Abstract
Rex1(Zfp42), GeneID 132625, is a gene whose expression is closely associated with pluripotency/multipotency in both mouse and human embryonic stem cells. To study the function of the murine Rex1 gene in vivo, we have used cre/lox technology to create Rex1(floxed) mice and mice deficient in Rex1 gene function. Rex1-/- males are characterized by an age-associated decrease in sperm counts, abnormal sperm morphology, and mild testicular atrophy. We characterized global patterns of gene expression in primary germ cells by microarray and identified the growth hormone responsive gene, GRTP1, as a transcript present at a 4.5 fold higher level in wild type (WT) compared to Rex1-/- mice. We analyzed immature germ cell (Dazl), proliferating (PCNA), and Sertoli cell populations, and quantitated levels of apoptosis in Rex1-/- as compared to WT testes. We evaluated the expression of proteins previously reported to correlate with Rex1 expression, such as STAT3, phospho-STAT3, p38, and phospho-p38 in the testis. We report a distinct cellular localization of total STAT3 protein in Rex1-/- affected testes. Our data suggest that loss of Rex1 leads to impaired testicular function.
Keywords: Testis, Spermatogenesis, germ cells, Stem Cells, Sertoli cells, Sox9
Introduction
Understanding the cell signaling networks commonly used in defining the pluripotent state is critical to understanding stem cell identity and differentiation. However, more must be learned about the functional role of a gene whose expression is associated with pluripotency, namely Rex1, in the context of adult and embryonic stem cell biology. The Rex1 (Zfp42) gene (GeneIDs: mouse, 22702; human, 132625), a member of the zinc finger protein family that also includes YY1 (Kim et al., 2007), was identified by our laboratory (Hosler et al., 1989) and is widely used as one of seven markers of human and murine embryonic stem (ES) cells (Brivanlou et al., 2003). We first identified Rex1 as an abundant transcript expressed in F9 teratocarcinoma stem cells; the transcription of Rex1 rapidly decreased upon addition of all-trans retinoic acid (RA), an active form of vitamin A (retinol) (Hosler et al., 1989; Hosler et al., 1993). Subsequently, we characterized Rex1 expression during mouse development and showed that Rex1 mRNA is primarily detected in trophoblast derived tissues and in the inner cell mass of the blastocyst (ICM), the source of embryonic stem cells in culture (Rogers et al., 1991). In adult mice, Rex1 mRNA is expressed in a subset of male germ cells, spermatocytes actively undergoing meiosis (Rogers et al., 1991). Rex1 (mRNA and protein) is selectively expressed in dividing cells of the human testes and ovaries (Kristensen et al., 2008), suggesting a conserved functional role. Rex1 transcripts are also detected in a variety of pluripotent cells, normal primary human keratinocytes (NHEK), human prostate epithelial cells (PrEC), renal parenchymal tissue, and some human neoplastic cell lines, such as MDA-MB-468 mammary carcinoma cells, SCC-15 head and neck squamous carcinoma cells, and NTERA2 teratocarcinoma cells (Kristensen et al., 2010; Mongan and Gudas, 2007; Mongan et al., 2006; Raman et al., 2006). Interestingly, we also showed that Rex1 expression increases in mouse digits during tissue regeneration/wound healing (Agrawal et al., 2010; Wang et al., 2010).
Most of what is functionally known about Rex1 comes from results obtained in cell differentiation studies, and/or analysis of the genes regulating its expression. First, our laboratory created Rex1 knockout (Rex1-/-) F9 teratocarcinoma stem cell lines by homologous recombination, and demonstrated that F9 Rex1-/- cells fail to differentiate completely into visceral endoderm (VE) in aggregate culture after RA treatment (Thompson and Gudas, 2002). We further demonstrated that Rex1 acts as a negative regulator of STAT3 during differentiation of F9 cells (Xu et al., 2008), identifying a possible mechanism behind the phenotype in F9 cells. We analyzed changes in gene expression during the differentiation of Rex1-/- murine embryonic stem cell (mES) lines, and showed that Rex1 affects differentiation and cell cycle progression in mES (Scotland et al., 2009). More recently, Bhandari et al investigated the relationship between Rex1 expression and p38 mitogen-activated protein kinase (MAPK) activation in human mesenchymal stem cells (hMSCs), and reported that Rex1 regulates hMSC proliferation/differentiation by suppressing the p38 MAPK signaling pathway (Bhandari et al., 2010). At the molecular level, murine Rex1 expression is reportedly regulated by the pluripotency genes Oct4, Sox2 and Nanog (Ben-Shushan et al., 1998; Hosler et al., 1993; Rosfjord and Rizzino, 1994; Shi et al., 2006). Not surprisingly, Rex1 is one of a few select genes shown to be re-expressed in induced pluripotent stem cells (iPS cells) (Chan et al., 2009). iPS cells hold enormous potential not only for regenerative medicine, but, also for studies in developmental biology, drug development, and the derivation of patient specific stem cell lines. Analysis of the promoter of the human Rex1 gene by our laboratory showed that human prostate cancer cells exhibited lower Rex1 promoter activity than normal prostate epithelial cells in culture (Lee et al., 2010).
Spermatogenesis, the proliferation and maturation of germ cells leading to formation of spermatozoa, occurs from puberty to death in male mammals. The testis is composed of germ cells as well as somatic cells, such as Sertoli cells and Leydig cells (Meng et al., 2000; O'Shaughnessy et al., 2009; Yoshinaga et al., 1991). Testicular germ cells consist of spermatogonial stem cells which can undergo self-renewal as well as differentiate into spermatocytes, spermatids, and finally into spermatozoa, the mature form of sperm. Sertoli cells produce growth factors, such as glial cell line-derived neurotrophic factor (GDNF), that maintain germ cell functions, and endocrine hormones such as inhibin, which acts on the hypothalamic-pituitary-gonadal axis (Hogarth and Griswold, 2010; Meng et al., 2000). Leydig cells are primarily responsible for testosterone production (Hogarth and Griswold, 2010). We wanted to understand the functions of Rex1 in stem cells in vivo and thus generated Rex1-/- mice by using a conditional knockout strategy (Cre-LoxP). We show here that Rex1-/- mice are viable, fertile, and grossly similar to WT littermates. Rex1 null mice were also generated by a conventional gene targeting strategy and while these mice were not characterized in depth, Rex1 was reported to be dispensable in development (Masui et al., 2008). Upon further characterization we discovered that Rex1-/- males showed an age associated decrease in sperm counts, abnormal sperm morphology, and mild testicular atrophy, phenotypes that could reflect a defect in the stem cell pool. Our data, collectively, indicate that Rex1 plays an important role in germ cell differentiation. Here we report experiments characterizing the germ cell compartment of Rex1 null mice in detail.
Materials and Methods
Reagents
Unless stated, all reagents were purchased from Sigma Aldrich (St. Louis, MO, USA).
Construction of the Rex1 Targeting Vector and Generation of Rex1-/- Mice
We previously generated a phage library with genomic DNA for the Rex1 gene (Hosler et al., 1993). To generate a targeted deletion of the protein coding exon, Rex1 exon 4, we first subcloned the LoxP sequence from the pBS64 plasmid (Baubonis and Sauer, 1993) into the BglII sites of plasmid pgRex1-1.38 (3′ of Rex1 exon 4) (Fig. 1A). We then ligated vectors pgRex1-1.37 and pgRex1-1.38 at their HindIII restriction sites (Fig. 1A). Subsequently, we subcloned the PGK-neo/MCI-TK cassette (pNEOTKLOX plasmid) into the NsiI site within intron 3′ of Rex1. This strategy created a targeting vector of approximately 12.7 kb (Fig. 1A); the 5′ region of this targeting vector contains 2.49 kb homologous to Rex1 intron 3, 3.86 kb of selection markers flanked by LoxP sites (PGK-neo, MCI-TK), 2.28 kb of the Rex1 gene (including all of exon 4), a LoxP site, and a 3′ arm consisting of a 4.07 kb genomic region 3′ of Rex1 exon 4.
Figure 1.

