Abstract
The obesity pandemic can be viewed as a result of an imbalanced reaction to changing environmental factors. Recent research has linked circadian arrhythmicity to obesity and related diseases; however, the underlying mechanisms are still unclear. In this study, we found that high-fat diet (HFD) feeding strikingly promoted daytime rather than nighttime caloric intake in mice, leading to feeding circadian arrhythmicity. Using scheduled feeding with a defined amount of daily HFD intake, we found that an increase in the ratio of daytime to nighttime feeding promoted weight gain, whereas a decrease of this ratio rebalanced energy expenditure to counteract obesity. In identifying the underlying mechanism, we found that hypothalamic release of anorexigenic neuropeptide oxytocin displayed a diurnal rhythm of daytime rise and nighttime decline, which negatively correlated with the diurnal feeding activities of normal chow-fed mice. In contrast, chronic HFD feeding abrogated oxytocin diurnal rhythmicity, primarily by suppressing daytime oxytocin rise. Using pharmacological experiments with hypothalamic injection of oxytocin or oxytocin antagonist, we showed that daytime manipulation of oxytocin can change feeding circadian patterns to reprogram energy expenditure, leading to attenuation or induction of obesity independently of 24-h caloric intake. Also importantly, we found that peripheral injection of oxytocin activated hypothalamic oxytocin neurons to release oxytocin, and exerted metabolic effects similar to central oxytocin injection, thus offering a practical clinical avenue to use oxytocin in obesity control. In conclusion, resting-stage oxytocin release and feeding activity represent a critical circadian mechanism and therapeutic target for obesity.
Keywords: oxytocin, obesity, feeding, circadian
obesity is an outcome of imbalanced interactions between environmental factors and the body's metabolic regulation (13, 38). Recent research has established that the hypothalamus in the brain plays an essential role in the regulation of appetite and body weight balance (9, 17, 37, 44, 57). The underlying molecular basis involves hormone-dependent and nutrient-dependent pathways in the mediobasal hypothalamus (1, 3, 4, 9, 12, 17, 25, 29, 37, 44). In addition to metabolic regulation, the hypothalamus governs the circadian rhythms of various physiological activities, primarily dictated by the circadian pacemaker in the hypothalamic suprachiasmatic nucleus (14, 40, 51, 52). The circadian pace in this hypothalamic region is predominantly exerted by the Clock-directed transcription-translation oscillation loop (16, 20, 52), which can synchronize the physiological activities of various organs, including peripheral metabolic tissues (2, 53, 54). A recent study revealed that knockout of the Clock gene led to the prominent metabolic dysregulations, including overeating, obesity, and glucose intolerance (50). In the same direction, it was recently found that mice fed on a high-fat diet (HFD) displayed altered diurnal activities (26). However, despite these emerging observations, it remains unclear how hypothalamic neuropeptide(s) are involved in the circadian control of feeding, energy expenditure, and body weight balance and, moreover, if a brain target for obesity intervention could be developed.
Physiological functions of the hypothalamus are ultimately directed by hypothalamic neuropeptides and neurotransmitters. Indeed, neuropeptide and neurotransmitter pathways in the hypothalamic control of metabolic physiology have been depicted significantly. An important example is the hypothalamic melanocortin system (9, 17, 37, 44, 57) in which neuropeptides, including α-melanocyte-stimulating hormone, agouti-related peptide, and neuropeptide Y, are released by the arcuate neurons to act on melanocortin 4 receptor (MC4R)-expressing neurons in the paraventricular nucleus (PVN). Notably, one group of MC4R-exprssing neurons in the PVN are oxytocin (OXT) neurons, so named because they release neuropeptide OXT (28, 47). Our recent study has revealed that the central action of OXT in the brain is critical for feeding and body weight balance, and dysfunction of OXT neurons can direct obesity development (56). Here it should be pointed out that the PVN receives afferent projections from both the arcuate nucleus, which is the first-order metabolic regulator (3, 11, 18), and the suprachiasmatic nucleus, which is the circadian pacemaker (23, 49). However, it remains unexplored whether and how neuropeptide(s) released by PVN neurons might coordinate the circadian and metabolic systems to control whole body metabolic physiology and disease. In this work, we focused on PVN neurons and discovered that OXT critically integrates circadian and metabolic controls of feeding. Our study provides a new mechanistic understanding for body weight control and obesity development.
MATERIALS AND METHODS
Animals and metabolic and biochemical profiling.
Adult male C57 BL/6 mice were obtained from the Jackson Laboratory. Mice were housed under the diurnal cycle of 12 h light (resting stage) and 12 h darkness (active stage) in standard conditions with free access to food and water. HFD feeding was initiated when mice were 10–12 wk old for the indicated periods by individual experiments. Age-matched mice maintained under chow feeding were used as dietary controls. Food intake of mice was recorded for the indicated diurnal periods on a daily basis. On each day at the same time, a laboratory technician supplied individually housed mice with a preweighed amount of food, and, at the same time on the following day, the food left over in each cage was weighed. Extra attention was paid to collecting food left over both on the cage frame as well as all visible food left in the bedding of each cage. New clean cages were used on a daily basis for each mouse. Body weight was measured at least two times a week. Oxygen consumption was measured using an open-circuit indirect calorimetry system (Columbus Instruments) and normalized by lean body mass, which was determined by magnetic resonance imaging (MRI). The calorimetry system also measured physical activities of mice based on the infrared beam interruptions in both horizontal and vertical directions. Glucose tolerance test was performed in overnight-fasted mice by intraperitoneal injection of glucose (2 g/kg body wt) and subsequent monitoring of tail vein glucose levels via a Glucometer (Bayer, Elkhart, IN) at the indicated time points. Serum OXT levels were measured using the Oxytocin EIA kit (Assay Design). HFD was purchased from Research Diets. All procedures were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine.
Scheduled feeding.
A defined amount of food was provided to individually housed mice immediately after the light was turned on or right before the light was turned off and maintained for 12 h daytime vs. nighttime. Two schemes of scheduled HFD feeding were simultaneously performed: 0.48 g for 12 h daytime and 1.92 g for 12 h nighttime; and 0.8 g for 12 h daytime and 1.6 g for 12 h nighttime. At the same time, matched mice under ad libitum HFD or chow feeding were included as positive and negative controls, respectively. The total, 24-h amount of HFD in the schedule feeding was 2.4 g, slightly lower than the amount of ad libitum HFD feeding, to ensure complete consumption of the designed HFD amount for the light vs. dark cycle. New clean cages were used every 12-h diurnal cycle so that food that mice had was accurate for both daytime and nighttime periods. Any mouse that did not consume all supplied food for >5% of experimental days were excluded from the final analysis.
