Abstract
The FoxO3-dependent increase in type II deiodinase (D2), which converts the prohormone thyroxine (T4) to 3,5,3′-triiodothyronine (T3), is required for normal mouse skeletal muscle differentiation and regeneration. This implies a requirement for an increase in D2-generated intracellular T3 under these conditions, which has not been directly demonstrated despite the presence of D2 activity in skeletal muscle. We directly show that D2-mediated T4-to-T3 conversion increases during differentiation in C2C12 myoblast and primary cultures of mouse neonatal skeletal muscle precursor cells, and that blockade of D2 eliminates this. In adult mice given 125I-T4 and 131I-T3, the intracellular 125I-T3/131I-T3 ratio is significantly higher than in serum in both the D2-expressing cerebral cortex and the skeletal muscle of wild-type, but not D2KO, mice. In D1-expressing liver and kidney, the 125I-T3/131I-T3 ratio does not differ from that in serum. Hypothyroidism increases D2 activity, and in agreement with this, the difference in 125I-T3/131I-T3 ratio is increased further in hypothyroid wild-type mice but not altered in the D2KO. Notably, in wild-type but not in D2KO mice, the muscle production of 125I-T3 is doubled after skeletal muscle injury. Thus, D2-mediated T4-to-T3 conversion generates significant intracellular T3 in normal mouse skeletal muscle, with the increased T3 required for muscle regeneration being provided by increased D2 synthesis, not by T3 from the circulation.
Keywords: type II deiodinase, skeletal muscle, regeneration, thyroid hormone action
thyroxine (t4), the principle secretory product of the thyroid gland, is a prohormone and must be monodeiodinated at the outer ring to form the active hormone 3,5,3′-triiodothyronine (T3). Virtually all of the physiological effects of thyroid hormone are generated by the interaction of T3 with its nuclear receptors. In humans, ∼80% of the T3 is derived from monodeiodination catalyzed by either the type I (D1) or the type II (D2) selenodeiodinase. The type I enzyme, highly expressed in liver, kidney, and the thyroid gland, is located in the plasma membrane with its active center in the cytosol. It is inhibited by the thiourea drug 6n-propylthiouracil (PTU) and provides much of the T3 for the circulation (10). On the other hand, D2 is expressed in pituitary, central nervous system, thyroid, bone, brown adipose tissue and skeletal muscle (1, 29). It is insensitive to PTU and is located in the endoplasmic reticulum, with its active center in the cytosol. Programmed or induced changes in D2 expression permit tissue-specific changes in intracellular T3 concentrations (1, 6, 10). Studies in rats have demonstrated that tissues expressing D2 contain nuclear thyroid receptor-bound T3 derived from two sources: the plasma, termed T3(T3), and D2-mediated intracellular T4-to-T3 conversion, termed T3(T4). The contribution of each to receptor occupancy varies among different tissues. The nuclear T3 in tissues not expressing D2, such as the D1-expressing liver and kidney, is almost exclusively T3(T3) (1, 10, 27). The importance of the distinction between the two sources is that T3(T4) production can be regulated in specific cells or tissues by various pathways such as cyclic AMP or FoxO3, in addition to being negatively regulated by T3 (transcriptionally) or T4 (postranslationally), whereas T3(T3) is regulated by the hypothalamic-pituitary-thyroid axis (1, 3, 4, 6). In addition, D2-generated T3(T4) allows for local increases in receptor occupancy, while circulating levels of T3(T3) remains unchanged.
D2 mRNA was identified in human skeletal muscle many years ago, but its physiological role has not been well defined because of its low activity (2, 20). Recent improvements in the assays of muscle D2 activity show that it is clearly present and higher in slow than in fast muscles (7, 13, 18).
Gene targeting techniques and studies using D2 inhibitors indicate that, despite the low expression of the D2 protein, D2-mediated T4-to-T3 conversion is required for normal differentiation of neonatal skeletal muscle and C2C12 mouse myoblast cells (4). After skeletal muscle injury in vivo, there is a marked increase in D2 activity, peaking 7–12 days after injury (4). Notably, in D2KO mice or mice haploinsufficient for FoxO3, muscle regeneration after injury is significantly delayed. Since circulating T3 concentrations are normal and not influenced by injury in D2KO mice, this suggests a critical role for D2-mediated T3(T4) during the regeneration process and perhaps even under basal conditions, but this has not been demonstrated. The goal of the present studies was to determine whether D2-mediated intracellular T3(T4) production occurs in mouse skeletal muscle, and if so, whether this increases during muscle regeneration in parallel with increased D2 activity.
MATERIALS AND METHODS
Reagents and Materials
Unless otherwise specified, all reagents were purchased from Sigma (St. Louis, MO). Outer ring-labeled 125I-T4 (specific activity 4,400 Ci/mmol) was purchased from PerkinElmer. 131I-T3 was prepared in the laboratory by iodination of 3,5-diiodothyronine, using chloramine T and purified by paper chromatography (25).