(A) Strategy and Structure of Targeting Vector and Strategy for Generating Rex1-/- mice. (B, C) Southern blot strategy verifying the accuracy of the targeting event. (B) 3′ Southern; genomic DNA from two WT, three heterozygous, and two knockout mice was digested with BglII, and the probe (0.9kb) was prepared by digesting pgRex1-1.46, a genomic fragment outside of the targeting construct, was prepared by digesting pgRex1-1.46 with HindIII/BglII. WT mice yield a band of 4.97 kb, heterozygous mice yield a 4.97 kb band and a 6.6 kb band, and knock out animals yield a 6.6 kb band. (C) 5′ Southern; genomic DNA from two WT, three heterozygous, and two knockout mice was digested with EcoRI, and the probe (0.3kb) was prepared by digesting pgRex1-1.51, a genomic fragment outside of the targeting construct, with BglII. WT mice yield a band of 8.12 kb, heterozygous mice yield a 8.12 kb band and a 5.6 kb band, and knock out animals yield only a 5.6 kb band. (C) Genotype by PCR: Genomic DNA from mouse tails is routinely genotyped by semi-quantitative PCR. We detect the recombination event by using a three primer PCR reaction: (1) specific forward primer homologous to an intragenic genomic region (P1F), in combination with two unique reverse primers: (2) a primer complementary to the Rex1 first exon (P2R); (3) and a primer complementary to the exogenous LoxP site (P4R). WT mice yield one band of approximately 350 bp, homozygous null animals yield a band of 470 bp, and heterozygous animals yield both bands. Primer sequences are listed in Supplemental Table 2. (D, E) RT-PCR: total RNA was isolated from testis and 2 μg of RNA was used to make cDNA using reverse transcriptase (Superscript, Invitrogen). 2 μl of 1:5 diluted cDNA was used to amplify mRex1 (D), and 36B4 (E) was used as a loading control. Rex1 primer pairs used for this are called Rex1F and Rex1R and result in a band of 670 bp; this band identity was verified by sequencing. 36B4 primers are called 36B4F and 36B4R and result in a 450 bp band. All primer sequences are listed in supplemental table 2.
This construct was linearized and introduced into the CJ7 mouse ES cell line (originally derived from 129S1/SvImJ) by electroporation using a Bio-Rad electroporator at a voltage of 230 mV and a capacitance of 500 microfarads. Drug resistant clones were isolated after G418 selection (300 μg/ml active G418), and a total of 116 cell clones were screened by Southern Blot analysis. Eight positive clones from cells that had undergone homologous recombination were obtained. One clone, # 66, was injected into C57BL/6 blastocysts to generate chimeric mice. Germ-line transmission was confirmed by mating chimeras to wild type C57BL/6 mice. Seven Rex1floxed/+ female mice were chosen to mate with CAG-cre male mice, which are on the C57BL/6J background, to excise exon 4 of the Rex1 gene and/or the PGK-neo-MCI-TK cassette. The Cre gene in these mice is under the control of the cytomegalovirus immediate early enhancer linked to the chicken beta-actin promoter so that the Cre gene is expressed early and ubiquitously (Sakai and Miyazaki, 1997). Recombination was detected by PCR-based genotyping (the primers used for this genotyping are in supplemental Table 2), and heterozygous mice (Rex1+/-) were inter-crossed to generate the Rex1-/- null mice. To determine the genotypes by PCR, two primer sets, P1F and P2R, and P1F and P4R were used. To confirm the genotype by Southern blot, genomic DNA was first digested with BglII. A 0.9 kb probe, located in genomic DNA 3′ of the targeting construct, was obtained by digesting pgRex1-1.46 plasmid DNA with HindIII/BglII, and hybridized to BglII digested genomic DNA. To prove homologous recombination we generated a second probe targeting the genomic DNA 5′ of the targeting construct. This second probe (0.3 kb) was obtained by digesting pgRex1-1.51 plasmid DNA with BglII, and it was hybridized to EcoRI digested genomic DNA. The locations of the primer sets are depicted in Fig. 1A. For these studies, mice were partially backcrossed into the C57BL/6J background, and thus they represent the N3 generation. Mice are now at the N12 generation with respect to the Rex1 knockout in the C57Bl/6J background.
Sperm Counts, Morphology Evaluation, and Testes Weight Measurements
Either 6-7 week or 4-6 month old male mice, both Rex1+/+ and Rex1-/-, were sacrificed by cervical dislocation. For sperm counts, our protocol was adapted from Yu et al (Yu et al., 2006). Briefly, the cauda epididymis was removed, minced in 3 ml of PBS (pH 7.4), and incubated for 5 min at room temperature by gently rocking. The samples were transferred to 15 ml tubes and centrifuged at 500 × g for 5 min. Then, the supernatants were used to count sperm with a hemocytometer. Three independent counts were performed for each mouse and the number of sperm was averaged. Briefly, sperm from 23 WT and 17 Rex1-/- mice were counted for the 6 week age group, and sperm from 10 WT and 20 Rex1-/- mice were counted for the four to six month age group. For sperm morphology evaluations, samples were prepared according to Ribeiro et al (Ribeiro et al., 1987). Briefly, 0.9 ml of the supernatant, prepared as above, was transferred to 0.1 ml of 1% Eosin Y in a 1.5 ml tube and incubated for 30 min at 22°C. The solution (10-15 μl) was used to prepare a smear on a slide, and this was air-dried. Mounting medium was added and sperm morphology was analyzed under the microscope. For testes weight measurements, both testes from each animal were removed, weighed, and the numbers were averaged.
Testicular Cell Preparation and Cell Cycle Analyses
Testicular cells were prepared for cell cycle analysis following a protocol adapted from Malkov et al. and Browning (Malkov et al., 1998). Briefly, testes were removed and decapsulated by making a small incision in the testis. The contents of the testes were collected through the incision into a 15 ml tube containing 5 ml ice-cold M199-BSA media (9.5 g tissue culture medium M199 (Invitrogen, Carlsbad, CA), 2.2 g NaHCO3, 1 g bovine serum albumin in 1 l distilled water, pH 7.3-7.4). 1-2 ml of 2 mg/ml collagenase was added to this tube, and the contents were incubated for 40 min at 37°C with vigorous shaking. Then, the tubes were placed on ice and incubated to allow the seminiferous tubules to settle. The supernatants were discarded and the seminiferous tubules were washed twice in 10 ml of M199-BSA medium. 5 ml of M199-BSA medium containing 2.5 μg/ml trypsin and 1U/ml DNase I was added to seminiferous tubules, and they were incubated for 20 min at 37°C. The reactions were stopped by transferring the tubes to ice and the cords were disaggregated by pipetting up and down with a Pasteur pipette several times. The resulting cell suspensions were filtered through a 50 μm nylon mesh and washed twice with M199-BSA medium and the cells were counted. A total of 2 × 106 cells were used for each cell cycle analysis. Cell cycle analyses were performed following the manufacturer's guide (Becton Dickinson Immunocytometry System, San Jose, CA). Cells (2 × 106) were resuspended in 875 μl of cold PBS containing 0.1% sodium azide and fixed by adding 125 μl of cold 2% paraformaldehyde in PBS and incubating for 1 hr at 4°C. Cells were centrifuged for 5 min at 250 g at 4°C and the supernatant was aspirated. Cells were then permeabilized by incubating them in 1 ml of PBS containing 0.05% Tween 20 for 15 min at 37°C. After washing the cells with 1 ml of PBS containing 0.1% sodium azide, cells were stained with propidium iodide (PI) staining solution (50 μl of 1 mg/ml of PI, 1 μl of 100 mg/ml RNase A in 1 ml PBS), and cell cycle analysis was performed on a Becton-Dickinson FACS Calibur in the Weill Cornell FACS Core.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) Assay
The TUNEL assay was performed following the manufacturer's guide (TUNEL enzyme, Ref: 11767305001, TUNEL label Ref: 11767291910, Roche, Basel, Switzerland); paraffin sections of testis (5 μm-thickness) were deparaffinized for 5 min in Histo-Clear (National Diagnostics, Atlanta, GA) twice, and then rehydrated in a series of serially diluted ethanol solutions (100%, 95%, 70%, and then H2O). Tissue sections were then treated with proteinase K (final concentration of 10 ug/ml in 10 mM Tris-HCl, pH 7.4-7.8) for 15 min at 22 °C. A positive control was prepared by incubating a tissue section with DNase I (3Unit/mL in 50 mM Tris-HCl, pH7.5, 1 mg/mL BSA) for 10 min at 22 °C before proteinase K treatment. A labeling reaction was prepared by mixing 45 μl of TUNEL-label solution with 5 μl of TUNEL-enzyme, added to tissue sections, and incubated for 60 min at 37°C in a humidified chamber in the dark. A negative control was prepared without TUNEL enzyme. Tissue sections were washed three times with PBS and analyzed under a fluorescence microscope using an excitation wavelength in the range of 450-550 nm and a detection wavelength in the range of 515-565 nm. Positive staining cells were manually counted from a section of each slide (100× magnification field).