Third-ventricle cannulation and pharmacological treatments.
As described previously (56, 57), we used a stereotaxic apparatus (David Kopf Instruments) to place a guide cannula in the third ventricle of anesthetized mice at the midline coordinates of 1.8 mm posterior to the bregma and 5.0 mm below the skull surface of bregma. Mice were allowed 2 wk for recovery and then verified for the success of surgery using the ANG II-induced drink response. The injection of OXT or OXT receptor antagonist [d-(CH2)5,Tyr(Me)2,Orn8]vasotocin (OVT) was performed at the beginning of daytime vs. nighttime. For the third-ventricle injection, individually housed mice were injected with 1 μg OXT (Bachem California) or 4 μg OVT (Bachem California) over the period of 5 min. Artificial cerebrospinal fluid was used as vehicle control. To evaluate the potential anti-obesity effect of OXT via peripheral administration (7, 8, 31, 33, 43), which was shown to partially penetrate across the blood-brain barrier in the literature (7, 8, 31, 33, 41, 43), mice with HFD-induced obesity received daily intraperitoneal OXT injection at the beginning of daytime using the dose (1 mg/kg) established in the literature. Similarly, to evaluate the potential obesogenic effect of blocking systemic OXT, normal chow-fed mice received daily intraperitoneal injection of OVT at the same dose (1 mg/kg). Body weight-matched mice with intraperitoneal injection of saline were used as controls.
Ex vivo OXT release assay.
A detailed protocol has been described previously (56). Briefly, PVN slices were dissected from the hypothalamus and incubated in the Locke solution supplied with 95% O2 and 5% CO2 at 37°C. Locke solution was changed every 5 min for 10 times. The 10th balancing solution was collected for the measurement of basal OXT release. The tissues were subsequently incubated in Locke solution containing 70 mM KCl for 5 min. The solution was collected and measured for the depolarization (KCl)-stimulated OXT release. An OXT EIA kit (Assay Design) was used to determine OXT concentrations in the solution. To evaluate the effect of intraperitoneal OXT injection on hypothalamic OXT release, the PVN slices were prepared at 30 min following intraperitoneal OXT injection and analyzed using the protocol above.
Immunofluorescence.
Chow-fed mice were injected OXT or saline intraperitoneally, and, at 30 min postinjection, mice were anesthetized and intracardially perfused with 4% paraformaldehyde (PFA). Brains were then dissected, further fixed in 4% PFA for 4 h, and sequentially incubated in 20 and 30% sucrose. Brains were sectioned under a cryostat at 20-μm thickness, and sections were rinsed, blocked, and incubated with guinea pig antibody against OXT (Bachem California), rabbit antibody against c-Fos (Santa Cruz Biotechnology), or monoclonal antibody against HuC/D (Invitrogen) overnight at 4°C. After subsequent reaction with Alexa fluor 488, 555, or 633 secondary antibodies (Invitrogen), sections were mounted with DAPI-containing Vectashield medium. Image acquisitions were performed using a confocal microscope.
Statistical analysis.
Data are presented as means ± SE. ANOVA and post hoc Bonferroni test were performed for data involving more than two groups, and Student's t-test was used for data that involved only two groups. P < 0.05 was considered significant.
RESULTS
Feeding circadian rhythm affects body weight independently of caloric intake.
Total 24-h caloric intake is obviously relevant to body weight and obesity, but the effect of circadian rhythm on 24-h caloric intake has received little attention. In mice maintained on a standard chow diet, we confirmed that a normal range of body weight was supported by a steady-state caloric intake ratio of 0.2 ± 0.05 between the daytime (resting) stage and the nighttime (active) stage. In contrast, in mice with HFD-induced obesity, we observed a striking and course-dependent increase in the daytime-to-nighttime caloric intake ratio (Fig. 1A). However, the nighttime caloric intake between HFD-fed and chow-fed mice differed only modestly, and the progressive increase of daytime caloric intake primarily accounts for the altered diurnal rhythm of caloric intake in HFD-fed mice (Fig. 1A). To examine the significance of feeding circadian rhythm in obesity, we performed scheduled HFD feeding to artificially manipulate the diurnal ratio of caloric intake based on a fixed amount of 24-h calorie intake. Over a 2-mo experimental period, two groups of mice consumed the same daily amount of calories, which is 14% higher than ad libitum chow feeding and 11% lower than ad libitum HFD feeding, but with a different daytime-to-nighttime ratio of calorie intake at 0.50 and 0.25 (defined as the daytime caloric intake divided by nighttime caloric intake), respectively (Fig. 1, B and C). We found that, despite the equal amount of 24-h caloric intake, mice with a normal daytime-to-nighttime ratio of calorie intake were resistant to obesity development, whereas mice with an elevated daytime-to-nighttime ratio of calorie intake were susceptible to obesity development (Fig. 1D). To understand the underlying metabolic physiology, we profiled energy expenditure levels of these mice using metabolic chambers. We found that a normal daytime-to-nighttime ratio of calorie intake is associated with elevated energy expenditure levels during both daytime and nighttime periods (Fig. 1E); however, the physical activities were not affected significantly (Fig. 1F). MRI analysis further confirmed that mice with a normal ratio of daytime to nighttime caloric intake were protected from fat mass expansion following the first 3∼4 wk of HFD feeding (Fig. 1G). Thus, the diurnal pattern of caloric intake is critical for body weight homeostasis, and this process can be dissociated from total 24-h caloric intake, with the underlying physiology involving reprogrammed energy expenditure homeostasis.
Fig. 1.
