Abstract
It has been very difficult to record from interneurons in acute slices of the lumbar spinal cord from mice >3 wk of age. The low success rate and short recording times limit in vitro experimentation on mouse spinal networks to neonatal and early postnatal periods when locomotor networks are still developmentally immature. To overcome this limitation and enable investigation of mature locomotor network neurons, we have established a reliable procedure to record from spinal cord neurons in slices from adult, behaviorally mature mice of any age. Two key changes to the established neonate procedure were implemented. First, we remove the cord by a dorsal laminectomy from a deeply anesthetized animal. This enables respiration and other vital functions to continue up to the moment the maximally oxygenated lumbar spinal cord is removed, improving the health of the slices. Second, since adult spinal cord interneurons appear more sensitive to the intracellular dialysis that occurs during whole cell recordings, we introduced perforated patch recordings to the procedure. Stable recordings up to 12 h in duration were obtained with our new method. This will allow investigation of changes in mature neuronal properties in disease states or after spinal cord injury and allow prolonged recordings of responses to drug application that were previously impossible.
Keywords: spinal cord
the spinal cord contains the central pattern generator (CPG) networks that organize the motor patterns for locomotion. These neuronal networks are composed of a set of interacting spinal interneurons that generate the timing, phasing, and intensity cues for the motoneurons to drive rhythmic leg movements (Grillner and Jessell 2009; Kiehn 2006; Pearson 1993). As with other neural networks, the output of the locomotor CPG depends on both the pattern of synaptic connectivity and the intrinsic electrophysiological properties of the component interneurons. Understanding these properties is a major area of current research because they may change in disease states or after spinal cord injury (SCI).
The intrinsic firing properties of spinal interneurons can be studied through a variety of techniques. Blind patch recordings or visually guided recording of genetically tagged interneurons have been used in the isolated largely intact spinal cord (Carlin et al. 2006; Gosgnach et al. 2006; Kiehn and Butt 2003; Kjaerulff and Kiehn 1996; Lanuza et al. 2004; Zhong et al. 2006, 2007). However, this is only feasible in neonatal and early postnatal animals: as the diameter of the spinal cord increases with age, adequate oxygenation of the ventromedial region of the cord, where the locomotor CPG is located, decreases (Wilson et al. 2003), leading to hypoxia-induced cell death. In principle, this can be avoided by studying the firing properties of interneurons in slices of the cord, where greater control over the extracellular environment typically can be achieved (Dougherty and Kiehn 2010; Wilson et al. 2005, 2010; Zhong et al. 2010). However, this has also proven to be very difficult in slices older than 1–2 wk because changes in the extracellular matrix and the perineuronal sheath seem to restrict access to the neuronal somata (Koppe et al. 1997; Milev et al. 1998), making stable intracellular recordings more difficult (Morales et al. 2004). Consequently, even though newborn mice are not able to walk in their 1st wk of life (Clarac et al. 2004), most in vitro studies of the cellular properties of spinal interneurons are conducted on postnatal days 1–5 (P1–P5) neonates (Dougherty and Kiehn 2010; Gosgnach et al. 2006; Lanuza et al. 2004; Zhang et al. 2008; Zhong et al. 2007, 2010). Since significant developmental changes occur between the neonatal period and the locomotor mature age (2–4 wk) (Jiang et al. 1999; Song et al. 2006), it is critically important to study mature interneurons in the investigation of spinal locomotor networks. In addition, studies of adult neurons are essential to analyze their responses to SCI, as these injuries occur most frequently in mature humans. Thus animal models of SCI should be performed with adults to separate injury-induced changes in neuronal properties from those evoked by interruption of the normal developmental schedule.
In this paper, we describe a method to record from identified spinal interneurons from mice in slices of any age. Establishing this protocol will allow studies of the developmental changes between neonatal stage and locomotor mature mice and open up new possibilities to investigate mid- and long-term changes after SCI on a cellular level.
MATERIALS AND METHODS
Animals.
Experiments were performed using adult (P43–P93) Chx10::CFP mice generated by Drs. Steven Crone and Kamal Sharma at the University of Chicago. The animal protocol was approved by the Institutional Animal Care and Use Committee at Cornell University and was in accordance with National Institutes of Health guidelines.
Spinal cord slice procedure.