Histology and Immunohistochemistry
Morphology of testicular and ovarian tissue was assessed by examination of Hematoxylin and Eosin (H&E) stained slides. Mice were sacrificed by cervical dislocation and testes was removed and fixed in 4% paraformaldehyde overnight at 4°C. Tissues were transferred to 70% ethanol and paraffin blocks were prepared. Tissue sections (7 μm thickness) were stained with H&E and tissue morphology was evaluated blindly by a pathologist (SM). For immunohistochemistry, tissue sections were deparaffinized for 5 min in Histo-Clear (National Diagnostics, Atlanta, GA) twice, and then rehydrated in serially diluted ethanol solutions (100%, 95%, 70%, and then H2O). Antigen retrieval was performed by cooking sections for 3 min in a pressure cooker containing antigen unmasking solution (pH 6.0, H-3300, Vector), and tissue sections were then blocked for 30 min in 5% goat serum in PBST (0.05% Tween 20 in PBS). After washing with PBST, the tissue sections were incubated with primary antibodies: GRTP1 (1:500, Sigma, PRS4589), Sox9 (1:200, H-90, sc-20095, Santa-Cruz Biotechnology, Santa Cruz, CA), DazL (1:400, ab34139, Abcam, Cambridge, MA), PCNA (1:400, M0879, Dako, Carpinteria, CA) overnight at 4°C for Sox9, or 1 hour at 22 °C for GRTP1, DazL and PCNA. Following three washes with PBST the sections were incubated with secondary antibodies, either anti-rabbit HRP for Sox9 and DazL (superpicture HRP polymer conjugated rabbit primary, 87-9263, Invitrogen, Carlsbad, CA) or anti-mouse HRP for PCNA (M.O.M kit, PK-2200, Vector Labs, Burlingame, CA) for 10 min or 30 min at 22 °C, respectively. DAB signals were generated and sections were counterstained with hematoxylin. We provided total Stat3 (Cell Signaling Technology, 9132), phospho-Stat3 (Cell Signaling Technology, 9145), p38 MAPK (Cell Signaling Technology, 9212, lot 16), and phospho-p38 MAPK (Cell Signaling Technology, 4631, lot 6) antibodies for automated staining at the Memorial Sloan Kettering Cell Biology Core Facility.
Germ Cell (GC) RNA Isolation, Labeling and Microarray Hybridization
Germ Cell RNA was isolated from whole testis following a protocol adapted from Malkov et al. (Malkov et al). Briefly, testes were decapsulated and digested with 0.1 mg/ml of collagenase type II (Invitrogen) for 40 min. After collagenase digestion, the dissociated seminiferous tubules were isolated by gravity sedimentation, and germ cells were further disconnected from tissue by digestion with 2.5 μg/mL trypsin (Lonza) supplemented with 1 U/mL DNase (Invitrogen). Single cells were filtered through a 50 μm nylon mesh, washed twice in separation medium (1) for 5 min at 300 g, and counted. Total germ cell RNA (GC RNA) was extracted using TRIZOL reagent (Invitrogen). GC RNA from two WT mice and three Rex1-/- mice were labeled and hybridized to oligonucleotide microarrays (GeneChip Mouse Gene 1.0 ST (Affymetrix)), according to standard protocols at the Weill Cornell Medical College Microarray Core Facility (the GC RNA of one wt mouse was analyzed twice).
Microarray Data Analysis
Raw data CEL files were normalized using the Robust Multi-Array (RMA-16) algorithm in GeneSpring GX 11.0.2 software (Agilent). Triplicate samples were grouped as wild type (wt) and homozygous null (HO), and statistical analysis was calculated with unpaired t-tests applied to log-transformed data.
Results
Rex1 null animals are characterized by premature age-associated testicular germ cell depletion, decreased sperm count, and abnormal sperm morphology
To investigate the functions of Rex1 in germ cell biology we have generated a mouse with a targeted deletion of the Rex1 gene (Fig. 1A). Initial attempts by our laboratory to create Rex1 null mice failed so we used Cre/Lox recombination to produce a conditional Rex1 knockout mouse. A LoxP site was inserted into the 3′ end of the Rex1 exon 4 while the selectable marker genes, the Neo-TK cassette, were flanked by LoxP sites as well (Fig. 1A). After germline transmission of the targeted allele, Rex1flox/wt mice were mated with a transgenic mouse line carrying the chicken β-actin promoter (Niwa et al., 1991) directing cre recombinase expression in all tissues (Sakai and Miyazaki, 1997). Rex1+/- mice were then identified, indicating excision of both the Neo-TK gene and exon 4 of the Rex1 gene (Fig. 1B, C, D). We obtained WT, heterozygous, and homozygous mutant Rex1 mice. Rex1-/- mice are viable and fertile and there appear to be no sex preferences among the progeny obtained (Table 1). As expected, Rex1 null mice failed to express Rex1 message as assayed by semi-quantitative PCR (Fig. 1E, F). We tested two commercially available antibodies against murine Rex1 (Millipore MAB4316, Abcam ab28141), and two custom generated Rex1 antibodies (Alpha Diagnostics), but failed to detect endogenous Rex1 protein by Western blot analysis of WT mouse testicular tissue, although the antibodies could detect mouse Rex1 overexpressed in Cos cells (data not shown).
Table 1. Genotype Analysis of Progeny from Crosses of Rex1+/- mice.
Distribution of male and female, wild type (WT), heterozygous (HT) and knock out (Rex1-/-) progeny from crosses between Rex1+/- mice. Primers P1F, P2R and P4R (sequences in supplemental Table 2) were used to genotype genomic DNA extracted from the mice tails.
| Genotype | Male | Female |
|---|---|---|
| WT (Rex1+/+) | 60 (0.32) | 50 (0.3) |
| HT (Rex1+/-) | 78 (0.41) | 68 (0.4) |
| KO (Rex1-/-) | 52 (0.27) | 51 (0.3) |
|
| ||
| Total | 190 | 169 |
To explore the physiological consequences of the loss of Rex1, the structure and function of the testes from Rex1 null mice were examined in detail. First, testis histology of six week (WT, n=23, KO, n=17) and 4-6 month old (WT, n=10, KO, n=20) mice was evaluated by H&E staining (Fig. 2B, 2D, 2C, 2E). We found that six of the Rex1-/- mutant testes showed a mild to moderate decrease in the number of germ cells in the seminiferous tubules (KO six week, n=5, KO 4-6 month old, n=1). None of the WT, either 6 week or 4-6 month old, showed this phenotype. This phenotype was seen most predominantly in spermatids (both early and late), but also occasionally in spermatocytes. In affected testes depleted seminiferous tubules were observed multifocally, admixed with normal tubules. We sacrificed two 1 year old animals for testis histology, at 13 months old (Supplemental Figure 1A, B). Germ cell depletion is known to naturally occur with increased age, and we observed mild germ cell depletion, admixed with physiologically normal tubules in both WT and Rex1-/- 1 year old male mice (Supplemental Figure 1). To investigate further the germ cell defect in Rex1-/- males, we performed sperm counts in animals of various ages. The same mice were evaluated for sperm counts (Fig. 2H) and testes weight (Fig. 2I). Both testes were removed from each mouse, weighed, and averaged. No significant difference was seen between six week old WT and Rex1-/- mice, though a decline in the number of spermatozoa was observed in Rex1-/- animals between four to six months of age (Fig. 2H). The average sperm count of WT mice in the four to six month age group was 502 × 104 ± 103/ml/epididymis for WT mice, and it was 301 × 104 ± 50/ml/epididymis in Rex1-/- mice (p=0.03). We also determined testis weights of six-week old WT mice and Rex1-/- mice. The tissue weights were 80 ± 4 mg and 77 ± 7 mg, respectively (Fig. 2G). No statistically significant differences were observed in testis weights between 6 week old WT vs. Rex1-/- mice (p=0.324), and the same is true for 4-6 month old mice.