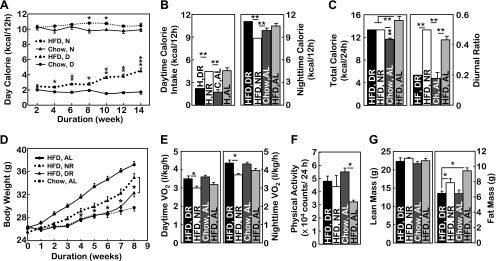
Feeding circadian rhythm affects body weight independently of caloric intake. A: longitudinal monitoring of daytime (D) vs. nighttime (N) caloric intake in C57BL/6 mice fed normal chow vs. a high-fat diet (HFD) starting at an adult age (∼10 wk old). The data at each time point represent the average daily food intake of each mouse during the week and then averaged per group. B–G: starting at an adult age (∼10 wk), subgroups of male C57BL/6 mice received HFD (H) feeding with daytime restriction (DR) vs. nighttime restriction (NR), whereas the 24-h HFD intake was controlled at the same level. Age-matched male mice under ad libitum (AL) chow (C) feeding or HFD feeding were used as negative and positive controls, respectively. B–D: average daytime (B, left) vs. nighttime (B, right) caloric intake, average total daily caloric intake (C, left), the diurnal ratio (daytime caloric intake divided by nighttime caloric intake) (C, right), and longitudinal follow-up of body weight (D) during the 8-wk intervention period. E–G: mice were measured for oxygen consumption (E) and physical activities (F) by metabolic chambers and lean vs. fat mass composition by magnetic resonance imaging (MRI, G) at 3–4 wk following scheduled feeding. Data on oxygen consumption shown in E were corrected based on the lean mass of mice. A–G: *P < 0.05, **P < 0.01, and ***P < 10−4; n = 6–11 (A–D) and n = 4 (E–G) mice/group. Statistics in A indicate the comparisons between HFD-fed mice and chow-fed mice with matched diurnal cycles. Statistics in D indicate the comparisons between HFD-fed mice under DR vs. NR. Error bars represent means ± SE. H, HFD; C, chow.
Diurnal OXT release correlates with feeding circadian patterns and body weight.
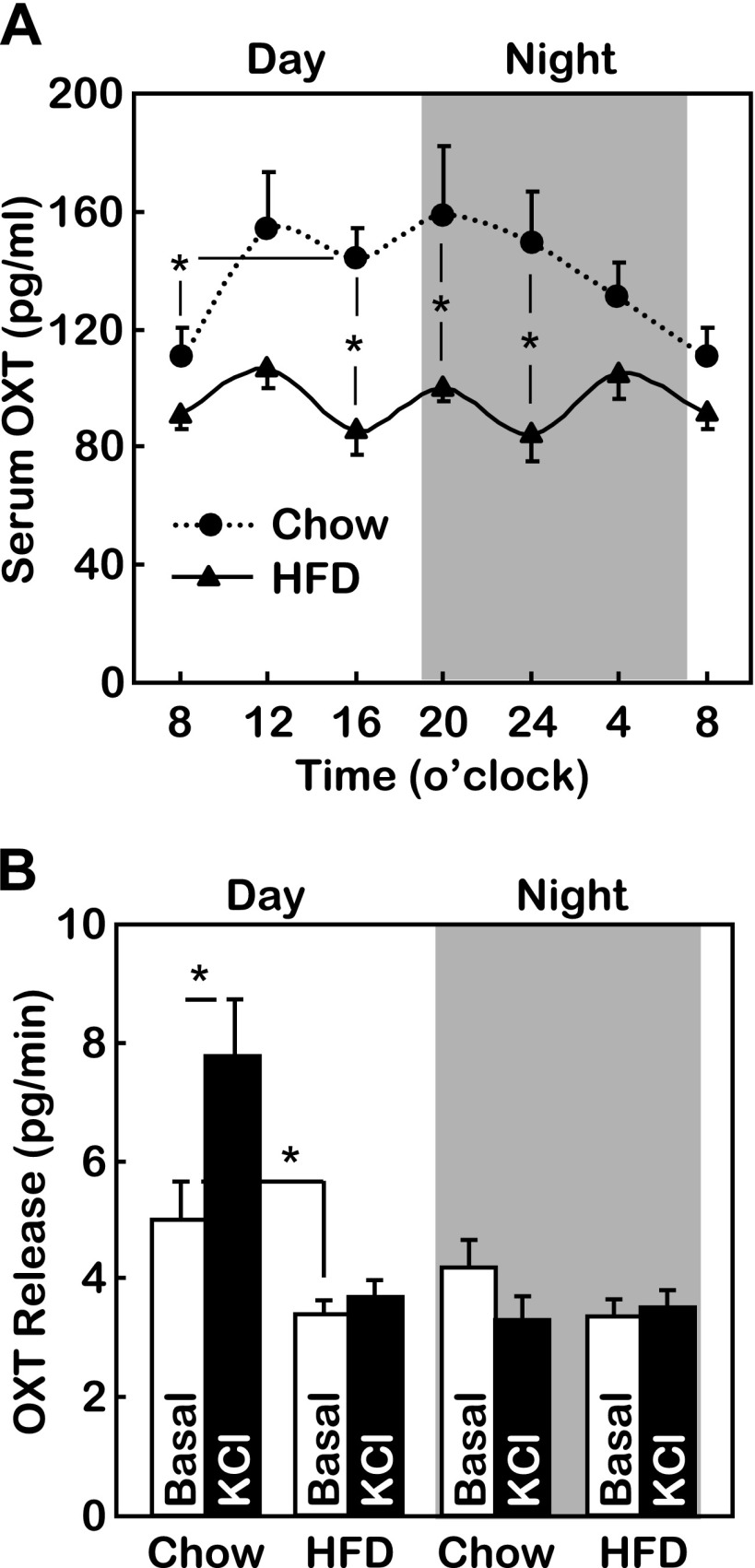
We subsequently explored the potential hypothalamic basis for the observations shown in Fig. 1. Our attention was directed to the PVN because it represents the converging site that integrates the metabolic regulatory neurons in the arcuate nucleus (3, 18) and the circadian pacemaker neurons in the suprachiasmatic nucleus (23, 49). Although diurnal rhythmicity of several PVN neuropeptides, such as vasopressin, thyrotropin-releasing hormone, corticotropin-releasing factor, and somatostatin, were documented in the literature (5, 21, 22, 24), the primary actions of these neuropeptides are to control various physiological activities other than feeding. Interestingly, we found that OXT, a PVN neuropeptide that we recently found to critically regulate feeding and body weight (56), exhibited diurnal rhythmicity in mice. The circulating concentrations of OXT in chow-fed mice displayed a rapid daytime rise and a gradual nighttime decline (Fig. 2A). The diurnal pattern of OXT change was correlated with the low-level daytime feeding vs. the high-level nighttime feeding activity in mice (Fig. 1A). This finding fits well with our recent study showing that OXT acts in the brain to restrict food intake and promote energy expenditure (56). In contrast, the diurnal rhythmic change of OXT was abolished in mice with HFD-induced obesity (Fig. 2A). Specifically, chronic HFD feeding abrogated the daytime rise of circulating OXT levels (Fig. 2A). To determine whether the abrogation of circadian OXT fluctuation was caused by a defect of daytime OXT release or nighttime OXT clearance, we examined ex vivo OXT release from PVN slices prepared from mice killed at midday vs. midnight. As shown in Fig. 2B, depolarization-induced hypothalamic OXT release was active during the daytime but completely absent during the nighttime in chow-fed mice. However, in mice with HFD-induced obesity, depolarization was unable to stimulate either daytime or nighttime hypothalamic OXT release (Fig. 2B). Altogether, diurnal OXT release is correlated with a feeding circadian pattern to control body weight balance, and loss of the diurnal OXT release rhythm by HFD feeding underlies feeding circadian arrhythmicity and obesity development.
