-A (ConA)-resolved LD fractions. Western blots loaded with samples from adipocyte LD homogenates (lane 1), nonbinding LD (lane 2), perilipin-enriched LD (lane 3), and protein standards (lane 4) were probed against the antibodies anti-perilipin (A), anti-ADRP (B), anti-TIP47 (C), anti-caveolin-1 (D), anti-flotillin (E), anti-hormone-sensitive lipase (HSL; F), anti-adipose triglyceride lipase (ATGL; G), and anti-comparative gene identification-58 (CGI58; H), as described in materials and methods. The protein standard was detected in homogenates of mouse adipose tissue.
Fig. 4.
Immunoprecipitation of proteins in ConA-resolved LD fractions. Perilipin-enriched LD fractions (A and B) and unfractionated LD (C and D) were immunoprecipitated (IP) with antibodies specific for perilipin (A), caveolin (B), ADRP (C), or caveolin (D) using the Catch and Release version 2.0 system, following the manufacturer's protocol. IP with a negative control of rabbit IgG (lane 2 in A–D) was performed to assess nonspecific binding. Samples were then analyzed by Western blot analysis, immunoblotted (IB) with caveolin (A), perilipin (B), caveolin (C), or ADRP (D), respectively.
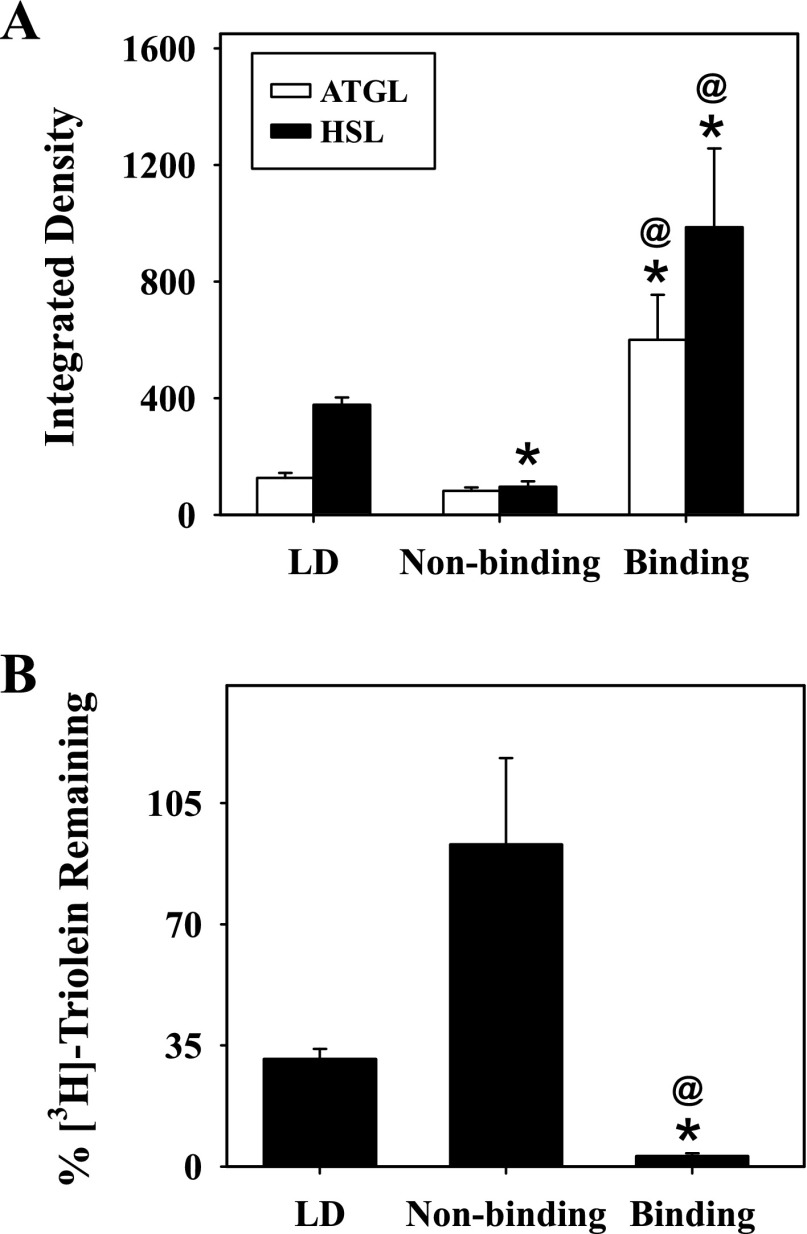
Since the perilipin-enriched LD fraction contained perilipin, it was essential to determine whether LD proteins associated with lipolysis such as HSL, ATGL, and CG-I58 coeluted with perilipin. HSL (Fig. 3F) and ATGL (Fig. 3G) were found primarily in the perilipin-enriched LD fraction, whereas CGI-58 (Fig. 3H) was not retained on the ConA resin. Since hydrolysis of TGs is a primary function of ATGL and HSL, the lipase activities of the isolated adipocyte LD and ConA-resolved fractions were determined by measuring the disappearance of [9,10-3H(N)]triolein, as described in materials and methods. After 30 min, isolated adipocyte LD retained 31 ± 3% of the labeled TG (Fig. 5B). In contrast, 93 ± 25% of the radiolabeled triolein was left in the nonbinding samples, indicating little to no lipase activity. The highest lipase activity was observed with the perilipin-enriched LD fraction, as seen by the small amount of triolein remaining (3 ± 0.8%) at the end of the experiment (Fig. 5B). It should be noted that, although ATGL requires the presence of CGI-58 for full activation, the lipase was still active under conditions without CGI-58, when similar assays were performed. Results from the labeled studies were consistent with the relative enrichment of ATGL and HSL in the perilipin-enriched LD fraction, where quantitative analysis of multiple Western blots revealed a 4.7- and 2.6-fold increase in levels of ATGL and HSL, respectively, compared with adipocyte LD before ConA resolution (Fig. 5A). Likewise, the lack of lipase activity closely approximated the little to no ATGL and HSL expression observed in the nonbinding LD fractions. In contrast, CGI-58 was not retained on the ConA resin, in keeping with studies suggesting that differential binding between lipases and their coactivators may regulate function and mobilization (43, 44).
Fig. 5.
Relative protein expression of ATGL and HSL and lipase activity in adipocyte LD and ConA-resolved LD fractions. Relative protein levels of ATGL and HSL (A) expressed as integrated density values and lipase activity expressed as %[3H]triolein remaining (B) were determined as described in materials and methods. Values represent means ± SE; n = 3–4. *P < 0.04 compared with adipocyte LD; @P < 0.05 compared with nonbinding LD fractions.