Figure 2. Measurements of Testes Weight, Sperm Counts and Sperm Morphology.
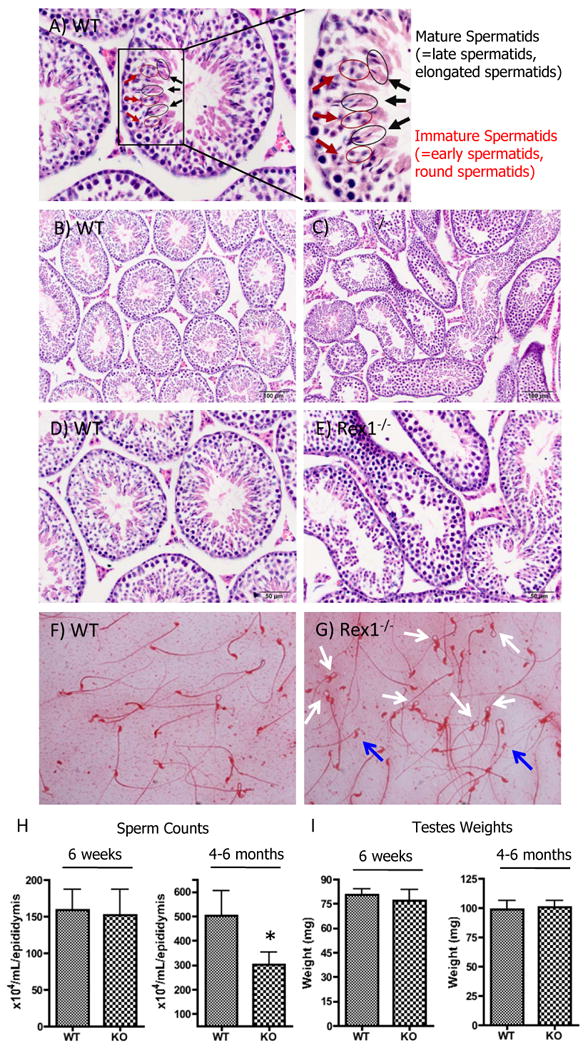
Immature spermatids (red arrows) and mature spermatids (black arrows) are highlighted in an H&E stained wild type reference slide (A). Testis histology of six week old and 4-6 month old mice evaluated by H&E staining (WT B, D; Rex1-/- C, E). Sperm from 4-6 month old mice were collected from a cauda epididymis and stained with 1% Eosin. Stained sperm were observed under a bright field microscope and evaluated for morphological abnormalities (WT F, Rex1-/- G). The white arrows highlight the kinks in the Rex1-/- sperm, while the blue arrows highlight a lighter eosin staining pattern (G). Evaluation of the same mice for sperm counts (H), and testes weights (I). For sperm counts, a cauda epididymis was removed from each mouse and minced in 3 ml of PBS. The supernatant was used to count sperm by the trypan blue exclusion assay. Three independent counts were performed for each sample and averaged. * p<0.05, t-test. Scale 100 μm (B, C), 50 μm (D, E).
We studied the sperm in 9 Rex1-/- and 10 WT mice between 4-6 months old, and noted the morphology to be different (Fig. 2F, G). In many Rex1-/- mice most sperm had bent tails just distal to the head (2G, white arrows). In addition, some sperm heads lacked cytoplasmic content, indicated by weaker staining (2G, blue arrows). The magnitude of this phenotype varied, suggesting incomplete penetrance. We conclude that Rex1 null animals are characterized by mild germ cell depletion, and a premature age-associated decrease in sperm counts.
Rex1-/- ovaries have similar numbers of follicles, corporea lutea, and ceroid pigment as compared to WT ovaries
In mammals the development of female germ cells entails a complex and tightly regulated process. At birth, the murine female ovaries contain primary oocytes arrested at stage I of mitosis (Pepling, 2006). Following puberty, a number of oocytes will periodically respond to environmental hormonal clues, resume mitosis, and mature further (Pepling, 2006). We previously failed to detect Rex1 transcript in murine ovaries (Rogers et al., 1991). Nevertheless Kristensen et al. reported human REX-1 staining specifically in the cycling oocytes of gestation week 40 (Kristensen et al., 2008). To investigate a role for Rex1 in female germ cell development we undertook a comparison of the number of follicles, corporea lutea and ceroid pigment in a group of mice across three distinct age groups, 21 days old, 6 weeks old, and 1 year old. We reasoned that if transient Rex1 expression in cycling cells affects the number of female germ cells we would be able to see a quantitative difference in the number of follicles and corporea lutea between WT and Rex1-/- mice. In general, ovary morphology was grossly similar between WT and Rex1-/- mice. At 21 days old the average number of follicles in the WT mice was 52.6 as compared to 75.5 in Rex1-/- mice (p = 0.06) (Figure 3A, B. Table 2). At 6 weeks old the average number of follicles in the WT mice was 37.6 as compared to 24.6 in Rex1-/- mice (p = 0.4), and there was an average of one corpus luteum per WT mice and 1.3 per Rex1-/- mice (Figure 3C, D. Table 2). At 1 year old, the average number of follicles in the WT mice was 8.5 as compared to 7.8 in Rex1-/- mice (p = 0.8), and there was an average of 7.25 corporea lutea per WT mice and 4.3 corporea lutea per Rex1-/- mice (p = 0.09) (Figure 3E, F. Table 2). We note mild lymphocytic infiltration in the ovarian bursa, which was observed in both WT and Rex1-/- mice (Table 2). Overall, we conclude that Rex1 expression does not affect the number or the morphology of follicles and corpus luteum in Rex1-/- mice.
Figure 3. Histological Evaluation of Follicles, Corporea Lutea, and Ceroid Pigment in WT vs Rex1-/-Mice.

Ovary histology of twenty one day, six week, and 1 year old mice was evaluated by H&E staining (WT A, C, E; Rex1-/- B, D, F). In panels A thru D black arrows highlight examples of follicles; in panels E and F black arrows highlight examples of corpus luteum. We analyzed ovaries across a range of different ages (WT, n=10, Rex1-/-, n=13 animals). Scale: Black bars denote 100 μm.
Table 2. Tabulation of the number of follicles, corporea lutea, and ceroid pigment in WT vs Rex1-/-mice.