In vitro Studies
Cell cultures.
C2C12 cells were obtained from ATCC. Primary cultures of skeletal muscle cells enriched in satellite cells (pp6 cells) were isolated from C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) as previously described (4, 17). The cells were cultured in DMEM containing 1 ng/ml β-FGF, 5 ng/ml IGF-I, 10% horse serum, and 10% FBS (proliferating conditions). They were induced to differentiate by removing the FBS, reducing the horse serum to 2%, and adding insulin, transferrin, and Na2SO3 (differentiation medium) (4). To assess the effect of differentiation-induced D2 on the ratio of 125I-T3(T4) to 131I-T3(T3) we incubated proliferating and differentiated cells overnight in 0.1% BSA containing free 125I-T4 (27 pM) and free 131I-T3 (4 pM) in 60-mm dishes for 22 h in 1.5 ml of DMEM (11). In some experiments, 30 nM reverse (r)T3 was included to block D2 activity (26).
Preparation of nuclei and cytosol and quantitation of 125I-T3, 131I-T3, and125I-T4.
Nuclei and cytosol were prepared using differential centrifugation as previously described (22). Nuclear pellets were extracted with 100% ethanol-ammonium hydroxide (99:1) and chromatographed on 3-mm Whatman chromatography paper using tertiary amyl alcohol-hexane-ammonium hydroxide for 24–48 h together with unlabeled iodide, T4, T3, and 10−3 M PTU as described (15, 24). The locations of labeled T3 and T4 on the strips were identified by colorimetry: appropriate segments of paper were counted in a dual-channel spectrometer. The counts of 125I-T3 were corrected for the 29% crossover of 131I-T3 appearing in the 125I-T3 window. The net 125I-T3 was multiplied by 2 to correct for loss of an 125I atom from the distal ring of 125I-T4 and the ratio of 125I-T3 to 131I-T3 determined. Aliquots of cytosol (∼30% of the total cytosol fraction) were treated in the same way.
D2 deiodination assays.
D2 activity was measured in cell homogenates as previously described (21).
In vivo Studies
Four to five 8- to 12-wk-old WT C57BL/6 males, age-matched within each experiment, were obtained from Jackson Laboratories. D2KO mice generated were back-crossed 11 times into the C57BL/6 line (23). In some experiments, mice were made hypothyroid by providing drinking water containing 0.1% methimazole (MMI) and 1% NaClO4 (MMI/ClO4) for 8 wk (13). Muscle regeneration was induced by cardiotoxin injection as described previously (4, 30). Briefly, 25 μl of 10 μM cardiotoxin (Calbiochem) was injected into the right anterior tibialis (AT) and 50 μl into the gastrocnemius (GC) muscles of isofluorane-anesthetized C57BL/6 age- and sex-matched WT and D2KO mice. The corresponding left AT and GC muscles were not injected and used as controls. Studies were performed 9 days after injury, which is the period of peak D2 expression using this approach (4). Serum T3 and T4 were unchanged in these mice (4).
Mice received an injection of 125I-T4 (∼20 μCi/animal ip) 24 h prior to being euthanized. On the day of the experiment each animal was given ∼5 μCi ip of 131I-T3 4 h prior to obtaining tissues. PTU (1 mg/100 g body wt ip) was administered 48, 24, and 6 h before tissue harvest to inhibit D1 activity in liver and kidney. Assays of liver homogenates confirmed 90% blockade of D1 activity in treated mice. Animals were anesthetized with isofluorane, and blood was collected via cardiac puncture, after which they were perfused with ice-cold 0.14 M NaCl containing 10−4 M PTU to remove residual blood. Tissues (skeletal muscles, liver, kidney, and cerebral cortex) were collected, homogenized in 10 vol wet/wt of Tris buffer, pH 7.5, 10−4 M PTU containing protease inhibitor (Roche). Cytosol was obtained via low-speed centrifugation (3,300 rpm × 5 min) at 4°C. All animal experimental protocols were approved by the Animal Research Committee of Harvard Medical School.
Statistics
Prism 4.0 software (GraphPad Software, San Diego, CA) was used for statistical analysis. When only two groups were analyzed, statistical significance was determined by an unpaired Student's t-test. Two-way ANOVA was used to compare the effects of different treatments on two different cell types (proliferating and differentiated cells) or on two different genotypes (WT vs. D2KO) followed by t-test when significant differences were found. Results shown are means ± SE. P < 0.05 was considered statistically significant.