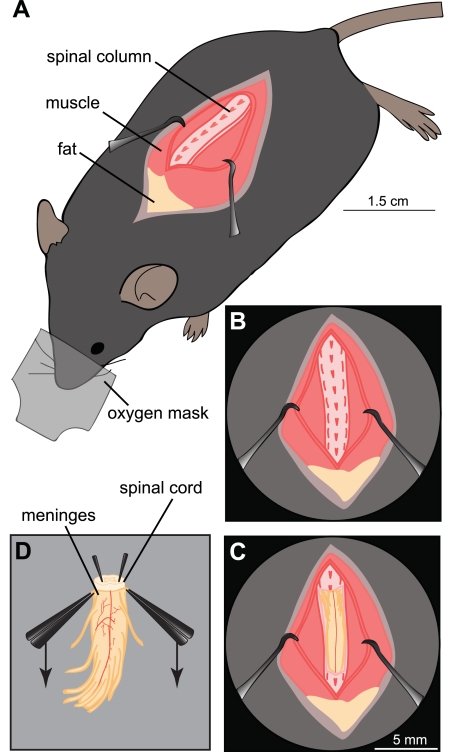
Animals were deeply anesthetized with ketamine (1.5 mg/10 g body wt) and xylazine (0.15 mg/10 g body wt). Nair (commercially available depilatory cream) was applied to remove the fur in the dorsal area between the neck and the sacral region of the spine. The mouse was placed on ice under a binocular microscope and administered pure oxygen (Fig. 1A). Access to the sacral midthoracic spinal column was gained from the dorsal surface (Fig. 1B). A midline incision along the thoracic vertebrae was made, and a dorsal laminectomy was performed (Fig. 1C). To cool further the spinal cord, it was constantly superfused with ice-cold (0–4°C), oxygenated (95% O2-5% CO2), glycerol-based modified artificial cerebrospinal fluid (GACSF; Ye et al. 2006), which contained (in mM): 222 glycerol, 3.08 KCl, 1.18 KH2PO4, 1.25 MgSO4, 2.52 CaCl2, 25 NaHCO3, and 11 d-glucose (∼300 mosmol/kgH2O). With fine scissors, the ventral and dorsal roots were transected. The cord was dissected from the lumbar spinal cord and quickly transferred to a Sylgard-coated (Dow Corning, Midland, MI) petri dish containing ice-cold GACSF and pinned with fine pins ventral side up. The ventral meninges and ventral roots were rapidly removed by pulling the meninges with two fine forceps from anterior to posterior (Fig. 1D). The cord was then turned over, and the dorsal meninges were removed. The spinal cord was transferred into a small, custom-built chamber filled with 34°C low melting point agarose (0.03 g/ml in GACSF; A0701; Sigma), which was immediately placed in ice-water slurry to cool rapidly and harden. The agarose block containing the lumbar enlargement was trimmed to shape with a razor blade and glued (Loctite 406; Henkel) on a metal vibratome disk. The disk was transferred into the microtome buffer tray, which was filled with ice-cold GACSF (kept cold with Microm CU65 cooling device; Thermo Scientific). Sections (250 μm) were sliced with a vibrating blade microtome (HM 650 V; Thermo Scientific; advance speed: 0.9 mm/s; vibratory frequency: 100 Hz; vibratory amplitude: 0.7 mm; blade angle: −15°; blade: Feather Double-Edge Blade, cat no. 121-9, TED PELLA). The slices were immediately transferred into 35°C oxygenated ACSF to recover for 45 min. ACSF contained (in mM): 111 NaCl, 3.08 KCl, 1.18 KH2PO4, 1.25 MgSO4, 2.52 CaCl2, 25 NaHCO3, and 11 d-glucose (280 mosmol/kgH2O). The incubation chamber was similar to the chamber used by Edwards et al. (1989). The slices were kept submerged under the ACSF while the solution was bubbled vigorously but not so strongly as to lead to damaging slice movements. It was essential for the steps from removal of the cord to slicing to be performed as rapidly as possible for good slice viability, optimally within 10 min. Afterward, the slices were allowed to cool down passively to room temperature and recover for 1 h. The spinal neurons were visualized with a fixed-stage upright microscope (BX51WI; Olympus) using a ×60 water-immersion objective (1 numerical aperture, 2-mm working distance; LUMPLFLN 60XW; Olympus) with infrared-differential interference contrast and fluorescence optics.
Fig. 1.