Histological analysis of the ovaries in WT and Rex1-/- mice ranging from 21 days old, 6 weeks old and 1 year old. * All types of follicles were counted, except primordial follicles. ** numbers correspond to scale measuring the level of ceroid pigment: 0: absent, 1: minimal, 2: mild, 3: moderate, 4: marked. At 21 days and 1 year two ovaries were evaluated per animal and the numbers listed are per pair of ovaries. At 6 weeks one ovary was examined per animal, and the numbers listed are per ovary.
| Age: 6 weeksOne ovary examined per animal, numbers are per ovary | |||
|---|---|---|---|
| Genotype | n follicles* | n corpora lutea | ceroid pigment** |
| WT | 31 | 1 | 0 |
| WT | 41 | 1 | 0 |
| WT | 41 | 1 | 0 |
| KO | 43 | 2 | 0 |
| KO | 15 | 0 | 0 |
| KO | 16 | 2 | 0 |
| Age: 21 daysTwo ovaries examined per animal, numbers are per pair of ovaries unless otherwise specified | |||
| Genotype | n follicles* | n corpora lutea | ceroid pigment** |
| WT | 55 | 0 | 0 |
| WT | 62 | 0 | 0 |
| WT | 41 | 0 | 0 |
| KO | 77 | 0 | 0 |
| KO | 80 | 0 | 0 |
| KO | 83 | 0 | 0 |
| KO | 62 | 0 | 0 |
| Age: 12 monthsTwo ovaries examined per animal, numbers are per pair of ovaries unless otherwise specified | |||
| Genotype | n follicles* | n corpora lutea | ceroid pigment**Other observations |
| WT | 10 | 5 | 1 |
| WT | 9 | 7 | 1 |
| WT | 12 | 7 | 2 |
| WT | 3 | 10 | 1 |
| KO | 5 | 8 | 1 |
| KO | 10 | 5 | 3 |
| KO | 3 | 6 | 1 |
| KO | 13 | 3 | 3 |
| KO | 12 | 4 | 2 |
| KO | 4 | 2 | 1 |
Depletion of the germ cell compartment in Rex1-/- males cannot be explained by defects in cell cycle distribution
Previously, our laboratory utilized microarray approaches to identify transcripts that were differentially expressed between wild type and Rex1-/- mouse ES cells (Scotland et al., 2009). One of the differentially expressed transcripts was cyclin D2, which showed a six fold decrease in Rex1-/- mES cells. To determine if alterations in cell cycle distribution, potentially indicating a block in cell cycle progression, could explain the decrease in sperm counts in Rex1-/- mice, we measured the DNA content of cells in the seminiferous tubules of 4-6 month old mice (Fig. 3). Comparing WT (mean ± S.E)(Fig. 4A, C), and Rex1-/- mice (mean ± S.E)(Fig. 4B, C), we observed no significant difference in the distribution of cells with 4N DNA content (19.2 ± 1.6 % vs 16.9 ± 0.8%) and 2N DNA content (9.7 ± 0.8 % vs 8.5 ± 0.4%). A small difference in the spermatid and spermatozoa populations was observed between WT and mutant mice; WT exhibited 68.9 ± 2 % of cells with 1N DNA content and Rex1 mutants 72.9 ± 0.8% (p=0.03). These results indicate that defects in cell cycle distribuition/progression cannot explain the reduction in sperm count in older Rex1-/- males.
Figure 4. Cell Cycle Analysis.

Testicular cells were prepared from the testes of 4-6 month old mice (WT, n=6, Rex1-/-, n=7) following the protocol described in Materials & Methods. ∼ 2 ×106 cells were stained with propidium iodide (PI) to analyze the cell cycle. * p<0.05, t-test.
Microarray analysis of WT and Rex1-/- primary germ cells reveals effects on the transcriptome
To investigate if Rex1 expression influences the transcriptome in testicular cells we performed microarray analyses on isolated primary germ cells from the seminiferous tubules of six week old WT and Rex1-/- mice. We were not able to detect Rex1 mRNA by RT-PCR in isolated primary germ cells from Rex1-/- mice using primers homologous to exons three and four (Fig. 5B). Three genes showed at least a four fold decrease in expression in Rex1 null mice. Two are unknown genes (Fig. 5A, and supplemental Table 1), whereas the third is the Growth Hormone Regulated TBC Protein-1, GRTP1, which shows decreased mRNA expression in germ cells of Rex1-/- mice (∼4.5 fold, Fig. 5B) (Lu et al., 2001). Although primary germ cells of Rex1 null mice express less GRTP1 mRNA than germ cells from WT mice (Fig. 5C), WT and Rex1-/- mES cells in culture (+LIF) exhibit the same GRTP1 transcript levels (data not shown).
Figure 5. Microarray Analysis.
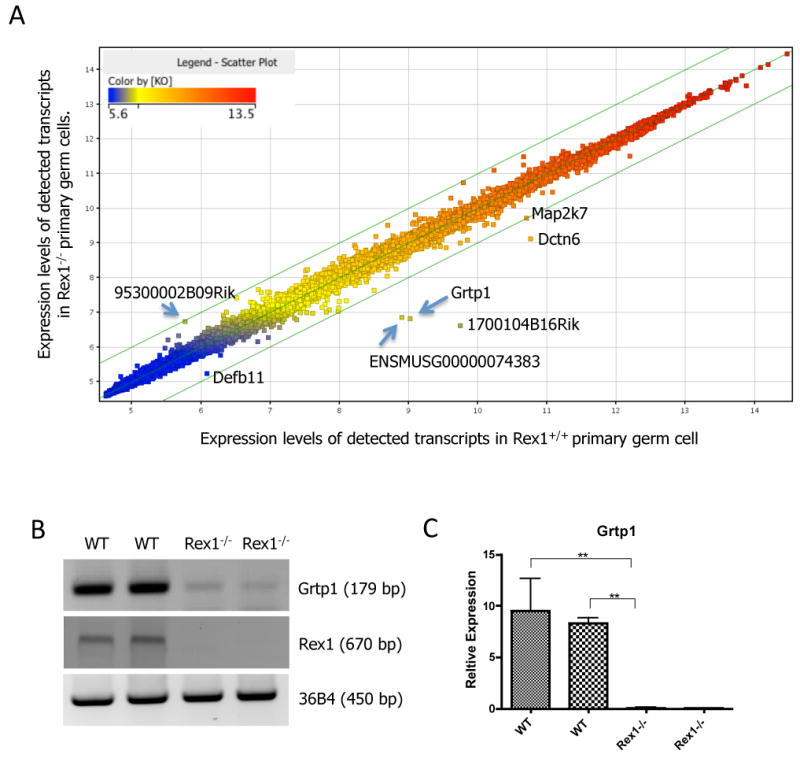
Primary germ cells were isolated from 6 week old WT and Rex1-/- mice. RNA from primary germ cells was isolated with Trizol. Normalized intensity value (Log scale) of WT (X-axis) and Rex1-/- (Y-axis) primary germ cell transcripts with a fold change >1.2 (A). Individual dots correspond to unique genes with a change in expression greater than 1.2 fold between WT and Rex1-/- primary germ cells. Semi-quantitative RT-PCR analysis of primary germ cell cDNA and mES cells (B). Validation of one of the differentially expressed genes identified in the array, GRTP1, was performed in another preparation of newly isolated primary germ cells by semi-quantitative RT-PCR (B), and real time quantitative PCR (C). (B) Levels of Grtp1, Rex1 and 36B4 transcripts shown in duplicate samples of newly isolated primary germ cells from 6 week old mice. (C) Quantitation of the signal from the samples described in (B) by real time PCR.
This is the first time that a microarray analysis of Rex1 expressing versus Rex1 deficient cells taken directly from mice has been performed. We compared the results of our microarray analysis to our previously published data from WT vs Rex1-/- null stem cells (Scotland et al., 2009; Xu et al., 2008) and that of others (Masui et al., 2008) to identify themes that might suggest conserved functions. GRTP1 transcript changes were only found in our analysis of WT vs Rex1-/- germ cells. The available data consist of two separately derived mouse embryonic stem cell lines (Masui et al., 2008; Scotland et al., 2009), a F9 teratocarcinoma stem cell line (Xu et al., 2008) and our primary germ cells (Fig. 5).
Characterization of apoptotic cells, STAT3, phospho-STAT3(Tyr705), p38 and phospho-p38 positive cells in the testes of Rex1-/- mice
Bhandari et al have recently shown that Rex1 acts as an inhibitor of the pro-apoptotic p38 mitogen-activated protein kinase (p38-MAPK) in human mesenchymal stem cells (Bhandari et al., 2010). The abnormal morphology of many seminiferous tubules and the lower sperm counts seen in Rex1-/- mutants may reflect an increase in apoptosis. To test this, we performed TUNEL assays to assess the level of apoptosis in the testicular cells of Rex1-/- mice. We defined a 100× magnification section of comparable slides as a field, and evaluated the TUNEL signal in the testes from six week old mice (WT, n=21, KO, n=19). The average number of apoptotic cells observed per field in WT testes was 39 ± 4 (Fig. 6A, C), while the average number of apoptotic cells in Rex1-/- testes was 56 ± 13 (Fig. 6B, C). We observed a large variance in the number of cells undergoing apoptosis within the testes of different WT and Rex1-/- mice, but with the exception of an outlier (Fig. 6B) most animals displayed comparable levels of apoptosis. The apoptotic cells were mostly immature germ cells located in the outer layers of the seminiferous tubule, and we found no correlation between the number/location of immature germ cells and germ cell depletion.