Fig. 2.
Diurnal profiles of oxytocin (OXT) release in chow-fed and HFD-fed mice. Adult male C57BL/6 mice received HFD vs. chow feeding from an adult age (∼10 wk) for 12 wk and then were measured for 24-h profile of serum OXT levels (A) or ex vivo OXT release under basal and depolarization (KCl) conditions as described in materials and methods (B). *P < 0.05; n = 8–10 (A) and n = 6–7 (B) mice/group. Error bars represent means ± SE. Statistics in A indicate the comparisons between HFD-fed mice and chow-fed mice at the matched time points.
Central OXT delivery normalizes feeding circadian rhythm to counteract obesity.
Having shown that daytime OXT release and the associated daytime caloric intake are critical factors in preventing obesity in mice, we next investigated the brain mechanism by which the daytime fraction of OXT contributes to the circadian regulation of feeding and body weight. To address this question, we first applied ∼12 wk HFD feeding to C57BL/6 mice to induce obesity. Following the development of HFD-induced obesity, mice received intrathird ventricle injection of OXT at the beginning of daytime or nighttime. As we previously observed (56), the effect of OXT injection on food intake lasted ∼6 h, so we were able to specifically control daytime vs. nighttime feeding of mice by daytime vs. nighttime OXT injection (Fig. 3, A and B). Although total 24-h caloric intake was reduced similarly between daytime vs. nighttime OXT treatment groups (Fig. 3C), the daytime-to-nighttime ratio of feeding was affected differentially by different OXT treatments, specifically, it was decreased by daytime OXT treatment but increased by nighttime OXT treatment (Fig. 3D). Such diurnal rhythmic change of feeding mimicked the model of scheduled feeding shown in Fig. 1. Similar to the effect of scheduled feeding, we found that daytime OXT administration exerted stronger anti-obesity effect compared with nighttime OXT administration (Fig. 3, E–G). We also observed that energy expenditure of mice was increased by daytime but not nighttime OXT treatment (Fig. 3, H and I), which further explains the potentiated anti-obesity effect of daytime OXT treatment. Taken together, OXT can act in the brain to affect the diurnal ratio of caloric intake and thereby readjusts the set point of energy expenditure to counteract against obesity.
Fig. 3.
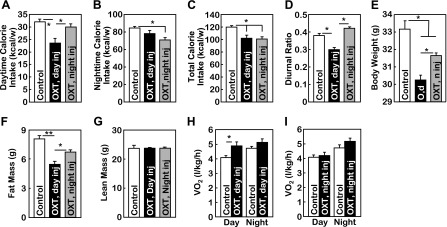
Central OXT delivery normalizes feeding circadian rhythm to counteract obesity. Adult male C57BL/6 mice received HFD feeding from an adult age (∼10 wk) for 12 wk. Subsequently, mice with matched body weight were subdivided into 3 groups to receive the following treatments. The group designated as “OXT, day injection (inj)” received OXT injection at the beginning of daytime and vehicle injection at the beginning of nighttime (n). The group designated as “OXT, night injection (inj)” received OXT injection at the beginning of nighttime and vehicle at the beginning of daytime. A matched control group received vehicle injection at the beginning of both daytime and nighttime. Mice received these treatments on a daily basis for 1 wk. During these treatments, mice continued to be maintained on HFD feeding. A–D: accumulated weekly daytime caloric intake (A), nighttime caloric intake (B), total caloric intake (C), and the diurnal ratio of feeding (daytime caloric intake divided by nighttime caloric intake) (D) were obtained from these mouse groups. E–I: body wt (E), fat vs. lean mass measured by MRI (F and G), and oxygen consumption measured by metabolic chambers (H and I) were obtained at the end of the 1-wk treatment. Data on oxygen consumption shown in H and I were corrected based on the lean mass of mice. A–I: *P < 0.05 and **P < 0.01; n = 5–6 (A–G) and n = 4 (H and I) mice/group. Error bars represent means ± SE.
Central delivery of OVT disrupts feeding circadian rhythm to promote obesity.
In addition to OXT gain-of-function experiments above, we tested whether blocking the brain action of OXT could have opposite metabolic effects. To do this, OVT, an effective antagonist of OXT (56), was delivered to the brain of chow-fed mice via the third ventricle at the beginning of daytime vs. nighttime. First, total caloric intake was similarly although slightly elevated by daytime vs. nighttime OVT treatment (Fig. 4, A–C). Importantly, the daytime-to-nighttime ratio of feeding was increased significantly by daytime but not nighttime OVT treatment (Fig. 4D), partially mirroring the observations from daytime vs. nighttime OXT treatment shown in Fig. 3. Notably, daytime OVT treatment significantly increased body weight and fat mass; however, these effects were not evident in the nighttime OVT treatment group (Fig. 4, E–G). Also, while mice receiving daytime OVT injection showed a reduction in energy expenditure, mice with nighttime OVT injection did not (Fig. 4, H and I). Overall, these findings further support the hypothesis that diurnal rhythmicity of feeding is critical for maintenance of normal body weight balance and prevention against obesity development.
Fig. 4.
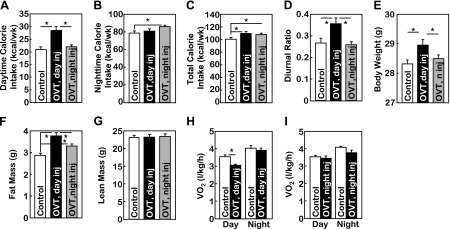
Central delivery of [d-(CH2)5,Tyr(Me)2,Orn8]vasotocin (OVT) disrupts feeding circadian rhythm to promote obesity. Normal chow-fed, adult (∼12 wk old) male C57BL/6 mice with matched body weight were subdivided into the 3 groups described in the legend for Fig. 3. During these treatments, mice continued to be maintained on chow feeding. A–D: accumulated weekly daytime caloric intake (A), nighttime caloric intake (B), total caloric intake (C), and the diurnal ratio of feeding (daytime caloric intake divided by nighttime caloric intake) (D) were obtained from these mouse groups. E–I: body wt (E), fat vs. lean mass measured by MRI (F and G), and oxygen consumption measured by metabolic chambers (H and I) were obtained at the end of the 1-wk treatment. Data on oxygen consumption shown in H and I were corrected based on the lean mass of mice. A–I: *P < 0.05; n = 5–9 (A–G) and n = 4 (H and I) mice/group. Error bars represent means ± SE.
