Abstract
Motor and sensory proprioceptive axons reinnervate muscles after peripheral nerve transections followed by microsurgical reattachment; nevertheless, motor coordination remains abnormal and stretch reflexes absent. We analyzed the possibility that permanent losses of central IA afferent synapses, as a consequence of peripheral nerve injury, are responsible for this deficit. VGLUT1 was used as a marker of proprioceptive synapses on rat motoneurons. After nerve injuries synapses are stripped from motoneurons, but while other excitatory and inhibitory inputs eventually recover, VGLUT1 synapses are permanently lost on the cell body (75–95% synaptic losses) and on the proximal 100 μm of dendrite (50% loss). Lost VGLUT1 synapses did not recover, even many months after muscle reinnervation. Interestingly, VGLUT1 density in more distal dendrites did not change. To investigate whether losses are due to VGLUT1 downregulation in injured IA afferents or to complete synaptic disassembly and regression of IA ventral projections, we studied the central trajectories and synaptic varicosities of axon collaterals from control and regenerated afferents with IA-like responses to stretch that were intracellularly filled with neurobiotin. VGLUT1 was present in all synaptic varicosities, identified with the synaptic marker SV2, of control and regenerated afferents. However, regenerated afferents lacked axon collaterals and synapses in lamina IX. In conjunction with the companion electrophysiological study [Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. J Neurophysiol (August 10, 2011). doi:10.1152/jn.01097.2010], we conclude that peripheral nerve injuries cause a permanent retraction of IA afferent synaptic varicosities from lamina IX and disconnection with motoneurons that is not recovered after peripheral regeneration and reinnervation of muscle by sensory and motor axons.
Keywords: spinal cord, axotomy, proprioception, muscle, monosynaptic stretch reflex
sensory dysfunction resulting from the reorganization of central circuits remains one of the major obstacles that impede full recovery of function after successful microsurgical reattachment and regeneration of peripheral nerve injuries (Lundborg 2003; Navarro et al. 2007). This is of special importance for the recovery of normal motor function following nerve regeneration. Muscle stretch reflexes are undetectable after peripheral nerve injuries even after successful reconnection in the periphery, and in the spinal cord regenerated motoneurons fail to respond or respond very weakly to muscle stretch (Cope et al. 1994; Haftel et al. 2005; Huyghues-Despointes et al. 2003; Maas et al. 2007). Nerve injury-induced areflexia and altered locomotor behaviors, for example, loss of interjoint coordination, are consistent with the loss of proprioceptive feedback from muscle spindle receptors (Abelew et al. 2000; Chang et al. 2003; Maas et al. 2007). These proprioceptive deficits are not due to lack of stretch signals reaching the spinal cord, because sensory afferents with normal IA conduction velocity and sensitivity to muscle stretch can be recorded from peripheral nerves and dorsal roots after peripheral regeneration (Brown and Butler 1976; Collins et al. 1986; Haftel et al. 2005). Therefore, the failure of stretch signals reaching the motoneurons most likely is due to abnormalities in the central connections between IA afferents and motoneurons. To investigate this possibility, we analyzed the structural stability of IA inputs on motoneurons after transection of the tibial or medial gastrocnemius nerves in situations in which regeneration was allowed by nerve reattachment or when peripheral regeneration was prevented.
It is well known that after peripheral nerve injuries synapses on axotomized motoneurons are lost, a phenomenon known as “synaptic stripping,” and many are recovered after motoneurons reconnect with muscle (Blinzinger and Kreutzberg 1968; Brannstrom and Kellerth 1998, 1999; Chen 1978; Cull 1974; de la Cruz et al. 1994; Sumner 1975, 1976; Sumner and Sutherland 1973; Svensson et al. 1991). It is unknown, however, whether all inputs are similarly competent for recovery. The possibility that this process results in major changes in synaptic composition is suggested by studies that reported different ratios of excitatory and inhibitory synapses following regeneration or in nonregenerating motoneurons normally or under different neurotrophic influences (Brannstrom and Kellerth 1998, 1999; Davis-López de Carrizosa et al. 2009; Linda et al. 1992, 2000; Novikov et al. 2000). The fate of IA synapses in particular has not been directly studied but assumed to follow that of other excitatory synapses and to recover after injury in parallel with the recuperation of the amplitude and time course of electrically evoked group I excitatory postsynaptic potentials (EPSPs) (Kuno and Llinas 1970; Mendell 1988; Titmus and Faber 1990). IA synapses represent a small proportion (∼2%) of all synapses on the motoneuron surface and mostly target the dendritic arbor (>90%), at least on cat motoneurons, where they have been more thoroughly studied (Burke and Glenn 1996; Fyffe 2001). In contrast, most studies of synaptic remodeling after peripheral nerve injuries have concentrated their analyses on the cell soma. Therefore, it is likely that the majority of excitatory synapses previously analyzed with light and electron microscopy over axotomized and regenerating motoneurons belong to excitatory inputs other than IA afferents. A different behavior for IA synapses compared with other excitatory synapses that target motoneurons should not be entirely unexpected because, of all inputs presynaptic to motoneurons, they alone arise from neurons directly injured in the periphery by nerve lesions.
In this study we analyzed the fate of IA afferent synapses by studying boutons containing isoform 1 of the vesicular glutamate transporter (VGLUT1) in combination with intra-axonal labeling of regenerated IA afferents that displayed normal stretch-evoked responses following peripheral regeneration. In the spinal cord, VGLUT1 is expressed by synaptic boutons from proprioceptors, cutaneous mechanoreceptors, and corticospinal tract fibers. The arguments for these origins are supported by multiple lines of evidence (see Alvarez et al. 2004; Betley et al. 2009; Llewellyn-Smith et al. 2007; Mentis et al. 2006; Oliveira et al. 2003; Persson et al. 2006; Todd et al. 2003; Wu et al. 2004). Briefly, these include findings that 1) the funicular and laminar distributions of VGLUT1 axons and varicosities correspond with the target regions of these three projection systems; 2) VGLUT1 is expressed by large dorsal root ganglion cells and pyramidal cells of the sensorimotor cortex and is absent in spinal cord neurons, with the exception of dorsal spinocerebellar tract neurons; 3) VGLUT1 is contained in the synapses of parvalbumin primary afferent axons in neonatal spinal cord (parvalbumin is a known proprioceptive axon marker in neonates; Arber et al. 2000); 4) VGLUT1 localizes in sensory afferents anterogradely labeled with cholera toxin b (CTb) subunit or fluorescent dextrans from either peripheral nerves or dorsal roots; 5) VGLUT1 is present in the annulospiral endings of muscle spindle afferents; 6) VGLUT1 varicosities are largely depleted from the ventral horn shortly after (1 wk) unilateral dorsal rhizotomies or genetic deletion of the ventral projections of proprioceptive afferents in Er81 knockout animals; and 7) VGLUT1 is present in corticospinal synaptic varicosities identified by genetic labeling. Given the known laminar distributions and synaptic interactions with motoneurons of these three sources of spinal VGLUT1 synapses (i.e., cutaneous mechanoreceptors, corticospinal axons, and proprioceptive sensory axons; Fyffe 1992; Yang and Lemon 2003), it is expected that the large majority of VGLUT1 varicosities in contact with motoneurons belong to IA afferents. Here we further show, for the first time, that the synaptic varicosities of electrophysiologically identified IA afferents in the adult rat always contain high levels of VGLUT1.