Figure 6. TUNEL Assay and STAT3 Analysis.

Apoptosis of cells in the testis was evaluated by the TUNEL assay. Testes from six week old mice (wt, n=21, ko, n=19) were removed and fixed in paraffin. Blocks were sectioned into 7μm thick sections and stained with the TUNEL assay kit following the Roche manufacturer's protocol (A, B). Positive staining was counted in an entire section of each mouse and averaged for each group to compare (C). Status of Phospho-Stat3 (Tyr705) was evaluated in WT (D), and Rex1-/- (E) testes. (E) Black arrows highlight strong P-STAT3 nuclear staining in Sertoli cells. Number of P-STAT3 positive staining cells in 25 WT and 26 Rex1-/- whole testis sections (F). Status of total Stat3 was evaluated in WT (G), and Rex1-/- testes (H). Black arrows (H) highlight strong cytoplasmic staining of total STAT3 in the Rex1-/- testes with marked germ cell depletion. Scale 50 μm.
The p38 mitogen activated protein kinases are a well-known class of proteins involved in responses to physiological stress (Han et al., 1994). Since human mesenchymal stem cells in culture show a direct relationship between Rex1 expression and activation of p38 by phosphorylation, we assessed primary germ cells from WT and Rex1-/- mice for phospho-p38 levels. We stained testes for phospho-p38 (P-p38) (WT, n=20, KO, n=19 animals) (Supplemental Fig. 3A, B), and for total p38 levels (WT, n=21, KO, n=19) (Supplemental Fig. 6C, D). There was a high variation in staining within both WT and Rex1-/- samples. Ten Rex1-/- mice displayed phospho-p38 expression as compared to seven WT mice. In most cases phospho-p38 was detected in Sertoli cells (Supplemental Fig. 3B, black arrows), and occasionally in germ cells (Supplemental Fig. 3B, white arrows). There was no statistically significant difference between the numbers of seminiferous tubules expressing phospho-p38 between WT and Rex1-/- mice (Supplemental Fig. 3C). Comparable total p38 levels were observed in the cytoplasm of both Sertoli and a range of immature germ cells in WT and Rex1-/- (Supplemental Fig. 3D, E). In mature spermatids, p38 staining was detected in the nucleus of unaffected seminiferous tubules in WT and Rex1-/- mice (Supplemental Fig. 3D, E).
Our laboratory previously examined the role of Rex1 in murine F9 teratocarcinoma stem cell during differentiation, and we discovered that F9 cells that lack Rex1 exhibited a large increase in transcription of the supressor of cytokine signaling 3 (SOCS3) gene (Xu et al., 2008). We found that this increase occurs via the signal transducer and activator of transcription (STAT3) binding sites located at - 99 to -60 of the SOCS3 promoter. We further showed that overexpression of a Stat3 dominant negative (DN) mutant diminished the increase of SOCS3 expression in F9 cells (Xu et al., 2008). To gain insight into possible mechanisms leading to increased apoptosis in the testes of Rex1-/- mice, we investigated the levels of active and total Stat3 protein in the testis. To this end, we stained testes for P-Stat3 (Tyr705) (WT, n=25, KO, n=26) (Fig. 6D, E, F), and for total Stat3 levels (WT, n=27, KO, n=24) (Fig. 6G, H). Most WT and Rex1-/- testes are negative for P-Stat3 (Tyr705) staining. However, strong P-Stat3 (Tyr705) nuclear staining was observed in the Sertoli cells of the six Rex1-/- testes displaying testicular atrophy (Fig. 6E). Although we observe a positive correlation between the degree of atrophy and the number of P-Stat3 (Tyr705) positive Sertoli cells in the Rex1-/- testes, it is unclear if activation of Stat3 by phosphorylation is the cause or a consequence of atrophy. We observe nuclear and cytoplasmic staining of total STAT3 in the Sertoli and germ cells of both WT and Rex1-/- mice (Fig. 6G, H). Rex1-/- mice are characterized by strong cytoplasmic staining of total Stat3, particularly in the seminiferous tubules of Rex1-/- mice with obvious, germ cell depletion (Fig. 6G). Our data suggest that loss of Rex1 may lead to depletion of the germ cell compartment via indirect activation of the JAK/STAT3 pathway.
Rex1 supports proper cell proliferation and proper distribution of the germ cell and Sertoli cell populations within the seminiferous tubules
The mature testis is composed of many different cell types, including germ cells and Sertoli cells. Germ cells are closely associated with Sertoli cells throughout their development and differentiation, and in wild type mice Rex1 is predominantly expressed in spermatocytes undergoing meiosis (Rogers et al., 1991). To examine how the lack of Rex1 affects testis architecture, histological sections of testicular tissues from WT and Rex1-/- mice were immunostained with markers of specific cell types. First, sections were stained with DazL, a protein known to be expressed in immature germ cells (Saunders et al., 2003). In both WT and Rex1-/- mice, nuclear staining was predominantly restricted to spermatogonia and was evenly distributed within this population along the outer edges of the seminiferous tubules (Fig. 6A, B). This indicates that Rex1 expression is not necessary for maintenance of the most immature type of germ cells, the spermatogonia.
To evaluate the proliferation of germ cells, sections were stained with proliferating cell nuclear antigen (PCNA) (Fig. 6C). In sections from both WT and Rex1-/- mice we observed positive staining for PCNA within well organized spermatogonia (Fig. 6D). Consistent with our observation that Rex1-/- mice display depletion of their germ cell compartment, PCNA staining was often absent from spermatogonia within the tubules of the sections from the Rex1-/- mice (Fig. 6D).
The structural design of the testis is well defined, with germ cell differentiation and maturation occurring in well synchronized steps. Sox9 expression appears to be tightly associated with Sertoli cell development (Morais da Silva et al., 1996), (Kent et al., 1996), (Vidal et al, 2001). Sox9 expression remains in Sertoli cells exclusively after testis cord formation and is used as a Sertoli cell marker (Morais da Silva et al., 1996), (Hemendinger et al., 2002). To investigate the status of Sertoli cells in Rex1-/- mice, we examined Sox9 protein in WT and Rex1-/- testes. As expected, Sox9 staining is observed in the nuclei of the Sertoli cells of WT mice (Fig. 6E). We also observe similar patterns of Sox9 staining in Rex1-/- mice (Fig. 6F). The staining of the immature germ cells (DazL), proliferating germ cells (PCNA), and Sertoli cells (Sox9) within WT and Rex1-/- seminiferous tubules, along with the observed depletion of mature spermatids (Hematoxylin and Eosin) and abnormal sperm morphology in Rex1-/- mice, suggest that Rex1 expression influences differentiation of mouse germ cells at later stages. This indicates that Rex1 mRNA may be necessary for proper differentiation or maturation of germ cells.
Discussion
Rex1 (Zfp42) is a zinc finger transcription factor (Hosler et al., 1989) and is expressed in human and murine stem cells (Boyer et al., 2006; Brivanlou et al., 2003; Loh et al., 2006; Masui et al., 2007; Mongan et al., 2006; Rogers et al., 1991). Although Rex1 is not necessary for the maintenance of ES cell pluripotency, Rex1-/- deficient ES cells do show a greater tendency to differentiate towards somatic lineages (Scotland et al., 2009; Toyooka et al., 2008). To understand the function of Rex1 in stem cells, we made a conditional knockout mouse that lacks this gene. We show here that mutant mice deficient in Rex1 are viable and fertile. This is the first conditional Rex1 knockout mouse line to be reported.