In summary, adipocyte LD were resolved by ConA sepharose affinity chromatography into two different LD populations. The perilipin-enriched LD fraction contained perilipin and caveolin along with several lipolytic proteins, including ATGL and HSL, and exhibited high lipase activity. In contrast, the nonbinding fraction contained ADRP and TIP47 and had little to no lipase activity, reflecting the lack of ATGL and HSL in these fractions.
Distribution of lipid markers in adipocyte LD fractions resolved by ConA affinity chromatography.
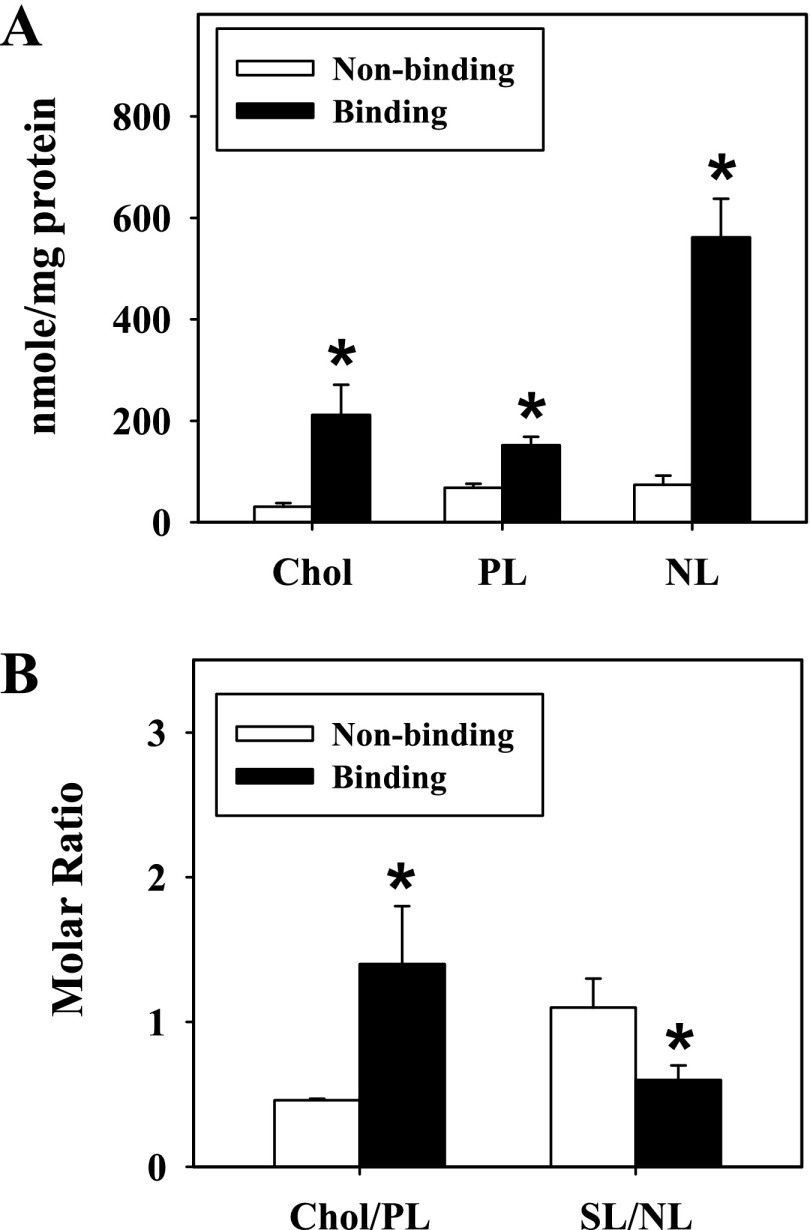
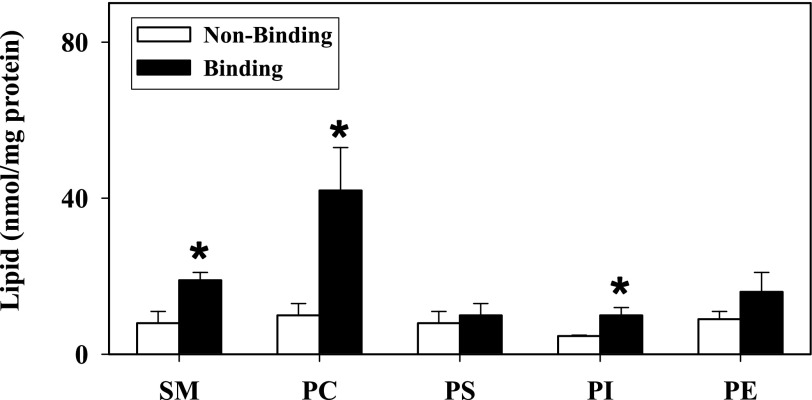
Because adipocytes are lipid storage cells and contain a large volume of lipids, especially TGs, it was essential to examine the lipid distribution in the different ConA-resolved LD fractions. Therefore, total lipids were extracted and analyzed as described in materials and methods. Compared with the nonbinding LD fraction, levels of cholesterol, PLs, and NLs in the perilipin-enriched LD fraction (normalized to total protein) were increased severalfold, with a 6.8-, 2.2-, and 6.2-fold increase observed, respectively (Fig. 6A). The molar ratio of cholesterol to PL was also significantly increased threefold, reflecting the high PL, and more so cholesterol, content in the perilipin-enriched LD fraction (Fig. 6B). The ratio of surface lipids (cholesterol, PLs) to NLs (CEs, TGs) was next determined to show a 1.8-fold increase in the nonbinding LD fraction (Fig. 6B). These results reflected the high TG content associated with the perilipin-enriched LD fraction. Moreover, because total PLs were increased in the perilipin-enriched LD fraction, it became necessary to examine levels of individual PL classes (Fig. 7). Levels (nmol/mg protein) of SM, PC, and PI were 2.4-, 4.2-, and 2.1-fold higher, respectively, in the perilipin-enriched LD fractions (P < 0.04, n = 4–7; Fig. 7). In addition, levels of PE exhibited a trend toward increasing in the perilipin-enriched fractions with phosphatidic acid just below the level of detection and not separable from PE (Fig. 7).
Fig. 6.
Distribution of lipids in adipocyte ConA-resolved LD fractions. The mass (A) and molar ratio (B) of lipids in nonbinding and perilipin-enriched LD fractions was determined as described in materials and methods. Values represent means ± SE (n = 3–7). *P < 0.05 compared with nonbinding LD fractions. NL, neutral lipids; SL, surface lipids.
Fig. 7.
Distribution of individual PLs in adipocyte ConA-resolved LD fractions. Levels of individual PLs, including sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylinositol (PI), and phosphatidylethanolamine (PE), were determined as described in materials and methods. Values represent means ± SE (n = 3–7). *P < 0.05 compared with resolved nonbinding LD fractions.
PL and TG fatty acyl composition of adipocyte LD and ConA-resolved LD fractions.