A previous study reported that VGLUT1 immunoreactivity decreased within lamina IX (LIX) 8 wk after sciatic nerve transections (Hughes et al. 2004). In the present study, we confirmed this decrease after peripheral nerve injury, but more importantly we analyzed the fates of the synapses at these and longer time intervals after injury and after peripheral regeneration. IA (VGLUT1)-motoneuron contacts were analyzed at various postinjury times on the somata and dendrites of lumbar motoneurons, and more specifically those in the medial gastrocnemius (MG) pool that successfully reinnervated the MG muscle. The results suggest that IA afferent fiber collaterals and their synapses retract from LIX after peripheral nerve injuries and these axons do not grow back, even long after peripheral regeneration has been successfully completed. As a result, the density of VGLUT1 synapses on somata and dendrites of motoneurons is greatly diminished. The functional consequences of this finding in terms of the ability of IA afferents to sustain high-frequency neurotransmission and maintain full connectivity with the motor pool are the focus of a companion electrophysiological study (Bullinger et al. 2011).
Preliminary data have been reported as conference summaries and abstracts (Alvarez et al. 2008, 2010).
METHODS
All animal procedures were performed according to National Institutes of Health guidelines and were reviewed by the local Laboratory Animal Use Committees at Wright State University. The data described in this article were obtained from 55 adult Wistar rats that underwent different combinations of the procedures explained below. All survival surgeries (i.e., nerve surgeries and tracer injections) and physiological recordings were obtained from rats deeply anesthetized by isoflurane inhalation (induction 4–5%; maintenance 1–3%, both in 100% O2). After survival surgeries, the animals received subcutaneous injections of buprenorphine (0.1 mg/kg) immediately and every 12 h after surgery prophylactically to alleviate any possible pain and distress. Pain and distress were closely monitored but not observed in any of the rats. In terminal experiments, rats were transcardially perfused with fixatives for histological analyses as described below. This procedure was performed under the effects of a lethal overdose of Nembutal (150 mg/kg ip injection) or Euthasol (>50 mg/kg ip) and confirmation of deep anesthesia.
Experimental Nerve Injuries and Summary of Analyses
Rats (n = 45) were anesthetized and subjected to sterile survival surgery in which a midline incision (∼2 cm) through skin and underlying connective tissue was made in the left hindlimb to expose the tibial nerve (TN) at midthigh or selected branches of TN, specifically the medial gastrocnemius nerve (MGN) and/or the adjoined lateral gastrocnemius and soleus nerves (LGSN) close to their muscle entries. The TN, the MGN, or the MGN and the LGSN were cut with scissors where exposed, and the cut ends were immediately either ligated with suture to prevent regeneration or surgically rejoined to promote regeneration. Surgical nerve reunion was achieved by standard epineurial repair, in which the cut fascicles were grossly aligned and the nerve was rejoined without tension by a few 10-0 suture ties passed through the epineurium. After washing with 0.9% sterile saline the wound was closed in layers, and the animals removed from anesthesia underwent postoperative care as explained above.
The effects of nerve injury and/or regeneration were studied in two histological and two combined electrophysiological and histological analyses. In a first study, 15 animals with TN surgery were prepared for histological analyses (see below) of VGLUT1 depletions at 3 days, 1, 2, 4, 6, and 12 wk, and 6 mo after injury. At each date one animal was prepared for either ligation or rejoined experiments, except for 12 wk, at which time an additional animal was prepared for nerve ligation. In another group of experiments animals underwent TN (n = 12) or MGN (n = 7) cut and nerve reunion surgeries and were used for analysis of regenerating MG motoneurons identified by retrograde tracing with fluorescently coupled CTb subunit (CTb-555, explained below). TN animals were killed in groups of four at 1 wk, 6 wk, and 6 mo after injury. MGN animals were prepared for histology at 1 wk (n = 1), 4 mo (n = 2), and 6 mo (n = 4) after surgery. All these animals were compared with a control group (n = 4) that received similar tracer injections without nerve surgeries. Finally, a third category of experiments included two types of terminal electrophysiological studies performed with an in vivo whole animal rat spinal cord preparation described in detail in our earlier reports (Bichler et al. 2007; Haftel et al. 2004, 2005) and in the companion paper (Bullinger et al. 2011). Electrophysiological experiments were performed from 6 to 16 mo after the nerve injury. In five rats with TN cut and reunion we intracellularly recorded EPSPs produced in tibial motoneurons by electrical stimulation of the TN distal to the injury. In 12 additional rats, either nonoperated rats (n = 7) or rats that had undergone MGN and LGSN cut and reunion (n = 5), group I sensory axons with responses to stretch of the MG muscle typical of primary spindle endings were penetrated intra-axonally in the dorsal roots and injected with neurobiotin (Vector Labs; 4–10% in 2 M potassium acetate). Their intraspinal trajectories were analyzed after fixation and spinal cord histological processing.
Retrograde Labeling of Medial Gastrocnemius Motoneurons
To identify MG motoneurons that reinnervated the MG muscle 6 wk, 4 mo, or 6 mo after nerve injuries we used a dual retrograde tracing procedure (n = 14 animals, 8 TN and 6 MGN). First we exposed the MG muscle, as explained above, and injected it with 2.5% Fast Blue (Polysciences, Eppelheim, Germany) 7 days before TN or MGN surgery. We made four or five 5-μl injections evenly distributed throughout the body of the MG muscle. Fast Blue is nontoxic to the cell and is stable for several months (Puigdellivol-Sanchez et al. 2002); however, labeling of dendrites is limited with this tracer. It was therefore solely used to identify, at long postinjury times, motoneurons that innervated the MG before the nerve injury. After nerve surgery and 1 wk before death and histological processing the MG muscle was exposed again and 1–5% CTb-555 (Invitrogen, Carlsbad, CA) was injected in four or five 2- to 5-μl injections to confirm reinnervation of MG by Fast Blue-labeled MG motoneurons. CTb-555 is a relatively rapidly transported retrograde tracer that consistently fills part of the dendritic arbor (see results). Thus CTb-555 fluorescence was utilized for analyses of VGLUT1 contact distributions on dendrites. One week survival animals (n = 5, 4 TN and 1 MGN) were not dual labeled (because no muscle reinnervation is expected at this short time frame), and therefore only CTb-555 injections were performed 7 days before nerve surgeries. Finally, four animals that did not undergo nerve injuries were injected with CTb 1 wk before perfusion-fixation and used as controls (these animals received no Fast Blue injections).
Electromyograms
Electromyograms (EMGs) were recorded from MG muscles bilaterally in all the rats used to analyze CTb-555-labeled control and regenerated motoneurons after TN or MGN surgeries. Before perfusion and tissue extraction, these rats were deeply anesthetized (see above) and the MG muscles and TN were surgically exposed. Compound muscle action potentials (CMAPs) were recorded across two wire electrodes, one inserted distally and the other proximally in the MG muscle. CMAPs were evoked electrically (square pulses 40 μs in duration repeated at 0.5 Hz) through a bipolar electrode positioned on TN at midthigh (proximal to the chronic nerve lesion in the left hindlimb). Stimulus strength was adjusted to a level producing the maximum CMAP. All hindlimb nerves except the MGN were acutely crushed to eliminate all EMG signals other than those evoked in the MG muscle. These procedures were applied to both the nerve-treated left and the untreated right hindlimb. CMAPs were digitized (20 kHz) and analyzed off-line with Spike-2 software (Cambridge Electronics Design). Recordings were compared in the MG muscle ipsilateral to the nerve injury with those recorded in the contralateral side. After the recording the animals were injected with an overdose of Euthasol (see below) and perfusion fixed.
Harvesting Tissues for Histological Analyses
Deeply anesthetized animals were transcardially perfused first with a vascular rinse and then with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.3 (PB). The spinal cords were then dissected and postfixed overnight in the same fixative. The next day the spinal cord blocks were placed in cryoprotectant (15% sucrose in 0.1 M PB, pH 7.4, containing 0.01% sodium azide) and stored at 4°C until being used. Transverse sections, 50 μm thick, were obtained from the lower lumbar spinal cord (lumbar segments 4 and 5) in a sliding microtome with a freezing stage and collected and processed free floating.
