Abstract
While the benefits of exercise are clear, many unresolved issues surround the optimal exercise prescription. Many organizations recommend aerobic training (AT) and resistance training (RT), yet few studies have compared their effects alone or in combination. The purpose of this study, part of Studies Targeting Risk Reduction Interventions Through Defined Exercise-Aerobic Training and/or Resistance Training (STRRIDE/AT/RT), was to compare the effects of AT, RT, and the full combination (AT/RT) on central ectopic fat, liver enzymes, and fasting insulin resistance [homeostatic model assessment (HOMA)]. In a randomized trial, 249 subjects [18–70 yr old, overweight, sedentary, with moderate dyslipidemia (LDL cholesterol 130–190 mg/dl or HDL cholesterol ≤40 mg/dl for men or ≤45 mg/dl for women)] performed an initial 4-mo run-in period. Of these, 196 finished the run-in and were randomized into one of the following 8-mo exercise-training groups: 1) RT, which comprised 3 days/wk, 8 exercises, 3 sets/exercise, 8–12 repetitions/set, 2) AT, which was equivalent to ∼19.2 km/wk (12 miles/wk) at 75% peak O2 uptake, and 3) full AT + full RT (AT/RT), with 155 subjects completing the intervention. The primary outcome variables were as follows: visceral and liver fat via CT, plasma liver enzymes, and HOMA. AT led to significant reductions in liver fat, visceral fat, alanine aminotransferase, HOMA, and total and subcutaneous abdominal fat (all P < 0.05). RT resulted in a decrease in subcutaneous abdominal fat (P < 0.05) but did not significantly improve the other variables. AT was more effective than RT at improving visceral fat, liver-to-spleen ratio, and total abdominal fat (all P < 0.05) and trended toward a greater reduction in liver fat score (P < 0.10). The effects of AT/RT were statistically indistinguishable from the effects of AT. These data show that, for overweight and obese individuals who want to reduce measures of visceral fat and fatty liver infiltration and improve HOMA and alanine aminotransferase, a moderate amount of aerobic exercise is the most time-efficient and effective exercise mode.
Keywords: aerobic training, liver fat, resistance training, weight lifting, homeostasis model assessment
while the benefits of being physically active are clear, many unresolved issues surround the optimal exercise prescription for these benefits. Many organizations recommend both aerobic training (AT) and resistance training (RT) for all adults. However, these recommendations are mainly based on the evaluation of each modality separately, as few studies have investigated the effects of combined AT and RT regimens compared with each modality individually. Furthermore, adherence to exercise recommendations of physicians is notoriously poor, and many patients cite lack of time as a reason for noncompliance. Understanding the effects of AT and RT is of critical importance if we are to apply evidence-based approaches to exercise recommendations to a wide population.
Visceral fat and liver fat are associated with type 2 diabetes, metabolic syndrome, and metabolic abnormalities (7, 10, 18, 27, 33). Visceral and liver fat are also independent risk factors for all-cause mortality (14). Elevated concentrations of circulating liver enzymes are also associated with type 2 diabetes, fatty liver, metabolic syndrome, and mortality (9, 12, 15, 16, 22, 32). In fact, alanine aminotransferase (ALT) is considered to be a marker of fatty liver infiltration (23). Elevated liver enzymes, even at concentrations considered to be within normal levels, are independent predictors of incident diabetes (9, 22) and nonalcoholic fatty liver disease (NAFLD) (2). The importance of insulin resistance [homeostatic model assessment (HOMA)] is well established, and aerobic exercise consistently improves insulin sensitivity (28). While the benefits of aerobic exercise on visceral adiposity and insulin sensitivity are well established, few studies have compared the effects of AT, RT, and AT + RT on these outcomes (5, 26), and, to our knowledge, none have examined the effect of RT on liver fat or concentrations of circulating liver enzymes.
The present study, STRRIDE-AT/RT (Studies Targeting Risk Reduction Interventions Through Defined Exercise-Aerobic Training and/or Resistance Training), was designed to address three major questions relating to exercise recommendations for overweight, sedentary adults. 1) What are the specific benefits of RT in this population? 2) How do these benefits compare with those that accrue when a similar amount of time is spent in AT? 3) What are the additive, synergistic, or possibly antagonistic effects of the combination of AT and RT (AT/RT)? These findings should improve the ability of clinicians, exercise professionals, and the lay public to more accurately understand the benefits of different exercise regimens, target exercise recommendations to specific outcomes, and more efficiently utilize precious exercise time. This report summarizes the effects of AT, RT, and AT/RT on visceral fat and fatty liver infiltration, as represented by liver density and the circulating liver-derived enzyme ALT, as well as fasting insulin resistance as presented by HOMA.
METHODS
Subjects: screening and inclusion and exclusion criteria.