Adult mouse spinal cord dissection. A: overview of the deeply anesthetized mouse receiving pure oxygen by a mask. The spinal column was exposed from the dorsal surface by removal of the overlying fat and muscle. B: detailed view of the spinal column after removing skin and muscles covering it. C: exposed intact spinal cord after performing the partial dorsal laminectomy. D: after removing the spinal cord, including the lumbar enlargement, the cord was pinned in an ice-cold Sylgard Petri dish filled with glycerol-based modified artificial cerebrospinal fluid (GACSF). The meninges were removed by pulling with 2 forceps toward the posterior end of the cord.
Perforated patch recordings.
Slices were transferred to a recording chamber (∼3-ml volume) and continuously superfused with oxygenated ACSF at a flow rate of ∼2 ml/min. To block most synaptic input in the slices, neurons were isolated from rapid synaptic inputs with a combination of dl-2-amino-5-phosphonopentanoic acid (AP-5; 10 μM; A5282; Sigma) and CNQX disodium salt hydrate (10 μM; C239; Sigma) to block glutamatergic synapses, picrotoxin (10 μM) to block GABAergic synapses, and strychnine (10 μM) to block glycinergic synapses. Perforated patch recordings (PPRs) (Horn and Marty 1988; Rae et al. 1991) were made with thick-walled, unfilamented borosilicate glass (1.5-mm outer diameter, 1.0-mm inner diameter; PG52151-4; WPI) on a vertical puller (PC-10; Narishige) with low resistances of 3–5 MΩ. The tip of the pipette was first filled with intracellular solution containing, in mM, 135 K-gluconate, 10 KCl, 10 HEPES, 0.1 EGTA, and 2 MgCl2 (adjusted to pH 7.2 with KOH, ∼270 mosmol/kgH2O) by placing the pipette tip side down into an 1.5-ml Eppendorf cap filled with ∼1.3-ml intracellular solution and applying 5–7 ml of negative pressure with a 10-ml syringe for 1 s. The pipette was then backfilled with a combination of intracellular solution, amphotericin B (A4888; Sigma) and Pluronic F-127 (P-2443; Sigma) (Herrington et al. 1995; Lovell and McCobb 2001). To prepare the solution, 1.2-mg amphotericin B was dissolved in 20-μl DMSO (D8418; Sigma) and added to 1-mg Pluronic F-127 dissolved in 40-μl DMSO. The 60-μl amphotericin B-Pluronic F-127-DMSO mix was added and vortexed in 1-ml intracellular solution. The ionophore mix was stored at room temperature and replaced every hour as needed. The cells were approached with application of very small positive air pressure (via the mouth) to the patch pipette to reach cells up to several layers below the surface but to avoid forcing the ionophore mix to the tip, which would impede seal formation. Close to the clearly visible membrane, the positive pressure was converted to a slight suction to obtain a gigaohm seal. The intact membrane patch in the pipette as well as the enhanced cyan fluorescent protein (eCFP) signal in the neuron were observed during the experiment to confirm the stability of the perforated patch configuration.
Data acquisition and analysis.
Current-clamp recordings were made with a MultiClamp 700B amplifier (Molecular Devices) controlled by Clampex (pCLAMP 9; Molecular Devices). Data were sampled at 10 kHz and low-pass filtered at 2 kHz. For action potential (AP) analysis, the membrane potential was adjusted to set the firing frequency at 1 Hz or lower to elicit temporally isolated APs. The voltage threshold for AP generation was measured as the peak of the second derivative of voltage with time during the rising phase of the AP. The spike amplitude was measured from the peak of the AP to the peak afterhyperpolarization. The AP half-width was established at the voltage halfway from the spike threshold to the peak of the AP. To measure the membrane input resistance and rheobase, all neurons were held below threshold at −60 mV with holding current (Ihold). Input resistance was estimated by averaging the response to small hyperpolarizing current pulses (1-s duration). The minimal amount of current necessary for spike generation was defined as the rheobase. To measure the spontaneous firing rate, the mean firing rate of 12 10-s bins was averaged.
Data analysis was performed with Clampfit (Axon Instruments), Spike2 (CED), IGOR Pro 6 (WaveMetrics), GraphPad Prism (GraphPad Software), and MATLAB (MathWorks). Data are given as means ± SD. To determine differences in parameter means, unpaired or paired t-tests were performed as appropriate. A significance level of 0.05 was accepted for all tests.
