Abstract
Focal cortical dysplasia is associated with the development of seizures in children and is present in up to 40% of intractable childhood epilepsies. Transcortical freeze lesions in newborn rats reproduce many of the anatomical and physiological characteristics of human cortical dysplasia. Rats with freeze lesions have increased seizure susceptibility and a region of hyperexcitable cortex adjacent to the lesion. Since alterations in hyperpolarization-activated nonspecific cation (HCN) channels are often associated with epilepsy, we used whole cell patch-clamp recording and voltage-sensitive dye imaging to examine alterations in HCN channels and inwardly rectifying hyperpolarization-activated currents (Ih) in cortical dysplasia. (L5) pyramidal neurons in lesioned animals had hyperpolarized resting membrane potentials, increased input resistances and reduced voltage “sag” associated with Ih activation. These differences became nonsignificant after application of the Ih blocker ZD7288. Temporal excitatory postsynaptic potential (EPSP) summation and intrinsic excitability were increased in neurons near the freeze lesion. Using voltage-sensitive dye imaging of neocortical slices, we found that inhibiting Ih with ZD7288 increased the half-width of dye signals. The anticonvulsant lamotrigine produced a significant decrease in spread of activity. The ability of lamotrigine to decrease network activity was reduced in the hyperexcitable cortex near the freeze lesion. These results suggest that Ih serves to constrain network activity in addition to its role in regulating cellular excitability. Reduced Ih may contribute to increased network excitability in cortical dysplasia.
Keywords: HCN channel, epilepsy, Ih, voltage-sensitive dye
focal cortical dysplasia is associated with the development of seizures in children (Krsek et al. 2009) and is present in up to 40% of intractable childhood epilepsies (Leventer et al. 2008). Current antiepileptic drugs are often ineffective in these patients (Mathern et al. 1999), leading to surgical treatment (Sisodiya 2000). Brain slices prepared from human dysplastic cortex display abnormal synaptic connections and increased excitability (Cepeda et al. 2006). Transcortical freeze lesions in the newborn rat (Dvorak and Feit 1977; Dvorak et al. 1978) reproduce many of the anatomical and electrophysiological characteristics of human focal cortical dysplasias (DeFazio and Hablitz 1998; Jacobs et al. 1996, 1999a, 1999b, 1999c; Luhmann and Raabe 1996). Such lesions also increase susceptibility to complex hyperthermic seizures (Scantlebury et al. 2004). Reduced inhibition (Zhu and Roper 2000) and alterations in glutamate receptors (DeFazio and Hablitz 2000) and transporters (Campbell and Hablitz 2008) have been shown to contribute to hyperexcitability in cortical dysplasia, possibly interacting with local changes in connectivity (Jacobs and Prince 2005) to further increase excitability. Although abnormalities in several voltage-dependent currents have been implicated in epilepsy (Avanzini et al. 2007; Becker et al. 2008; Catterall et al. 2008), changes in intrinsic excitability in cortical dysplasia have not been extensively investigated.
Hyperpolarization-activated, nonselective cation (HCN) channels are encoded by four mammalian genes, termed HCN1–4. Distinct patterns of activation and inactivation and varying sensitivities to cyclic nucleotides are displayed by each subunit (Santoro et al. 2000; Wainger et al. 2001). Depending on the cell type and brain region, the inwardly rectifying hyperpolarization-activated current Ih contributes to generation of rhythmic activity (McCormick and Pape 1990), determination of the resting membrane potential (Robinson and Siegelbaum 2003), and synaptic integration (Magee 2000; Berger et al. 2001). Alterations in Ih and HCN expression occur in a variety of seizure models including kainic acid- and pilocarpine-induced epilepsy (Jung et al. 2007; Shin et al. 2008), early-life hyperthermia (Chen et al. 2001), temporal lobe kindling (Powell et al. 2008), and absence seizures (Kole et al. 2007; Schridde et al. 2006; Strauss et al. 2004). HCN1 subunit-specific knockout mice have a reduced seizure threshold (Huang et al. 2009), whereas HCN2 knockout mice exhibit an absence epilepsy phenotype (Ludwig et al. 2003). Paradoxically, hyperexcitability has been associated with both up- and downregulation of HCN channels (reviewed by Dyhrfjeld-Johnsen et al. 2009). Modifications in Ih have not been examined in cortical dysplasia.
Despite the relatively well-characterized role of Ih in cellular excitability, its contribution to network activity is not well understood. Maturation of rhythmic slow-wave sleep activity patterns is dependent on the density and the properties of Ih during development (Kanyshkova et al. 2009). Working memory networks are strengthened by inhibition of HCN channel signaling in prefrontal cortex (Wang et al. 2007). Theta activity in hippocampus (Hu et al. 2002; Marcelin et al. 2009; Xu et al. 2004) and subthreshold oscillations in entorhinal cortex (Dickson et al. 2000) are disrupted by Ih blockers. The timing of interictal bursts in the neonatal rat hippocampus is positively modulated by Ih (Agmon and Wells 2003). The contribution of Ih to network hyperexcitability in cortical dysplasia has not been established. In the present study, we have used whole cell patch-clamp recordings and voltage-sensitive dye imaging to determine the effect of HCN channel alterations on intrinsic excitability of individual cells and activity in local circuits. Results suggest that Ih contributes significantly to the normal pattern of spread of activity across the cortical mantle. Decreases in Ih in cortical dysplasia augment network excitability, possibly contributing to the hyperexcitability seen in malformed cortex.
METHODS
Animals.
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Every effort was made to minimize pain and discomfort. Focal freeze lesions were induced in postnatal day (PN)1 Sprague-Dawley rats. In brief, newborn rat pups were anesthetized by hypothermia, and a small incision was made to expose the skull. A 2-mm copper rod cooled to approximately −50°C was placed on the surface of the skull for 3 s. Sham-operated animals received similar treatment without cooling of the probe. After the scalp was sutured, the animals were warmed and returned to their home cage. Rats were allowed to recover for 21–27 days before recordings were made.
Preparation of in vitro brain slices.
Rats were anesthetized and decapitated. The brain was quickly removed and placed in ice-cold cutting solution consisting of (in mM) 135 N-methyl-d-glucamine, 1.5 KCl, 1.5 KH2PO4, 23 choline HCO3, 0.4 ascorbic acid, 0.5 CaCl2, 3.5 MgCl2, and 25 d-glucose (Tanaka et al. 2008). The solution was bubbled with 95% O2-5% CO2 to maintain a pH around 7.4. Coronal brain slices (300 μm thick) were cut with a vibratome (Microm, Walldorf, Germany). Slices were obtained from an area of somatosensory neocortex containing the microgyrus in freeze-lesioned animals and a corresponding location in sham-operated control animals. The slices were stored for 40–60 min at 37°C in oxygenated recording solution containing (in mM) 124 NaCl, 2.5 KCl, 10 d-glucose, 26 NaHCO3, 2.0 Ca2+, and 2.0 Mg2+ and then kept at room temperature. For recording, individual slices were transferred to a recording chamber and continuously perfused (4 ml/min) with oxygenated recording solution.