Peripheral OXT administration stimulates OXT release in the PVN.
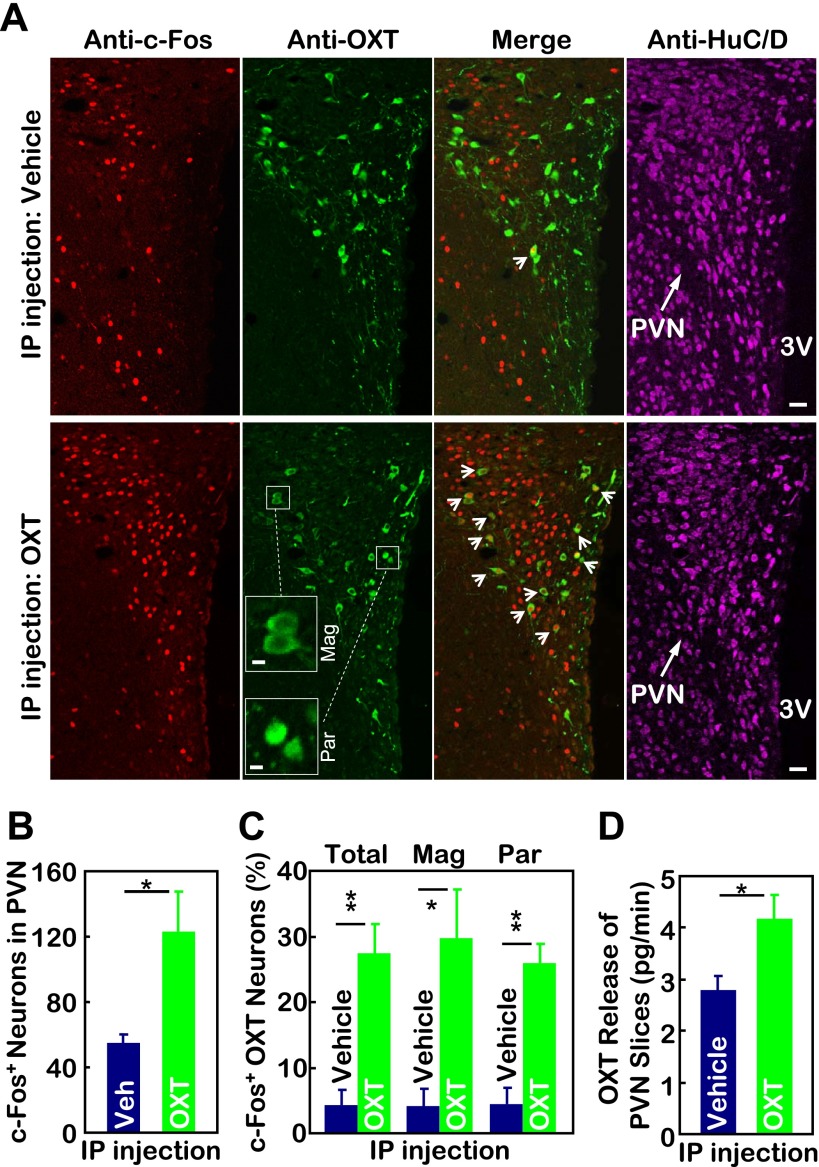
In conjunction with the studies employing brain OXT injection, we examined whether alternative peripheral OXT administration could have metabolic effects via the central nervous system. To answer this question, we injected OXT vs. control vehicle (saline) intraperitoneally in normal chow-fed C57BL/6 mice. Using c-Fos induction as a neuronal activity marker, we examined if peripheral OXT delivery could potentially activate some brain neurons. Data revealed that a single intraperitoneal injection of OXT markedly induced c-Fos expression in magnocellular and parvocellular OXT neurons within the PVN (Fig. 5, A–C), indicating that peripheral OXT treatment can stimulate OXT neurons in the PVN. To test the functional significance of this activation, we performed ex vivo OXT release assay using PVN slices prepared from mice that had received intraperitoneal injection of OXT vs. saline. Results showed that OXT release in the PVN was enhanced significantly by peripheral OXT injection compared with vehicle injection (Fig. 5D). To summarize, peripheral OXT treatment can stimulate the neuronal activity and neuropeptide release of OXT neurons in the PVN. This finding can suggest a useful strategy to manipulate central OXT actions via peripheral OXT injection. Such a strategy may be clinically significant since intranasal delivery of OXT has been recently proven successful in treating neurological diseases such as autism (27).
Fig. 5.
Peripheral OXT injection activates OXT neurons in the paraventricular nucleus (PVN) to release OXT. A: chow-fed adult male C57BL/6 mice received a single intraperitoneal (IP) injection of OXT vs. control vehicle. At 30 min postinjection, mice were trans-heart perfused and fixed, and brains were removed and sectioned for coimmunostaining of c-Fos (red) and OXT. Merged micrographs demonstrate cytoplasmic OXT and nuclear c-Fos. Immunostaining for neuronal marker HuC/D (pink) demonstrates the overall anatomy of the PVN. Bar=20 μm. 3V, third ventricle. Insets: high-magnification images of representative magnocellular (Mag) and parvocellular (Par) OXT neurons; bar=5 μm. B and C: average nos. of c-Fos-positive cells in the PVN (B) and averaged percentage of total, magnocellular (Mag), and parvocellular (Par) populations of OXT neurons in the PVN sections that were positive for c-Fos immunostaining (C). Data represent the analyses for median PVN sections. *P < 0.05 and **P < 0.01; n = 3–5/group. Error bars represent means ± SE. D: chow-fed adult male C57BL/6 mice received a single ip injection of OXT vs. control vehicle. At 30 min postinjection, mice were killed, and PVN slices were prepared from the brain and subjected to ex vivo OXT release assay as described in materials and methods. *P < 0.05, n = 6–7/group. Error bars represent means ± SE.
Peripheral OXT treatment activates brain neural pathway downstream of OXT.