RESULTS
D2 is Expressed in Muscle Precursor Cells and Increases Nuclear and Cytosolic T3
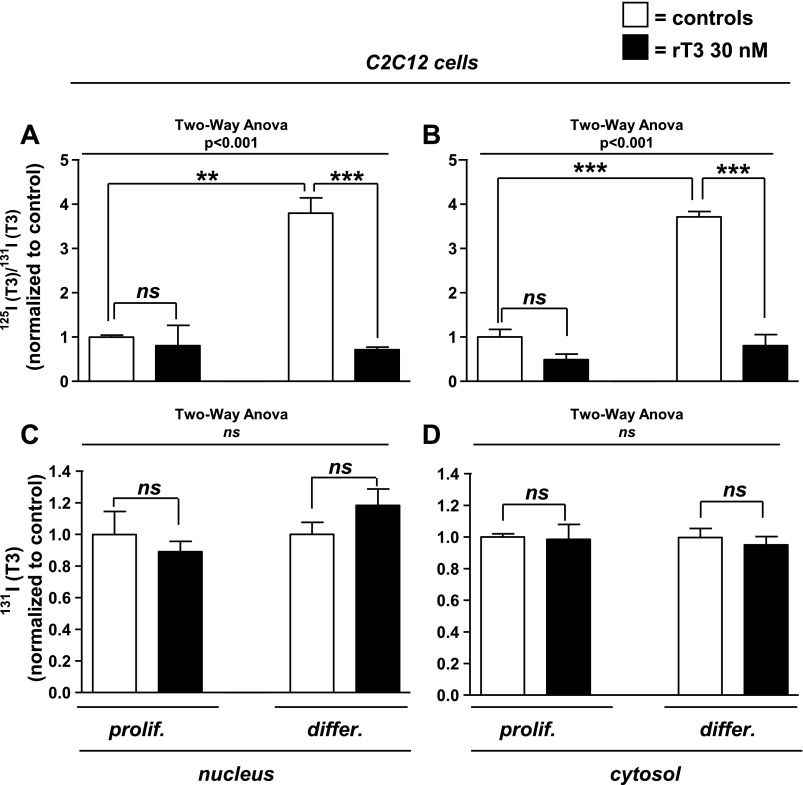
D2 mRNA is increased during differentiation in C2C12 cells and in neonatal mouse skeletal myoblasts (4). After 48 h of incubation in differentiation media, D2 activity in C2C12 cell sonicates increased fourfold, from 0.35 ± 0.01 to 1.7 ± 0.01 fmol·min−1·mg protein−1 (P < 0.0001), in differentiated compared with proliferating cells. To determine whether the increase in D2 was reflected in changes in intracellular T3(T4), proliferating and differentiated C2C12 cells were incubated overnight in 125I-T4 and 131I-T3 containing media supplemented with unlabeled hormone at physiological concentrations, and nuclear and cytosolic 125I-T3 and 131I-T3 were isolated. The ratio 125I-T3/131I-T3 was fourfold higher in differentiated vs. proliferating C2C12 cells in both the nucleus and the cytosol (Fig. 1, A and B). In both proliferating and differentiated cells, nuclear T3 binding of both isotopically labeled T3 molecules was blocked by high concentrations of unlabeled T3, indicating that nuclear T3 binding was saturable and specific (data not shown).
Fig. 1.
Nuclear and cytosolic 125I-T3/131I-T3 (triiodothyronine) ratio or total 131I-T3 in C2C12 cells after overnight incubation with 125I-T4 (thyroxine) and 131I-T3. Proliferating (prolif) and differentiated (differ) C2C12 cells were incubated in physiological concentrations of free 131I-T3 and 125I-T4 in DMEM containing 0.1% BSA and treated with vehicle or 30 nM reverse (r)T3 to block type II deiodinase (D2). Nuclear extracts (A) and cytosol fractions (B) were isolated, and labeled T3 was identified by paper chromatography. The basal ratio of 125I-T3(T4) to 131I-T3(T3) in proliferating C2C12 cells treated with vehicle were arbitrarily set as 1 (1st column on the left), and the remaining results were normalized to that value. 131I-T3 in nuclear extracts (C) or cytosol (D) were normalized as in A and B. Data are means ± SE of triplicates of 3 independent experiments. **P < 0.01; ***P < 0.001; ns, not significant.
rT3 (3,3′,5′-triiodothyronine) is a competitive inhibitor of D2-mediated T4-to-T3 conversion, which also selectively reduces D2 activity by promoting its ubiquitination, thus accelerating its proteasomal degradation (26). We found that the inhibitory effect of rT3 is dependent on cell differentiation (two-way ANOVA interaction, P < 0.001). Treatment with 30 nM rT3 reduced the 125I-T3/131I-T3 ratio in the nuclei of differentiated cells to levels not different from that in nuclei of proliferating C2C12 cells (Fig. 1, A and B). The same treatment had no effect on the 125I-T3/131I-T3 ratio in proliferating C2C12 cells, consistent with much lower D2 activities in the proliferative phase. These observations establish a role for D2 in this model of mouse skeletal muscle cell precursors in generating intracellular T3, which in turn is bound to the thyroid nuclear receptors. Reverse T3 had no effect on the nuclear 131I-T3 (Figs. 1, C and D), indicating that the reduction in the 125I-T3/131I-T3 ratio with rT3-treated cells was not due to enhanced cellular 131I-T3 uptake. Thus, the increased 125I-T3 in differentiated C2C12 cells reflects an increase in D2-mediated T3(T4) production.