Based on studies with model and biological membranes, cholesterol-enriched areas within the plasma membrane are regions with increased rigidity due to the ability of cholesterol to pack tightly with PL FA chains, especially saturated FAs (47, 95). Since it was evident from the previous section that the perilipin-enriched, ConA-binding LD fractions were enriched in several PLs, it became necessary to determine the saturation and individual PL FA content in these fractions. Therefore, total PLs were separated by TLC and converted into FAME, as described in materials and methods. The mole (%) of esterified PL FAs in both LD fractions was determined as shown in Table 2. Compared with nonbinding LD fractions, levels of unsaturated FAs and monounsaturated FAs were decreased significantly (P < 0.05, n = 3–4) in the perilipin-enriched LD fractions (Table 2). Differences reflected significant decreases in C20:2 (1.5-fold), C22:4 (1.4-fold), and C22:6 (1.7-fold). In contrast, saturated FAs exhibited an increase that did not reach significance in the adipocyte perilipin-enriched LD fractions, due mainly to increases observed in C16:0, C18:0, and C20:0 (Table 2). The ratios of the individual groups of saturated, unsaturated, monounsaturated, and polyunsaturated FAs were also examined as shown in Table 3. The ratios of saturated to unsaturated FAs (Sat/Unsat) and saturated to monounsaturated FAs (Sat/MUFA) were 1.4- and 1.5-fold higher, respectively (P < 0.02, n = 3–4), in adipocyte perilipin-enriched LD fractions compared with the nonbinding LD fraction. Similar results were observed when the perilipin-enriched LD fraction was compared with the LD from which it was derived (Table 3). Since unsaturation in the fatty acyl chains increases rotational mobility (fluidity) upon insertion of the first double bond (27, 54, 100), enrichment of saturated FAs and depletion of unsaturated FA and monounsaturated FAs, along with increased cholesterol and sphingolipid content in the perilipin-enriched LD fraction, suggested increased lipid order (rigidity) in the PL monolayer.
Table 2.
Phospholipid and triacylglyerol fatty acyl composition of ConA-resolved LD fractions
| Phospholipid |
Triacylglycerol |
|||
|---|---|---|---|---|
| FAME | Nonbinding LD | Perilipin-enriched LD | Nonbinding LD | Perilipin-enriched LD |
| C16:1 (n-7) | 3.8 ± 0.5 | 2.5 ± 0.4 | 1.6 ± 0.5 | 5.2 ± 0.9* |
| C18:1 (n-9) | 9.4 ± 0.7 | 7.9 ± 0.9 | 11 ± 2 | 7 ± 1 |
| C20:1 (n-9) | 3.2 ± 0.3 | 2.4 ± 0.3 | 1.9 ± 0.4 | 2.4 ± 0.5 |
| C22:1 (n-9) | 3.0 ± 0.4 | 2.3 ± 0.3 | 3.2 ± 0.9 | 1.3 ± 0.2 |
| C24:1 (n-9) | 3.4 ± 0.5 | 3.8 ± 0.7 | 0.8 ± 0.2 | 0.25 ± 0.06* |
| C18:2 (n-6) | 7 ± 2 | 8 ± 1 | 1.3 ± 0.1 | 9 ± 3 |
| C18:3 (n-6) | 4.1 ± 0.3 | 3.7 ± 0.8 | 2.6 ± 0.6 | 2.4 ± 0.3 |
| C20:2 (n-6) | 3.7 ± 0.4 | 2.4 ± 0.3* | 9 ± 2 | 0.21 ± 0.04* |
| C20:3 (n-6) | 3.4 ± 0.3 | 2.9 ± 0.4 | 6 ± 1 | 2.5 ± 0.3* |
| C20:4 (n-6) | 2.6 ± 0.2 | 2.8 ± 0.3 | 4.7 ± 0.6 | 1.6 ± 0.1* |
| C22:2 (n-6) | 2.9 ± 0.4 | 2.8 ± 0.4 | 0.3 ± 0.1 | 0.5 ± 0.1 |
| C22:3 (n-3) | 2.8 ± 0.2 | 3.0 ± 0.4 | 0.3 ± 0.1 | 0.32 ± 0.06 |
| C22:4 (n-6) | 3.6 ± 0.3 | 2.5 ± 0.2* | 1.1 ± 0.1 | 0.8 ± 0.2 |
| C22:6 (n-3) | 5.9 ± 0.3 | 3.4 ± 0.4* | 0.5 ± 0.1 | 1.1 ± 0.1* |
| C16:0 | 12 ± 2 | 14 ± 2 | 44 ± 9 | 52 ± 8 |
| C18:0 | 17 ± 3 | 23 ± 3 | 9 ± 1 | 11 ± 1 |
| C20:0 | 2.6 ± 0.2 | 3.7 ± 0.6 | 1.0 ± 0.1 | 1.1 ± 0.2 |
| C22:0 | 3.6 ± 0.7 | 3.7 ± 0.6 | 0.10 ± 0.01 | 0.5 ± 0.1* |
| C24:0 | 6.5 ± 0.9 | 5 ± 1 | 1.2 ± 0.2 | 0.4 ± 0.1* |
| Unsat | 59 ± 2 | 50 ± 2* | 44 ± 4 | 35 ± 4 |
| MUFA | 23 ± 1 | 19 ± 1* | 18 ± 2 | 17 ± 2 |
| Sat | 41 ± 4 | 50 ± 3 | 56 ± 10 | 65 ± 8 |
| PUFA | 36 ± 2 | 31 ± 2 | 26 ± 3 | 18 ± 3 |
Values are expressed as mole% and represent means ± SE; n = 3-4. FAME, fatty acid methyl esters; Unsat, unsaturated fatty acids; MUFA, monounsaturated fatty acids; Sat, saturated fatty acids; PUFA, polyunsaturated fatty acids.
Significance, P ≤ 0.05 compared with nonbinding LD fractions.
Table 3.
Fluorescence polarization and lipid ratios of adipocyte membrane fractions
| Parameter | LD | Nonbinding LD | Perilipin-Enriched LD | PM |
|---|---|---|---|---|
| Fluorescence polarization | ||||
| Sterol Structure | ||||
| NBD-Chol | 0.124 ± 0.009 | 0.11 ± 0.01 | 0.153 ± 0.009*† | 0.16 ± 0.01* |
| PL structure | ||||
| NBD-SM | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.01*† | 0.13 ± 0.01* |
| DPH-TMA | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.31 ± 0.01*† | 0.30 ± 0.01 |
| PL lipid ratios | ||||
| Chol/PL | 0.48 ± 0.07 | 0.46 ± 0.01 | 1.4 ± 0.4*† | 0.51 ± 0.06‡ |
| Sat/Unsat | 0.77 ± 0.04 | 0.71 ± 0.07 | 1.0 ± 0.1*† | - |
| Sat/MUFA | 1.8 ± 0.1 | 1.8 ± 0.2 | 2.7 ± 0.1*† | 0.7 ± 0.1*‡ |
Values represent means ± SE, n = 3–8. PL, phospholipid; Chol, cholesterol; SM, sphingomyelin; NBD-Chol, {22-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-23,24-bisnor-5-cholen-3b-ol}; NBD-SM, 6-{[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-hexanoyl}sphingosyl-phosphocholine; DPH-TMA, 1,6-diphyenyl-1,3,5-hexatrienyl-trimethylammonium.