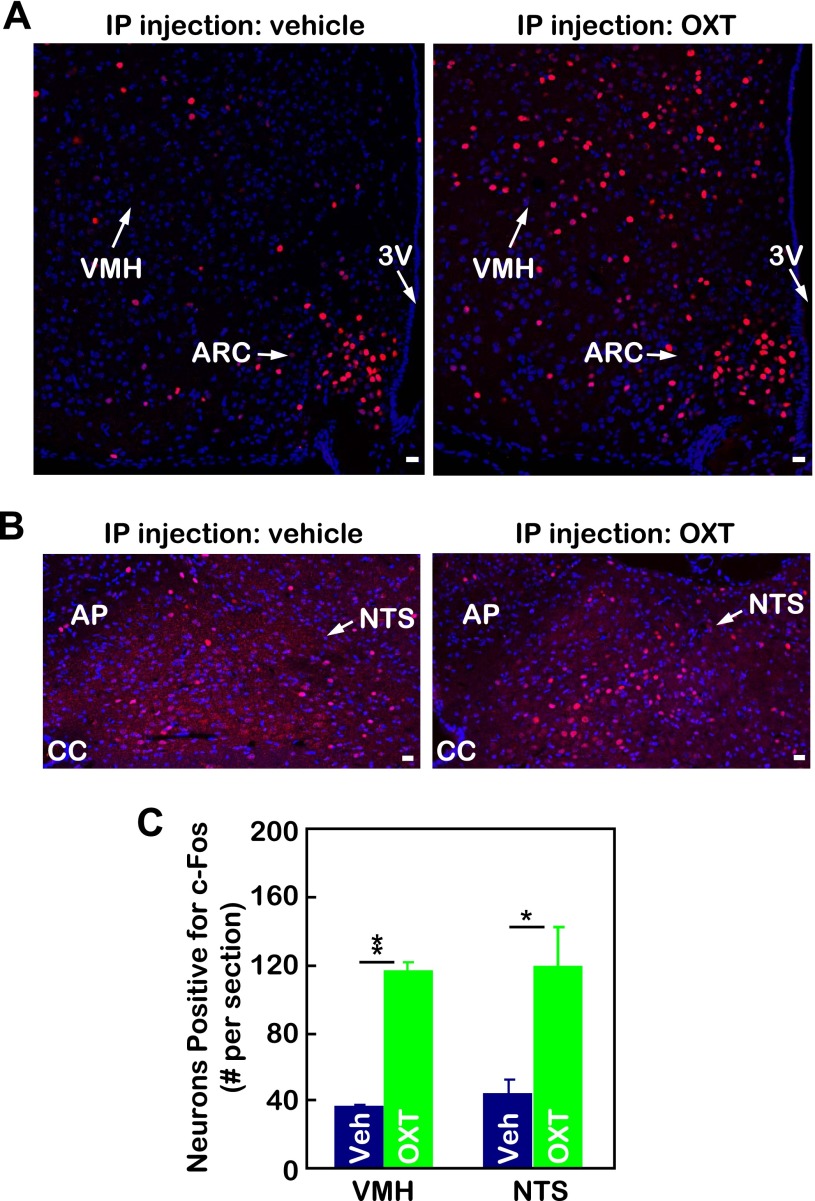
Based on the results that peripheral OXT can stimulate hypothalamic OXT release (Fig. 5), we further asked whether peripheral OXT delivery can mimic the central action of OXT to activate OXT-targeting neurons in the brain. OXT-targeting neurons are widely distributed in the brain, and two major regions that are directly innervated by OXT neurons are the ventral medial nucleus in the hypothalamus (VMH) (48, 55) and the nucleus of solitary tract (NTS) in the brain stem (32). Using c-Fos induction to report neuronal activation, we found that a significant portion of neurons in the VMH and NTS was activated by peripheral OXT injection (Fig. 6, A–C). For control purposes, we also examined a few other brain regions, such as the dorsal medial nucleus in the hypothalamus, which has no neural connections with OXT neurons, and found no induction of c-Fos in these regions by peripheral OXT treatment (data not shown). Thus, the activation of OXT-targeting neurons was not a nonspecific reaction to peripheral drug delivery. In summary, by enhancing central OXT release, peripheral OXT delivery can reproduce, at least partially, the biological effects of central OXT action.
Fig. 6.
Peripheral OXT injection activates the brain pathway of OXT neurons. Chow-fed adult male C57BL/6 mice received a single ip injection of OXT vs. control vehicle. At 30 min postinjection, mice were trans-heart perfused and fixed, and the brains were removed and sectioned for immunostaining of c-Fos (red) in the ventromedial hypothalamic (VMH) nucleus (A) and nucleus of the solitary tract (NTS, B). Nuclear staining by DAPI reveals all cells in the sections. Bar graphs (C) present the average nos. of c-Fos-positive cells in the VMH and NTS. Data represent the cell nos. across the median section of the VMH or NTS. *P < 0.05 and **P < 0.01; n = 3–5/group. Error bars represent means ± SE. ARC, arcuate nucleus; AP, area postrema; CC, central canal; Veh, vehicle. Bar=20 μm.
Counteracting peripheral OXT distorts feeding circadian rhythm to promote obesity.