RESULTS
In earlier work, we used the standard neonatal procedure to attempt recordings from adult (P50 and older) interneurons. However, the recordings were very difficult to obtain, very unstable, and of short duration. Thus we developed a new procedure that improved the protocol in several ways to allow reliable recording from adult spinal interneurons of any age for long durations. Two key changes made the difference. First, the dissection of the spinal cord from a dorsal approach in a physiologically functioning mouse increased the health of the slices and the viability of the interneurons. The dorsal approach was previously described for spinal motoneurons in adult rats by Carp et al. (2008). Second, we introduced the PPR technique, which avoids replacement of the intracellular contents with the electrode solution and enabled us to make stable long-term recordings from the healthy neurons.
Comparison with the standard “neonate spinal cord slice procedure.”
To illustrate the benefits of the modified procedure, we compared recordings from adult (P56–P60) V2a interneurons using the standard method [ventral dissection from decapitated animal and whole cell recordings (WCRs)] (Dougherty and Kiehn 2010; Wilson et al. 2005, 2010; Zhong et al. 2010) and the new method (dorsal dissection from living animal, P43–P93, and PPRs) and give examples of the stability of long-term recordings only possible with PPR. In the standard procedure, deeply anesthetized animals were killed by decapitation and eviscerated, and their spinal cords were ventrally dissected from the gut and isolated under ice-cold (4°C) oxygenated (95% O2-5% CO2) modified sucrose-based ACSF (in mM: 206 sucrose, 2 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 MgCl, 2 MgSO4, 1 CaCl2, and 20 d-glucose). Patch-clamp WCRs (Hamill et al. 1981) were performed with pipette solution containing, in mM, 138 K-gluconate, 10 HEPES, 5 ATP-Mg, 0.3 GTP-Li, and 0.0001 CaCl2 (pH 7.4 with KOH, ∼240 mosmol/kgH2O). With 29 approaches to cells (3 preparations) using the standard neonatal slice procedure, we were able to form 14-GΩ seals, resulting in 11 successful WCRs. In comparison, using the PPR and a dorsal spinal cord dissection, 26 pipette approaches (12 preparations) resulted in 20-GΩ seals, of which 11 led to stable recordings.
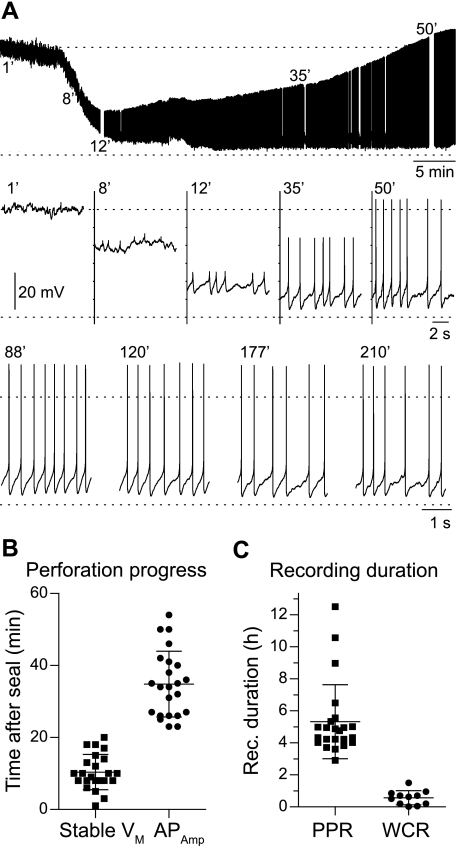
Using the new method, PPRs were performed as described in materials and methods. After ensuring a gigaohm seal, the perforation progress was monitored by the membrane potential and the amplitude of the APs (Fig. 2A). The recorded membrane potential was initially 0 mV, but over time the amphotericin B diffused into the pipette tip and inserted into the membrane patch. This enabled recording of the membrane potential after 10 ± 5 min (n = 23; Fig. 2B). Initially, the APs were small, as the initial very high series access resistance (≫100 MΩ) results in low-pass filtering of the measured signal (Fig. 2A). With further perforation of the membrane, the series access resistance dropped to lower values (<100 MΩ); after 35 ± 9 min (n = 23), the APs began to overshoot 0 mV. Over the period of ∼1 h, the continuously dropping series access resistance eventually stabilized at 15–25 MΩ. Thus the neuron was ready for recording ∼1 h after gigaohm seal formation.