Subjects recruited for the STRRIDE-AT/RT study were used in this analysis. The protocol was approved by the institutional review boards at Duke University Medical Center and East Carolina University. Subjects (n = 3,145) responded to local advertisements and were screened by phone. Of these, 234 met inclusion criteria and were recruited into the study. Inclusion criteria were as follows: age 18–70 yr, sedentary (physically active <2 times per week), body mass index 26–35 kg/m2, and mild-to-moderate dyslipidemia (LDL cholesterol 130–190 mg/dl and/or HDL cholesterol ≤40 mg/dl for men or ≤45 mg/dl for women). Subjects were nonsmokers without a history of diabetes, hypertension, or coronary artery disease. After providing informed written consent, subjects were asked to maintain their current lifestyle during a 4-mo run-in period followed by randomization into one of three exercise-training groups. The purpose of the run-in period was to discourage individuals who were not serious about the study commitment and, thus, reduce the dropout rate that occurs after randomization. Of the 234 subjects recruited, 38 dropped out during the run-in period, leaving 196 subjects for randomization. Of the subjects who were randomized, 73.5% (n = 144) completed the study.
CT, cardiopulmonary exercise testing, and strength evaluations.
Body weight of subjects dressed in light clothing without shoes was measured to the nearest 0.1 kg on a digital scale. The average of three weights taken over 2 wk, on different days, was used for each time point. Height was measured once, to the nearest 0.5 cm. CT scans were performed by a radiological technologist who was blinded to the subject's study status. With subjects in the supine position, a single, 10-mm axial image was taken of the abdomen at the level of the L4 pedicle. A second, single 10-mm axial image was taken at the best visual location of the liver (determined by a scout image frontal radiograph taken prior to the liver scan). The spleen was also captured in 67% of these scans. As a result, the liver-only analyses are based on a higher total number of subjects, whereas analyses of the spleen only and the liver-to-spleen ratio are based on smaller numbers of subjects. The CT images were analyzed using OsiriX imaging software, an advanced open-source picture archiving-and-communication system (PACS) workstation DICOM viewer (OsiriX Foundation, Geneva, Switzerland), to determine the surface area of the visceral, subcutaneous, and total abdominal adipose tissue. With this program, once the parameters are set (e.g., definition of adipose tissue density range was set at −30 to −190 Hounsfield units), the program is largely automated. Test-retest reliability correlations for surface areas obtained are generally nearly perfect (r = 0.98 or 0.99), as the methodology is extremely reproducible. To obtain liver density, in the liver image, three 3.0-cm2 circular regions of interest were manually selected, with care taken to avoid visible vessels, bile ducts, bordering surfaces, and motion artifact, and averaged to estimate liver density. In the spleen image, three 2.0-cm2 circular regions of interest were manually selected using the same analytic guidelines and averaged to estimate spleen density. Prior to the beginning of the exercise interventions, CT tests of 117 subjects were done, and the test-retest correlation for liver density values was 0.910 (P < 0.0001), with no significant difference between test 1 and test 2 means (P = 0.79).
A maximal cardiopulmonary exercise test with a 12-lead ECG and expired gas analysis were performed on a treadmill using a TrueMax 2400 Metabolic Cart (ParvoMedics, Sandy, UT) before and after the exercise interventions.
In RT and AT/RT subjects, the total amount of weight lifted during a single RT session was recorded each week by a supervising personal trainer at the East Carolina University site or electronically by the FitLinxx Strength Training Partner system (FitLinxx, Norwalk, CT) at the Duke University site. The total amount of weight lifted in pounds from a typical single session during week 5 or 6 was used as the baseline measure of overall strength, and the total from a typical, single session at the end of training was used as the end-of-training measure of overall strength.
Exercise training: protocols, ramp period, duration, modes, verification, and adherence.
The exercise groups were as follows: 1) RT (3 days/wk, 3 sets/day, 8–12 repetitions/set, 8 exercises), 2) AT [calorically equivalent to 19.2 km/wk (∼12 miles/wk) at 75% peak O2 uptake (V̇o2)], and 3) AT + RT (AT/RT), i.e., the full AT regimen + the full RT regimen.
A ramp period of 8–10 wk, designed to gradually increase the amount of aerobic exercise over time, was prescribed to all subjects in the AT and AT/RT groups. Details about the prescribed and actual exercise-training amounts, intensity, and frequency are provided in Table 1. The aerobic exercise modes included treadmill, elliptical trainers, cycle ergometers, or any combination of these. As the intensity of the AT program was based on and maintained using heart rate zones, it was important for the subjects in the AT/RT group to perform the AT program first, followed by the RT program.
Table 1.