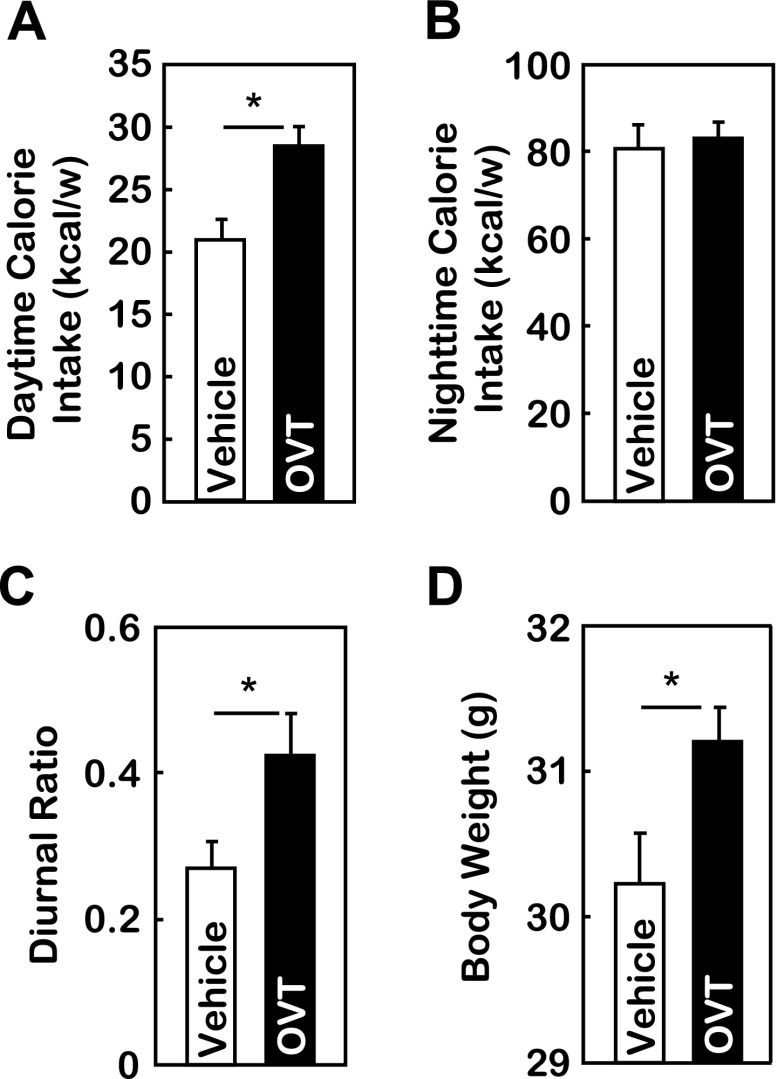
Subsequently, we asked if the endogenous peripheral OXT has physiological significance in maintaining diurnal feeding rhythm and body weight balance. To answer this question, we performed intraperitoneal injection of OXT antagonist OVT or control vehicle to body weight-matched, chow-fed C57BL/6 mice. Injection was given at the beginning of the daytime period. As shown in Fig. 7, A and B, OVT significantly increased daytime but not nighttime caloric intake. The 24-h total caloric intake was only slightly altered, but the ratio of daytime to nighttime food intake was elevated significantly (Fig. 7C). Thus, peripheral OVT injection partially recapitulated the changes of feeding circadian pattern in response to central OVT injection, as shown in Fig. 4. Similar to the obesogenic effect of central OVT injection (Fig. 4E), mice receiving peripheral OVT treatment gained more body weight compared with vehicle treatment (Fig. 7D). These data further confirmed that the resting-stage caloric profile determines the circadian homeostasis of feeding and body weight control, and the underlying regulatory mechanism is the circadian programming of the central-peripheral OXT axis.
Fig. 7.
Peripheral delivery of OVT distorts feeding circadian rhythm to promote obesity. Chow-fed adult male C57BL/6 mice with matched body weight were divided into two groups to receive ip injection of OVT vs. vehicle at the beginning of daytime on a daily basis for 1 wk. Data shown are accumulated weekly daytime (A) vs. nighttime (B) caloric intake and diurnal ratio of feeding (C) during the 1-wk treatment and body weight (D) following the completion of the 1-wk treatment. *P < 0.05; n = 5–7/group. Error bars represent means ± SE.
Peripheral OXT treatment normalizes the feeding circadian pattern to counteract obesity.
Finally, we examined if peripheral administration of OXT can mimic central OXT treatment to normalize the feeding circadian pattern and therefore counteract obesity. HFD-fed, adult C57BL/6 mice received intraperitoneal injection of OXT vs. control vehicle at the beginning of daytime on a daily basis for 6 wk. Results showed that OXT administration significantly reduced daytime but not nighttime caloric intake (Fig. 8, A and B). Thus, despite the HFD feeding, peripheral OXT treatment normalized the feeding circadian rhythm compared with vehicle treatment (Fig. 8C). Over a 6-wk follow-up period, OXT-injected mice showed a significant protection against HFD-induced obesity (Fig. 8D). In addition to body weight monitoring, mice were subjected to a glucose tolerance test at the end of the 6-wk treatment period. As shown in Fig. 8E, control mice developed glucose intolerance, a major obesity comorbidity that often leads to the development of type 2 diabetes. In contrast, OXT-treated mice showed a decreased extent of glucose intolerance at 30 min postglucose challenge in HFD-fed mice. This observation can lend support to the metabolic benefit of OXT treatment in obesity control.
Fig. 8.
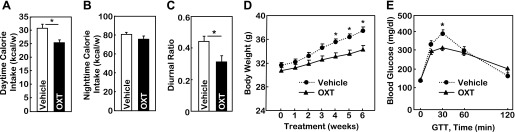
Peripheral delivery of OXT normalizes feeding circadian pattern to counteract obesity. Adult male C57BL/6 mice received HFD feeding from an adult age (∼8 wk) for 6 wk. Subsequently, mice with matched body weight were divided into two groups to receive ip injection of OXT vs. vehicle at the beginning of daytime on a daily basis for 6 wk. Mice continued to be maintained on HFD feeding during the entire treatment period. Data shown are accumulated weekly daytime (A) vs. nighttime (B) caloric intake, the diurnal ratio of feeding (C) and longitudinal follow-up of body wt (D) and glucose tolerance test (GTT) (E) at the completion of the 6-wk treatment. *P < 0.05; n = 5–8/group. Error bars represent means ± SE.
DISCUSSION
OXT-directed resting-stage feeding mediates circadian control of body weight balance.
The 24-h rotation of the earth generates daily circadian rhythms. To maintain a healthy body weight, living organisms have evolutionally developed a strategy to synchronize levels of caloric intake with the day-night cycle and the concurrent physical activity-resting cycle. In this study, we discovered that circadian release of OXT from the hypothalamic PVN directs hypothalamic regulation of feeding circadian rhythms to maintain body weight homeostasis. Disturbance of this control system underlies obesity development. Indeed, environmental overnutrition, such as chronic fat-enriched diet, is a major contributor to an impaired circadian release pattern of OXT, leading to altered feeding rhythms and body weight imbalance. However, using pharmacological or nutritional (scheduled feeding) models, we were able to treat HFD-induced body weight changes roughly by 50% through normalizing feeding circadian rhythm even without much change in the total, 24-h caloric intake. More importantly, advancing from recent observations that both caloric excess (26) and caloric shortage (19) impact feeding circadian rhythms, we further proved that the resting stage (daytime for mice, nighttime for humans), the period that is characterized by limited caloric intake and physical activity, is the critical component for controlling feeding circadian rhythms. Our finding is corroborated by previous research showing that a phase delay in eating is associated with obesity despite the relatively normal amount of total 24-h caloric intake (39). From a medical viewpoint, such phase delay of eating is most often seen in patients with night-eating syndrome, who typically develop morbid obesity and need medicinal or surgical interventions to lose weight (10). Another human subject-based study showed that scheduled nighttime meal intake resulted in reduced diet-induced thermogenesis compared with morning meal intake (42), indicating that energy expenditure status can be entrained by the feeding circadian pattern to cause or prevent obesity. We found that resting-stage release of hypothalamic OXT underlies diurnal feeding rhythmicity to maintain normal body weight. Conversely, impairment of resting-stage hypothalamic OXT release mediates diurnal feeding arrhythmicity to cause obesity. We recognize that several other PVN neuropeptides, such as vasopressin, thyrotropin-releasing hormone, corticotropin-releasing factor, and somatostatin, were known to have diurnal rhythmicity (5, 21, 22, 24). While the primary actions of these neuropeptides are to control various physiological activities other than feeding, they may secondarily contribute to the action of OXT in the regulation of metabolic rhythmicity. Overall, our data suggest that deviation from the normal circadian eating pattern, a phenomenon prevalent with modern society's lifestyle, is an important risk factor for obesity independent of total daily caloric intake, and the underlying neurobiological basis is disrupted hypothalamic OXT rhythmicity.