Significance, P ≤ 0.04 compared with the LD fraction;
significance, P ≤ 0.05 compared with the nonbinding LD fraction;
data cited in Ref. 96.
Because adipocytes represent important reservoirs of TGs and FAs, the TG fatty acyl composition was also determined for each ConA-resolved LD fraction. Total TGs were resolved from lipid extracts and converted into FAME, as described materials and methods. In both fractions the major FA present was C16:0, representing a respective 44 ± 9 and 52 ± 8% of the total when expressed as mole% composition. The major TG FA groups were not significantly different between the two LD fractions, but levels of several unsaturated FAs were decreased significantly in the perilipin-enriched LD fraction, including C24:1, C20:2, C20:3, and C20:4, whereas C22:6 was increased (Table 2). It should be noted that the presence of C22:6 and other long-chain FAs has been found in the TG pool of adipose tissue, representing an active pool of FAs for use by the body (103, 104). In the nonbinding LD, levels of the FAs were in the following order: C16:0 >> C18:1 > C18:0, C20:2 > C20:3, C20:4 > C22:1 > C18:3, with the rest <2% of the total. In the perilipin-enriched LD fraction, FAs >2% were in the following order: C16:0 >> C18:0 > C18:2 > C18:1 > C16:1 >> C20:1, C18:3. These results were consistent with published values where levels of C16:0, C18:1, C18:0, and C18:3 represented the majority of FAs found in mouse adipose tissue (20, 72).
Lipid fluidity of adipocyte LD and ConA-resolved LD fractions.
Adipocyte LD and ConA-resolved LD fractions were treated with DPH-TMA, NBD-cholesterol, and NBD-sphingomyelin to examine the microenvironment of the probes in each LD fraction. Because of the presence of the cationic TMA group, DPH-TMA localizes to the outer leaflet of the plasma membrane bilayer (96). In the LD molecule, the TMA group anchors the probe to the lipid monolayer, preventing internalization to the NL core. DPH-TMA fluorescence polarization in adipocyte perilipin-enriched LD fractions was significantly higher than in LD or nonbinding LD fractions, results that are consistent with the presence of more order (less fluidity) in the perilipin-enriched LD fraction (Table 3). DPH-TMA fluorescence polarization in the adipocyte plasma membrane was comparable with that observed with the perilipin-enriched LD fraction, indicating that the environment the probe observed was of similar rigidity. The sterol and PL environment in the adipocyte LD fractions was next probed using NBD-labeled cholesterol and sphingomyelin as described (38, 40, 69, 89). Fluorescence polarizations of NBD-cholesterol and NBD-sphingomyelin were both significantly higher in the perilipin-enriched LD fraction compared with the nonbinding LD fraction (Table 3). Moreover, the fluidity of the plasma membrane fractions labeled with NBD-cholesterol and sphingomyelin was similar to that observed with perilipin-enriched LD fraction, indicating that both were in a less fluid state compared with the nonbinding LD fractions (Table 3). These results were in close agreement with the increased cholesterol/PL, Sat/Unsat, and Sat/MUFA ratios observed in the perilipin-enriched LD fractions, again suggesting that the LD PL monolayer associated with these fractions contained more ordered, rigid structures than that observed with the adipocyte LD or nonbinding LD fractions (Table 3). In summary, the results from the fluorescent polarization experiments revealed substantial differences in the microenvironment of PL monolayers containing different LD proteins.
DISCUSSION
Despite growing interest in understanding the molecular mechanisms governing lipid storage in adipocyte LD, major questions remain unanswered regarding the influence of the LD phospholipid membrane structure on LD protein targeting and function. Excessive adipocyte lipid storage can result in obesity (82, 94) and promote cardiovascular disease (17, 32, 92), diabetes (67), and several lipid disorders, including neutral lipid storage disease (51). Under normal conditions, cells utilize lipids stored within LD for energy by oxidizing the fatty acids stored as triacylglycerols, for membrane synthesis using stored phospholipids and cholesterol, and in steroidogenesis using cholesterol and cholesteryl esters (4, 22, 51, 65). Thus, the importance of regulating and maintaining normal cellular lipid levels is clear, yet relatively little is known about how protein/lipid interactions on the LD surface influence lipid uptake/efflux from the neutral lipid interior core. Even less is known about how changes in the LD phospholipid hemimembrane with respect to membrane fluidity, lipid composition, and lipid order may affect LD protein targeting and function.
To begin to address these issues, a ConA sepharose affinity chromatography method (5, 7, 38, 87, 97, 98) was employed to isolate two populations of adipocyte LD: 1) functionally active LD containing perilipin and caveolin-1, along with several lipolytic proteins, including ATGL and HSL, that by structure and organization closely resembled the adipocyte PM; and 2) LD enriched in ADRP and TIP47 that exhibited little to no lipase activity. The selectivity of the ConA resin for caveolin that allowed resolution of two LD fractions was based on the specificity of the ConA protein for certain glycolipids and glycoproteins associated with caveolae, caveolin/cholesterol-enriched areas of the plasma membrane (5, 7, 38, 87, 97, 98). Carbohydrate receptor structures, including simple sugars and oligosaccharides with a d-manno- or d-glucopyranoside configuration and glycopeptides with a core structure of Manα1 → 3[Manα1 → 6]Manβ1 → 4GlcNAcβ1 → 4GlcNAc → Asn (10), are present in several proteins and lipids, including SR-B1 and glucosylceramides (7), that are found in caveolin-enriched caveolae plasma membranes. The observed selective partitioning of perilipin with caveolins was reported previously (23, 83). Likewise, ADRP and TIP47 have been shown to reside on LD of the same size and location in the cell (23, 83). In the present study, coimmunoprecipitation experiments confirmed the close association between perilipin and caveolin in the perilipin-enriched LD fraction and suggested that the proteins could colocalize on the same LD. The validity of the ConA isolation method was assessed further by performing coimmunoprecipitation experiments on unfractionated LD to determine whether ConA-bound proteins (caveolin) interacted with nonbound proteins (ADRP) prior to the ConA procedure. No evidence of interaction between bound caveolin and nonbound ADRP was found, in keeping with results from the ConA separation process and suggesting that the nonbound LD represented a physiologically distinct fraction of LD. It should be noted that a small population of perilipin (perilipin B) was found recently in a specialized subgroup of plasma membrane caveolin-containing caveolae that was the site of exogenous fatty acid uptake and fatty acid conversion to triacylglyerol (1, 74). In other experiments, results from freeze-fracture immunogold labeling experiments with adipocytes and macrophages revealed that PAT family members not only localized around and in the LD core, but under conditions of LD formation, these proteins became organized into plasma membrane clusters or domains, in close contact with underlying LD (84). On the basis of these results it was suggested that a plasma membrane/LD interface would facilitate rapid shuttling of lipids from the plasma membrane to the LD for storage or usage as necessary (84). In the present study, PAT proteins were not found in the plasma membrane, but selective partitioning on the ConA resin led to two LD populations, one enriched in perilipin and caveolin (designated as the perilipin-enriched LD fraction) that closely resembled the adipocyte plasma membrane over several parameters and the other containing ADRP and TIP47 (designated as nonbinding). Both populations were examined further to reveal the following novel insights.