Immunofluorescence Methods
Three different immunocytochemical experiments were performed in this study. All the sections were first blocked with normal donkey serum diluted 1:10 in phosphate-buffered saline (PBS) with 0.1% of Triton X-100 (PBS-TX) before being placed in different combinations of primary antibodies (see Table 1 for a list of sources, characteristics, and dilutions of primary antibodies). All primary antibodies used in this study have been amply characterized in the past by us and other labs as to their specific labeling patterns in the spinal cord (see, for example, Alvarez et al. 1999, 2004). Incubations in primary antibodies were carried out overnight at 4°C, and the following day immunoreactive sites were revealed with the use of species-specific secondary antibodies raised in donkey and conjugated to FITC, Cy3, or Cy5 (always diluted 1:50; Jackson Labs). After a 2- to 4-h incubation in secondary antibodies the sections were thoroughly washed in PBS and mounted on slides and coverslipped with Vectashield (Vector Labs, Burlingame, CA).
Table 1.
Antibodies used in this study
| Antigen | Immunogen | Host/Type | Manufacturer | Dilution |
|---|---|---|---|---|
| VGLUT1 | Rat VGLUT1 | Rabbit polyclonal | Synaptic Systems | 1:1,000 |
| (aa 456–560) Strep-Tag fusion protein | (Goettingen, Germany) | 1:2,000 | ||
| catalog no. 135 302 | ||||
| VGLUT2 | Rat VGLUT2 | Rabbit polyclonal | Synaptic Systems | 1:1,000 |
| (aa 510–582) Strep-Tag fusion protein | (Goettingen, Germany) | |||
| catalog no. 135 402 | ||||
| VAChT | Synthetic peptide from cloned rat VAChT | Goat polyclonal | Chemicon | 1:1,000 |
| (Temecula, CA) | ||||
| catalog no. AB1578 | ||||
| VGAT | Rat VGAT | Rabbit polyclonal | Synaptic Systems | 1:500 |
| (aa 75–87) synthetic peptide coupled to keyhole limpet hemocyanin | (Goettingen, Germany) | |||
| catalog no. 131 002 | ||||
| SV2 | Purified synaptic vesicles from ommata electric organ | Mouse monoclonal | Developmental Hybridoma Bank | 1:200 |
| (University of Iowa, Iowa City, IA) | ||||
| NeuN | Purified cell nuclei from mouse brain | Mouse monoclonal | Chemicon | 1:500 |
| (Temecula, CA) | ||||
| catalog no. MAB377B |
VGLUT, vesicular glutamate transporter; VAChT, vesicular acetylcholine transporter; VGAT, vesicular GABA/glycine transporter.
In the first series of experiments we investigated the density of various synaptic markers around the cell bodies of axotomized and regenerating motoneurons located in lumbar regions that contain TN motor pools. In this case, sections of the lumbar 4 and 5 segments were dual immunolabeled for neuronal nuclear protein (NeuN) and either VGLUT1 or VGLUT2, the vesicular acetylcholine transporter (VAChT), or the vesicular GABA/glycine transporter (VGAT). NeuN immunoreactivity was always revealed with Cy3-conjugated secondary antibodies, while the synaptic markers were revealed with FITC-conjugated secondaries.
In the second series of experiments we analyzed VGLUT1 contacts on the soma and dendrites of MG motoneurons retrogradely labeled with Fast Blue and CTb-555 injected before or after the nerve lesion. VGLUT1 contacts were visualized with FITC-conjugated secondaries, and all the sections were counterstained with deep red (640 nm) Neurotrace Nissl (Invitrogen) to confirm laminar locations.
In the third series of experiments we analyzed VGLUT1 or VGLUT2 content inside the synaptic varicosities of neurobiotin-filled IA afferents. In this case neurobiotin was revealed first by incubating the sections overnight in a 1:75 dilution of streptavidin conjugated to FITC (Jackson Labs). Sections containing labeled axon collaterals were then placed in primary antibodies against synaptic vesicles (SV2; Buckley and Kelly 1985) (to reveal neurobiotin-filled varicosities with synaptic vesicles) and either VGLUT1 or VGLUT2. SV2 was labeled with Cy5-conjugated secondary antibodies and VGLUTs with Cy3 antibodies. We performed these immunolabelings in spinal cord sections from four control and four experimental animals with regenerated MG nerves.
Electrophysiology
Intra-axonal recordings and neurobiotin labeling.
Rats were prepared for in vivo intra-axonal recordings of MG stretch-responsive IA afferents with sharp ∼10-MΩ resistance micropipette electrodes, as described previously (Haftel et al. 2004, 2005) and in the companion article (Bullinger et al. 2011; in these animals the lateral gastrocnemius nerve was also cut and sutured). Sensory axons with IA-type responses to stretch (see Bullinger et al. 2011) were then intracellularly injected through the recording pipette with neurobiotin (Vector Labs; 4–10% in 2 M potassium acetate). Recordings and injections were done in dorsal roots ∼3–4 mm from the dorsal root entry zone. Positive current pulses were used to aid neurobiotin passage into the fiber (4–30 nA, most commonly 11–15 nA delivered by 400-ms-long pulses at 2 Hz for 4–30 min; good labeling of central collaterals was more frequently found in experiments that included injections that lasted for >12 min). After retraction of the electrode a minimum of 6-h waiting period was allowed for anterograde transport and labeling of central collaterals inside the spinal cord.
After the waiting period the animals were perfusion fixed as explained above and postfixed for 16–28 h, and transverse frozen sections (50 μm thick) were obtained from lumbar 4 and 5 segments. Sections containing neurobiotin-labeled collaterals were used for immunofluorescence labeling for VGLUT1 (3 control and 1 experimental) or triple fluorescence labeling for quantitative analysis of VGLUT1 and VGLUT2 content in SV2-immunoreactive (IR) and neurobiotin-filled varicosities (4 control and 4 experimental animals; see above).
EPSP properties of regenerated motoneurons.
In five animals we recorded 20 motoneurons that underwent regeneration after 24–26 wk (i.e., ∼6 mo) transection and reunion of the TN. Intracellular recordings were performed with sharp 7- to 10-MΩ resistance electrodes as described in detail in the companion article (Bullinger et al. 2011). Regeneration was confirmed by antidromic action potentials evoked from the TN (i.e., distal to the original nerve transection), and reinnervation was sometimes confirmed by recording a motor unit twitch in the MG in response to firing the motoneuron with a brief (50 μs) current pulse applied through the recording electrode. Electrically evoked EPSPs were elicited from bipolar electrodes placed in the peripheral nerve distal to the injury (50-μs pulses delivered at 0.5 Hz) and were isolated in the intracellular record from action potential contamination by adjusting stimulus strength just below the level for firing threshold for antidromic action potentials and/or below EPSPs of amplitudes that elicit orthodromic action potentials in the motoneuron.