Therapeutic potentials of OXT mimetics for obesity and related diseases.
OXT is well known for its actions in mediating female reproductive activities during parturition and lactation. However, OXT is similarly abundant in the brain and circulation of males and females, suggesting that OXT has functions beyond female reproduction. Recent literature has linked OXT to promoting social behaviors (15) and anxiolytic effects (30). More recently, we have reported that OXT can act in the brain to restrict food intake and promote energy expenditure in mice (56). Importantly, these metabolic effects of central OXT treatment did not cause adverse behavioral changes, rationalizing OXT as an ideal candidate as an anti-obesity therapeutic (56). In this context, the current research aimed to gain further mechanistic understanding of central OXT actions in metabolic regulation. We discovered that brain OXT employs a circadian system to control the diurnal feeding pattern and body weight balance. This metabolic regulation by OXT is in line with the recent appreciation that obesity can result from circadian arrhythmicity (50). It is also well supported by the obesity phenotype of OXT (6) or OXT receptor (45) knockout mice. As a matter of fact, circadian rhythmicity is a shared feature of many social behaviors recently found to be regulated by OXT (15), further supporting the circadian component of OXT actions. Our current study demonstrated that central OXT treatment can counteract obesity by normalizing diurnal feeding rhythmicity. This finding can inspire future studies regarding OXT and diseases associated with circadian arrhythmicity. One limitation of our study was that we have not been able to test the potential effects of multiple OXT injections within 24 h or continuous OXT delivery through osmotic minipump. In addition to central administration, this study demonstrated that the peripheral OXT delivery can effectively mimic the central OXT delivery to affect feeding diurnal rhythm, body weight, and obesity. The underlying processes might involve several mechanisms. First, a portion of the peripherally delivered OXT may cross the blood-brain barrier. According to the literature (33), peripheral OXT injection at pharmacological doses can deliver a small fraction of OXT in the central nervous system across the blood-brain barrier, and, even though the absolute transferred amount was small (∼0.002%), it transiently elevated OXT concentrations in the cerebrospinal fluid by approximately fivefold. Also importantly, we found in the current study that exogenously provided OXT further promoted hypothalamic OXT release presumably via the autocrine feedforward system of OXT neurons, which was also indicated by observations in the literature (36, 46). Second, peripherally injected OXT might employ afferent neural pathway(s) to activate various brain regions, including the hypothalamic OXT neurons, resulting in elevated OXT release from OXT neurons. Regardless of the underlying mechanisms, our data showed that peripherally delivered OXT indeed upregulated OXT release by OXT neurons in the PVN in mice. Overall, while native OXT does have limitations for direct use in patients with obesity, such as the short half-life and possible off-target effects, our study can provide a preclinical basis for next-step development of OXT analogs with optimized pharmacological properties and target actions as an approach for treating human obesity or related diseases.
GRANTS
This study was supported by the internal support of Albert Einstein College of Medicine, National Institutes of Health Grants R01 DK-078750 and R01 AG-031774 (all to D. Cai).
DISCLOSURES
The authors state that they have no competing financial interests.
ACKNOWLEDGMENTS
We thank D. Cai's laboratory members for technical assistance.
REFERENCES
- 1. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 330: 1349–1354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Burhol PG, Lygren I, Jenssen TG, Florholmen J, Jorde R. Somatostatin release and plasma molecular somatostatin components in man. Acta Physiol Scand 121: 223–228, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009 [DOI] [PubMed] [Google Scholar]
- 7. Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58: 38–43, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol 15: 448–463, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell 129: 251–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colles SL, Dixon JB, O'Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 31: 1722–1730, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med 54: 453–471, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends Cogn Sci 11: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Dunlap JC. Molecular bases for circadian clocks. Cell 96: 271–290, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science 304: 63–64, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Friedman JM. Modern science versus the stigma of obesity. Nat Med 10: 563–569, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science 320: 1074–1077, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148, 2007 [DOI] [PubMed] [Google Scholar]
- 21. George CP, Messerli FH, Genest J, Nowaczynski W, Boucher R, Kuchel Orofo-Oftega M. Diurnal variation of plasma vasopressin in man. J Clin Endocrinol Metab 41: 332–338, 1975 [DOI] [PubMed] [Google Scholar]
- 22. Hiroshige T, Sakakura M. Circadian rhythm of corticotropin-releasing activity in the hypothalamus of normal and adrenalectomized rats. Neuroendocrinology 7: 25–36, 1971 [DOI] [PubMed] [Google Scholar]
- 23. Kalsbeek A, La FS, Van HC, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24: 7604–7613, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerdelhue B, Palkovits M, Karteszi M, Reinberg A. Circadian variations in substance P, luliberin (LH-RH) and thyroliberin (TRH) contents in hypothalamic and extrahypothalamic brain nuclei of adult male rats. Brain Res 206: 405–413, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 435: 673–676, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kublaoui BM, Holder JL, Jr, Gemelli T, Zinn AR. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol 20: 2483–2492, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, guilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: the great facilitator of life. Prog Neurobiol 88: 127–151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol 428: 95–108, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res 262: 143–149, 1983 [DOI] [PubMed] [Google Scholar]
- 36. Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol 102: 63–72, 1984 [DOI] [PubMed] [Google Scholar]
- 37. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 444: 854–859, 2006 [DOI] [PubMed] [Google Scholar]
- 38. O'Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature 462: 307–314, 2009 [DOI] [PubMed] [Google Scholar]
- 39. O'Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, Stunkard AJ. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res 12: 1789–1796, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 185: 218–225, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr 57: 476–480, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci 30: 8274–8284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19: 951–955, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Theodosis DT. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nucleus. Nature 313: 682–684, 1985 [DOI] [PubMed] [Google Scholar]
- 47. Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci 30: 3803–3812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6: 384–390, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci 24: 2983–2988, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms 13: 100–112, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72: 551–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318: 1786–1789, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology 133: 1239–1246, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69: 523–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]