Based on protein content and enzyme activity, the perilipin-enriched LD represented a small pool of lipolytically active adipocyte LD containing 12% of total LD protein. Proteins retained on the ConA resin included enzymes involved in LD lipolysis such as perilipin, ATGL, and HSL. The fact that the lipolytically active pool was a small percentage of the whole may be based on the fact that the mice the LD were isolated from were not fasted or overfed. It should be expected that the percentage of lipolytically active LD would vary under conditions of fasting or overfeeding given the proclivity of adipocytes to readily adapt to the changing energy requirements of the cell and body. Moreover, despite the relatively decreased protein content of the perilipin-enriched LD pool, the lipase active fraction was lipid rich, with a 4.6-fold increase in total lipids, reflecting the large amount of triacylglyerols associated with this fraction. These results were in contrast to similar studies with fibroblasts (7) and primary cultured hepatocytes (6), where ∼30% of total plasma membrane protein and 60% total lipids were found in caveolin-enriched, ConA-Binding fractions. Thus, the lipolytically active perilipin-enriched LD fraction contained fewer proteins yet more total lipids than observed in similar fractions isolated from the plasma membrane, a reflection of the different cellular functions for LD (storage) vs. plasma membrane (structure).
The lipid content of the perilipin-enriched LD was increased significantly in levels of cholesterol, suggesting that surface lipids (phospholipids, cholesterol) in the monolayer membrane of the perilipin-enriched LD were in a more rigid environment. Moreover, surface lipid (cholesterol, phospholipids) to neutral lipid (triacylglyerols, cholesteryl ester) ratios were decreased significantly. Using a simple model of the LD as a sphere with a uniformly smooth surface, the surface area of the LD phospholipid monolayer can be described by the lipids in the phospholipid monolayer (cholesterol, phospholipids), whereas the LD volume can be defined essentially by the neutral lipids in the LD interior core (triacylglyerols, cholesteryl esters) such that the ratio of surface lipids to neutral lipids also represents the ratio of surface area to volume (SA/vol) if surface and volume effects of intercalated proteins are neglected. Despite the potential loss of lipids, especially triacylglyerols, during the isolation process, the decrease in the SA/vol ratio corroborated results showing that the lipid-rich, protein-poor, perilipin-enriched LD exhibited significantly increased cholesterol/phospholipid ratios. Moreover, the selective and consistent partitioning of perilipins and functional lipases to a membrane monolayer of specific lipid composition with more tightly packed lipids and/or less protein intercalation was highly suggestive of the membrane domains that would be found surrounding these proteins, enabling their stability and specific function in LD. It should be noted that the driving force for tight packing between cholesterol and phospholipids (especially sphingolipids) in membranes is derived from the preferential partitioning of cholesterol with phospholipid saturated fatty acyl chains (78, 89). As a result, increased incorporation of cholesterol and phospholipids with saturated fatty acids is associated with increased lipid order, such as is found in plasma membrane lipid domains (99). In keeping with this, levels of total phospholipid unsaturated fatty acids and monounsaturated fatty acids were significantly lower in perilipin-enriched LD fractions due primarily to decreased levels of the following fatty acids: C16:1, C18:1, C20:2 and C22:4, and C22:6. Based on studies with model and biological membranes, rigidity of the lipid bilayer depends not only on cholesterol content but also upon the saturation level of the phospholipid fatty acyl chain (47, 95). The ability of cholesterol to pack tightly with phospholipid unsaturated fatty acyl chains diminishes with each double bond addition due to the kinked conformation found in polyunsaturated fatty acid chains with the largest decrease in lipid order (rigidity) experienced upon insertion of the first double bond and subsequent additions showing less change (reviewed in Refs. 27, 54, 89, and 100). It should be noted that although changes in the levels of individual saturated fatty acids were small, levels were increased significantly in the perilipin-enriched LD fraction and contributed to the overall increased membrane rigidity observed in the phospholipid monolayer. In contrast, although several individual polyunsaturated fatty acids exhibited greater changes and were decreased in the perilipin-enriched LD fraction, the mole% of total PUFA was not significantly different from the nonbinding LD fraction. Consequently, as a result of significantly decreased levels of unsaturated and monounsaturated phospholipid fatty acids, the perilipin-enriched LD exhibited higher saturated fatty acid to unsaturated fatty acid and saturated fatty acid to monounsaturated fatty acid ratios. The triacylglyerol fatty acyl composition of both ConA-resolved LD was also determined, but although it was established that both LD fractions were significant reservoirs of C16:0, C18:1, C18:0, and C18:3, in keeping with literature on mouse adipose tissue (20, 72), no consistent trend was otherwise observed. Taken together, these results indicated an increased rigidity or higher lipid order in the phospholipid monolayer found in perilipin-enriched LD (60). It was also noteworthy that levels of several phospholipids such as SM, PC, and PI were significantly enriched in the perilipin-enriched LD fraction, with little to no differences observed in PE or PS levels compared with nonbinding LD fractions. Thus, in the perilipin-enriched LD, the ratio of anionic/neutral zwitterionic phospholipids [i.e., (PS + PI)/(PC + PE + SM)] was more than twofold lower than in the nonbinding LD population. Since zwitterionic phospholipids, including PC and SM, are typically enriched in the exofacial leaflet of the plasma membrane bilayer, whereas PE and anionic phospholipids such as PS, PI, and PA populate the cytofacial leaflet (24, 25), these results indicated that the LD phospholipid monolayer associated with the perilipin-enriched LD most closely resembled the exofacial leaflet of the plasma membrane bilayer.