Baseline demographics, baseline calorie and alcohol intake, and exercise prescriptions
| Variables | RT (n = 52) | AT (n = 48) | AT/RT (n = 44) |
|---|---|---|---|
| Age, yr | 49.7 (11.4) | 49.5 (9.8) | 46.9 (10.0) |
| Body mass index, kg/m2 | 30.5 (3.4) | 30.4 (3.2) | 30.7 (3.4) |
| Race | |||
| Caucasian | 43 | 42 | 37 |
| African American | 8 | 6 | 6 |
| Other | 1 | 0 | 1 |
| Sex | |||
| Female | 30 | 26 | 25 |
| Male | 22 | 22 | 19 |
| Food intake, kcal/day | 2,168 (773) | 1,971 (733) | 2,104 (559) |
| Alcohol intake, g | 4.7 (8.8) | 2.9 (6.5) | 5.9 (11.6) |
| Resistance exercise | |||
| Rx frequency, sessions/wk | 3 | 3 | |
| Intensity | Progressive | Progressive | |
| Rx amount, sets/wk | 72 | 72 | |
| Rx time, min/wk | 135–180 | 135–180 | |
| Adherence, % | 83.0 (13) | 81.4 (14) | |
| Actual frequency, sessions/wk | 2.5 (0.4) | 2.46 (0.4) | |
| Actual amount, sets/wk | 59.7 (9) | 58.6 (10) | |
| Aerobic exercise | |||
| Intensity, %peak V̇o2 | 75 | 75 | |
| Rx amount, kcal·kg−1·wk−1 | 14 | 14 | |
| Rx time, min/wk | 132 (24) | 133 (25) | |
| Adherence, % | 89.8 (10) | 82.2 (17) | |
| Actual frequency, sessions/wk | 3.0 (0.5) | 2.9 (0.6) | |
| Actual time, min/wk | 117 (20) | 109 (27) |
Values are means (SD). AT, aerobic training; RT, resistance training; AT/RT, AT + RT; V̇o2, O2 uptake; Rx, prescribed. There were no significant baseline differences between groups. Rx amount (72 sets/wk) = 3 days/wk, 3 sets of 8–12 repetitions, on 8 different machines. Actual amount (min/wk) = approximate range of the number of minutes per week to complete the prescribed sets/wk. Actual amount (sets/wk) = Rx amount × adherence. Rx amount (14 and 23 kcal·kg−1·wk−1) is approximately calorically equivalent to 12 and 20 miles of jogging per week, respectively. Actual time (min/wk) = Rx time × adherence.
For subjects randomized to RT, the ramp period began with one set during weeks 1–2 and two sets during weeks 3–4, with build-up to the prescribed three sets on week 5. For subjects in the RT group, three sessions per week (on nonconsecutive days) of three sets of 8–12 repetitions on eight Cybex weight-lifting machines, designed to target all major muscle, groups were prescribed. Throughout the training intervention, the amount of weight lifted was increased by 5 pounds each time the participant performed 12 repetitions with proper form on all three sets on two consecutive workout sessions to ensure a progressive RT stimulus.
All aerobic exercise sessions were verified by direct supervision and/or with a heart rate monitor that provided recorded, downloadable data (Polar Electro, Woodbury, NY). Aerobic compliance percentages were calculated each week and were equal to the number of minutes completed within the prescribed heart rate range divided by the number of total weekly minutes prescribed. All RT sessions were verified by direct supervision and/or the FitLinxx Strength Training Partner.
Liver enzymes, insulin, and glucose.
Plasma samples were taken at each time point, and ALT and aspartate aminotransferase (AST) were measured by conventional spectrophotometric methodology using a DxC 600 autoanalzyer (instrument and reagents from Beckman Coulter, Fullerton, CA). These tests conform to International Federation of Clinical Chemistry standardization, utilizing pyroxidal 5-phosphate as a cofactor in the enzymatic determination. Insulin was determined with immunoassay (Access Immunoassay System, Beckman Coulter), and glucose was determined with an oxidation reaction (model 2300 Stat Plus, Yellow Springs Instrument, Yellow Springs, OH). All samples were centrifuged, and plasma was frozen at −80°C. All posttraining samples were collected within 16–24 h of the last training session.
Statistical analyses.
Data were analyzed using Statview (SAS Institute, Cary, NC). Two-tailed, paired t-tests were used to determine if the posttraining-pretraining difference within each group was significant. P < 0.05 was considered significant. ANOVA was used to determine if there were significant differences between groups. When the ANOVA was significant, a Fisher's post hoc analysis was performed to determine differences between groups. Post hoc P < 0.05 was considered significant.
RESULTS
Baseline characteristics, daily caloric and alcohol intake, and the exercise programs are described in Table 1. There were no differences in caloric intake or ethanol intake between groups at baseline. During the 4-mo run-in period, subjects experienced small, but significant, increases in body weight, abdominal subcutaneous fat area, and total abdominal fat area (data not shown). Caloric intake changes as a result of exercise training ranged from +13.3 to +72.2 kcal/day, all nonsignificant (P > 0.30 for all 3 within-group change comparisons). Daily alcohol consumption also did not change as a result of exercise training, as changes in consumption ranged from −1.1 to +1.7 g [all changes were nonsignificant (P > 0.30 for all 3 groups)].