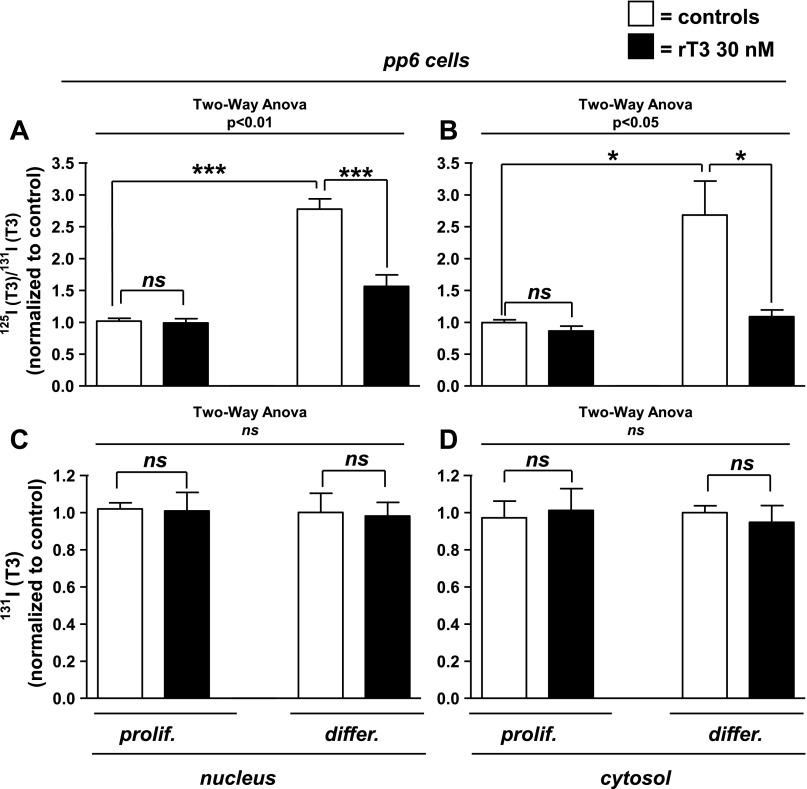
pp6 cells are dispersed neonatal (P3-P4) mouse myoblasts enriched with satellite cells expressing Pax7 (16). The expression of D2 mRNA, while detectable in the proliferative phase, markedly increases after differentiation (4). In agreement with this, in pp6 cells, D2 activity increases eightfold, from 0.01 ± 0.01 to 0.08 ± 0.2 fmol·min−1·mg protein−1 (P < 0.01). In experiments analogous to those performed in C2C12 cells, the ratio of 125I-T3 to 131I-T3 was nearly threefold greater in differentiated than in proliferating cells [2.8 ± 0.2 vs. 1.0 ± 0.05 (P < 0.001) and 2.7 ± 0.5 vs. 1.0 ± 0.05 (P < 0.05)] in nuclei and cytosol, respectively (Fig. 2, A and B). Exposure to rT3 eliminated this difference (Fig. 2, A and B), confirming that the effect of treatment depended on the cell differentiation status (two-way ANOVA interaction, P < 0.001 and P < 0.05 in nuclei and cytosol, respectively). As in C2C12 cells, no differences were found in 131I-T3 in either nuclei or cytosol in proliferating and differentiated cells (Fig. 2, C and D), confirming that the higher ratios of 125I-T3 to 131I-T3 in the differentiated cells is due to an increase in D2-mediated T3 production. Also, rT3 did not reduce cytosolic 125I-T4 in pp6 cells, indicating that its effect to decrease 125I-T3 formation was not due to a reduction of 125I-T4 uptake (data not shown).
Fig. 2.
Nuclear and cytosolic 125I-T3/131I-T3 ratio or total 131I-T3 from pp6 cells after overnight incubation with 125I-T4 and 131I-T3. Proliferating (prolif) and differentiated (differ) pp6 cells were incubated in physiological concentrations of free 131I-T3 and 125I-T4 and treated with vehicle or 30 nM rT3 to block D2, as labeled (see Fig. 1 legend). Data are means ± SE of triplicates of 3 independent experiments. *P < 0.05, ***P < 0.001.
D2 Increases Intracellular T3(T4) In Vivo in Adult Mouse Skeletal Muscle and Cerebral Cortex
We next examined whether D2-mediated T4-to-T3 conversion contributed to intracellular T3 in vivo using a similar dual isotopic approach. Since there have been no previous studies of this issue in the mouse, we studied two D2-expressing tissues (skeletal muscle and cerebral cortex) and compared these with the D1-expressing (but D2-null) liver and kidney. The latter two tissues should not form intracellular T3(T4), since the T3 formed by D1 rapidly exits the cell (10).