Polarization studies were performed to examine the sterol and phospholipid environment of the phospholipid monolayer in LD resolved by ConA. Membrane fluidity was assessed by measuring the fluorescence polarizations of NBD-cholesterol, NBD-sphingomyelin, and DPH-TMA to measure the motional freedom of lipids in the phospholipid monolayer of each LD pool. The use of NBD-labeled lipids was based on numerous studies in living cells (L cells, intestinal enterocytes, CaCo2 cells) that showed that the intracellular distribution, trafficking, binding, and metabolism of NBD-labeled lipids was similar to natural analogs (5, 9, 19, 26, 33, 38, 102). With NBD-sphingomyelin, the probe did not undergo transbilayer movement (58, 59) but instead localized to the outer leaflet of the lipid bilayer. In similar fashion, DPH-TMA localized to the outer leaflet of the plasma membrane lipid bilayer due to the presence of the cationic TMA group (96). However, although polarization assays allowed examination of changes in fluidity within the surface monolayer, performing time-resolved DPH fluorescence lifetime measurements to estimate rotational correlational times would also allow assessment of the phospholipid acyl chain order (31). In the present work, the use of DPH as a probe in these studies would be problematic because it would intercalate deep within the triacylglyerol core wherein the bulk of the fluorescent signal lies, giving little information about the surface membrane monolayer. The ability of the probes to anchor to one leaflet of the lipid bilayer was important when working with the adipocyte LD phospholipid monolayers so that internalization to the neutral lipid core was minimized. Probes such as DPH that intercalated between the acyl chains of the plasma membrane lipid bilayer were not useful for this reason. With all three probes, higher fluorescent polarizations were found in the perilipin-enriched LD fraction compared with nonbinding LD fractions, indicating that the environment surrounding the probe was more restricted (more rigid). When these experiments were repeated with adipocyte plasma membrane, fluidity measurements were similar to those observed with the perilipin-enriched LD regardless of the probe used, suggesting that probes in the LD phospholipid monolayer and the plasma membrane lipid bilayer experienced similar microenvironments. Since the lipid bilayer of the plasma membrane is asymmetric with sphingomyelin residing mainly on the exofacial side, the cholesterol and phospholipid (sphingolipid)-enriched areas of the plasma membrane and LD present a similar lipid “face” to their external environment whether it is the extracellular (plasma membrane) or cytoplasmic (LD) face. This was important when considering the effect of plasma membrane and LD phospholipid membrane fluidity (rigidity) in lipid uptake/efflux into and throughout the cell. Similar fluorescent polarizations observed in the perilipin-enriched LD vs. plasma membrane fractions emphasized the contribution of surface lipids and proteins to lipid membrane function, contributions that often depend on membrane structure to guide function.
The consequence of a more highly organized, rigid phospholipid monolayer in association with perilipin and LD lipolytic proteins gives rise to a model of lipolysis wherein select proteins are localized to select areas on the LD surface to promote uptake/efflux of lipids from the interior LD core. This hypothesis, suggesting that the LD phospholipid monolayer may direct lipolytic function based on the structure and order of lipids within it, has its basis in similar observations with the plasma membrane, where cholesterol and fatty acid uptake/efflux occurs within highly organized, caveolin/cholesterol-enriched domains (89). Moreover, changes in surface hydrophobicity based on phospholipid packing have been used to explain the differential binding affinity and displacing ability of select LD proteins in studies where increased expression of ADRP was shown to reduce the association of other proteins for the LD surface (63). In addition, the presence of perilipin in highly organized caveolae structures in the plasma membrane where triacylglyerols are synthesized (74) indicates a preference of perilipin for rigid membranes and highlights the importance of membrane structure in cellular function. The sequestering of lipolytic proteins such as perilipin, HSL, and AGTL to rigid areas of the membrane may be critical to enzyme function and define how lipolysis is controlled at the molecular level. Moreover, by creating a lipid zip code in the monolayer membrane that is a target for proteins involved in lipolysis, a novel mechanism to regulate lipid flux is in place, depending on the needs of the cell.
In summary, two populations of LD were analyzed for protein/lipid content and membrane fluidity to show that the perilipin-enriched LD pool, enriched in lipids and proteins involved in lipolysis, exhibited increased rigidity (less fluidity) as assessed by DPH-TMA, NBD-cholesterol, and NBD-sphingomyelin fluorescence polarization studies and lipid analysis where cholesterol/phospholipid, Sat/Unsat, and Sat/MUFA ratios were increased, indicating increased lipid order. In contrast, the nonbinding LD fraction, enriched in ADRP, TIP47, and CGI58, was relatively lipid poor, had more protein, and exhibited increased SA/vol ratios, reflecting the observed decreased order and increased fluidity. Taken together, these results indicate that perilipin and lipolytic LD proteins target areas in the cholesterol/phospholipid monolayer that are highly organized and rigid, similar in structure to localized areas of the plasma membrane where cholesterol and fatty acid uptake and efflux occur. In all, these results provide new insights into the role of the LD phospholipid monolayer in regulating lipid metabolism in the cell.
GRANTS
This work was supported by the US Public Health Service and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-70965.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance of Meredith Dixon and Ashley R. Stone is much appreciated.
REFERENCES
- 1.Aboulaich N, Vener AV, Stralfors P. Hormonal control of reversible translocation of perilipin B to the plasma membrane in primary human adipocytes. J Biol Chem 281: 11446–11449, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Allen JA, Yu JZ, Donati RJ, Rasenick MM. β-Adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol Pharm 67: 1493–1504, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Arner P. Human fat cell lipolysis: biochemistry, regulation, and clinical role. Best Pract Res Clin Endocrinol Metab 19: 471–482, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Atshaves BP, Storey SM, McIntosh AL, Peterscu AD, Lyuksyutova OI, Greenberg AS, Schroeder F. Sterol carrier protein 2 expression modulates protein and lipid composition of lipid droplets. J Biol Chem 276: 25324–25335, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Atshaves BP, Jefferson JR, McIntosh AL, Gallegos AM, McCann BM, Landrock KK, Kier AB, Schroeder F. Effect of sterol carrier protein-2 expression on sphingolipid distribution in plasma membrane lipid rafts/caveolae. Lipids 42: 871–884, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock KK, Maeda N, Kier AB, Schroeder F. SCP-2/SCP-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res 48: 2193–2211, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Atshaves BP, Gallegos AM, McIntosh AL, Kier AB, Schroeder F. Sterol carrier protein-2 selectively alters lipid composition and cholesterol dynamics of caveolae/lipid raft vs nonraft domains in L-cell fibroblast plasma membranes. Biochemistry 42: 14583–14598, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Atshaves BP, Petrescu AD, Starodub O, Roths JB, Kier AB, Schroeder F. Expression and intracellular processing of the 58 KDa SCP-x/3-oxoacyl-CoA thiolase in transfected mouse L cells. J Lipid Res 40: 610–622, 1999 [PubMed] [Google Scholar]