Confocal Analyses of Synaptic Varicosities on NeuN-Immunoreactive or Retrogradely Labeled Cell Somata
Dual NeuN and VGLUT1 immunofluorescence was imaged at low [20×1; numerical aperture (NA) 0.70] and high (60×2; oil objective NA 1.35) magnification in an FX Olympus confocal microscope using simultaneous excitation with the 488 (argon) and 568 (krypton) laser lines. Large NeuN-IR profiles were randomly sampled from LIX regions known to contain motoneuron pools sending axons in the TN (Nicolopoulos-Stournaras and Iles 1983; Swett et al. 1986). Each NeuN-IR profile and associated synaptic varicosities were imaged through series of confocal images separated by 0.5-μm z-steps. NeuN immunoreactivity provides excellent definition of the somatic cellular surface, and there is little ambiguity in defining somatic contacts from immunofluorescent varicosities. From these image stacks the midsomatic region was identified by the presence of a well-defined nucleolus and from this center image three to five optical sections separated by at least 2 μm in the z-axis (to avoid sampling the same terminals) were chosen for quantification. The number of immunofluorescent varicosities in contact with the surface of NeuN cell bodies was counted and the cellular perimeter in each image measured, excluding the origins of primary dendrites. Measurements were obtained with Fluoview software. Densities were estimated as the number of contacts per 100 μm of linear perimeter. An average density estimate was obtained for each cell. For each synaptic marker and animal we sampled 8–12 cells in 4–6 different lumbar sections in comparable regions of the control and experimental sides. Care was taken that images used for counting corresponded to cell body cross sections viewed in an orthogonal plane, avoiding optical sections with tangential views of the cell surface and that render erroneous estimates of cell perimeter and synaptic coverage. We found significant interanimal variability for VGLUT1 densities in controls; therefore the percentage change in synaptic coverage was estimated always between the control and experimental sides within individual animals. After measurements were done, we noted that VGLUT1 terminals were smaller in the experimental side. This might have resulted in undersampling compared with control boutons using the method described above based on few cross sections. To estimate this error we recounted selected experiments and considered all the VGLUT1 terminals in contact with the motoneuron and contained in the confocal stack. Then we traced the somatic contours with Neurolucida software and estimated the sampled somatic surface to obtain a surface density (number of contacts per 100 μm2 of surface area). From here we calculated a percentage loss that was compared with estimates from sampling selected optical sections and calculation of linear densities.
Similar soma analyses were performed in MG motoneurons identified with retrograde labeling in animals after TN cut and suture and studied at 1 wk, 6 wk, and 6 mo after injury (n = 4 in each group). These were then compared with four animals that did not undergo nerve surgeries but only retrograde tracing with CTb-555. In this analysis we sampled per animal 6–14 motoneurons retrogradely labeled with either CTb (control and 1 wk animals) or Fast Blue (6 wk and 6 mo animals) only or dual labeled with Fast Blue and CTb-555 (6 wk and 6 mo animals). Finally, we analyzed with the same methods 6–18 motoneurons in four animals in which the MG nerve was cut and rejoined and studied 1 wk, 4 mo, and 6 mo after surgery. In this situation MG injured motoneurons self-reinnervate the MG muscle and only homonymous primary afferents are injured, leaving intact heteronymous afferents from, for example, the lateral gastrocnemius muscle.
Confocal Analyses of VGLUT1 Varicosities on Fast Blue- and CTb-Labeled Motoneurons
Imaging of CTb- and Fast Blue-labeled MG motoneurons was performed with confocal microscopy in an Olympus FV1000 system, and the sections were excited “sequentially” with 405 (Fast Blue and blue Nissl)-, 488 (VGLUT1-FITC)-, 568 (CTb 555)-, and 647 (deep red Nissl)-nm laser lines. Low-magnification images were obtained with a ×10 objective (NA 0.30) and stacks of confocal optical sections separated by 2-μm z-steps collected throughout the thickness of the tissue section. High-magnification confocal stacks of individual CTb and/or Fast Blue motoneurons and their dendrites were obtained with a ×60 oil objective (NA 1.35) digitally zoomed X1 or X2 and a z-step of 0.5 μm. To image the whole CTb-labeled dendritic arbor at a magnification high enough for unambiguous identification of VGLUT1 contacts on dendrites, it was necessary to tile four to six image stacks in the x-y plane to cover the neuropil region occupied by all dendritic branches.
These images were imported into Neurolucida (v. 8.0) with the confocal module (Microbrightfield Bioscience, Willinston, VT). Neuron tracing was performed on cell bodies and dendrites by moving up and down through the z-stack of tiled confocal images while plotting the positions of VGLUT1 contacts on the cell body and dendritic arbor. The cell soma was traced with every 1 μm z-steps and the dendrites every 0.5 μm z-steps. Not all motoneuron cell bodies were fully contained within the 50-μm section. We selected for analysis of somata, motoneurons in which 75% or more of the cell body could be reconstructed. From these reconstructions the Neurolucida software calculated the somatic surface contained within the section. The total number of VGLUT1-IR contacts on the somata was divided by the total amount of surface sampled to obtain a contact density. Dendritic trees were manually traced from their cell body origins to the point where CTb fluorescence was lost. Dendritic segments were entered by individual points more or less separated depending on the orientation of the dendrite and the tortuosity of its path. Dendrite thickness was entered at each individual point by adjusting the thickness of the cursor. From these traces we obtained estimates of dendritic length and surface for each dendrite, segment, or whole dendritic arbor. VGLUT1 contacts plotted along the dendritic arbor were used for calculation of surface densities (number of VGLUT1 contacts per 100 μm2 of dendritic surface). Sholl analysis was performed with Neurolucida software and used to estimate linear and surface density of VGLUT1 contacts on dendritic segments located within concentric spheres at increasing 50-μm distance steps from a reference point that was always centered in the cell nucleolus.
Using these methods, we analyzed 10 motoneurons in each of four experimental groups (control, 1 wk postsurgery, 6 wk, and 6 mo), sampling two or three motoneurons per animal in each group (n = 4 animals per group). Overall or Sholl linear and surface densities were statistically compared between control values and the three different postsurgery times (see below).
Confocal Analyses of VGLUT1 and VGLUT2 Content in Injured and Control IA Afferents
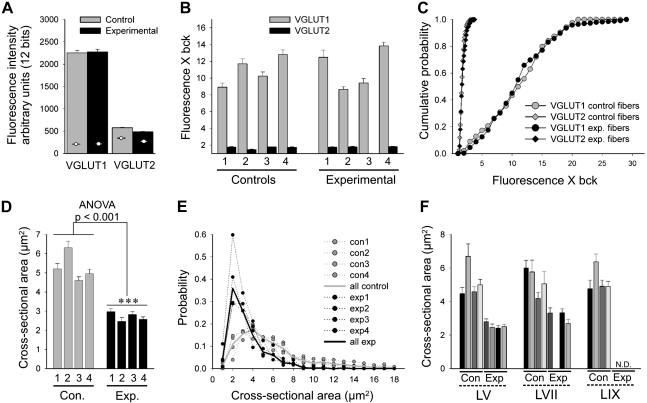
All sections containing filled collaterals of sensory afferents with IA-like responses to muscle stretch were imaged with epifluorescence at low magnification and recorded with a digital camera (RT-SPOT, Diagnostic Instruments, Sterling Heights, MI). Sections with segments of IA-like fibers were divided into three groups and prepared for VGLUT1 or VGLUT2 analyses or no immunolabeling (see above). For confocal analysis low magnification (10×1) confocal stacks of neurobiotin-labeled fiber segments were first obtained, and all synaptic varicosities in the section were identified and their z-positions recorded. An intensity profile of changes in VGLUT immunofluorescence in the z-axis was obtained for each section. Tissue depths showing decreased immunofluorescence were discarded. Synaptic varicosities at z-positions with optimal VGLUT1 immunofluorescence were imaged at 60 × 2,5 in all three channels to record neurobiotin labeling with streptavidin-488 (argon, 488), VGLUTs Cy3 (krypton, 568), and SV2 Cy5 (HeNe, 647) immunofluorescence. Two or three fields of synaptic varicosities were imaged at high magnification in each animal for VGLUT1 and VGLUT2 immunostains. Between 217 and 335 neurobiotin varicosities with SV2 immunoreactivity were sampled for analyses of VGLUT2 or VGLUT1 intensities in each group (4 control and 4 experimental animals); 1,209 varicosities were analyzed in total (see Table 3). Each varicosity was imaged at the focal plane of maximum size and outlined with Fluoview software tools. Imaging conditions for VGLUT1 and VGLUT2 were maintained constant in all experiments. The area and average immunofluorescence intensity (VGLUT1 or VGLUT2) were recorded for each varicosity, which was then classified according to laminar location and experimental animal (see Table 3). The varicosity outline was then moved in the x-y plane, outside the neurobiotin-labeled varicosity and avoiding VGLUT-immunolabeled clusters, to obtain three estimates of neighboring background fluorescence. Intensity measurements were performed at 12-bit resolution (0 black, 4,095 maximum intensity). VGLUT immunofluorescence intensities were normalized for each varicosity by either subtracting or dividing the average local background. In the first case this was equal to subtracting a background offset and the units were arbitrary fluorescence units. In the second case the values were multiples of the background. Similar results were obtained with both normalization procedures, and we chose to present data as multiples of background level. In this case a value of 1 equals background fluorescence. We then constructed histograms and cumulative probability functions of normalized immunofluorescence for each VGLUT in each animal or for all control and experimental data pooled together.