Fig. 2.
Perforated patch recording (PPR) on a spinal V2a interneuron of a 7-wk-old mouse. A, top trace: current-clamp recording (53-min duration) showing the process of perforated patch formation after sealing onto a V2a interneuron. See results for description. B: time course of the perforation process. The data points show the time for individual neurons to reach stable membrane potential (VM) and overshooting action potentials (APAmp). C: duration of recordings (Rec.) is much longer using the new PPR method than the standard whole cell recording (WCR) method. Most PPRs were voluntarily terminated after 4–5 h, so the average recording duration is artificially shortened.
The PPRs lasted much longer than the traditional WCR measurements. Most of the PPRs we conducted with the new method were discontinued by the investigator after ∼5 h, resulting in an average recording time of 5.3 ± 2.3 h (n = 23; Fig. 2C). However, in cases where we did not terminate the recording, it was possible to record as long as 12 h from a single spinal interneuron (Fig. 2C). In contrast to this, using the standard neonatal dissection, the WCRs lasted only 35 ± 27 min (n = 11); this is consistent with our many adult WCR recordings from other interneuron types (data not shown).
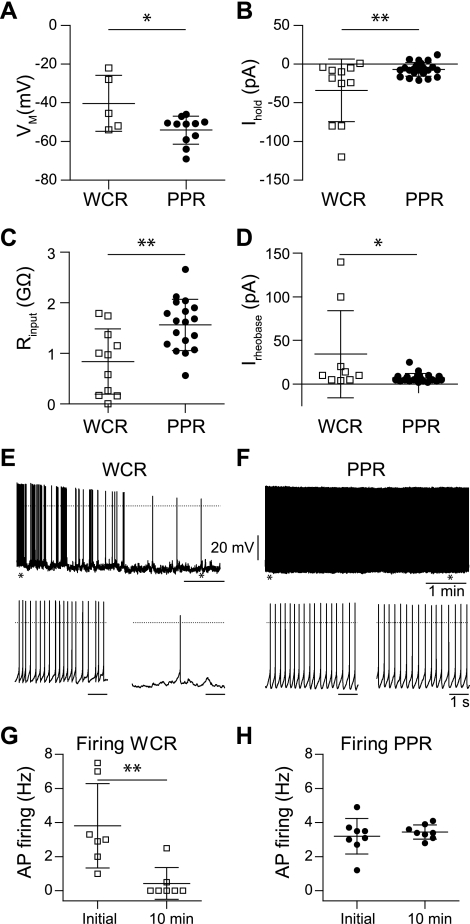
In addition to the relatively short recording duration using the old WCR approach, the quality of the recording was poorer than with the PPRs. For example, using the standard method, the initial membrane potential was more depolarized. In V2a neurons for which the firing frequency was low enough to measure accurately the membrane potential (AP frequency ≤ 1 Hz), the potential was −40.3 ± 14.5 mV in WCRs (n = 5), whereas using PPRs it was in a more hyperpolarized range with a smaller SD (−54.1 ± 7.3 mV; n = 11; P = 0.02, t-test; Fig. 3A). The depolarized membrane potential in WCRs was also reflected by significantly greater negative current necessary to hold the neuron at −60 mV (P = 0.0045, t-test; Fig. 3B). Whereas in the WCR the Ihold at −60 mV was −34 ± 40 pA (n = 11), the PPRs only needed −6.7 ± 8.8 pA (n = 22; again less variability) to hold the neuron at −60 mV. This difference is in part explained by the significantly lower input resistances measured in the neurons using the WCR procedure (WCR, 0.8 ± 0.6 GΩ, n = 11; PPR, 1.6 ± 0.5 GΩ, n = 18; P = 0.002, t-test; Fig. 3C). Reflecting the difference in input resistance, the rheobase, or current required to evoke an AP from −60 mV, was larger using the standard WCR procedure than the new PPR procedure (Fig. 3D). Both the rheobase (34 pA) and its variability (±50 pA SD) were larger using WCR than with PPR (7 ± 5 pA).
Fig. 3.