In Table 2, baseline and change scores are shown for the three intervention groups. In addition, Table 2 includes P values for the two-tailed t-tests that indicate which within-group change scores were significant. AT provided a substantial stimulus, as peak V̇o2 increased by 14% in AT and 16% in AT/RT. RT resulted in a smaller, but significant, increase of 5.5% in peak V̇o2. The RT stimulus was also successful, as the amount of weight lifted per session increased by 55% in RT and 41% in AT/RT. In addition to the highly significant increases in weight lifted, the RT and AT/RT programs resulted in highly significant increases in lean body mass of 1.1 and 0.8 kg, respectively (both P < 0.001), whereas the AT group experienced no change in lean body mass (P = 0.63).
Table 2.
Baseline values and change scores for key variables
| RT (n = 52) |
AT (n = 48) |
AT/RT (n = 44) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Change | P value | Baseline | Change | P value | Baseline | Change | P value |
| Body weight, kg | 88.6 (16) | 0.7 (2.4) | 0.042* | 88.5 (11) | −2.0 (3.8) | 0.001* | 90.4 (12) | −2.1 (3.2) | <0.0001* |
| V̇o2 peak, ml·kg−1·min−1 | 26.6 (6) | 1.4 (3) | 0.001* | 27.7 (6) | 3.6 (3) | <0.0001* | 27.2 (6) | 4.0 (3) | <0.0001* |
| Strength, kg/session | 8780 (495) | 4064 (357) | <0.0001* | NA | NA | NA | 8574 (402) | 3492 (386) | <0.0001* |
| Visceral fat,‡ cm2 | 156 (82) | 0.8 (19) | 0.8 | 190 (106) | −15.9 (34) | 0.007* | 154 (66) | −10.9 (33) | 0.057 |
| Subcutaneous fat,‡ cm2 | 321 (124) | −8.2 (30) | 0.095 | 307 (83) | −25.1 (54) | 0.010* | 348 (132) | −28.7 (37) | <0.0001* |
| Total abdominal fat,‡ cm2 | 471 (141) | −7.2 (37) | 0.23 | 497 (132) | −35.2 (69) | 0.004* | 503 (162) | −41.0 (61) | 0.0003* |
| AST,‡ U/l | 27.4 (16.9) | 0.7 (8.8) | 0.57 | 26.7 (8.3) | −0.0 (9.7) | 0.99 | 26.8 (8.7) | 0.7 (6.6) | 0.48 |
| ALT,‡ U/l | 29.3 (13.7) | −2.8 (12) | 0.20 | 31.7 (17.7) | −4.3 (11) | 0.009* | 31.5 (13.9) | −4.4 (10) | 0.008* |
| Liver density,‡ HU | 59.2 (7.6) | 0.4 (4.9) | 0.64 | 55.7 (11.0) | 2.5 (5.7) | 0.012* | 56.6 (12.0) | 1.8 (5.9) | 0.079 |
| Liver fat score,†‡ HU | 40.9 (7.6) | −0.4 (4.9) | 0.64 | 44.3 (11.0) | −2.5 (5.7) | 0.012* | 43.4 (12.0) | −1.8 (5.9) | 0.079 |
| Spleen density,‡ HU | 50.2 (5.0) | 1.9 (5.0) | 0.063 | 51.8 (4.27) | −1.5 (2.8) | 0.009* | 50.9 (5.26) | 0.7 (4.2) | 0.42 |
| Liver-to-spleen ratio‡ | 1.17 (0.15) | −0.02 (0.11) | 0.44 | 1.07 (0.24) | 0.08 (0.12) | 0.001* | 1.13 (0.24) | 0.04 (0.12) | 0.19 |
| HOMA,‡ mg·dl−1·μU−1·ml−1 | 2.08 (1.1) | −0.09 (1.3) | 0.63 | 2.37 (1.6) | −0.40 (0.8) | 0.004* | 2.12 (1.2) | −0.50 (0.9) | 0.002* |
Values are means (SD). NA, not available; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HU, Hounsfield, units; HOMA, homeostatic model assessment.
Number of subjects for RT, AT, and AT/RT, respectively, for visceral, subcutaneous, and total abdominal fat (n = 39, 36, and 35); liver enzymes (n = 47, 46, and 44), liver density and liver fat score (n = 36, 36, and 35), spleen density and liver-to-spleen ratio (n = 24, 28, and 22), and HOMA (n = 48, 45, and 42). There were no significant baseline differences between groups. Fat and density measures are from CT.
Liver fat score = 100 − liver density. HOMA = [(fasting glucose (mg/dl) ∗ fasting insulin (μU/ml)]/405, which is a measure of fasting insulin sensitivity, with lower numbers being more sensitive.
Significant change (post vs. pre value) using a paired t-test.