Intracellular T3(T4) is Present in D2-Expressing Tissues of Adult Mice
To evaluate the role of D2 in vivo, we injected WT and D2KO mice with 125I-T4 and 131I-T3, 24 and 4 h, respectively, prior to harvesting cerebral cortex, skeletal muscle, liver, and kidney for isolation of labeled T3. The sera of these mice contain 131I-T3 and 125I-T3, the latter generated from 125I-T4 by either D1- or D2-mediated deiodination. To minimize the contribution of D1 to the 125I-T3 production and facilitate identification of the D2-generated product, all mice were treated with PTU to inhibit D1 activity prior to tracer T4 injection (see materials and methods). Because hypothyroidism increases D2 activity and reduces that of D1 in liver and kidney, further reducing the D1-generated 125I-T3, we first examined hypothyroid WT and D2KO mice (Fig. 3). The 125I-T3/131I-T3 ratio in WT sera was 2.85 ± 0.05-fold higher than that in the sera of D2KO mice (P < 0.001), indicating a significant contribution of D2-generated 125I-T3 to the sera of hypothyroid mice. Since T3 derived from serum will enter the tissues, this difference in isotope ratios between the WT and D2KO mice must be taken into account when the ratio of 125I-T3 to 131I-T3 in each tissue is analyzed. To correct for this difference, the 125I-T3/131I-T3 ratio in each tissue was divided by the serum 125I-T3/131I-T3 ratio in the same animal. If D2 provides significant intracellular 125I-T3 to a tissue, the 125I-T3/131I-T3 ratio in WT tissues (after correction for that in the serum) will be significantly higher than in tissues from D2KO mice. The 125I-T3/131I-T3 ratio in the hypothyroid WT cerebral cortex is 14-fold higher than that in D2KO mice (Fig. 3A and Table 1). The corrected 125I-T3/131I-T3 ratio in skeletal muscle is 3.5-fold greater in WT than in D2KO mice (Fig. 3B). In contrast, in WT liver and kidney, the 125I-T3/131I-T3 ratio is not different from that in D2KO mice (Fig. 3, C and D, and Table 1). Thus, there is a substantial contribution of T3(T4) only in the D2-expressing tissues of the WT mice.
Fig. 3.
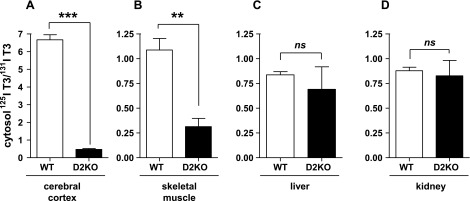
125I-T3(T4)/131I-T3(T3) ratio is higher in cytosol of cerebral cortex (A) and skeletal muscle (B) from hypothyroid wild-type (WT) than from D2 knockout (D2KO) mice but do not differ in liver (C) and kidney (D) cytosol. Assessment of 125I-T3/131I-T3 ratios in extracts of tissue cytosol from WT and D2KO mice that received 125I-T4 and 131I-T3 for 24 and 4 h, respectively, prior to obtaining tissues. Observed tissue ratios were divided by the 125I-T3/131I-T3 ratio in the serum of each mouse to correct for 125I-T3 serum contribution to intracellular T3. Results are means ± SE of 4 animals per group. **P < 0.01, ***P < 0.001.
Table 1.
Effect of hypothyroidism on the ratio of T3(T4)/T3(T3) in wild-type vs. D2KO in D2- and D1-expressing tissues
| Deiodinase Expressed | Hypothyroid | Euthyroid | P Value | |
|---|---|---|---|---|
| Cerebral cortex | D2 | 14 ± 0.6 | 3.0 ± 0.6 | P < 0.0001 |
| Skeletal muscle | D2 | 3.5 ± 0.4 | 2.2 ± 0.2 | P < 0.05 |
| Liver | D1 | 1.2 ± 0.1 | 1.0 ± 0.3 | P = NS |
| Kidney | D1 | 1.1 ± 0.1 | 0.8 ± 0.3 | P = NS |
Results are means ± SE of the ratios of 125I-T3/131I-T3 in each wild-type mouse divided by the corresponding ratio for the D2KO mouse in that same group.
T3, triiodothyronine; T4, thyroxine; D1 and D2, types I and II iodothyronine deiodinase; NS, not significant.