Recording quality is superior using the new PPR method. A: the mean membrane potential in standard WCRs was more depolarized with a higher variability than the membrane potential with the newly introduced PPRs. B: the holding current (Ihold) to maintain the neuron at −60 mV was significantly more negative and variable in standard WCRs than using the new PPR. C: the membrane input resistance (Rinput) with the standard approach WCRs was significantly lower compared with the PPRs. D: the rheobase was significantly larger and more variable using the standard approach than the new approach. Irheobase, rheobase current. E: V2a neuron recording using the standard WCR approach. During the 1st 10 min, the spontaneous firing rate decreased, and the membrane potential hyperpolarized. F: V2a neuron recording using the new PPR approach. Firing frequency was typically very stable. For each panel, the bottom recordings are made at the points of the asterisks in the top recordings. G: using the standard WCR approach, the firing frequency dropped significantly after 10 min, and most neurons fell silent. Asterisks indicate levels of significance, *P < 0.05, **P < 0.005. H: using the new PPR approach, the firing rate did not change significantly after 10 min.
Whereas the rate of spontaneous AP firing in the new procedure was very stable over hours, the spontaneous firing rate using the standard WCR procedure significantly decreased during the 1st 10 min of recording (Fig. 3, E and G). In neurons where the initial firing rate was >0.5 Hz, the WCR showed a significant drop in firing rate from initial (membrane breakthrough) 3 ± 2.7 to 0.3 ± 0.8 Hz 10 min later (n = 9; P = 0.004, paired t-test; Fig. 3, E and G). The reduction in basal firing rate was often associated with either a variable hyperpolarization (Fig. 3E) or a depolarization (data not shown) of the neuron. The hyperpolarization may arise in part from activation of KATP currents (see discussion). Using the perforated patch method, the firing rate was very stable over time (Fig. 3, F and H). The initial firing rate when the APs became overshooting was 3.2 ± 1.1 Hz and 10 min later was essentially the same (3.5 ± 0.4 Hz; n = 7; P = 0.4, paired t-test; Fig. 3H).
Stability in recordings with adult slice preparation and perforated patch.
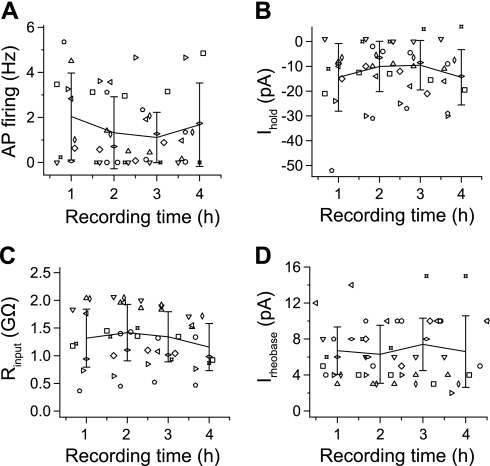
In addition to the greater length of recording using PPR in adult spinal interneurons, the excitability was generally very stable for many hours. The spontaneous firing frequency (with no Ihold) remained constant between 1 and 2 Hz for ≥4 h (Fig. 4A); individual neurons had different initial firing rates but maintained them at a similar frequency for the duration of the recordings. Intrinsic parameters such as membrane input resistance, the Ihold at −60 mV, and the rheobase also remained constant over time. The amount of current needed to hold the cells at −60 mV remained stable over the course of the recordings (−14.4 ± 13.6 to −14.4 ± 11.1 pA after 4 h; Fig. 4B). Once the series access resistance had stabilized, the input resistance stayed approximately the same over many hours, reflecting the healthiness of the recorded neuron and the noninvasive nature of the PPR. The mean input resistance after stable perforation of 1.3 ± 0.5 GΩ was essentially the same 4 h later (1.2 ± 0.4 GΩ; Fig. 4C). The neuronal excitability, as reflected by the stability of the rheobase value, did not change significantly over the 1st 4 h (6.7 ± 2.6 pA after 1 h vs. 6.6 ± 3.9 pA at 4 h; Fig. 4D).
Fig. 4.
Recording stability is superior using the new PPR method. A–D: using the new PPR approach, the AP firing rate (A), Ihold at −60 mV (B), Rinput (C), and rheobase values (D) remained very stable for ≥4.5 h. Symbols represent 11 individual interneurons per plot, with 3–4 data points measured from each neuron during the 1st 4.5 h of recording. The average ± SD graphs represent 60-min bins.