Since the testis expresses a high level of Rex1 RNA (Rogers et al., 1991), we examined Rex1-/- mutant mice testes and found mild to moderate germ cell depletion (Fig. 2B, C, D, E), with early/late spermatids being the population mostly affected. Additionally, Rex1-/- mutant mice displayed abnormal sperm morphology. These data could indicate defects in DNA replication or chromosome segregation in meiosis, a phase during which differentiation/maturation of germ cells occurs and when Rex1 is highly expressed (Kristensen et al., 2008; Rogers et al., 1991). Alternatively, this phenotype could indicate that Rex1-/- mice are undergoing accelerated aging (Zhang et al., 2006). Yet another possibility is that the phenotypes can be attributed to defects in X chromosome inactivation. X chromosome inactivation is an example of an epigenetic regulatory mechanism used to equalize the X linked gene dosage. During mammalian germ cell development the X chromosome is transiently silenced at meiosis (Namekawa et al., 2006; Nguyen and Disteche, 2006), presumably to equalize the X linked gene dosage during those critical steps of development. In cultured mES cells Rex1 was recently identified as a required factor for efficient elongation of Tsix, a key non-coding transcript involved in X-inactivation (Navarro et al., 2010), and we and others have identified key regulators of X-inactivation as differentially expressed genes in global gene expression analysis (Masui et al., 2008; Scotland et al., 2009). Kim et al recently characterized a mouse line in which the β-geo gene trap vector was inserted into the third intron of the Rex1 gene; since the entire open reading frame for Rex1 protein is present in the fourth exon (Hosler et al., 1989; Hosler et al., 1993), we would predict that the entire Rex1 protein would still be produced in this β-geo gene trap mouse line (Kim et al., 2011).
Spermatogenesis is a highly organized process that takes place in three distinct phases in the seminiferous epithelium: spermatogonial proliferation (spermatogoniogenesis), meiosis of spermatocytes, and differentiation of haploid round spermatids into elongated spermatids (spermiogenesis) (Leblond and Clermont, 1952). Each phase is accompanied by morphological and biochemical changes (Johnston et al., 2008) and changes in regulation of the cell cycle (Kierszenbaum, 2006). The entire process can be subdivided into 12 stages. Although we have not analyzed each step in depth, we studied cellular pathways relevant to the observed phenotype. We did not observe statistically significant differences between populations with 1N, 2N and 4N DNA content within the testes of 4-6 month old mice, indicating that Rex1 ablation does not interfere with cell cycle distribution in male germ cells (Fig 3). Similarly, the evaluation of apoptosis in the testis does not show a statistically significant difference between WT and Rex1-/- mice, although there was high variability within individual testes. The above phenotypes of Rex1-/- mice are variable, suggesting incomplete penetrance. One possible explanation for this is that the ubiquitous polycomb transcription factor Yin Yang 1 (YY1) may be compensating for Rex1 to some extent (Kim et al., 2007; Mongan et al., 2006). A high degree of homology exists between Rex1 and YY1, and although YY1 gene knockout results in embryonic lethality during peri-implantation (Donohoe et al., 1999), a conditional knockout of YY1 showed defects in spermatogenesis (Wu et al., 2009). When YY1 expression was lost in the testes, testis size was reduced to a third the size of YY1 heterozygous mice (YY1f/+/Cre), and the testes had abnormal and vacuolated seminiferous tubules and less pachytene spermatocytes (Wu et al., 2009).
Stat3 is an essential mammalian protein with well-defined roles in the maintenance of mES cell pluripotency (Raz et al., 1999). Phosphorylation of the STAT3 protein leads to the formation of active STAT3 dimers, which translocate to the nucleus and work as active transcription factors (Zhang et al., 1995). Rex1-/- mice show strong cytoplasmic staining of total Stat3 in Sertoli cells, spermatogonia, and spermatocytes, particularly in the seminiferous tubules with obvious atrophy/germ cell depletion (Fig. 5G). Characterization of the more immature germ cells (DazL), proliferating germ cells (PCNA), and Sertoli cells (Sox9) (Fig. 6A-F) indicates that those populations are unaffected by the lack of Rex1 expression. These results, along with the abnormal sperm morphologies (Fig 2G) and spermatid depletion in Rex1-/- mice, indicate that Rex1 expression affects the later stages of spermatogenesis.
Lastly, from the microarray analysis, we identified a growth hormone regulated gene, GRTP1, that was transcriptionaly downregulated in Rex1-/- germ cells. Although hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone have all been shown to play important roles in germ cell biology, it is generally accepted that hormone triggered physiology effects occur via signaling from the neighboring Sertoli cells, as germ cells do not contain receptors for hormones (Ruwanpura et al., 2010). It is unclear if the lack of Rex1 leads to a cell intrinsic defect in germ cell hormonal response. In mouse ES cells the key regulators of mammalian X-innactivation, Tsix and Xist, were respectively identified as a 3.6 fold downregulated gene in Rex1-/- (Masui et al., 2008), and a ∼2.9 fold upregulated gene in the presence and abscense of LIF (Scotland et al., 2009). We have previously studied in detail the mechanisms underlying the transcriptional activation of the supressor of cytokine signaling 3 (SOCS3) gene by a combination treatment of retinoic acid, theophylline and dibutyryl cyclic AMP in F9 cells. By promoter deletion, mutation and transient tranfection analysis we identified this transcriptional increase to be mediated by STAT3 DNA binding elements in the SOCS-3 promoter. We also observed activation of phospho-STAT3 in the context of differentiation when a retinoic acid, theophylline and dibutyryl cyclic AMP combination treatment was used; we concluded that Rex1 plays an indirect role in the Janus Kinase (JAK) / signal transducer and activator pathway.
Our results provide in vivo genetic evidence demonstrating that Rex1 plays an important role within the testis. We are currently investigating if loss of Rex1 leads to defects in other adult stem cell compartments and/or misregulation of adult stem cell differentiation.
Supplementary Material
Figure 7. Evaluation of Germ Cell, Proliferating, and Sertoli Cell Populations Immunohistological Evaluation.

Six week old mice were assessed by immunohistochemistry using antibodies to Dazl, PCNA and Sox9. (A, B) Dazl staining of both wild type and Rex1-/- mice (33 wt, 28 Rex1-/-). Black arrows highlight examples of the most immature, Dazl positive germ cells within the WT and Rex1-/- testis. (C, D) PCNA staining of WT and Rex1-/- testes (33 wt, 28 Rex1-/-). Black arrows highlight examples of proliferating germ cells. (E, F) Sox9 staining of WT and Rex1-/- testes (34 wt, 26 Rex1-/-). Black arrows highlight examples of Sertoli cells of both WT and Rex1-/- mice.
Research Highlights.
A transgenic (knock-out) mouse model of the stem cell transcription factor Rex1 (Zfp42)
Rex1 (Zfp42) null mice display testicular atrophy
Early/late spermatids are the most affected population in Rex1-/- mice
Potential mechanisms behind the phenotype have been analyzed
Acknowledgments
We thank all of the members of the Gudas laboratory for scientific discussions, Tamara Weissman for editorial assistance, the WCMC Genomics Core Facility and the Histology Core of Weill Cornell Medical College for section preparation, and the Transgenic Core Facility for ES cell injections into blastocysts. We would like to thank Sergei Rudchenko and Stanka Semova for their assistance with the FACS analysis in the WCMC FACS Core Facility, and Jacqueline Bromberg from Memorial Sloan Kettering Cancer Center for guidance with STAT3 and p-STAT3 staining. This research was supported in part by NIH R01CA043796 to L.J.G., and by Weill Cornell funds. M.Y.L was supported for a portion of this research by DOD grant W81XWH-06-1-0109. N.C.R. is a Howard Hughes Medical Institute Gilliam Fellow.