In euthyroid mice, the 125I-T3/131I-T3 ratio in the serum of the WT mice is only 1.4 ± 0.12 times that in the D2KO mice, reflecting the lower expression of D2 in euthyroid tissues. The differences in the 125I-T3/131I-T3 ratios (corrected for that in the serum) between WT and D2KO mice are smaller but qualitatively the same as in the hypothyroid mice (Fig. 4 and Table 1). In the cerebral cortex, the corrected 125I-T3/131I-T3 ratio is threefold higher in WT and in skeletal muscle twofold higher than in the D2KO mice (Fig. 4, A and B, and Table 1; P < 0.05). The 125I-T3/131I-T3 ratios in the liver and kidney of WT mice are not different from those in the D2KO mice, indicating no significant production of T3(T4) in these D1-expressing tissues (Fig. 4, C and D). To compare the results of the same tissue between euthyroid and hypothyroid mice, it is necessary to normalize the WT results by dividing the corrected ratios by the average 125I-T3/131I-T3 ratio in the same tissues of the D2KO mice (Table 1). That is required because the amounts of labeled T3 and T4 given to the mice varied slightly between animals and experiments. The magnitude of that ratio is a rough estimate of the net D2-mediated T3 production in a given tissue. The 125I-T3/131I-T3 ratio is significantly higher in the hypothyroid cerebral cortex than in skeletal muscle compared with same tissues in euthyroid animals (Table 1), consistent with the higher D2 expression in hypothyroidism.
Fig. 4.
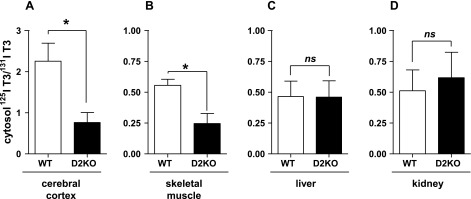
125I-T3(T4)/131I-T3(T3) ratio is higher in cytosol of cerebral cortex (A) and skeletal muscle (B) from euthyroid WT than from D2KO mice but not different in liver (C) and kidney (D) cytosol. Assessment of 125I-T3/131I-T3 ratios in tissue cytosol extracts from WT and D2KO mice that received 125I-T4 and 131I-T3 for 24 and 4 h, respectively, prior to obtaining tissues (see Fig. 3 legend). As in Fig. 3, observed ratios were corrected by dividing by the serum 125I-T3/131I-T3 ratio. Results are means ± SE of 4 animals per group. *P < 0.05.
The Increase in D2 During Muscle Regeneration Increases Local T3(T4) in Injured Tissue
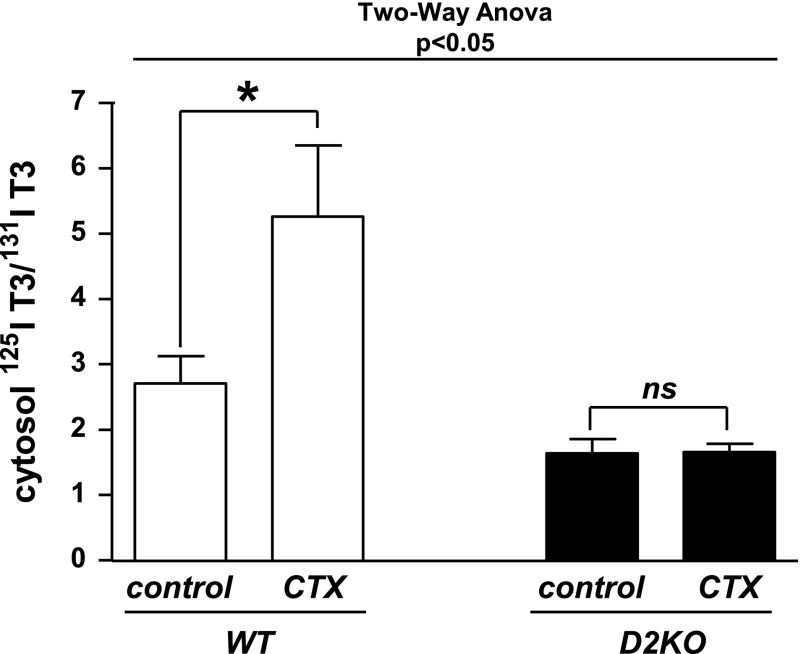
Injury to skeletal muscle causes an increase in D2 mRNA and activity, which peaks six- to eightfold above basal levels at about 8 days after the injury (4). In D2KO mice, regeneration is impaired, and the normal postinjury increase in the T3-dependent myoD does not occur. We hypothesized that the increase in D2 provided additional T3(T4), which was necessary for the transcriptional increase in myoD and its downstream targets (4). We tested this by examining the 125I-T3/131I-T3 ratio in cytosol of AT and GC muscle in mice given labeled iodothyronines 8 days after cardiotoxin injection (Fig. 5). Results were compared with the corresponding uninjected muscle in the contralateral leg in both WT and D2KO mice. The effect of the injury was D2 dependent (two-way ANOVA interaction, P < 0.05). In WT animals, there was a twofold higher 125I-T3/131I-T3 ratio in the injured skeletal muscles of WT animals than in the uninjured controls (5.3 ± 1 vs. 2.7 ± 0.4, P < 0.01; Fig. 5). The normal skeletal muscles of D2KO animals have lower baseline 125I-T3/131I-T3 ratios than do WT mice, and these do not change after injury. These results were confirmed in a second experiment.