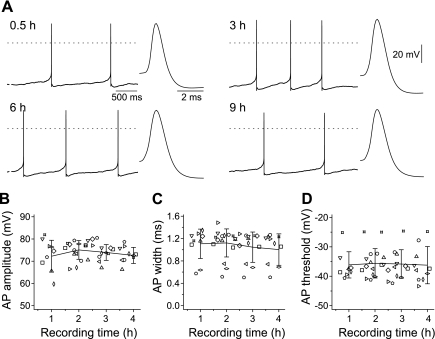
Once the ionophore penetration into the membrane reached steady state, in most recordings the properties of the APs (amplitude, half-width, and threshold) remained stable over many hours (Fig. 5). Most of the experiments were terminated after about 4–5 h, but some showed stable firing properties for as long as 9 h (Fig. 5A). For 11 V2a interneurons, the AP properties were plotted over time. Means of 60-min bins around 1, 2, 3, and 4 h illustrate the stability of the AP properties (Fig. 5, B–D). The AP amplitude remained stable over many hours, averaging 72.2 ± 7.2 mV after 1 h and 72.6 ± 3.6 mV after 4 h (Fig. 5B). The AP half-width also remained very stable, with virtually no change over time (1.1 ± 0.3 ms after 1 h to 1.0 ± 0.3 ms after 4 h; Fig. 5C). Another extremely stable parameter was the AP threshold, remaining around −36 mV for the duration of the recordings (−36.1 ± 4.5 mV after 1 h; −36.2 ± 6.3 mV after 4 h; Fig. 5D).
Fig. 5.
Stability of AP properties. A: examples of spontaneous APs in a single V2a interneuron at different recording times between 0.5 and 9 h after recording began. Insets show averaged AP waveforms (averaged by aligning peaks from 10 to 12 APs) at a higher sweep speed. B–D: AP amplitude, half-width, and threshold remained very stable for ≥4.5 h. Symbols represent 11 individual interneurons per plot, with 3–4 data points measured during the 1st 4.5 h of recording. The average ± SD graphs represent 60-min bins.
DISCUSSION
A major current focus of our laboratory is to understand better how transection of the spinal cord alters the intrinsic properties of neurons below the lesion site. In pursuing this goal, we found it necessary to improve on the procedures used to record intracellularly from adult spinal interneurons in vitro. Whereas the commonly used procedures enable reliable recordings from neonatal tissue with relative ease (Carlin et al. 2006; Dougherty and Kiehn 2010; Gosgnach et al. 2006; Lanuza et al. 2004; Wilson et al. 2005; Zhong et al. 2010), obtaining similarly stable recordings in mature tissue (i.e., tissue from mice old enough to be developmentally mature and with enough time postinjury for homeostatic changes in neuronal properties to occur) proved problematic using the standard neonatal dissection and recording methods. By modifying the neonatal approach, we have successfully overcome the “age barrier” in intracellular recordings from spinal interneurons in vitro. Although we made many changes to the procedure (outlined in detail in materials and methods), there were two major changes that made the biggest impact: a dorsal approach to dissecting the spinal cord and using PPRs.
In switching to a dorsal approach to the spinal cord dissection as previously described by Carp et al. (2008) for spinal rat motoneurons, we were able to maintain normal blood flow to the cord for as long as possible before it was finally removed from the animal. Ventral dissections, which require initially killing the animal followed by evisceration before extracting the spinal cord, induce hypoxia in the spinal cord much earlier in the dissection. Hypoxia is particularly problematic in adult tissue where neurons appear to be less capable of recovering than similar tissue from neonates (Haddad and Donnelly 1990). Another advantage of the dorsal approach is that ice-cold GACSF (Ye et al. 2006) can be bathed over the exposed spinal cord while it is intact and receiving normal blood flow. Cooling the tissue provides a neuroprotective effect by reducing the rate of metabolism and slowing the effects of hypoxia associated with the remainder of the dissection (Erecinska et al. 2003). Thus this step optimizes the capacity of the tissue to recover after the slicing process.