The RT program resulted in a significant increase in body mass. However, abdominal subcutaneous fat trended toward a decrease (P = 0.10), suggesting that the body mass gain was not due to fat mass gain. No other changes were observed, with the exception of an unexpected trend toward an increase in spleen density (P = 0.06).
In contrast, AT resulted in significant improvements in nearly every parameter, including a reduction in body mass, visceral fat area, subcutaneous and total abdominal fat area, and, presumably, in fatty liver infiltration as represented by the combination of a reduction in liver fat score (liver attenuation on CT) and the liver enzyme ALT. AT resulted in an unexpected reduction in spleen density (P < 0.01), which was in the opposite direction of and significantly different from the outcome of the RT program (P < 0.01). Spleen density was included to serve as a standard compared with liver density, as it is expected that spleen density would remain constant. While we report liver-to-spleen ratio changes, the observed enigmatic changes in spleen density for AT and RT led us to believe that the liver-to-spleen ratio may not be an accurate indicator of changes in liver fat.
AT/RT subjects generally experienced the additive effects of AT and RT for the outcomes in this study. Where RT had little or no effect, the effect of AT/RT reflected that of AT alone. This was true for visceral fat area, ALT, and liver density. Where RT had a positive effect, the effect of AT/RT resembled the additive effects of RT + AT, as in subcutaneous and total abdominal fat area.
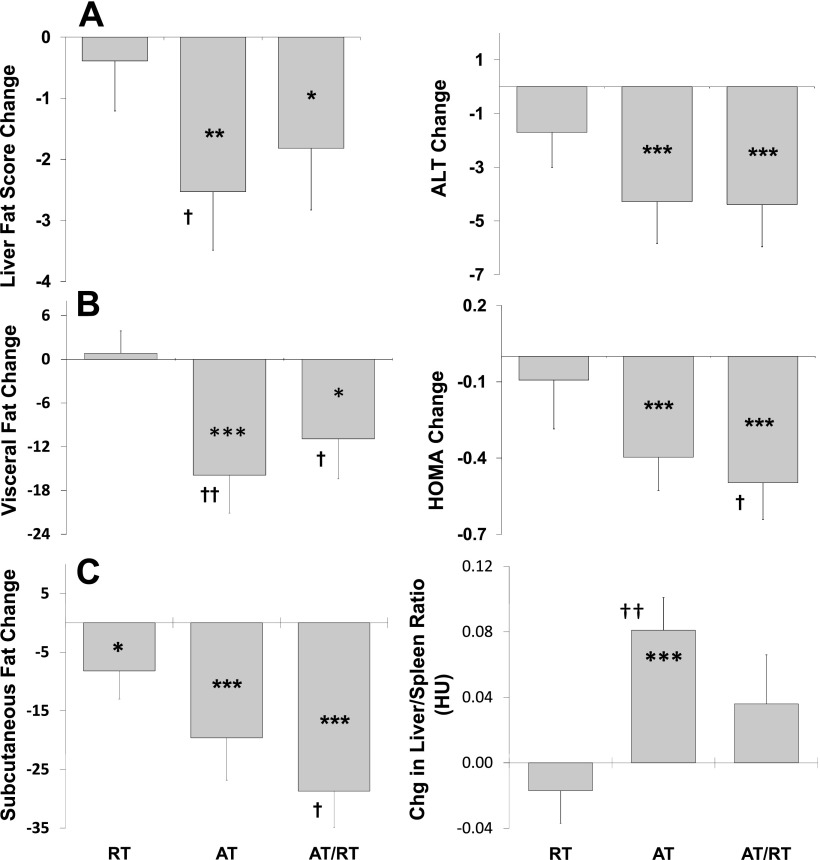
Figure 1 details the results of group comparisons for key variables. AT led to larger improvements in visceral fat and liver-to-spleen ratio (P < 0.05) and also trended toward a greater reduction in liver fat score (P < 0.10) than did RT. AT/RT trended toward larger improvements in visceral fat, abdominal subcutaneous fat, and fasting insulin resistance (HOMA) (all P < 0.10) than did RT. However, AT/RT was not different from AT for any of these key variables.
Fig. 1.
Effects of exercise mode(s) [aerobic training (AT), resistance training (RT), and AT + RT (AT/RT)] on changes in liver fat score [A; 100 − liver density in Hounsfield units (HU)], visceral fat (B; cm2), subcutaneous abdominal fat (C; cm2), liver-specific enzyme alanine aminotransferase (D; ALT, mg/dl), fasting insulin resistance (E; HOMA), and liver-to-spleen ratio (F). †Trend toward difference from RT (P < 0.10). Significant difference from RT: ††P < 0.05; †††P < 0.01. *Trend toward significant within-group change score (P < 0.10). Significant within-group change score: **P < 0.05; ***P < 0.01.