Whole cell recording.
A Zeiss Axioskop FS (Carl Zeiss, Thornwood, NY) microscope, equipped with Nomarski optics, a ×40 water immersion lens, and infrared illumination, was used to view neurons in the slices. L5 pyramidal neurons were identified by their pyramidal shape and size, presence of a prominent apical dendrite, distance from the pial surface, and spiking properties. In addition, cells were intracellularly labeled with biocytin to confirm identification. Labeled cells were processed as described previously (Zhou and Hablitz 1996).
Whole cell recordings were obtained from visually identified L5 pyramidal neurons. Signals were acquired with a MultiClamp 700A amplifier (Molecular Devices, Sunnyvale, CA) controlled by Clampex 8.0 software via a Digidata 1322A interface (Molecular Devices). Responses were filtered at 5 kHz, digitized at 10–20 kHz, and analyzed off-line with Clampfit 8.0 software. Tight seals (>2 GΩ before breaking into whole cell mode) were obtained with patch electrodes that had an open tip resistance of ∼3 MΩ. Series resistance during recording varied from 9 to 20 MΩ. Under voltage-clamp conditions, series resistance was compensated 50–70% and continually monitored throughout the experiment. Recordings were terminated whenever significant increases (>20%) in series resistance occurred. In current-clamp recordings, the Bridge Balance control of the MultiClamp amplifier was used to compensate for the voltage drop across the electrode. All current-clamp records were visually checked for proper compensation during analysis. The intracellular solution for recording contained (in mM) 125 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 0.5 EGTA. pH and osmolarity were adjusted to 7.3 and 290 mosM, respectively. Bicuculline methiodide (10 μM; Sigma-Aldrich, St. Louis, MO) was present during all whole cell recording experiments in order to block GABAA receptors. Synaptic responses were evoked with a bipolar stimulating electrode (twisted pair of 25-μm Formvar-insulated nichrome wires) positioned 150–200 μm above the recording pipette. Stimuli were current pulses 50–200 μA in amplitude and 50 μs in duration. A stimulation frequency of 0.05 Hz was used. All traces of synaptic currents shown are the average of 10 consecutive responses. Recordings were done at 32 ± 1°C.
Data are expressed as means ± SE. Statistical analysis of response amplitudes from control and freeze-lesioned animals was carried out with two-tailed Student's t-test or one-way ANOVA. P < 0.05 was considered significant.
Voltage-sensitive dye imaging.
Imaging experiments were conducted with the voltage-sensitive fluorescent dye N-[3-(triethylammonium)propyl]-4-[4-(p-diethylaminophenyl)butadienyl]pyridinium dibromide (RH 414). Individual slices were stained with 30 μM RH 414 for at least 60 min at room temperature and then placed in the recording chamber on the stage of the microscope (Axiovert 135TV, Zeiss) used for optical recording. Slices were continuously perfused with recording saline at a rate of 4 ml/min for at least 30 min before recording in order to wash out excess dye. A bipolar stimulating electrode was positioned intracortically in middle cortical layers. Activity was evoked with single shocks 40–100 μA in amplitude and 190 μs in duration. A hexagonal photodiode array containing 464 diodes (Neuroplex, Red Shirt Imaging, Fairfield, CT) was used to detect activity-dependent changes in fluorescence. Excitation of the dye was achieved with a stabilized power supply (Hewlett-Packard, Palo Alto, CA), a 100-W halogen lamp, and a 535 ± 40-nm filter. The emitted light passed through a 590-nm long-pass filter. Optical signals were amplified and stored on a computer for later analysis. The resting light intensity measured for each diode was used to normalize fluorescent measurements. Correction for dye bleaching was done by using measurements taken in the absence of stimulation. All optical signals are represented as changes in fluorescence with stimulation divided by resting fluorescence (ΔF/F, where F is the fluorescence measured in the absence of stimulation and ΔF is the change in fluorescence following stimulation). Responses to three stimulations were averaged. RH 414 responds to membrane depolarization with a decrease in fluorescence. This is plotted as an upward deflection in all figures. Using fixed scaling for individual figures, pseudocolor images were generated to visualize spatiotemporal patterns of activity in the slice. A digital image of the slice in the recording chamber was taken with a CCD camera attached to a dissecting microscope in order to document the position of the photodiode array with respect to cortical layers.
Data analysis.
For analysis of changes in amplitude and duration of dye signals, a region of interest containing 18 diodes showing significant dye signals before drug application was selected. The peak amplitudes and half-widths of these responses were compared before and during drug administration. To examine changes in spread of activity, the number of diodes showing peak signal amplitudes three times the baseline noise levels was determined. The baseline noise level was determined from 10 diodes that exhibited no obvious activity. A two-way ANOVA was used for statistical comparison, with differences being considered significant if P < 0.05. Data are expressed as means ± SE.
Drugs.
Drugs were stored in frozen stock solution and dissolved in the recording solution prior to each experiment. After recording control responses, drugs were bath applied for 20 min. Lamotrigine and ZD7288 were obtained from Tocris Bioscience (Ellisville, MO).
RESULTS
Membrane properties of L5 pyramidal neurons.
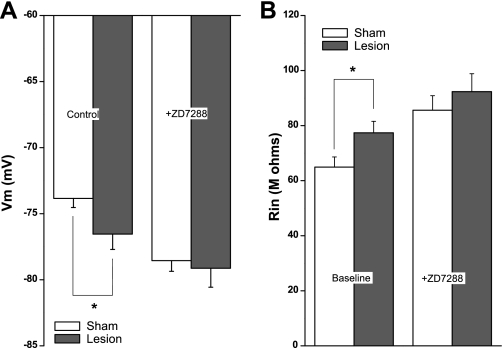
To observe the impact of Ih changes on L5 pyramidal neurons, we obtained somatic whole cell patch-clamp recordings from 22- to 28-day-old sham-operated and lesioned animals. Recordings in lesioned animals were obtained 1–2 mm lateral to the lesion. Consistent with a reduction in the expression level of Ih, the somatic resting membrane potential (Vm) in L5 pyramidal neurons was more hyperpolarized in slices from lesioned (−76.5 ± 1.0 mV, n = 24) than sham-operated (−73.8 ± 0.9 mV, n = 26; P < 0.05) animals (Fig. 1A, left). Furthermore, L5 pyramidal neurons in slices from lesioned animals had a significantly larger somatic input resistance (Rin) (lesioned: 77.4 ± 4.2 MΩ, n = 24; sham-operated: 64.9 ± 3.7 MΩ, n = 26; P < 0.05) (Fig. 1B, left). These differences in membrane properties were no longer significant after bath application of the Ih channel blocker ZD7288 (10 μM) (Vm: lesioned −79.1 ± 1.4 mV; sham operated −78.5 ± 0.81 mV; Rin: lesioned 92.3 ± 6.5 MΩ; sham operated 85.6 ± 5.3 MΩ), suggesting that the initial differences arose from alterations in Ih expression (Fig. 1, A and B, right).