- 9.Atshaves BP, Starodub O, McIntosh A, Petrescu A, Roths JB, Kier AB, Schroeder F. Sterol carrier protein-2 alters high density lipoprotein-mediated cholesterol efflux. J Biol Chem 275: 36852–36861, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Baenziger JU, Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem 254: 2400–2407, 1979 [PubMed] [Google Scholar]
- 11.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791: 419–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Brasaemle D, Bolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem 279: 46835–46842, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Brasaemle DL, Robertson RD, Attie AD. Transbilayer movement of cholesterol in the human erythrocyte membrane. J Lipid Res 29: 481–489, 1988 [PubMed] [Google Scholar]
- 16.Brasaemle DL, Barber T, Wolins N, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997 [PubMed] [Google Scholar]
- 17.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 232: 34–47, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164: 103–114, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Cai TQ, Qiu G, Birming W, Milot D, Liwen Z, Wright SD. Protein-disulfide isomerase is a component of an NBD-cholesterol monomerizing protein complex from hamster small intestine. Biochim Biophys Acta 1581: 100–108, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carraro R, Lu Z, Li ZH, Johnson JE, Gregerman RI. Adipose tissue islets: tissue culture of a potential source of fat cells in the adult rat. FASEB J 4: 201–207, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Chanderbhan R, Noland BJ, Scallen TJ, Vahouny GV. Sterol carrier protein2. Delivery of cholesterol from adrenal lipid droplets to mitochondria for pregnenolone synthesis. J Biol Chem 257: 8928–8934, 1980 [PubMed] [Google Scholar]
- 23.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53: 1261–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 44: 233–242, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Daleke DL. Phospholipid flippases. J Biol Chem 282: 821–825, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Davies J, Scott C, Oishi K, Liapis A, Ioannou Y. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Cell Physiol 280: 12710–12720, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Demiel RA, Guerts van Kessel WS, van Deenen LL. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta 266: 26–40, 1972 [DOI] [PubMed] [Google Scholar]
- 28.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Eckert GP, Igbavboa U, Müller WE, Wood WG. Lipid rafts of purified mouse brain synaptosomes prepared with or without detergent reveal different lipid and protein domains. Brain Res 962: 144–150, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ehehalt R, Füllekrug J, Pohl J, Ring A, Herrmann T, Stremmel W. Translocation of long chain fatty acids across the plasma membrane—lipid rafts and fatty acid transport proteins. Mol Cell Biochem 284: 135–140, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barceló-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol 25: 10190–10201, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res 38: 1503–1521, 1997 [PubMed] [Google Scholar]
- 33.Frolov A, Petrescu A, Atshaves BP, So PT, Gratton E, Serrero G, Schroeder F. High density lipoprotein-mediated cholesterol uptake and targeting to lipid droplets in intact L-cell fibroblasts. A single- and multiphoton fluorescence approach. J Biol Chem 275: 12769–12780, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Caveolin-2 is targeted to lipid droplets, a new membrane domain in the cell. J Cell Biol 152: 1079–1085, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta 1644: 47–59, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Schroeder F. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry 40: 6493–6506, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Gallegos AM, Atshaves BP, Storey SM, Schoer J, Kier AB, Schroeder F. Molecular and fluorescent sterol approaches to probing lysosomal membrane lipid dynamics. Chem Phys Lipids 116: 19–38, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Gallegos AM, McIntosh AL, Atshaves BP, Schroeder F. Structure and cholesterol domain dynamics of an enriched caveolae/raft isolate. Biochem J 382: 451–461, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallegos AM, Storey SM, Kier AB, Schroeder F, Ball JM. Structure and cholesterol dynamics of caveolae/raft and nonraft plasma membrane domains. Biochemistry 45: 12100–12116, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Gallegos AM, Storey SM, Kier AB, Schroeder F, Ball JM. Stucture and cholesterol dynamics of caveolae/raft and nonraft plasma membrane domains. Biochemistry 45: 12100–12116, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem 274: 16825–16830, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Goldberg AA, Bourque SD, Kyryakov P, Boukh-Viner T, Gregg C, Beach A, Burstein MT, Machkalyan G, Richard V, Rampersad S, Titorenko VI. A novel function of lipid droplets in regulating longevity. Biochem Soc Trans 37: 1050–1055, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (plin5) with adipose triglyceride lipase. J Biol Chem 286: 5126–5135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284: 34538–34544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harder T, Schieffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 141: 929–942, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan J, Feighery C, Whelan A. Staining of molecular weight markers on nitrocellulose using Ponseau S. J Clin Lab Immunol 24: 104, 1987 [PubMed] [Google Scholar]
- 47.Herring FG, Tatischeff I, Weeks G. The fluidity of plasma membranes of Dictyostelium discoideum. The effects of polyunsaturated fatty acid incorporation assessed by fluorescence depolarization and electron paramagnetic resonance. Biochim Biophys Acta 602: 1–9, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Hood LF, Patton S. Isolation and characterization of intracellular lipid droplets from bovine mammary tissue. J Dairy Sci 56: 858–863, 1973 [DOI] [PubMed] [Google Scholar]
- 49.Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 55: 3394–3402, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Huggins KW, Camarota LM, Howles PN, Hui DY. Pancreatic triglyceride lipase deficiency minimally affects dietary fat absorption but dramatically decreases dietary cholesterol absorption in mice. J Biol Chem 278: 42899–42905, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J Biol Chem 271: 16644–16651, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Igbavboa U, Avdulov N. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem 66: 1717–1725, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Ishii I, Onozaki R, Takahashi E, Takahashi S, Fujio N, Harada T, Morisski N, Shirai K, Saito Y, Hirose S. Regulation of neutral cholesterol esterase activity by phospholipids containing negative charges in substrate liposome. J Lipid Res 36: 2303–2310, 1995 [PubMed] [Google Scholar]
- 54.Keough KM, Giffin B, Kariel N. The influence of unsaturation on the phase transition temperatures of a series of heteroacid phosphatidylcholines containing twenty-carbon chains. Biochim Biophys Acta 902: 1–10, 1987 [DOI] [PubMed] [Google Scholar]
- 55.Kier AB, Sweet WD, Cowlen MS, Schroeder F. Regulation of transbilayer distribution of a fluorescent sterol in tumor cell plasma membranes. Biochim Biophys Acta 861: 287–301, 1986 [PubMed] [Google Scholar]
- 56.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51: 468–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudson AG. Inborn errors of sphingolipid metabolism. Am J Clin Nutr 9: 55–62, 1961 [DOI] [PubMed] [Google Scholar]