Table 3.
Number of SV2-immunoreactive synaptic varicosities in intracellular filled afferents analyzed for VGLUT1 or VGLUT2 content
| Control (4 animals) |
Regenerated (4 animals) |
Total |
||||
|---|---|---|---|---|---|---|
| VGLUT1 | VGLUT2 | VGLUT1 | VGLUT2 | Total | Per Fiber | |
| No. of synaptic boutons | ||||||
| Lamina V | 110 | 141 | 259 | 126 | 636 | 31–117 |
| Lamina VII | 93 | 73 | 72 | 91 | 329 | 0–70 |
| Lamina IX | 132 | 112 | 0 | 0 | 244 | 0–80 |
| All laminae | 335 | 326 | 331 | 217 | 1,209 | 112–192 |
Finally, bouton sizes were estimated as the cross-sectional two-dimensional projection area of neurobiotin-labeled synaptic varicosities imaged though all optical planes. Bouton sizes were compared for each individual animal and lamina, and probability size distributions were constructed for each animal or all control and experimental samples pooled together.
Statistics
All statistics were performed with Sigma Stat (v. 3.1, Jandel). t-Tests were used for comparisons of VGLUT1, VGLUT2, VGAT, and VAChT densities between the control and experimental sides within animals. Comparisons of average VGLUT1 densities in different control and experimental animals after different postsurgery times were performed with one-way ANOVAs followed by pairwise comparisons using the Holm-Sidak test. Comparisons between two groups of motoneurons, for example, Fast Blue labeled only versus Fast Blue and CTb-555 dual labeled, were done with t-tests. Comparisons of different experimental groups versus a single control value (i.e., VGLUT1 densities in different dendritic compartments) were performed with a one-way ANOVA test followed by Bonferroni corrected multiple t-tests. Finally, comparisons of cumulative probability distributions were performed with a Kolmogorov-Smirnov test. In all cases significance was set at P < 0.05.
RESULTS
VGLUT1 Synaptic Boutons Are Permanently Removed from Lamina IX and Motoneuron Cell Bodies After Peripheral Nerve Injury
VGLUT1 immunoreactivity was compared between the control and experimental sides of spinal cord lumbar 4 and 5 segments from rats with unilateral (left side) TN cuts that were either surgically rejoined (to allow regeneration) or ligated (to prevent regeneration). To study the time course of changes in VGLUT1-IR synapses, several postinjury times were analyzed (3 days, 1, 2, 4, 6, and 12 wk, and 6 mo). VGLUT1 immunoreactivity was profoundly depleted in LIX regions containing TN motoneuron pools 1 wk after injury and did not recover at longer survival times whether in rats with rejoined or ligated nerves (Fig. 1, Fig. 2).
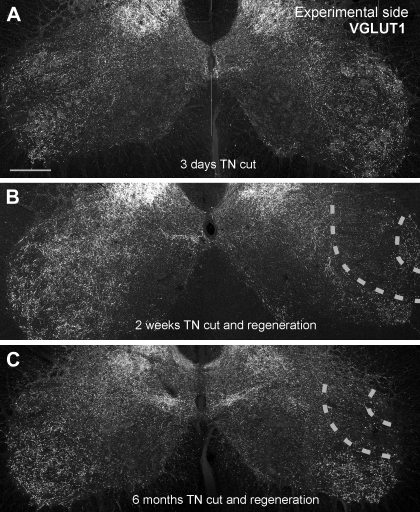
Fig. 1.
VGLUT1 immunoreactivity is permanently depleted in lamina IX regions containing motoneurons and central projections of IA afferents injured peripherally. A: low-magnification confocal image of VGLUT1 immunoreactivity in the ventral horns of the spinal cord in a rat with a unilateral tibial nerve (TN) transection performed 3 days earlier. VGLUT1-immunoreactive (IR) puncta are very dense in the lateral regions of lamina IX. VGLUT1-IR puncta at this location preferentially label the synaptic varicosities of IA afferents. No differences can be appreciated between the sides at this short postinjury time. B: image similar to A but from a rat whose TN was transected 2 wk earlier and allowed to regenerate and reinnervate the muscles. A large depletion is observed in the region corresponding to motoneuron pools sending axons through the TN (region marked between dashed lines). C: same as in B, but in an animal that fully regenerated its peripheral muscle 6 mo after the injury. VGLUT1-IR puncta remained depleted around TN motor pools in lamina IX (region between dashed lines). Scale bar in A is 250 μm. All images are at the same magnification.
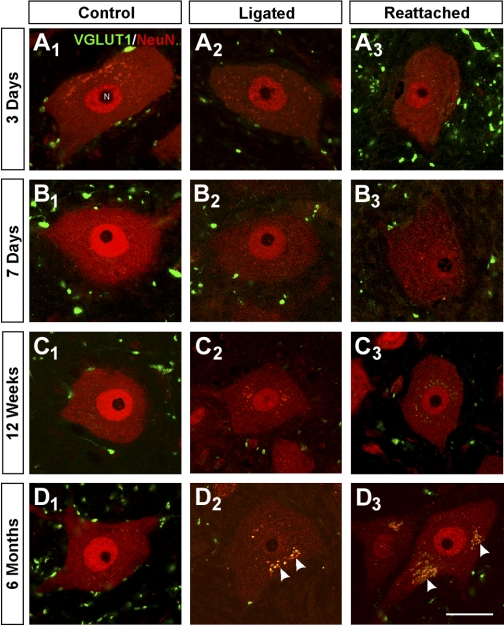
Fig. 2.
VGLUT1-IR contacts on neuronal nuclear protein (NeuN)-IR motoneuron cell bodies are permanently depleted after nerve injury and do not recover after muscle reinnervation. A–D: high-magnification, single plane, confocal images of VGLUT1-IR varicosities (FITC, green) and NeuN-IR cell bodies (Cy3, red) sampled from lamina IX regions containing motoneurons with axons injured in the TN at various times after injury: 3 days (A), 7 days (B), 12 wk (C), and 6 mo (D). Control motoneurons (A1, B1, C1, D1) show strong NeuN immunoreactivity in the cell nucleus and are surrounded by VGLUT1 varicosities in the neuropil, some of which are in contact with the cell body. Injured motoneurons whose axons are not allowed to regenerate (ligated; A2, B2, C2, D2) show progressive downregulation of NeuN, especially in the cell nucleus. VGLUT1 varicosities are reduced in density in the adjacent neuropil, and they make fewer contacts with motoneuron cell bodies. VGLUT1-IR varicosities also appear smaller. Injured motoneurons whose axons have been rejoined in the periphery and are undergoing regeneration (A3, B3) also lose NeuN immunoreactivity initially, but this is recovered after reconnection with muscle (C3, D3). VGLUT1-IR varicosities are depleted and reduced in size on regenerating (A3, B3) or reconnected (C3, D3) motoneurons to a degree similar to that observed on motoneurons that do not regenerate. Motoneurons in older animals (usually at longer survival times) display autofluorescent lipofucsin granules in their somata (arrowheads in D series). Scale bar in D3 is 25 μm. All other panels are at the same magnification.