Abbreviations
- ES
embryonic stem
- mES
mouse embryonic stem cell
- RA
all-trans retinoic acid
- hMSCs
human mesenchymal stem cells
- RT-PCR
reverse transcription PCR
- HET
heterozygote
- KO
knockout
- H & E
Hematoxylin & Eosin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, Hirschi KK, Braunhut S, Gudas LJ, Badylak SF. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A. 2010;107:3351–3355. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baubonis W, Sauer B. Genomic targeting with purified Cre recombinase. Nucleic Acids Res. 1993;21:2025–2029. doi: 10.1093/nar/21.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, Thompson JR, Gudas LJ, Bergman Y. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol. 1998;18:1866–1878. doi: 10.1128/mcb.18.4.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari DR, Seo KW, Roh KH, Jung JW, Kang SK, Kang KS. REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells. PLoS One. 2010;5:e10493. doi: 10.1371/journal.pone.0010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J. Stem cells. Setting standards for human embryonic stem cells. Science. 2003;300:913–916. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, Hartung O, Rho J, Ince TA, Daley GQ, Schlaeger TM. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- Donohoe ME, Zhang X, McGinnis L, Biggers J, Li E, Shi Y. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol Cell Biol. 1999;19:7237–7244. doi: 10.1128/mcb.19.10.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hemendinger RA, Gores P, Blacksten L, Harley V, Halberstadt C. Identification of a specific Sertoli cell marker, Sox9, for use in transplantation. Cell Transplant. 2002;11:499–505. [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler BA, LaRosa GJ, Grippo JF, Gudas LJ. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989;9:5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler BA, Rogers MB, Kozak CA, Gudas LJ. An octamer motif contributes to the expression of the retinoic acid-regulated zinc finger gene Rex-1 (Zfp-42) in F9 teratocarcinoma cells. Mol Cell Biol. 1993;13:2919–2928. doi: 10.1128/mcb.13.5.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, Jelinsky SA. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc Natl Acad Sci U S A. 2008;105:8315–8320. doi: 10.1073/pnas.0709854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL. Cell-cycle regulation and mammalian gametogenesis: a lesson from the unexpected. Mol Reprod Dev. 2006;73:939–942. doi: 10.1002/mrd.20536. [DOI] [PubMed] [Google Scholar]
- Kim JD, Faulk C, Kim J. Retroposition and evolution of the DNA-binding motifs of YY1, YY2 and REX1. Nucleic Acids Res. 2007;35:3442–3452. doi: 10.1093/nar/gkm235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JD, Kim H, Ekram MB, Yu S, Faulk C, Kim J. Rex1/Zfp42 as an epigenetic regulator for genomic imprinting. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Kalisz M, Dalgaard MD, Audouze K, Larsen ME, Jacobsen GK, Horn T, Brunak S, Skakkebaek NE, Leffers H. OCT4 and downstream factors are expressed in human somatic urogenital epithelia and in culture of epididymal spheres. Mol Hum Reprod. 2010 doi: 10.1093/molehr/gaq008. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Skakkebaek NE, Graem N, Jacobsen GK, Rajpert-De Meyts E, Leffers H. Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008;23:775–782. doi: 10.1093/humrep/den010. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lee MY, Lu A, Gudas LJ. Transcriptional regulation of Rex1 (zfp42) in normal prostate epithelial cells and prostate cancer cells. J Cell Physiol. 2010;224:17–27. doi: 10.1002/jcp.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Lu C, Kasik J, Stephan DA, Yang S, Sperling MA, Menon RK. Grtp1, a novel gene regulated by growth hormone. Endocrinology. 2001;142:4568–4571. doi: 10.1210/endo.142.10.8527. [DOI] [PubMed] [Google Scholar]
- Malkov M, Fisher Y, Don J. Developmental schedule of the postnatal rat testis determined by flow cytometry. Biol Reprod. 1998;59:84–92. doi: 10.1095/biolreprod59.1.84. [DOI] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Masui S, Ohtsuka S, Yagi R, Takahashi K, Ko MS, Niwa H. Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev Biol. 2008;8:45. doi: 10.1186/1471-213X-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Martin KM, Gudas LJ. The putative human stem cell marker, Rex-1 (Zfp42): structural classification and expression in normal human epithelial and carcinoma cell cultures. Mol Carcinog. 2006;45:887–900. doi: 10.1002/mc.20186. [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, Griswold MD, Lee JT. Postmeiotic sex chromatin in the male germline of mice. Curr Biol. 2006;16:660–667. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Navarro P, Oldfield A, Legoupi J, Festuccia N, Dubois A, Attia M, Schoorlemmer J, Rougeulle C, Chambers I, Avner P. Molecular coupling of Tsix regulation and pluripotency. Nature. 2010;468:457–460. doi: 10.1038/nature09496. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: data from mutant and genetically modified mice. Mol Cell Endocrinol. 2009;306:2–8. doi: 10.1016/j.mce.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Raman JD, Mongan NP, Liu L, Tickoo SK, Nanus DM, Scherr DS, Gudas LJ. Decreased expression of the human stem cell marker, Rex-1 (zfp-42), in renal cell carcinoma. Carcinogenesis. 2006;27:499–507. doi: 10.1093/carcin/bgi299. [DOI] [PubMed] [Google Scholar]
- Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96:2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LR, Salvadori DM, Pereira CA, Becak W. Activity of ethylene oxide in the mouse sperm morphology test. Arch Toxicol. 1987;60:331–333. doi: 10.1007/BF01234675. [DOI] [PubMed] [Google Scholar]
- Rogers MB, Hosler BA, Gudas LJ. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- Rosfjord E, Rizzino A. The octamer motif present in the Rex-1 promoter binds Oct-1 and Oct-3 expressed by EC cells and ES cells. Biochem Biophys Res Commun. 1994;203:1795–1802. doi: 10.1006/bbrc.1994.2395. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–131. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Turner JM, Ruggiu M, Taggart M, Burgoyne PS, Elliott D, Cooke HJ. Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction. 2003;126:589–597. doi: 10.1530/rep.0.1260589. [DOI] [PubMed] [Google Scholar]
- Scotland KB, Chen S, Sylvester R, Gudas LJ. Analysis of Rex1 (zfp42) function in embryonic stem cell differentiation. Dev Dyn. 2009;238:1863–1877. doi: 10.1002/dvdy.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Wang H, Pan G, Geng Y, Guo Y, Pei D. Regulation of the pluripotency marker Rex-1 by Nanog and Sox2. J Biol Chem. 2006;281:23319–23325. doi: 10.1074/jbc.M601811200. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Gudas LJ. Retinoic acid induces parietal endoderm but not primitive endoderm and visceral endoderm differentiation in F9 teratocarcinoma stem cells with a targeted deletion of the Rex-1 (Zfp-42) gene. Mol Cell Endocrinol. 2002;195:119–133. doi: 10.1016/s0303-7207(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- Wang G, Badylak SF, Heber-Katz E, Braunhut SJ, Gudas LJ. The effects of DNA methyltransferase inhibitors and histone deacetylase inhibitors on digit regeneration in mice. Regen Med. 2010;5:201–220. doi: 10.2217/rme.09.91. [DOI] [PubMed] [Google Scholar]
- Wu S, Hu YC, Liu H, Shi Y. Loss of YY1 impacts the heterochromatic state and meiotic double-strand breaks during mouse spermatogenesis. Mol Cell Biol. 2009;29:6245–6256. doi: 10.1128/MCB.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sylvester R, Tighe AP, Chen S, Gudas LJ. Transcriptional activation of the suppressor of cytokine signaling-3 (SOCS-3) gene via STAT3 is increased in F9 REX1 (ZFP-42) knockout teratocarcinoma stem cells relative to wild-type cells. J Mol Biol. 2008;377:28–46. doi: 10.1016/j.jmb.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- Yu Z, Dadgar N, Albertelli M, Scheller A, Albin RL, Robins DM, Lieberman AP. Abnormalities of germ cell maturation and sertoli cell cytoskeleton in androgen receptor 113 CAG knock-in mice reveal toxic effects of the mutant protein. Am J Pathol. 2006;168:195–204. doi: 10.2353/ajpath.2006.050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ebata KT, Robaire B, Nagano MC. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74:119–124. doi: 10.1095/biolreprod.105.045591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