- 58.Koval M, Pagano RE. Lipid recycling between the plasma membrane and intracellular compartments: transport and metabolism of fluorescent sphingomyelin analogues in cultured fibroblasts. J Cell Biol 108: 2169–2181, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koval M, Pagano RE. Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts. J Cell Biol 111: 429–442, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kutchai H, Chandler LH, Zavoico GB. Effects of cholesterol on acyl chain dynamics in multilamellar vesicles of various phosphatidylcholines. Biochim Biophys Acta 736: 137–149, 1983 [DOI] [PubMed] [Google Scholar]
- 61.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Lavie Y, Liscovitch M. Changes in lipid and protein constituents of rafts and caveolae in multidrug resistant cancer cells and their functional consequences. Glycoconj J 17: 253–259, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacyglycerol turnover. J Lipid Res 48: 2751–2761, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 279: 3787–3792, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol 10: 51–58, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Marzo A, Ghirardi P, Sardini D, Meroni G. Simplified measurement of monoglycerides, diglycerides, triglycerides, and free fatty acids in biological samples. Clin Chem 17: 145–147, 1971 [PubMed] [Google Scholar]
- 67.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity, and insulin secretion. Diabetologia 42: 128–138, 1999 [DOI] [PubMed] [Google Scholar]
- 68.McIntosh AL, Storey SM, Atshaves BP. Intracellular lipid droplets contain dynamic pools of sphingomyelin: ADRP binds phospholipids with high affinity. Lipids 45: 465–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee S, Raghuraman H, Dasgupta S, Chattopadhyay A. Organization and dynamics of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: a fluorescence approach. Chem Phys Lipids 127: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants, and microorganisms. Prog Lipid Res 40: 325–438, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Murphy EJ, Stiles T, Schroeder F. Sterol carrier protein-2 expression alters phospholipid content and fatty acyl composition in L-cell fibroblasts. J Lipid Res 41: 788–796, 2000 [PubMed] [Google Scholar]
- 72.Nakamura MT, Phinney SD, Tang AB, Oberbauer AM, German JB, Murray JD. Increased hepatic delta 6-desaturase activity with Growth hormone expression in the MG101 transgenic mouse. Lipids 31: 139–143, 1996 [DOI] [PubMed] [Google Scholar]
- 73.Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol Immunol 43: 607–612, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Ost A, Ortegren U, Gustavsson J, Nystrom FH, Stralfors P. Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J Biol Chem 280: 5–8, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Ostermeyer AG, Paci JM, Zeng Y, Lublin DM, Munro S, Brown DA. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol 152: 1071–1078, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ostermeyer AG, Ramcharan LT, Zeng Y, Lublin DM, Brown DA. Role of the hydrophobic domain in targeting caveolin-1 to lipid droplets. J Cell Biol 164: 69–78, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parmryd I, Adler J, Patel R, Magee AI. Imaging metabolism of phosphatidylinositol 4,5-bisphosphate in T-cell GM1-enriched domains containing Ras proteins. Exp Cell Res 285: 27–38, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Pohl J, Ring A, Ehehalt R, Herrmann T, Stremmel W. New concepts of cellular fatty acid uptake: role of fatty acid transport proteins and of caveolae. Proc Nutr Soc 63: 259–262, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Prattes S, Horl G, Hammer A, Blaschitz A, Graier WF, Sattler W, Zechner R, Steyrer E. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci 113: 2977–2989, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Rajendran L, Le Lay S, Illges H. Raft association and lipid droplet targeting of flotillins are independent of caveolin. Biol Chem 388: 307–314, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med 339: 953–959, 1998 [DOI] [PubMed] [Google Scholar]
- 83.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res 46: 1331–1338, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem 280: 26330–26338, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Rodbell M. Metabolism of isolated fat cells. J Biol Chem 239: 375–380, 1964 [PubMed] [Google Scholar]
- 86.Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Schroeder F, Fontaine RN, Kinden DA. LM fibroblast plasma membrane subfractionation by affinity chromatography on ConA-sepharose. Biochim Biophys Acta 690: 231–242, 1982 [DOI] [PubMed] [Google Scholar]
- 88.Schroeder F, Nemecz G, Wood WG, Joiner C, Morrot G, Ayraut-Jarrier M, Devaux PF. Transmembrane distribution of sterol in the human erythrocyte. Biochim Biophys Acta 1066: 183–192, 1991 [DOI] [PubMed] [Google Scholar]
- 89. Schroeder F, Atshaves BP, Gallegos AM, McIntosh AL, Liu JC, Kier AB, Huang H, Ball JM. Lipid rafts and caveolae organization. In: Advances in Molecular and Cell Biology, edited by Lisanti MP, Frank PG. Boca Raton, FL: CRC, 2005, p. 3–36 [Google Scholar]
- 90.Schroeder F, Jefferson JR, Kier AB, Knittell J, Scallen TJ, Wood WG, Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med 196: 235–252, 1991 [DOI] [PubMed] [Google Scholar]
- 91.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tomqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281: 40236–40241, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Shekelle RB, Shryock AM, Paul O, Lepper M, Stamler J, Liu S, Raynor WJ., Jr Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N Engl J Med 304: 65–70, 1981 [DOI] [PubMed] [Google Scholar]
- 93. Smart EJ, van der Westhuyzen DR. Scavenger receptors, caveolae, caveolin, and cholesterol trafficking. In: Intracellular Cholesterol Trafficking, edited by Chang TY, Freeman DA. Boston, MA: Kluwer Academic, 1998, p. 253–272 [Google Scholar]
- 94.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNF alpha. Cell 73: 625–627, 1993 [DOI] [PubMed] [Google Scholar]
- 95.Storch J, Schachter D. Dietary induction of acyl chain desaturases alters the lipid composition and fluidity of rat hepatocyte plasma membranes. Biochemistry 23: 1165–1170, 1984 [DOI] [PubMed] [Google Scholar]
- 96.Storch J, Schulman SL, Kleinfeld AM. Plasma membrane lipid order and composition during adipocyte differentiation of 3T3F442A cells. J Biol Chem 264: 10527–10533, 1989 [PubMed] [Google Scholar]
- 97.Storey SM, Gallegos AM, Atshaves BP, McIntosh AL, Martin GG, Parr RD, Landrock KK, Kier AB, Ball JM, Schroeder F. Selective cholesterol dynamics between lipoproteins and caveolae/lipid rafts. Biochemistry 46: 13891–13906, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Storey SM, Gibbons TF, Williams CV, Parr RD, Schroeder F, Ball JM. Full-length, glycosylated NSP4 is localized to plasma membrane caveolae by a novel raft isolation technique. J Virol 81: 5472–5483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Straume M, Litman BJ. Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry 26: 5121–5126, 1987 [DOI] [PubMed] [Google Scholar]
- 100.Stubbs CO, Kouyama T, Kinosita K, Ikegami A. Effect of double bonds on the dynamic properties of the hydrocarbon region of lecithin bilayers. Biochemistry 20: 4257–4262, 1981 [DOI] [PubMed] [Google Scholar]
- 101.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem 277: 44507–44512, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Tomás M, Durán JM, Lázaro-Diéguez F, Babià T, Renau-Piqueras J, Egea G. Fluorescent analogues of plasma membrane sphingolipids are sorted to different intracellular compartments in astrocytes; Harmful effects of chronic ethanol exposure on sphingolipid trafficking and metabolism. FEBS Lett 563: 59–65, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 43: 1809–1817, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta 1791: 494–500, 2009 [DOI] [PubMed] [Google Scholar]