DISCUSSION
To our knowledge, this is the first randomized trial to investigate the effects of RT, AT, and RT/AT on visceral fat area and fatty liver infiltration (indicated by liver density on CT and substantiated by circulating ALT levels) in overweight and obese inactive adults. Although RT and AT are vastly different in terms of the nature of the training stimulus (i.e., intermittent vs. continuous contractions, time skeletal muscle is under load, metabolic pathways utilized, and others), the basis for comparison was that the prescriptions utilized were consistent with national recommendations for the general population (31). Thus, information gathered in this study should be useful when considering the optimal exercise prescription for improving visceral and liver fat, as well as improving fasting insulin resistance (HOMA).
In this respect, there were several important findings. 1) A RT program, even a very substantial one, did not significantly reduce body mass, visceral fat, liver fat, or ALT liver enzyme levels. RT also did not reduce total abdominal fat, nor did it improve fasting insulin resistance. 2) In contrast to RT, a typical vigorous AT program resulted in significant reductions in visceral fat, liver fat, and abdominal subcutaneous fat and also led to improvements in circulating ALT and HOMA (fasting insulin resistance). The decrease in liver fat in AT subjects is an important finding, and this is the first time, to our knowledge, such a decrease has been observed. 3) In a direct comparison of AT with RT, AT more effectively reduced visceral fat and improved the liver-to-spleen ratio than did RT (P < 0.05), and AT trended toward a larger reduction in liver fat (P < 0.10). 4) AT/RT was not superior to AT alone, as there were no statistical differences between these groups for any of these variables. That is, for these important physiological variables, there was no additional statistically significant advantage to adding a substantive RT program (and spending approximately twice as much time exercising) to a moderate AT program. It is the overall consistency of these findings of a greater AT than RT effect on visceral fat and fatty liver infiltration, rather than any one finding, that is so compelling. We believe that these data convincingly show that, for overweight and obese individuals who want to lose body weight, visceral fat, and liver fat and improve liver enzymes and fasting insulin resistance, AT alone would be the most time-efficient and effective exercise modality.
It is important to point out that RT is known to result in significantly lower caloric expenditure than a similar amount time spent in vigorous AT. Davidson et al. (5) estimated that the typical RT program expended ∼45% of maximal V̇o2. This compares with 75% of maximal V̇o2 used in the AT program from the present study. The result is that ∼67% more calories were likely expended in the AT than the RT program. We would hypothesize that much of the difference in the effects on ectopic fat is due to the differences in caloric expenditure between the two training programs.
NAFLD is directly associated with obesity and hepatic insulin resistance and is an important emerging metabolic risk factor (4, 30). An elevated liver fat level is an independent predictor of type 2 diabetes, dyslipidemia, metabolic syndrome, and cardiometabolic abnormalities (4, 18, 33). It is important to point out that, in this study, it would appear that visceral and liver fat are not the best markers of fasting insulin resistance, since visceral and liver fat tend not to decrease as much in the AT/RT group, whereas HOMA tends to decrease more. However, liver enzymes, in particular ALT, even within the normal reference limits, are correlated with and predictive of incident NAFLD and type 2 diabetes (2, 9, 22). Evidence from cross-sectional studies suggests that physical activity will likely reduce liver fat (29). However, we are aware of only three exercise studies in humans that have examined the effects of aerobic exercise on liver fat, and none of the studies has reported a significant effect (3, 8, 25). This is likely explained by the short duration (6, 10, and 12 wk) and small number of subjects in all three of these studies, which resulted in a limited exercise stimulus and a limited statistical ability to detect an effect. Evidence from the present study demonstrates that a moderate amount of aerobic exercise training over 8 mo leads to statistically significant reductions in liver fat that are clinically meaningful as indicated by significant improvements in ALT. As this is the first study of the effect of RT on liver fat or liver enzymes, the data would suggest that RT alone or in addition to AT is not an effective modality for improving these variables.
While we measured ALT and AST, only one of these enzymes was affected by training. It is not entirely clear why only ALT was improved. However, according to a position stand by the American Association of the Study of Liver Diseases, ALT is a better indicator of health and disease (10). While most of this position stand is about ALT, and not AST, the authors indicate that ALT is a better indicator of chronic liver injury than is AST because of its longer half-life (47 h vs. 17 h). Furthermore, Chang et al. (2) suggest that of the liver enzymes AST, ALT, and γ-glutamyltransferase (GGT), ALT is most closely related to liver fat accumulation, and their finding of a higher correlation between ALT and the development of NAFLD might be due to the higher specificity of ALT for liver injury and/or its contribution as a glucogenic enzyme. They also indicate that their findings “agree with several previous studies that ALT is more closely associated than either AST or GTT with both hepatic insulin resistance and later decline in hepatic insulin sensitivity.”