To quantify the coverage of VGLUT1-IR contacts on motoneuron somata we used NeuN as a marker of α-motoneuron cell bodies in LIX (see Friese et al. 2009; Shneider et al. 2009). NeuN immunoreactivity was decreased in axotomized motoneuron cell bodies 1 and 2 wk after injury (Fig. 2), in agreement with a similar report in facial motoneurons (McPhail et al. 2004). NeuN immunoreactivity recovered in animals undergoing peripheral regeneration (Fig. 2). On the basis of the occupancy of vacated bungarotoxin-labeled end-plates (not shown) and EMG recordings (see below) we estimated that rather complete motor axon peripheral reinnervation of the MG muscle occurs between 4 and 6 wk after TN cut and reunion, suggesting that NeuN expression in α-motoneurons is regulated by target innervation. NeuN-IR motoneuron cell bodies were imaged at high magnification to estimate VGLUT1-IR contact densities (i.e., contacts per 100 μm of soma perimeter, see methods; for this analysis we used a 60×2, NA 1.4 objective, with a theoretical depth of field between 0.65 and 0.75 μm; thus 100 μm of soma perimeter corresponds to ∼70 μm2 of somatic surface membrane). As expected, there was a clear reduction in the number of VGLUT1-IR contacts surrounding NeuN-IR motoneuron cell bodies at 1 wk after injury. The apparent size of VGLUT1-IR clusters found in LIX was also smaller (see below). These contacts did not recover at later times in animals with nerves either ligated or rejoined and undergoing successful muscle reinnervation (Fig. 2). VGLUT1-IR contact density was estimated in 8–12 NeuN-IR motoneuron cell bodies per animal. Compared with other synaptic markers (see below) the density of VGLUT1-IR contacts on the cell body was rather low, and it was necessary to pool data from five optical planes per motoneuron to obtain representative samples of VGLUT1-IR contacts around cell bodies. No statistically significant differences in VGLUT1 densities were found between experimental and control side motoneurons at 3 days after injury in animals with either ligated (P = 0.99; t-test) or reattached (P = 0.18; t-test) nerves (Fig. 3). At all other postinjury times, VGLUT1-IR contact density in the experimental side was significantly decreased compared with the control side (P < 0.05, t-tests) and did not recover at later postinjury times. Permanent depletions in VGLUT1-IR contact density were similar in rats with ligated cut nerves (Fig. 3A) and in rats with rejoined cut nerves undergoing peripheral regeneration (Fig. 3B). Percent depletions plateaued at 4 wk after injury, and the loss of VGLUT1-IR contacts (estimated as linear densities; see below comparison with surface densities) ranged between 75% and 95% at all later postinjury times, being similar in animals undergoing regeneration or prevented from regeneration (Fig. 3C).
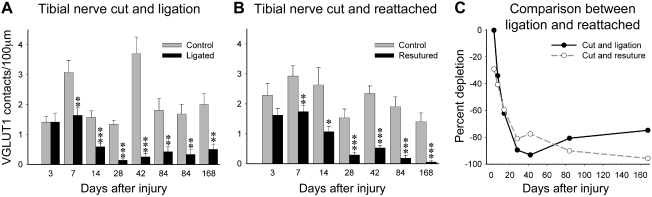
Fig. 3.
Reductions on VGLUT1 contact density on the cell bodies of NeuN-IR motoneurons axotomized in the periphery are quantitatively similar when axon regeneration is prevented or allowed. A: VGLUT1-IR contact densities around NeuN-IR motoneuron cell bodies after nerve cut and ligation to prevent regeneration at different postinjury times. Pairs of gray (control side) and black (experimental side) bars are shown for each animal at each survival day. Progressive depletions occur in the experimental side compared with the control side 1 and 2 wk after injury (7 and 14 days, respectively). This depletion becomes very large from 4 wk (28 days) to 6 mo (168 days) after injury [2 animals were analyzed 12 wk (84 days) after injury]. Asterisks denote significant differences when comparing control and experimental sides by t-tests (*P < 0.05, **P < 0.01, ***P < 0.001). B: similar to A, but from animals in which the TN was rejoined and allowed to regenerate. As before, VGLUT1 depletions in the experimental side are progressive in the first 2 wk and then very profound from 4 wk to 6 mo, with no evidence of recovery. Significant variability was observed in the control sides of the different animals analyzed in A and B. This might represent normal intrinsic variability in the number of contacts reaching the cell body in different animals, a possible sampling bias in immunoreactions necessarily carried out at different times in different animals, or an adaptation after injury in the control side. However, on average the control side of all animals in the nerve ligation experiments was not significantly different from control animals undergoing regeneration or from the estimated average densities in 4 animals with no nerve injuries (see Fig. 5A). C: % depletions within each animal comparing average VGLUT1 densities in control vs. experimental sides (the point at 84 days for cut and ligation contains average depletions calculated in 2 animals; for all other points, each point represents 1 animal). A similar sharp decline in VGLUT1 contacts on the cell soma of axotomized motoneurons, either regenerating (grey dashed line, open circles) or not (black line and filled circles), occurs during the first 2 wk. The % depletion plateaus at 4 wk, and there is no significant recovery in animals that regenerate and reconnect with muscle or in those prevented from regeneration.
The previous analysis might have included motoneurons with axons in the TN that during regeneration could have reinnervated the original or a different muscle. Some motoneurons could also fail to find a neuromuscular junction, although we consider this a minor outcome because by 6–8 wk after injury most vacated neuromuscular junctions recover motor axon innervation after regeneration (i.e., presynaptic axons immunoreactive for the VAChTs and highly phosphorylated neurofilaments; data not shown) and electrophysiologically recorded motoneurons elicited muscle twitches when tested by evoking a single action potential through the recording electrode (Bullinger et al. 2011). In addition, given the partial overlap with motor pools from other nerves, some uninjured motoneurons may have been included in the samples. To exclude these possibilities, we labeled motoneurons innervating the MG muscle with fluorescent retrograde tracers both before and after nerve section (Fig. 4; see methods). In this experiment we prepared 12 animals in which the TN was cut and rejoined, and 4 animals were analyzed at each of three selected postinjury dates: 1 wk (before significant regeneration), 6 wk (when MG reinnervation is almost complete), and 6 mo (long after peripheral reinnervations has been completed). The extent of muscle reinnervation was confirmed in each animal before histology by EMG recording of the CMAPs evoked by stimulation of the nerve above the injury. One week after surgery the MG muscle ipsilateral to the nerve injury showed partial atrophy in all four animals and only in one of four animals was there a detectable, but small CMAP in the EMG traces. At 6 wk and 6 mo after injury all MG muscles had recovered apparent normal sizes and evoked CMAPs were comparable ipsilateral (experimental) and contralateral (control) to the injury.
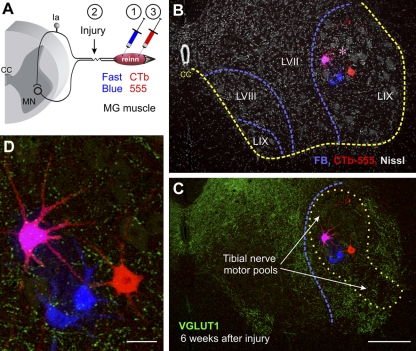
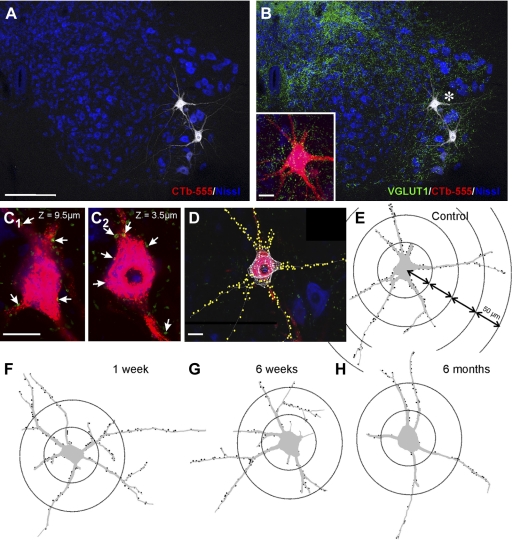
Fig. 4.