Fig. 1.
L5 pyramidal neurons from freeze-lesioned rats have depolarized membrane potentials (Vm) and increased input resistances (Rin). A: the resting Vm of pyramidal neurons near the freeze lesion is significantly hyperpolarized compared with sham-operated control animals. This difference is not significant after hyperpolarization-activated nonspecific cation (HCN) channel inhibition with ZD7288. B: the Rin of pyramidal neurons near the freeze lesion is significantly higher than that of sham-operated control animals. This difference is not significant after HCN channel inhibition. *P < 0.05.
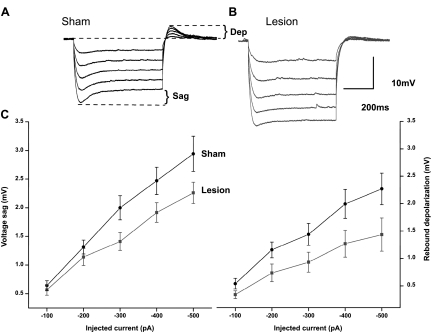
When neurons exhibit a prominent Ih, hyperpolarizing current pulses evoke a voltage response that reaches a peak and then “sags” back toward rest (Berger et al. 2001; Maccaferri et al. 1993; Sutor and Hablitz 1993). Figure 2A shows responses to a series of hyperpolarizing current pulses in an L5 neuron from a sham-operated animal. Sag responses were prominent. When the same currents were applied to a neuron from a lesioned animal, sag responses were reduced (Fig. 2B), indicating a decreased Ih. For example, when a current pulse of −400 pA was employed, the sag response was 2.26 ± 0.3 mV (n = 9) in control animals and 1.91 ± 0.2 mV (n = 9) in lesioned animals. These differences were statistically significant (P < 0.05, 1-way ANOVA). At the end of the current pulse, the smaller deactivating Ih in cells from lesioned animals led to a smaller rebound depolarization. With a −400 pA current pulse, rebound amplitudes were 1.99 ± 0.2 and 1.26 ± 0.2 pA in control and lesioned animals, respectively. The differences between control and lesioned animals were significant (P < 0.05, 1-way ANOVA). A summary plot of the changes in responses to hyperpolarizing current pulses is shown in Fig. 2C. It can be seen that that sag responses (Fig. 2C, left) and rebound depolarizations (right) were significantly reduced in neurons from lesioned animals.
Fig. 2.
Reduction in hyperpolarization-activated current Ih-dependent voltage changes in L5 pyramidal neurons from lesioned animals. A: specimen records showing that membrane hyperpolarization in sham-operated animals is associated with a depolarizing “sag” in membrane voltage caused by Ih activation. Rebound depolarizations (Dep) are also seen. B: superimposed specimen records showing that sag responses are reduced in a pyramidal neuron near the freeze lesion. Rebound depolarizations upon current offset are also reduced. C: summary graphs showing a significant reduction in the amplitude in the voltage sag (left) and rebound depolarization (right) in pyramidal neurons from freeze-lesioned animals.
Intrinsic excitability changes in L5 pyramidal neurons.
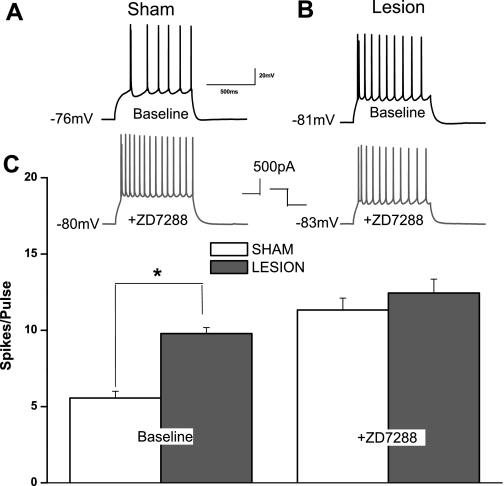
The changes described above, a more hyperpolarized Vm and an increased Rin, make it difficult to predict the net effect on intrinsic excitability of L5 neurons (Dyhrfjeld-Johnsen et al. 2009). We therefore examined the relationship between somatic current injection and AP firing in neurons from sham-operated and lesioned animals. At least 5 min after a whole cell recording was obtained, cells were stimulated, at their resting potential, with 500-pA depolarizing current pulses. Figure 3A, top left, shows a typical response in a neuron from a sham-operated animal. The depolarizing current pulse evoked a train of action potentials. In L5 pyramidal neurons from lesioned animals (Fig. 3B, top right), the number of action potentials was significantly higher than in sham-operated animals (lesioned: 9.8 ± 0.4 spikes/pulse, n = 9; sham operated: 5.6 ± 0.4 spikes/pulse, n = 9; P < 0.05) despite the fact that Vm was more hyperpolarized. When 10 μm ZD7288 was bath applied, the number of action potentials in the sham-operated neuron was markedly increased, whereas the cell from the lesioned animal showed a smaller increase. A summary plot of the results from a group of cells is shown in Fig. 3C. It can be seen that neurons from lesioned animals are more excitable under baseline conditions. This difference was no longer significant in the presence of ZD7288 (lesioned 12.4 ± 0.9 spikes/pulse; sham operated 11.3 ± 0.8 spikes/pulse, n = 9; P > 0.05). These results suggest that decreased Ih in neurons from lesioned animals results in increased intrinsic excitability of L5 pyramidal cells.
Fig. 3.
L5 pyramidal neurons from freeze-lesioned rats have increased intrinsic excitability. A: recordings showing a somatically evoked train of action potentials in a neuron from a sham-operated animal (top). In the same cell during bath application of ZD7288, Vm is hyperpolarized and the number of action potentials is increased (bottom). B: records obtained from a pyramidal neuron near a lesion. The same current injection resulted in a greater number of spikes under control conditions (top). After ZD7288, Vm and number of evoked action potentials are virtually unchanged. C: summary graphs showing difference in number of action potentials (APs) between sham-operated and lesioned animals before (left) and during (right) ZD7288. The difference in AP number is not significant after Ih inhibition. *P < 0.05.
Voltage-clamp analysis of Ih.