Even with healthier tissue slices, we found that our whole cell recordings were not as stable as we routinely achieved in neonatal tissue, and obtaining adequate seals was also more difficult. It is likely that spinal interneurons from older mice are more susceptible to dialysis of the intracellular milieu that occurs with whole cell recordings. In particular, we suspect that rundown in intracellular ATP levels leads to activation of the KATP-type ATP-dependent potassium current, hyperpolarizing the neuron and dramatically altering its intrinsic properties and overall health of the neurons (Seino and Miki 2003). This contributes to the poor health of adult spinal cord neurons, but, since not all neurons hyperpolarized during the 10 min after breakthrough of the patch, additional consequences of intracellular dialysis must also occur. To overcome this sensitivity to intracellular dialysis, we switched to PPRs, which minimize the exchange of solutions between the pipette and the recorded neuron (Horn and Marty 1988). Using amphotericin B as our ionophore, we were able to obtain long-lasting recordings during which the intrinsic properties of the recorded neurons remained remarkably stable. Indeed, the recordings were most often terminated not because of a decline in the health of the cell but at the discretion of the experimenter.
Together, the dorsal dissection combined with the use of PPRs (and other minor methodological changes; see materials and methods for complete details) enable long-lasting and highly stable recordings from spinal V2a interneurons of developmentally mature mice (as old as 93 days). The ability to perform these recordings is an important step for future studies to understand how spinal networks generate particular behaviors (such as locomotion) and the progression of disease/injury states, which can only be investigated in relatively old preparations. In addition, because the recordings are extremely stable, it is possible to discern even subtle effects of neuromodulators on the intrinsic properties of neurons, which were previously difficult to measure due to the rundown of various intrinsic currents in whole cell recordings. We have not tested our procedure on other interneurons or motoneurons, but in our slices these cells also appear healthy and should be amenable to PPRs. We believe that the ability to record from mature spinal interneurons provides an additional and valuable tool in understanding fundamental questions of how the nervous system generates behaviors and studying the neuronal consequences of disease or injury.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-057599 to R. M. Harris-Warrick and Deutsche Forschungsgemeinschaft (DFG) Grant HU 1963/1-1 to A. Husch.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.H., N.C., and R.M.H.-W., conception and design of research; A.H. and N.C. performed experiments; A.H. and N.C. analyzed data; A.H., N.C., and R.M.H.-W. interpreted results of experiments; A.H. prepared figures; A.H. and N.C. drafted the manuscript; A.H., N.C., and R.M.H.-W. edited and revised the manuscript; A.H., N.C., and R.M.H.-W. approved the final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kamal Sharma at the University of Chicago for kindly providing Chx10::CFP mouse line and Drs. Shelby Dietz and Bruce Johnson for valuable comments on earlier versions of the manuscript. We also thank V. Patel and C. Benton for outstanding technical assistance.
REFERENCES
- Carlin KP, Dai Y, Jordan LM. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol 95: 1278–1284, 2006 [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Mongeluzi DL, Dudek CJ, Chen XY, Wolpaw JR. An in vitro protocol for recording from spinal motoneurons of adult rats. J Neurophysiol 100: 474–481, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res 143: 57–66, 2004 [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci 30: 24–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch 414: 600–612, 1989 [DOI] [PubMed] [Google Scholar]
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 23: 513–530, 2003 [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006 [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572–586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol 429: 411–428, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Herrington J, Solaro CR, Neely A, Lingle CJ. The suppression of Ca2+- and voltage-dependent outward K+ current during mAChR activation in rat adrenal chromaffin cells. J Physiol 485: 297–318, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci 11: 3481–3487, 1999 [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol 70: 347–361, 2003 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res 288: 33–41, 1997 [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004 [DOI] [PubMed] [Google Scholar]
- Lovell PV, McCobb DP. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J Neurosci 21: 3429–3442, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, Margolis RK, Margolis RU. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun 247: 207–212, 1998 [DOI] [PubMed] [Google Scholar]
- Morales E, Fernandez FR, Sinclair S, Molineux ML, Mehaffey WH, Turner RW. Releasing the peri-neuronal net to patch-clamp neurons in adult CNS. Pflügers Arch 448: 248–258, 2004 [DOI] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16: 265–297, 1993 [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 37: 15–26, 1991 [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003 [DOI] [PubMed] [Google Scholar]
- Song ZM, Hu J, Rudy B, Redman SJ. Developmental changes in the expression of calbindin and potassium-channel subunits Kv3.1b and Kv3.2 in mouse Renshaw cells. Neuroscience 139: 531–538, 2006 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Blagovechtchenski E, Brownstone RM. Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. J Neurosci 30: 1137–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25: 5710–5719, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Chersa T, Whelan PJ. Tissue PO2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience 117: 183–196, 2003 [DOI] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods 158: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci 26: 6509–6517, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci 30: 170–182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 27: 4507–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]