Dual retrograde labeling for identification of medial gastrocnemius (MG) motoneurons that reinnervate the MG muscle. A: experimental design. The left MG muscle was injected with Fast Blue (1) prior to transection and reattachment of the TN (2). Then 1 wk before the end of the survival period (6 wk or 6 mo) the MG muscle was injected again (3) with cholera toxin b coupled to Alexa 555 (CTb-555). B and C: low-magnification confocal images (all optical planes through a 50-μm-thick section were superimposed) of Fast Blue (FB) and CTb-555 retrograde labeling from the MG, respectively, before and after the nerve lesion (from a 6 wk survival animal). B shows the location of labeled motoneurons within lamina (L)IX in a lumbar 5 segment section counterstained with fluorescent Nissl (gray, 640-Neurotrace) to delineate lamination (dashed blue lines). The yellow line indicates the boundary between the gray and white matter [central canal (CC)]. Motoneurons were labeled either for Fast Blue or CTb-555 only or dual labeled. Dual-labeled motoneurons (pink, asterisk) represent MG motoneurons confirmed as reinnervating the MG muscle. Neurons labeled only with Fast Blue or CTb-555 might be MG motoneurons that failed to uptake the tracer in one of the two injections or, alternatively, represent MG motoneurons reinnervating a different muscle after regeneration (Fast Blue) or non-MG motoneurons that after regeneration reinnervate the MG muscle (CTb-555). The analysis compared Fast Blue only vs. dual-labeled motoneurons since there were relatively few motoneurons labeled only with CTb in most experiments. C shows the same section but with VGLUT1 immunoreactivity superimposed (FITC, green). As before, a large depletion in VGLUT1 varicosities is found 6 wk after injury in the lamina IX region (dotted yellow outline) occupied by motoneurons and the central arborizations of IA afferents with peripheral axons in the injured TN. MG motoneurons are located at relatively mid-dorsoventral positions within this region (blue dashed line indicates the lamina IX boundary from B). D: high-magnification image of retrogradely labeled motoneurons showing VGLUT1 contacts on dendrites and cell bodies. Scale bars: 250 μm in C (B is at the same magnification), 50 μm in D.
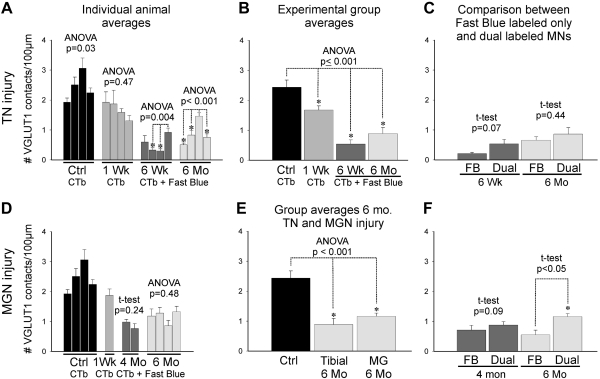
VGLUT1-IR contact densities in regenerating motoneurons were compared with motoneurons sampled from four control animals injected only with CTb-555 and that did not undergo nerve surgery. We analyzed in each animal, on average, 10.5 retrogradely labeled motoneurons (range 6–14). Small motoneurons (putative γ-motoneurons) were excluded from the analysis. The density of VGLUT1-IR boutons in contact with MG motoneuron cell bodies 1 wk after TN surgery (regenerating but not yet reinnervating muscle) was slightly, but significantly, reduced (Fig. 5, A and B). This depletion was much larger on dual-labeled motoneurons (MG motoneurons that reinnervated the MG) 6 wk and 6 mo after injury. Within individual animals, motoneurons that were confirmed as reinnervating the MG (dual labeled with Fast Blue and CTb-555) tended to display slightly higher densities of VGLUT1-IR contacts on their cell bodies compared with MG motoneurons that were labeled with Fast Blue before the injury but not by CTb-555 after the injury (not shown). These differences reached statistical significance in only one of four animals in each postinjury time group (6 wk and 6 mo; P < 0.01, t-tests). These animals correspond to the two animals that showed significantly higher VGLUT1-IR contact densities around dual-labeled motoneurons in, respectively, the 6 wk and 6 mo groups (Fig. 5A; P < 0.001, ANOVA followed by post hoc Holm-Sidak pairwise comparisons) and perhaps represent small interanimal differences in their responses to injury or during regeneration. No significant interanimal variability was observed in the non-reinnervating 1 wk group. In control animals, some interanimal variability was also detected in the somatic coverage by VGLUT1-IR boutons when the four control animals were compared (P = 0.03; 1-way ANOVA), but this could not be confirmed with post hoc pairwise comparisons (Holm-Sidak post hoc test). VGLUT1 contact density estimates for each animal were then averaged together for each survival group (n = 4 in each group) and compared with control animals (n = 4 animals). In these group averages VGLUT1-IR linear density was reduced by 32% in the 1 wk group and more profoundly in reinnervating motoneurons (dual labeled) at 6 wk (78% decrease) and 6 mo (64%). All these decreases were statistically significant (P < 0.001, 1-way ANOVA and post hoc Bonferroni tests for each survival date average vs. control). When dual-labeled (Fast Blue + CTb-555) and single-labeled (Fast Blue only) motoneurons are pooled together, with no assumptions on target reinnervation (more similar to the NeuN study), the percentages of depletion increase to 91% at 6 wk and 72% at 6 mo, comparable to deletions estimated in NeuN-labeled motoneurons from 4 wk to 6 mo after injury. Differences in VGLUT1 density between 6 wk and 6 mo in CTb-labeled motoneurons did not reach significance.
Fig. 5.
Quantitative analysis of changes in VGLUT1-IR contacts on the cell bodies of MG motoneurons at different postinjury times after tibial or MG nerve transections and reunion. A: average VGLUT1-IR contact density on CTb-labeled (control and 1 wk) or Fast Blue and CTb dual-labeled MG motoneurons (6 wk and 6 mo) in individual animals (6–14 motoneurons analyzed per animal) from the control (black bars), 1 wk survival (medium gray bars), 6 wk (dark gray bars), and 6 mo (light gray bars) groups. Despite some interanimal variability (depicted as significance in ANOVA tests run for each group; asterisks indicate pairwise significant differences tested post hoc), overall VGLUT1 densities were, in most animals, slightly depleted 1 wk after injury and very significantly depleted 6 wk and 6 mo after injury. B: group averages (n = 4 animals/group) indicated significant differences (ANOVA, P < 0.001). The control group was significantly different from all nerve injury groups (asterisks indicate P < 0.05, Holm-Sidak post hoc analysis). The 1 wk survival group (i.e., at the start of reinnervation) was less depleted than the 6 wk and 6 mo groups (times at which peripheral reinnervation has been completed). In contrast, differences between 6 wk and 6 mo were not significant. C: motoneurons labeled only with Fast Blue (FB) were not significantly different from dual-labeled motoneurons (confirmed MG reinnervating motoneurons) at 6 wk or 6 mo. D: as in A for individual animal averages in the 1 wk (medium gray bars), 4 (dark gray bars), and 6 mo (light gray bars) groups after MG nerve injury and reattachment [control uninjured group (black bars) is the same as in A]. In this case there was no significant interanimal variability within the 4 and 6 mo groups. E: comparison of MG motoneurons axotomized in the TN or in the MG nerve (MGN) and reinnervating the MG muscle (thus dual labeled) analyzed 6 mo after injury (n = 4 animals/group). VGLUT1 density depletions compared with control were similar in both groups. F: comparison between Fast Blue labeled only (FB) and dual-labeled MG motoneurons after MG nerve injury and reunion. No significant differences were detected at 4 mo, but a significant difference was found at 6 mo.