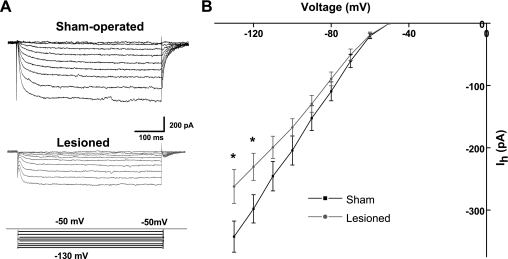
Somatic voltage-clamp recordings were performed to examine Ih currents. Cells were held at −50 mV in the presence of TTX (1 μM). Voltage steps 500 ms in duration were given from −50 to −130 mV in 10-mV increments to activate Ih currents. ZD7288 (10 μM) was then bath applied to block HCN channels. Currents evoked after a 10-min perfusion with ZD7288 were subtracted from control to obtain the ZD7288-sensitive current. Specimen records of ZD7288-sensitive currents from a sham-operated animal are shown in Fig. 4A. The ZD7288-sensitive currents recorded in a neuron from a lesioned animal were significantly smaller in amplitude (Fig. 4B). Currents began to activate around −60 mV. When the membrane potential was held at −120 and −130 mV, Ih currents showed a significant decrease in lesioned compared with sham-operated animals. [holding potential (Vh) = −120 mV: sham operated −298.1 ± 23 pA (n = 9), lesioned −230.6 ± 22 pA (n = 9), P < 0.05; Vh = −130 mV: sham operated −342.6 ± 25 pA, lesioned −262.1 ± 27.2 pA, P < 0.05]. However, because of space-clamp errors, which result in the incomplete control of dendritic membrane potential, it is likely that these somatic voltage-clamp data underestimated the HCN channel conductance, in particular, at more hyperpolarized potentials. The higher Rin in lesioned animals is expected to reduce this potential confound. Currents evoked at −130 mV were fitted to single exponential functions to determine activation time constants. There were no significant differences between sham-operated and lesioned groups [sham operated: 24.7 ± 4 ms (n = 9), lesioned 29.6 ± 4 ms (n = 9); P > 0.05]. This value is in the range for Ih in thalamic neurons (Santoro et al. 2000), hippocampal interneurons (Santoro et al. 2000), and neocortical pyramidal cells (Williams and Stuart 2000) and is consistent with mediation by HCN1–HCN2 subunits.
Fig. 4.
Voltage-clamp recordings of Ih in neurons from sham-operated and lesioned animals. A, top: ZD7288-sensitive somatic Ih currents obtained by subtracting obtained before and after bath application of ZD7288 in a L5 pyramidal neurons from a sham-operated animal. Slowly activating Ih currents are observed. Bottom: recordings from a neuron near the freeze lesion revealed a significant decrease in Ih amplitude following membrane hyperpolarization. B: summary diagram showing current-voltage plots for a group of neurons in sham-operated and lesioned animals. *P < 0.05.
Alterations in excitatory postsynaptic potential temporal summation.
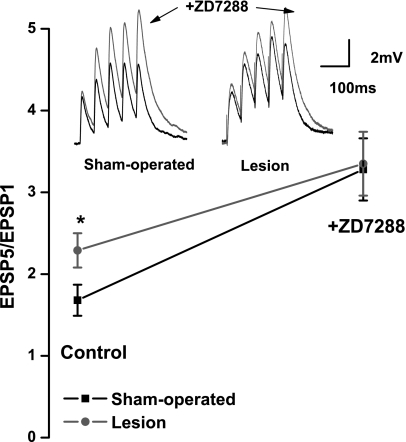
During a train of evoked excitatory postsynaptic potentials (EPSPs) in L5 pyramidal neurons, summation is reduced or prevented by the presence of Ih (Berger et al. 2001). To determine whether the observed Ih decreases in lesioned animals altered synaptic integration, distal EPSPs were evoked by a bipolar stimulating electrode positioned 150–200 μm above the recording pipette. A train of five stimuli at 20 Hz was used to evoke EPSPs in L5 neurons. As shown in Fig. 5, sublinear temporal summation was observed in neurons from both sham-operated and lesioned animals under control conditions. When the ratio of the amplitude of the fifth to the first EPSP in the train (EPSP5/EPSP1) was calculated, a significantly increased ratio was observed in the lesioned group [sham operated 1.7 ± 0.2 (n = 26), lesioned 2.3 ± 0.2 (n = 24); P < 0.05], indicative of an increased summation in the latter group due to a decreased Ih.
Fig. 5.
Effects of ZD7288 on excitatory postsynaptic potential (EPSP) summation in sham-operated and lesioned animals. Top left: specimen records of EPSPs evoked by a train of stimuli at 20 Hz. In a slice from a sham-operated animal, under control conditions EPSPs show weak facilitation. After ZD7288 amplitudes of EPSPs in this neuron were increased. Top right: similar experiment in a neuron from a slice from a lesioned animal. EPSPs evoked at 20 Hz summated to a significantly greater degree in pyramidal neurons from freeze-lesioned rats. EPSPs showed increased facilitation in presence of ZD7288. Bottom: graph of EPSP5-to-EPSP1 ratios shows that under control conditions ratios were significantly higher in sham-operated group. This difference was not significant after ZD7288. *P < 0.05.
The effect of Ih blockade on synaptic activation was further examined with the use of ZD7288 (10 μM). In the presence of the Ih channel blocker, temporal summation during the EPSP train was significantly increased in both sham-operated and lesioned groups. However, in the presence of ZD7288, the groups were no longer statistically different from each other (sham operated 3.28 ± 0.4, lesioned 3.35 ± 0.4 mV; P > 0.05). These results suggest that dendritic Ih is reduced but not abolished in the lesioned animals.
Spatiotemporal spread of activity in dysplastic cortex.
Multielectrode field potential recordings of paroxysmal discharges in freeze-lesioned cortex have demonstrated propagation over long distances in the horizontal direction (Jacobs et al. 1996; Luhmann and Raabe 1996). Voltage-sensitive dye studies of evoked activity in normal neocortex have shown that the time courses of dye signals are similar to those of locally recorded field potentials. Dye signal responses peak rapidly (Yuste et al. 1997) and spread horizontally over relatively short distances (Langenstroth et al. 1996). Using voltage-sensitive dye imaging, we have shown that spread of activity in lesioned animals was greater in upper cortical layers in the paramicrogyral area relative to sham-operated control animals (Bandyopadhyay and Hablitz 2006). More persistent activation of local cortical circuits was also seen in dysplastic cortex. Experiments described below examine the role of Ih in regulating spread of activity in dysplastic neocortex.
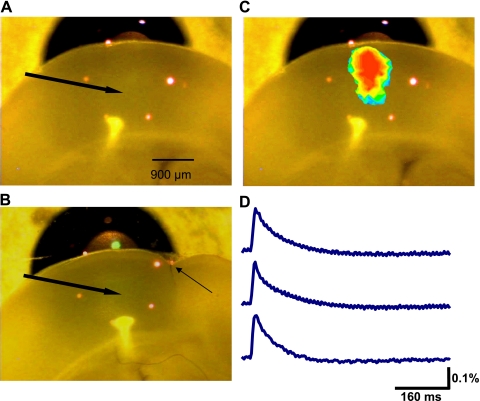
The voltage-sensitive dye RH 414 and optical imaging were used to quantify how alterations in Ih modify spatiotemporal patterns of activity. The hexagonal photodiode array used for this purpose covered an area of ∼1.8 × 1.8 mm of the slice at the magnification (×10) used. Figure 6A shows the typical positioning of the photodiode array over the neocortex. The arrow indicates the location of the stimulating electrode. Figure 6B shows the typical position of the array over slices from lesioned animals. The small arrow shows the location of the microgyrus. Four stimulus intensities were tested in each slice (40, 60, 80, and 100 μA). A typical pseudocolored voltage-sensitive dye response is shown superimposed on an image of the cortex in Fig. 6C. Examples of individual diode responses from a slice from a sham-operated animal are shown in Fig. 6D. Fluorescence changes had a rapid rising phase and a slower decay (Fig. 6D).