Fig. 5.
Ratio of 125I-T3(T4) to 131I-T3(T3) is increased in injured skeletal muscle of WT mice but not in D2KO mice. 125I-T3/131I-T3 ratio was assessed by paper chromatography in cytosol of regenerating muscles in 8- to 9-wk-old WT and D2KO mice that had received cardiotoxin injection (see materials and methods) into the right anterior tibialis and gastrocnemius 8 days earlier. Contralateral homologous muscles were not injected and served as controls. WT and D2KO mice received 125I-T4 and 131I-T3 for 24 and 4 h, respectively, prior to obtaining tissues. Results are means ± SE of 5 animals per group. *P < 0.05.
DISCUSSION
We are not aware of other studies of the relative contribution of T3(T3) and T3(T4) in mouse tissues. Previous molecular physiological results with C2C12 myoblasts, pp6 cells, and whole animals suggest that skeletal muscle is T3 deficient when D2 is chemically inhibited or the dio2 gene is inactivated (4). Consistent with this, in vitro results in cultured cells show decreased expression of T3-dependent genes, such as myoD and its downstream T3-dependent gene products. This corresponds with a delay in differentiation, which can be rescued by overexpression of D2 or by exposing the cells to pharmacological T3 concentrations (30 nM) (4). In agreement with this, in D2KO animals, or if D2 expression is reduced due to a haploinsufficiency of the FoxO3 gene, the postinjury regeneration process is impaired (4, 8). The present experiments directly show that D2 provides intracellular T3(T4) to the nuclei of skeletal muscle cells in vitro and that its inhibition in vitro or inactivation in vivo reduces the intracellular T3, despite normal circulating serum or media T3 concentrations. This work directly addresses the role of D2 in normal differentiation and regeneration.
In both C2C12 and pp6 cells, differentiation resulted in morphological changes in the cells, as previously documented, and a six- to eightfold increase in D2 expression, suggesting there is a relationship between these two events (5). We found that in proliferating cells the 125I-T3/131I-T3 ratio in nuclei and cytosol incubated with physiological free concentrations of T4 and T3 was much lower than after differentiation when D2 is increased (Figs. 1 and 2A). When differentiated C2C12 or pp6 cells were incubated with rT3 to accelerate proteasomal degradation of D2, only 125I-T3 was reduced, resulting in a marked decrease in the 125I-T3/131I-T3 ratio. rT3 treatment did not either block the 125I-T4 uptake or increase the amount of 131I-T3 bound to the nuclei. Thus, the decrease in the 125I-T3/131I-T3 ratio reflected a decrease in D2-mediated T4-to-T3 conversion (Figs. 1 and 2).
Results in vivo confirm that T3(T4) contributes to intracellular T3 in D2-expressing tissues but not in those expressing only D1, since the 125I-T3/131I-T3 ratio in each D1-containing tissue is the same as that found in the serum. This is analogous to the situation in the rat (10). A comparison of the tissue-to-serum ratios of 125I-T3/131I-T3 in euthyroid and hypothyroid WT as opposed to D2KO mice further validates this strategy. In hypothyroid mice, in which D2 activity is elevated, these ratios were 14- and 3-fold higher in cerebral cortex and skeletal muscle, respectively, of WT vs. D2KO mice but not different in D1-containing liver or kidney (Fig. 3 and Table 1). The higher values for the tissue-to-serum ratios in the hypothyroid D2-expressing tissues illustrates that there is a quantitative relationship between the amount of T3(T4) produced and D2 expression(13).
While more tracer T3(T4) is formed in hypothyroid than in euthyroid tissues, it should be kept in mind that the serum T4 is much lower in hypothyroidism, so that the higher 125I-T3/131I-T3 ratio indicates that the tracer, not the gravimetric, T3 production rates are increased in hypothyroid D2-expressing tissues. This contrasts with the situation in regenerating skeletal muscle, where there is up to a sixfold increase in D2 activity, which peaks about 8–9 days after skeletal muscle injury in euthyroid animals (4). Since this increase occurs in the presence of a normal circulating T4, the amount of T3 generated is significantly increased (Fig. 5), confirming the physiological role of D2 in inducing myoD expression and other changes during the healing process (4). No change of 125I-T3/131I-T3 above the basal ratio was observed in D2KO mice after injury, as expected. D2KO mice are insulin resistant and susceptible to diet-induced obesity, and bile acids stimulate UCP3 mRNA in skeletal muscle through a T4-dependent process (12, 19, 28). These features may reflect other physiologically relevant actions of the intracellular T3 supplied by skeletal muscle D2.