The importance of visceral fat and insulin resistance (HOMA) to cardiometabolic health and disease risk is well established. It is also known that aerobic exercise training can significantly reduce visceral fat (5, 11, 20, 21, 27) and consistently improves insulin sensitivity (28). Far fewer randomized controlled studies have investigated the effects of RT on visceral fat in overweight or obese subjects. Davidson et al. (5) observed no effect of RT on visceral fat. However, their study design involved only 20 min of RT, three times per week, and therefore cannot rule out that a more substantial program might have had a significant response. Sigal et al. (26) did not find that RT had a significant effect on visceral fat; however, nor did AT in their study. The reason for the difference between their findings and ours is not clear but may be due to differences in study populations. Schmitz et al. (24), in a 2-yr study, reported that visceral fat increased by 7% with RT, but this was significantly less than the 21% increase observed in their inactive controls. This is an important finding and indicates the need for more research in this area.
Relevant to and supportive of the findings in this report, we recently published results from the same cohort of the effects of AT, RT, and AT/RT on metabolic syndrome score and the individual components (HDL-cholesterol, triglycerides, waist circumference, fasting glucose, and blood pressure) (1). RT did not significantly improve metabolic syndrome or any of the individual components. AT resulted in a trend toward an improved metabolic syndrome score (P = 0.067) and a trend toward reducing waist circumference. Perhaps particularly relevant to the findings of improved visceral and liver fat observed in this report, AT significantly reduced fasting triglyceride levels, whereas RT had no effect. Interestingly, the AT/RT group, while not statistically better than AT group for any variable, experienced the most robust and widespread benefits and significantly improved metabolic syndrome score, waist circumference, triglycerides, and diastolic blood pressure. Whether this robust response was due to a synergistic effect of AT/RT or simply the greater total amount of exercise cannot be determined from this study design.
Important strengths of this study include 1) the randomized study design, 2) the inclusion of three training programs in the same study, 3) direct verification of exercise amount, intensity, and, therefore, exposure, for nearly all AT, RT, and AT/RT training sessions, 4) the inclusion of a substantial RT program that reduces the likelihood that negative findings are due to an inadequate RT stimulus, 5) a significant proportion of women and minorities in the study population, 6) the additive nature of the combination program, permitting the assessment of additive or interacting effects of AT and RT, and 7) the inclusion of two important ectopic fat depots (liver and visceral fat) and two major metabolic plasma risk markers (insulin resistance and ALT).
An important limitation of this study is that liver density measures obtained from CT, while highly correlated to liver fat measures from MRS and liver biopsy studies (13, 17), are not direct measures of liver fat. However, the changes in liver density are very similar to the patterns of change across the groups in visceral fat, ALT, HOMA, subcutaneous fat, and body weight changes. Another limitation is that iron status was not measured, and increased or decreased iron levels can affect liver density. However, numerous studies have shown that increases in physical activity (the studies are predominantly on aerobic activities) are related to decreases in hemoglobin concentration, hematocrit, and serum ferritin (19). The effect, in the case of AT, would be to decrease liver density, which is the opposite of the effect in this study. There are far fewer studies of the effect of RT on iron status, but the few studies that do exist suggest that the outcome is the same, i.e., a similar reduction, not an increase, in iron status (6). Furthermore, as with AT, these effects appear to be due to an increase in plasma volume, and not an actual decrease in total hemoglobin or iron status (6).
Finally, while AT and RT are very different training modes and comparisons between them should be done with this in mind, it is still important to determine which mode is most effective for benefiting which specific risk factors.
Future research directions for comparing RT with AT could include two programs matched for caloric expenditure. This could be accomplished by comparing a lower-intensity aerobic exercise-training program than was used in the present study and a RT program similar to that used in the present study. These would be similar in total time required. The benefit of this approach would be that changes in ectopic fat would likely be very similar for the AT and RT programs (as previous research has shown that AT effects on ectopic fat follow a dose-response relationship). This would allow a more direct comparison of the RT benefits, which are specific to muscle mass increases, and the AT benefits, which are specific to the oxidative/mitochondrial changes.
Conclusions.