Fig. 6.
Voltage-sensitive dye imaging of evoked activity. A: photograph showing the typical position of the brain slice over the diode array. The red dots indicate the borders of the hexagonal photodiode array. The array was positioned so that the upper limit was approximately in line with the pial surface. The arrow indicates the approximate position of the stimulating electrode. B: similar picture showing the typical position of the array relative to the freeze lesion. Small arrow indicates location of lesion. C: a pseudocolored image of peak activity is shown superimposed on the image of a slice. D: typical responses from selected individual diodes showing time course of fluorescence change.
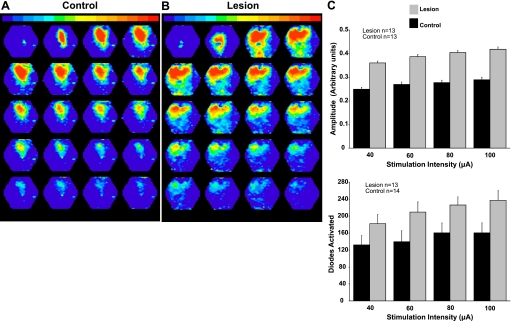
Stimulation in slices from sham-operated control animals evoked responses near the site of stimulation with subsequent vertical and horizontal spread. Figure 7A is a montage of 20 pseudocolor maps showing the spatial distribution of dye signals (ΔF/F) at given points in time. The first panel displays activity 2–5 ms after stimulation, and additional panels are shown at 3-ms intervals. Warm colors represent larger-amplitude dye signals, i.e., high levels of activity. The pial surface is up in each panel. Activity first spread to more superficial layers and then laterally. When the same stimulation intensity was used in a slice from a lesioned animal, activity rapidly spread across large portions of the superficial layers and was more persistent (Fig. 7B), as described previously (Bandyopadhyay and Hablitz 2006). To quantify these results, the average peak amplitude from selected diodes (see methods) and the number of diodes activated (indicative of activity spread) were determined. Peak amplitudes and the number of diodes activated were significantly increased at all stimulus intensities in slices from lesioned animals (Fig. 7C; P < 0.05, 2-way ANOVA).
Fig. 7.
Comparison of voltage-sensitive dye signals in control and lesioned animals. A: specimen record of a typical network response evoked from control cortex. B: a typical network response evoked in the hyperexcitable region adjacent to the freeze lesion. Evoked activity near the malformation in freeze-lesioned rats spreads further and is of higher amplitude. C: summary diagrams showing differences in response amplitude (top) and number of diodes activated (bottom) in lesioned vs. control animals.
Ih and spread of activity.
Ih has significant effects on dendritic excitability and attenuation of EPSPs in L5 pyramidal cells (Berger et al. 2001; Day et al. 2005; Williams and Stuart 2000). Blockade of Ih results in enhanced temporal summation (Berger et al. 2001) and increased dendritic calcium action potential generation (Tsay et al. 2007). Given these changes, it was reasoned that Ih blockade should result in enhanced spatiotemporal spread of activity.
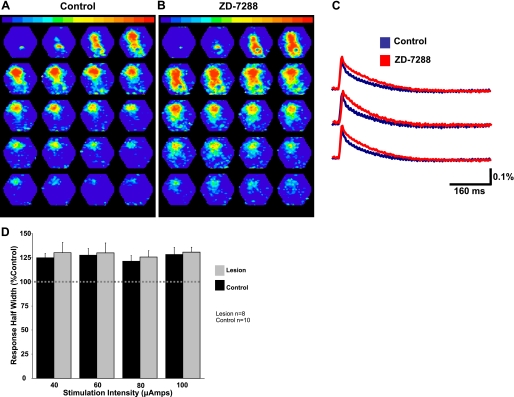
The spatiotemporal distribution of evoked activity from a sham-operated animal under control conditions is shown in Fig. 8A. The first panel shows activity ∼2–5 ms after stimulation. Subsequent panels show responses at 3-ms intervals. The control images acquired after intracortical stimulation show an area of activity that appears first near the stimulating electrode and was generally constrained to a columnar shape. Responses following application of the HCN channel blocker ZD7288 (10 μM) are shown in Fig. 8B. Activity was seen to persist longer in the presence of ZD7288. Pseudocolor scaling was the same for all conditions. Superimposed dye signals from three different diodes under control conditions and in the presence of ZD7288 are shown in Fig. 8C. Response half-widths were significantly increased when the Ih blocker was present at all stimulation intensities (Fig. 8D). We did not observe a significant difference in the effect of ZD7288 on response half-width between lesioned and control animals (P > 0.05, 2-way ANOVA). We also observed a slight decrease in response amplitude following 20 min of ZD7288 that did not differ between lesioned and control animals (data not shown). ZD7288 did not significantly change the number of diodes activated (indicating spread of activity) in either group.
Fig. 8.
HCN channel inhibition increases the duration of evoked network activity. A: typical network response evoked before HCN channel inhibition in a control animal. B: the same response after HCN channel inhibition with 10 μM ZD7288. C: responses from individual diodes before (blue) and after (red) HCN channel inhibition are shown superimposed. HCN channel inhibition increased the half-width of these responses. D: bar graphs showing that Ih inhibition increases the duration of evoked activity in both control and lesioned animals.
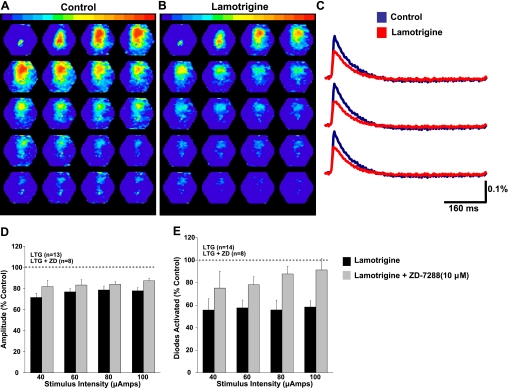
Anticonvulsant drugs such as lamotrigine (Peng et al. 2010; Poolos et al. 2002) and gabapentin (Surges et al. 2003) have been shown to enhance Ih. The effect of enhancing Ih on network behavior has received little attention. We therefore tested the effect of bath application of lamotrigine (100 μM) on spatiotemporal spread of activity in neocortical slices. A montage of 20 pseudocolored maps under control conditions is shown in Fig. 9A. Activity 2–5 ms after stimulation is shown in the first panel. Panels are subsequently shown at 2.5-ms intervals. Figure 9B shows responses to the same stimulation 20 min after bath application of lamotrigine (100 μM). Lamotrigine altered the spatiotemporal pattern of evoked neocortical activity. When individual responses before and after lamotrigine were superimposed, a decrease in amplitude was observed (Fig. 9, C and D). Lamotrigine also decreased the number of diodes activated (indicating spread of activity) (Fig. 9E). Additionally, we observed a small, but significant, decrease in diode half-width after lamotrigine (data not shown). This is in contrast to the increase observed after ZD7288.