With respect to humans, about 80% of daily T3 production derives from peripheral T4 monodeiodination. The relative roles of D2 vs. D1 in serum T3 production have not been defined, although there are significant reductions in the serum T3/T4 ratio in humans with a mutation in the selenocysteine insertion sequence (SBP-2), which is required for both D1 and D2 synthesis (5). It has not been possible to quantitate the degree of impairment of D1 in those individuals, but D2 expression is significantly reduced in cultured fibroblasts. A decrease of D2 in the hypothalamic-pituitary axis can also explain the compensatory increases in TSH and T4 secretion in those patients. In individuals with high D2-expressing metastatic thyroid carcinoma, the ratio of circulating T3 to T4 is markedly increased, again confirming that D2-mediated generation of T3 from T4 can contribute significantly to circulating T3 (9, 14).
Taken together, our data establish that the D2 present in mouse skeletal muscle provides a significant contribution to the intracellular T3 under basal circumstances. Increases in D2, such as occur in the postnatal period or following injury, are sufficient to provide an increase in intracellular T3 in this tissue, as long as serum T4 remains constant. Thus, skeletal muscle, like the pituitary, central nervous system, and brown adipose tissue, is a tissue in which alterations in D2 activity can influence the local T3 concentration and thereby alter the expression of T3-dependent genes.
GRANTS
This work was supported by National Institutes of Health Grants DK-44128 and T32-DK-007529 to P. R. Larsen and DK-076117 to A. M. Zavacki. A. Marsili was partially supported by a fellowship stipend from the Department of Endocrinology and Kidney, University Hospital of Pisa, Italy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Special thanks to Elena Gianetti for help with the figure preparation and statistical analysis.
REFERENCES
- 1. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23: 38–89, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98: 405–417, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104: 14466–14471, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120: 4021–4030, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37: 1247–1252, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29: 898–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heemstra KA, Soeters MR, Fliers E, Serlie MJ, Burggraaf J, van Doorn MB, van der Klaauw AA, Romijn JA, Smit JW, Corssmit EP, Visser TJ. Type 2 iodothyronine deiodinase in skeletal muscle: effects of hypothyroidism and fasting. J Clin Endocrinol Metab 94: 2144–2150, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Hu P, Geles KG, Paik JH, DePinho RA, Tjian R. Codependent activators direct myoblast-specific MyoD transcription. Dev Cell 15: 534–546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim BW, Daniels GH, Harrison BJ, Price A, Harney JW, Larsen PR, Weetman AP. Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J Clin Endocrinol Metab 88: 594–598, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Larsen PR, Silva JE, Kaplan MM. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev 2: 87–102, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115: 2524–2533, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marsili A, Aguayo-Mazzucato C, Chen T, Kumar A, Chung M, Lunsford E, Harney J, Van-Tran T, Gianetti E, Ramadan W, Chou C, Bonner-Weir S, Larsen P, Silva J, Zavacki A. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS One 6(6): e20832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsili A, Ramadan W, Harney JW, Mulcahey M, Castroneves LA, Goemann IM, Wajner SM, Huang SA, Zavacki AM, Maia AL, Dentice M, Salvatore D, Silva JE, Larsen PR. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology 151: 5952–5960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyauchi A, Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Amino N, Toyoda N, Nomura E, Nishikawa M. 3,5,3′-Triiodothyronine thyrotoxicosis due to increased conversion of administered levothyroxine in patients with massive metastatic follicular thyroid carcinoma. J Clin Endocrinol Metab 93: 2239–2242, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Oppenheimer JH, Schwartz HL, Surks MI. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology 95: 897–903, 1974 [DOI] [PubMed] [Google Scholar]
- 16. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23: 3430–3439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 142: 1257–1267, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramadan W, Marsili A, Huang S, Larsen PR, Silva JE. Type-2 iodothyronine 5′deiodinase in skeletal muscle of C57Bl/6 mice. I. Identity, subcellular localization, and characterization. Endocrinology 2011. May 31; doi: 10.1210/en.2011-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramadan W, Marsili A, Larsen PR, Zavacki AM, Silva JE. Type-2 iodothyronine 5′ deiodinase (D2) in skeletal muscle of C57BL/6 mice. II. Evidence for a role of D2 in the hypermetabolism of thyroid hormone receptor-alpha deficient mice. Endocrinology 152: 3093–3102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salvatore D, Bartha T, Harney JW, Larsen PR. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137: 3308–3315, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest 98: 962–968, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samuels HH, Tsai JS, Casanova J, Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest 54: 853–865, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15: 2137–2148, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Silva JE, Larsen PR. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest 61: 1247–1259, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva JE, Larsen PR. Pituitary nuclear 3,5,3′-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science 198: 617–620, 1977 [DOI] [PubMed] [Google Scholar]
- 26. Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J Clin Invest 102: 1895–1899, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Doorn J, van der Heide D, Roelfsema F. Sources and quantity of 3,5,3′-triiodothyronine in several tissues of the rat. J Clin Invest 72: 1778–1792, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Williams GR, Bassett D. Local control of thyroid hormone action—role of type 2 deiodinase. J Endocrinol 209: 261–72, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003 [DOI] [PubMed] [Google Scholar]