While RT reliably improves strength and increases lean body mass, its effects on central ectopic fat depots are less clear. The major finding in this study was that, in sedentary, overweight and obese adults, AT consistently more effectively improved visceral fat, total abdominal fat, liver fat, and the liver-derived enzyme ALT than did RT. When RT was added to AT, there was no additional beneficial effect on these variables. These data show that, for overweight and obese individuals who want to reduce body weight and measures of visceral fat and fatty liver infiltration and also improve fasting insulin resistance and liver enzymes, a moderate amount of aerobic exercise is the most time-efficient and effective exercise mode.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant 2R01-HL-057354 (clinical trial registration no. NCT00275145).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bateman L, Slentz C, Willis L, Shields A, Piner L, Bales C, Houmard J, Kraus W. Comparison of aerobic vs. resistance training effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention Through Defined Exercise-STRRIDE-AT/RT). Am J Cardiol 108: 838–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict NAFLD. Clin Chem 53: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Chen S, Liu C, Li S, Huang H, Tsai C, Jou H. Effects of therapeutic lifestyle program on ultrasound-diagnosed NAFLD. J Chin Med Assoc 71: 551–557, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Clark J, Brancati F, Diehl A. Nonalcoholic fatty liver disease. Gastroenterology 122: 1649–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Davidson L, Hudson R, Kilpatrick K, Kuk J, McMillan K, Janiszewski P, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults. Arch Intern Med 169: 122–131, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Deruisseau K, Roberts L, Kushnick M, Evans A, Austin K, Haymes E. Iron status of young males and females performing weight-training exercise. Med Sci Sports Exerc 36: 241–248, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Despres JP, Coillard C, Gagnon J, Bergeron J, Leon A, Rao D, Skinner J, Wilmore J, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women (HERITAGE). Arterioscler Thromb Vasc Biol 20: 1932–1938, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Devries M, Samjoo I, Hamadeh M, Tarnopolsky M. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 16: 2281–2288, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Ford E, Schulze M, Bergmann M, Thamer C, Joost H, Boeing H. Liver enzymes and incident diabetes. Diabetes Care 31: 1138–1143, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Frayn K. Visceral fat and insulin resistance—causative or correlative? Br J Nutr 83: S71–S77, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Irwin M, Yassui Y, Ulrich C, Bowen D, Schwartz R, Yukawa M, Aiello E, Potter J, McTiernan A. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized, controlled trial. JAMA 289: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Kim W, Flamm S, Di Bisceglie A, Bodenheimer H. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease: a policy statement of the American Association for the Study of Liver Disease. Hepatology 47: 1363–1370, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Kodama Y, Ng C, Wu T, Ayers G, Curely S, Abdalla E, Vauthey J, Charnsangavej C. Comparison of CT methods for determining fat content of the liver. Am J Roentgenol 188: 1307–1312, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kuk J, Katzmarzyk P, Nichaman M, Church T, Blair S, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 14: 336–341, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Lee D, Ha M, Christiani D. Body weight, alcohol consumption and liver enzyme activity—a 4-year follow-up study. Int J Epidemiol 30: 766–770, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Lee T, Kim W, Benson J, Therneau T, Melton L. Serum aminotransferase activity and mortality risk in a US community. Hepatology 47: 880–887, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Longo R, Ricci C, Masutti F, Vivimari R, Croce L, Bercich L, Tiribelli C, Palma D. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 28: 297–302, 1993 [PubMed] [Google Scholar]
- 18. Nguyen-Duy TB, Nichaman M, Church T, Blair S, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 284: E1065–E1071, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Pate R. Iron status of female runners. Int J Sports Nutr 3: 222–231, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Ross R, Dagnone D, Jones P, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med 133: 92–103, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Ross R, Janssen I, Dawson J, Kungl A, Kuk J, Wong S, Nguyen-Duy T, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized, controlled trial. Obesity (Silver Spring) 12: 789–798, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Sattar N, Scherbakova O, Ford I, O'Reilly D, Stanley A, Forrest E, MacFarlane P, Packard C, Cobbe S, Shepard J. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and CRP in the West of Scotland Coronary Prevention Study. Diabetes 53: 2855–2860, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Schindhelm R, Diamant M, Dekker J, Tushuizen M, Teerlink T, Heine R. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes and cardiovascular disease. Diabetes Metab Res Rev 22: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Schmitz K, Hannan P, Stovitz S, Bryan C, Warren M, Jensen M. Strength training and adiposity in premenopausal women: Strong Healthy, and Empowered Study. Am J Clin Nutr 86: 566–572, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Shojaee-Moradic F, Baynes K, Pentecost C, Bell J, Thomas E, Jackson N, Stolinski M, Whyte M, Lovell D, Bowes S, Gibney J, Jones R, Umpleby A. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 50: 404–413, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Sigal R, Kenny G, Boule N, Wells G, Prud'homme D, Fortiere M, Reid R, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training or both on glycemic control in type 2 diabetes. Ann Intern Med 147: 357–369, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Slentz C, Aiken L, Houmard J, Bales C, Johnson J, Tanner C, Duscha B, Kraus W. Inactivity, exercise and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 99: 1613–1618, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 164: 31–39, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Spassiani N, Kuk J. Exercise and the fatty liver. Appl Physiol Nutr Metab 33: 802–807, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Targher G, Day C, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363: 1341–1350, 2010 [DOI] [PubMed] [Google Scholar]
- 31. US Department of Health and Human Services. Physical Activity Guidelines for Americans (Online). http://wwwhealthgov/paguidelines/guidelines/2008 [2008].
- 32. Vento S, Nobili V. Aminotransferases as predictors of mortality. Lancet 371: 1822–1823, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann Med 37: 347–356, 2005 [DOI] [PubMed] [Google Scholar]