Fig. 9.
Effects of the anticonvulsant lamotrigine on evoked network activity. A: typical network response evoked in a control animal before lamotrigine. B: response to the same stimulation 20 min after application of lamotrigine. C: responses from individual diodes before (blue) and after (red) lamotrigine. D: lamotrigine (LTG) reduced the amplitude of diode responses in control in control animals. This effect was significantly attenuated by coapplication of ZD7288 (ZD). E: lamotrigine reduced the number of diodes activated (indicating spread of activity) in control animals. This effect was also significantly attenuated by coapplication of ZD7288.
Lamotrigine is known to have effects on ion channels other than Ih (Thompson et al. 2011). We therefore tested the effect of lamotrigine when applied in the presence of ZD7288. Figure 9, D and E, show that lamotrigine had a significantly reduced effect on the amplitude of voltage-sensitive dye signals and number of diodes activated, respectively, in the presence of ZD7288 (P < 0.05, 2-way ANOVA). This suggests that a significant portion of lamotrigine's effect on network activity is mediated via an action on Ih.
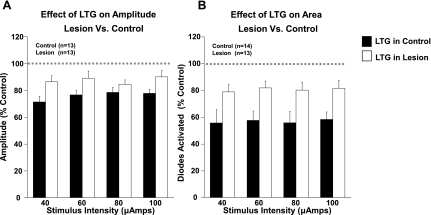
The effects of lamotrigine on response amplitude in slices from sham-operated and lesioned animals are summarized in Fig. 10. In both groups, bath application of lamotrigine produced a significant reduction in amplitude at all intensities (P < 0.05, 2-way ANOVA) (Fig. 10A). The effect of lamotrigine on response amplitude was significantly decreased in lesioned compared with control animals (P < 0.05, 2-way ANOVA). The effect of lamotrigine on the number of diodes activated is shown in Fig. 10B. A significant decrease in the number of diodes reaching threshold levels of activation was also observed in both groups. Again, this effect was significantly reduced in the lesion group (P < 0.05, 2-way ANOVA). A decrease was observed in the ability of lamotrigine to reduce half-width in lesioned animals compared with control animals.
Fig. 10.
Sensitivity of network activity to lamotrigine. A: the ability of lamotrigine (LTG) to decrease voltage-sensitive dye signal amplitude was significantly reduced in lesioned animals (open bars). B: the ability of lamotrigine to reduce the spread of the voltage-sensitive dye signal was significantly reduced in lesioned animals (open bars).
We also examined the ability of lamotrigine to alter the Ih-dependent voltage sag and rebound (as seen in Fig. 2) in control and lesioned animals. Lamotrigine significantly (P < 0.05, 2-way ANOVA) increased both the voltage sag (−250 pA injection, 1.48 ± 0.82 mV increase, P < 0.05) and rebound depolarization (−250 pA injection, 1.31 ± 0.86 mV increase, n = 7; P < 0.05) in neurons from control animals. Lamotrigine did not have a significant effect on Ih-dependent voltage sag or rebound in neurons in dysplastic cortex (sag: −250 pA current injection, −0.29 ± 0.6 mV change, P < 0.05; rebound: −0.27 ± 0.8 mV change, n = 7, P < 0.05). The lack of a significant effect of lamotrigine on sag and rebound in lesioned animals further suggests that animals with freeze lesions have reduced Ih. This reduction may contribute to the reduced effectiveness of lamotrigine in constraining network activity in lesioned animals.
DISCUSSION
In the present study, we used whole cell patch-clamp recording and voltage-sensitive dye imaging to examine alterations in HCN channels and Ih in the rat freeze-lesion model of cortical dysplasia. L5 pyramidal neurons in lesioned animals demonstrated hyperpolarized resting membrane potentials, increased Rin, and a reduction in the voltage “sag” associated with Ih activation. Temporal EPSP summation and intrinsic excitability were increased in neurons near the freeze lesion. These differences became nonsignificant after application of the Ih blocker ZD7288. Furthermore, we demonstrated a role for Ih in constraining network activity, finding that this effect was reduced in dysplastic cortex.
Ih changes in epilepsy.
Alterations in Ih have been described in several animal models of epilepsy. A progressive, persistent downregulation of dendritic HCN channels is seen in the rat pilocarpine model of epilepsy (Jung et al. 2007). Rats with pilocarpine-induced epilepsy exhibit increases in input resistance and dendritic excitability. A reduction in Ih and increased dendritic EPSP summation also have been observed after status epilepticus induced by kainic acid (Shin et al. 2008). Similarly, the spontaneously epileptic WAG/Rij rat exhibits reduced Ih associated with increased Rin and enhanced synaptic summation (Kole et al. 2007; Strauss et al. 2004). Perinatal seizures induced by hypoxia are also accompanied by a downregulation of Ih (Zhang et al. 2006). The present study indicates that reductions in Ih associated with increases in cellular excitability and enhanced EPSP summation are found in a nonchemically induced malformation epilepsy model. This suggests that persistent Ih downregulation associated with increased excitability may be a pervasive finding in many types of epilepsy.
Genetic reduction in HCN channels is strongly associated with epilepsy. HCN2 knockout animals exhibit spontaneous absence-type seizures (Ludwig et al. 2003), whereas HCN1 knockouts have enhanced seizure susceptibility (Huang et al. 2009). Additionally, Apathetic mice, which possess spontaneously truncated HCN2 channels, display an absence epilepsy phenotype (Chung et al. 2009). Whereas our findings suggest a decrease in Ih as one potential mechanism for hyperexcitability in cortical dysplasia, increases in Ih have been reported to produce increased excitability in a febrile seizure model (Chen et al. 2001). Although differential effects on Ih may occur depending on the initial insult, it appears that proper network function can be perturbed by up- or downregulation of HCN channels.
Ih has a well-characterized role in regulating dendritic excitability. Ih activation increases resting membrane conductance, depolarizes the resting membrane potential, and decreases dendritic excitability (Magee 1998; Poolos et al. 2002; Robinson and Siegelbaum 2003). In the present study, L5 pyramidal neurons from lesioned animals have significantly reduced Ih, increased Rin, and hyperpolarized Vm. Despite the membrane hyperpolarization, depolarizing current pulses of the same amplitude elicited more spikes from neurons near the lesion compared with sham-operated controls. This counterintuitive inhibitory effect of Ih on action potential firing in the sham-operated group has previously been attributed to HCN channels active at the resting membrane potential decreasing Rin (Poolos et al. 2002; Robinson and Siegelbaum 2003). In addition to changes in intrinsic excitability, Ih blockade also enhances temporal summation of distal excitatory inputs (Magee 1999; Williams and Stuart 2000). Our observed increase in EPSP summation coupled with enhanced intrinsic excitability may be an underlying mechanism contributing to the hyperexcitability seen in dysplastic cortex. HCN channels are highly expressed in the apical dendrites of L5 pyramidal neurons (Lorincz et al. 2002), where they regulate excitability (Berger et al. 2001). Somatic recordings, like those employed here, do not faithfully reproduce dendritic responses (Williams and Mitchell 2008). Computational modeling studies have shown that somatic measurements underestimate dendritic Ih (Day et al. 2005). Changes observed at the somatic level are nonetheless informative since they can potentially influence neuronal output. Spike initiation in L5 pyramidal neurons occurs in the distal portion of the axon initial segment (Palmer and Stuart 2006). Somatic membrane potential changes resulting from alterations in dendritic Ih could influence action potential generation. Our results indicate that, despite the presumptive dendritic localization of HCN channels in L5 pyramids, Ih modulates Vm, intrinsic excitability, and synaptic responses at the somatic level.
Presynaptic HCN channels have been reported in hippocampus (Aponte et al. 2006; Bender et al. 2007; Notomi and Shigemoto 2004), brain stem (Cuttle et al. 2001), and entorhinal cortex (Huang et al. 2011). Such presynaptic channels have been reported to affect both GABA (Aponte et al. 2006; Southan et al. 2000) and glutamate (Huang et al. 2011) release. If present in neocortex, presynaptic HCN channels would increase the repertoire of mechanisms whereby HCN channels could influence network excitability.
The factors responsible for Ih alterations in cortical dysplasia are unclear. A single seizure episode can decrease Ih (Shah et al. 2004), and long-term downregulation of total Ih has previously been shown to occur independent of repeated seizure activity in the pilocarpine model of epilepsy (Jung et al. 2007). Although spontaneous seizures are not typically seen in the freeze-lesion model, increases in synaptic activity have been observed (Jacobs and Prince 2005) and high-frequency stimulation is known to downregulate Ih in CA1 pyramidal neurons (Campanac et al. 2008). Increased extracellular glutamate levels have been shown to be present in dysplastic cortex (Campbell and Hablitz 2008). In cultured hippocampal neurons, activation of AMPA and NMDA receptors is capable of acutely augmenting HCN1 surface expression while diminishing channel trafficking (Noam et al. 2010). It is currently unclear whether the observed decreases in Ih are activity dependent or result from the initial cortical injury.
Regulation of activity in local circuits by Ih.
Although numerous studies have characterized the role of Ih in regulating the excitability of individual neurons (George et al. 2009; Magee 1998; Rosenkranz and Johnston 2006; Williams and Stuart 2000), the functional outcome on network activity has received less attention. The ability of Ih to constrain synaptic excitability suggests that Ih also could serve to restrict activity across networks of neurons. Using voltage-sensitive dye imaging to quantify cortical circuit organization and dynamics, we have found significant changes in network activity in sham-operated animals following either Ih blockade or enhancement, effects that were altered in dysplastic cortex. As previously reported, intracortical stimulation elicited synchronized, horizontally restricted areas of activity extending from L1 to L5 (Bandyopadhyay and Hablitz 2006; Kubota et al. 1999; Yuste et al. 1997). In the presence of ZD7288, activity persisted significantly longer. A similar increase in half-width has previously been described for distally evoked EPSPs in single cells (Williams and Stuart 2000). It is tempting to hypothesize that the network effect of ZD7288 is simply due to a net increase in the time constant of EPSPs. Enhancement of Ih with the anticonvulsant lamotrigine decreased response half-width, dampened network excitability, and reduced the spatiotemporal spread of activity.
The ability of the anticonvulsant lamotrigine to constrain network activity was significantly reduced in lesioned animals. This suggests that the ability of Ih to constrain network activity in dysplastic cortex was reduced. We did not observe a similar decrease in the ability of ZD7288 to enhance the duration of activity. The hyperexcitability in dysplastic cortex, the low concentration of ZD7288 used, remaining Ih, and variability in epileptiform events may mask subtle alterations in the ability of ZD7288 to enhance the duration of network activity. Although HCN staining is prominent in L5 pyramidal neurons, Ih have been reported in L2/3 pyramidal cells (Strauss et al. 2004; Sutor and Hablitz 1993) and GABAergic neurons (Wu and Hablitz 2005). How Ih properties in these cells are altered in cortical dysplasia has not been established.
The decrease in HCN channel staining (Hablitz and Yang 2010), total Ih, and accompanying voltage sag observed in the freeze-lesioned animals is associated with increases in synaptic integration and intrinsic excitability. These changes are mimicked in sham-operated control animals when Ih is blocked. This includes increased summation, increased spiking following current injection, as well as decreased membrane conductance. We also observed greatly increased network activation following electrical stimulation. Decreased Ih may contribute to the excitability changes observed in cortical dysplasia and malformation epilepsy. Blockade of Ih increased the duration of network activity, whereas enhancement of Ih limited the spread of network activity. The ability of the anticonvulsant lamotrigine to limit network activity was significantly reduced in freeze-lesioned rats. These novel observations lead us to hypothesize that Ih serves to constrain network activity in addition to its role in constraining cellular excitability. Reduced Ih in rats with cortical malformations may contribute to the increased network excitability.
GRANTS
This work was supported by National Institutes of Health Grants NS-22373, P30-NS-47466, P30-HD-38985, and P30-NS-57098.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank K. Alison Margolies for technical assistance.
REFERENCES
- Agmon A, Wells JE. The role of the hyperpolarization-activated cationic current Ih in the timing of interictal bursts in the neonatal hippocampus. J Neurosci 23: 3658–3668, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574: 229–243, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini G, Franceschetti S, Mantegazza M. Epileptogenic channelopathies: experimental models of human pathologies. Epilepsia 48: 51–64, 2007 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Hablitz JJ. NR2B antagonists restrict spatiotemporal spread of activity in a rat model of cortical dysplasia. Epilepsy Res 72: 127–139, 2006 [DOI] [PubMed] [Google Scholar]
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci 28: 13341–13353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Kirschstein T, Kretz O, Brewster AL, Richichi C, Ruschenschmidt C, Shigemoto R, Beck H, Frotscher M, Baram TZ. Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J Neurosci 27: 4697–4706, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Lüscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855–868, 2001 [DOI] [PubMed] [Google Scholar]
- Campanac E, Daoudal G, Ankri N, Debanne D. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J Neurosci 28: 8635–8643, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Hablitz JJ. Decreased glutamate transport enhances excitability in a rat model of cortical dysplasia. Neurobiol Dis 32: 254–261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci 28: 11768–11777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Levine MS, Salamon N, Miyata H, Vinters HV, Mathern GW. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav 9: 219–235, 2006 [DOI] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med 7: 331–337, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WK, Shin M, Jaramillo TC, Leibel RL, LeDuc CA, Fischer SG, Tzilianos E, Gheith AA, Lewis AS, Chetkovich DM. Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2. Neurobiol Dis 33: 499–508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle MF, Rusznak Z, Wong AYC, Owens S, Forsythe ID. Modulation of a presynaptic hyperpolarization-activated cationic current (Ih) at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol 534: 733–744, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci 25: 8776–8787, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio T, Hablitz JJ. Zinc and zolpidem modulate mIPSCs in rat neocortical pyramidal neurons. J Neurophysiol 80: 1670–1677, 1998 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Hablitz JJ. Alterations in NMDA receptors in a rat model of cortical dysplasia. J Neurophysiol 83: 315–321, 2000 [DOI] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransén E, Hasselmo ME, Alonso A. Properties and role of Ih in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol 83: 2562–2579, 2000 [DOI] [PubMed] [Google Scholar]
- Dvorak K, Feit J. Migration of neuroblasts through partial necrosis of the cerebral cortex in newborn rats: contribution to the problems of morphological development and developmental period of cerebral microgyria. Acta Neuropathol (Berl) 38: 203–212, 1977 [DOI] [PubMed] [Google Scholar]
- Dvorak K, Feit J, Jurankova Z. Experimentally induced focal microgyria and status verrucosus deformis in rats: pathogenesis and interrelation histological and autoradiographical study. Acta Neuropathol (Berl) 44: 121–129, 1978 [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan R, Soltesz I. Double trouble? Potential for hyperexcitability following both channelopathic up- and downregulation of Ih in epilepsy. Front Neurosci 3: 25–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K+ channels. Nat Neurosci 12: 577–584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz JJ, Yang J. Abnormal pyramidal cell morphology and HCN channel expression in cortical dysplasia. Epilepsia 51: 52–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol 545: 783–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. Presynaptic HCN1 channels regulate CaV3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci 14: 478–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci 29: 10979–10988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Gutnick MJ, Prince DA. Hyperexcitability in a model of cortical maldevelopment. Cereb Cortex 6: 514–523, 1996 [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Hwang BJ, Prince DA. Focal epileptogenesis in a rat model of polymicrogyria. J Neurophysiol 81: 159–173, 1999a [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Kharazia VN, Prince DA. Mechanisms underlying epileptogenesis in cortical malformations. Epilepsy Res 36: 165–188, 1999b [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Mogensen M, Warren E, Prince DA. Experimental microgyri disrupt the barrel field pattern in somatosensory cortex. Cereb Cortex 9: 733–744, 1999c [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Prince DA. Excitatory and inhibitory postsynaptic currents in a rat model of epileptogenic microgyria. J Neurophysiol 93: 687–696, 2005 [DOI] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci 27: 13012–13021, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci 29: 8847–8857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MHP, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol 578: 507–525, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsek P, Pieper T, Karlmeier A, Hildebrandt M, Kolodziejczyk D, Winkler P, Pauli E, Blumcke I, Holthausen H. Different presurgical characteristics and seizure outcomes in children with focal cortical dysplasia type I or II. Epilepsia 50: 125–137, 2009 [DOI] [PubMed] [Google Scholar]
- Kubota M, Nasu M, Taniguchi I. Layer-specific horizontal propagation of excitation in the auditory cortex. Neuroreport 10: 2865–2867, 1999 [DOI] [PubMed] [Google Scholar]
- Langenstroth M, Albowitz B, Kuhnt U. Partial suppression of GABAA-mediated inhibition induces spatially restricted epileptiform activity in guinea pig neocortical slices. Neurosci Lett 210: 103–106, 1996 [DOI] [PubMed] [Google Scholar]
- Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues Clin Neurosci 10: 47–62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185–1193, 2002 [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22: 216–224, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Raabe K. Characterization of neuronal migration disorders in neocortical structures. I. Expression of epileptiform activity in an animal model. Epilepsy Res 26: 67–74, 1996 [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol 69: 2129–2136, 1993 [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 848, 1999 [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 1: 181–190, 2000 [DOI] [PubMed] [Google Scholar]
- Marcelin B, Chauviére L, Becker A, Migliore M, Esclapez M, Bernard C. h Channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol Dis 33: 436–447, 2009 [DOI] [PubMed] [Google Scholar]
- Mathern GW, Giza CC, Yudovin S, Vinters HV, Peacock WJ, Shewmon DA, Shields WD. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia 40: 1740–1749, 1999 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol 431: 319–342, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ, Baram TZ. Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem 285: 14724–14736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol 471: 241–276, 2004 [DOI] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci 26: 1854–1863, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Justice JA, Zhang K, He X, Sanchez RM. Increased basal synaptic inhibition of hippocampal area CA1 pyramidal neurons by an antiepileptic drug that enhances IH. Neuropsychopharmacology 35: 464–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci 5: 767–774, 2002 [DOI] [PubMed] [Google Scholar]
- Powell KL, Ng C, O'Brien TJ, Xu SH, Williams DA, Foote SJ, Reid CA. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia 49: 1686–1695, 2008 [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci 26: 3229–3244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264–5275, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury MH, Ouellet PL, Psarropoulou C, Carmant L. Freeze lesion-induced focal cortical dysplasia predisposes to atypical hyperthermic seizures in the immature rat. Epilepsia 45: 592–600, 2004 [DOI] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Brauer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci 23: 3346–3358, 2006 [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron 44: 495–508, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis 32: 26–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain 123: 1075–1091, 2000 [DOI] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B. Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol 526: 91–97, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci 19: 3048–3058, 2004 [DOI] [PubMed] [Google Scholar]
- Surges R, Freiman TM, Feuerstein TJ. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia 44: 150–156, 2003 [DOI] [PubMed] [Google Scholar]
- Sutor B, Hablitz JJ. Influence of barium on rectification in rat neocortical neurons. Neurosci Lett 157: 62–66, 1993 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tanaka Y, Furuta T, Yanagawa Y, Kaneko T. The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices of adult mice. J Neurosci Methods 171: 118–125, 2008 [DOI] [PubMed] [Google Scholar]
- Thompson CH, Kahlig KM, George J. SCN1A splice variants exhibit divergent sensitivity to commonly used antiepileptic drugs. Epilepsia 52: 1000–1009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56: 1076–1089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411: 805–810, 2001 [DOI] [PubMed] [Google Scholar]
- Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci 27: 11334–11342, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008 [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J Neurophysiol 83: 3177–3182, 2000 [DOI] [PubMed] [Google Scholar]
- Wu J, Hablitz JJ. Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J Neurosci 25: 6322–6328, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Datta S, Wu M, Alreja M. Hippocampal theta rhythm is reduced by suppression of the H-current in septohippocampal GABAergic neurons. Eur J Neurosci 19: 2299–2309, 2004 [DOI] [PubMed] [Google Scholar]
- Yuste R, Tank DW, Kleinfeld D. Functional study of the rat cortical microcircuitry with voltage-sensitive dye imaging of neocortical slices. Cereb Cortex 7: 546–558, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang K, Peng BW, Sanchez RM. Decreased IH in hippocampal area CA1 pyramidal neurons after perinatal seizure-inducing hypoxia. Epilepsia 47: 1023–1028, 2006 [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J Comp Neurol 376: 198–213, 1996 [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Roper SN. Reduced inhibition in an animal model of cortical dysplasia. J Neurosci 20: 8925–8931, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]