In conclusion, MG motoneurons that reinnervate the MG muscle showed, overall, depletions similar to those detected over NeuN-IR motoneurons, confirming that VGLUT1-IR boutons contacting the cell soma of motoneurons are largely removed after injury and are not significantly recovered after the motoneurons successfully reinnervate the same muscles. In other words, peripheral motor axon regeneration and muscle reinnervation is not associated with recovery of somatic VGLUT1-IR contacts.
In a second series of four animals the MGN was transected and rejoined. In these experiments there was no possibility of inappropriate target muscle reinnervation, and, by difference to TN injuries, only homonymous IA connections were injured by MGN sections, while heteronymous IA connections arising from synergistic muscles were preserved. These animals were analyzed 1 wk (n = 1), 4 mo (n = 2), and 6 mo after the injury (n = 4). EMG recordings showed normal CMAPs in all animals 4 and 6 mo after MGN surgery, while the 1 wk animal did not show functional reinnervation. We analyzed 10.6 retrogradely labeled motoneuron cell bodies per animal (range 6–18), and these were compared with the same uninjured control group as above and with the TN-injured groups. Analysis of motoneurons in the MGN self-reinnervation model indicated that the loss of somatic VGLUT1-IR contacts was similar to that observed after TN injuries (Fig. 5, D and E), suggesting that somatic VGLUT1-IR contacts are dominated by homonymous IA inputs. Also similar to the TN injury experiment, small differences were observed between motoneurons labeled only with Fast Blue (in this case this group cannot include wrongly targeted motoneurons, only those projecting to the MG but that failed to uptake the second tracer) or dual labeled (reinnervating) 4 or 6 mo after injury (Fig. 5F). These differences were significant in the 6 mo group. In conclusion, the small differences in the density of VGLUT1-IR contacts detected between single- and dual-labeled motoneurons seems better explained by diminished efficiency in CTb uptake by axons from motoneurons with fewer VGLUT1-IR contacts than by innervation of wrong targets in the periphery.
We noted that VGLUT1-IR clusters on injured motoneurons were usually smaller than on control motoneurons. Twenty to forty VGLUT1-IR clusters were measured (maximum length) in each animal over control and experimental motoneurons, and their sizes always showed nonnormal distributions with long tails of relative large clusters. Many of these large-size clusters were not present on injured motoneurons. The median size of VGLUT1-IR clusters on control motoneurons in different animals ranged from 2.6 to 3.3 μm, and the differences among animals were not statistically significant (P = 0.062, ANOVA on ranks). However, VGLUT1-IR clusters on injured motoneurons were always significantly smaller compared with the control side (P < 0.05, rank sum tests) at postinjury times of 2 wk and greater. Percent decreases in average median length ranged from 25% to 49% of the control length in different animals, with an average of 34.4 ± 7.5 (±SD). No clear trend was detected in relation to nonregenerating versus regenerating afferents or postinjury time after 2 wk. At earlier times decreases in size were smaller (range 8–18% decrease of control size) and, with the exception of the 1 wk regenerating animal, the differences with their respective controls were not statistically significant (P > 0.05, rank sum tests). These decreases in size could be large enough to result in undersampling of the smaller VGLUT1-IR contacts compared with larger VGLUT1-IR clusters (which extend through more optical sections) in analyses performed at selected confocal optical sections separated by 2 μm or more in the z-axis. Therefore, we reanalyzed some experiments by fully reconstructing the somatic cell surfaces contained within the histological 50-μm-thick section and counting all VGLUT1 contacts detected on their surface to obtain a surface density (number of contacts per 100 μm2 of somatic surface). Although within this section thickness full cell body reconstructions were rarely obtained, we analyzed in all motoneurons >50% of their surface. We selected for reanalysis animals at 2 and 12 wk and 6 mo postinjury after either cut and ligation or cut and reunion. Percentage depletions comparing linear densities measured in selected optical sections were always larger than when comparing experimental and control motoneuron surface densities obtained by analyzing the whole somata contained in the histological section. At 2 wk after injury we estimated 59% (cut and reunion) and 56% (cut and ligation) depletions based on linear density and 44% depletions in both animals when analyzing surface densities. At 12 wk estimated depletions in linear density were of 90% (cut and reunion) and 80% (cut and ligation), and these decreased to 85% and 76%, respectively. Finally, at 6 mo after injury linear density depletions were estimated at 96% (cut and reunion) and 75% (cut and ligation), and after surface analyses this was reduced to 81% and 51%, respectively.
In conclusion, total surface reconstructions allowed us to detect a few more of the smaller terminals, and this slightly reduced the estimated percentage reductions in density of VGLUT1-IR boutons around cell somata of experimental motoneurons. However, the estimated change in synaptic covertures was rather similar with either method and did not change the overall conclusions. The reasons for the relatively small differences between both methods are likely due to the fact that most of the bouton size change involves the disappearance after injury of the larger VGLUT1-IR clusters and these represent a relatively small proportion of all clusters (see further analyses on bouton size changes below).
Other Synapses on the Cell Soma of Motoneurons Recover at Different Rates With or Without Peripheral Muscle Innervation
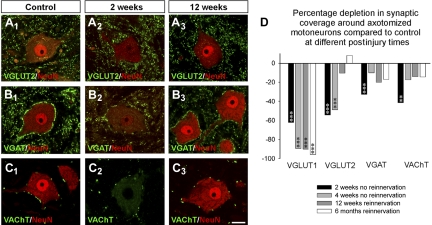
The lack of recovery of VGLUT1-IR synapses on the cell bodies of axotomized motoneurons after peripheral muscle reinnervation was a surprise and contrasted with the commonly held view that synaptic coverage largely recovers after peripheral reconnection with muscle. To confirm that this was the case also in our experiments, we analyzed at selected dates, 2 and 4 wk with regeneration prevented (TN cut and ligation) and 12 wk and 6 mo with reinnervation allowed (TN cut and rejoin), the linear density coverage on NeuN-IR motoneurons by VGLUT2-IR contacts (originated mostly from spinal interneurons; Todd et al. 2003), VGAT-IR contacts (originated in local inhibitory interneurons) and VAChT-IR boutons (which labels C-terminals originated in spinal cholinergic interneurons; Miles et al. 2007; Zagoraiou et al. 2009) (Fig. 6, A–C). In this case we analyzed 6–12 NeuN-IR motoneurons from one animal at each survival date and marker and compared with the depletions to VGLUT1 changes described previously in the same animals (Figs. 2 and 3). Each motoneuron was sampled through two to five optical planes depending on the cell body coverage by each synaptic marker (VGAT > VGLUT2 > VAChT; all display higher contact density on motoneuron cell bodies than VGLUT1). All synaptic markers were significantly depleted in the experimental side compared with the control side 2 wk after the injury, although depletions were less intense for inhibitory VGAT-IR terminals (32% depleted from control values) compared with VGLUT2-IR (54.3% loss) and VGLUT1-IR (62.2%; Fig. 6D) terminals. Four weeks after the injury and with no regeneration allowed (ligation), VGAT-IR terminals were partially recovered (10% depletion) while VGLUT2-IR synapses remained similarly depleted (48.8% loss) with no significant recovery. This result is in agreement with previous reports suggesting that inhibitory terminals are more resistant to synaptic stripping after axotomy and recover faster than glutamatergic excitatory terminals in experimental situations in which motor axons do not reinnervate muscle (Linda et al. 2000; Novikov et al. 2000). VGLUT1-IR terminals were, in contrast, further depleted (89.5% of synapses were lost). At longer survival times and with reinnervation allowed, VGLUT2-IR terminals showed better recovery than inhibitory VGAT-IR synapses at 12 wk and 6 mo after injury, also confirming a conclusion from a previous study using electron microscopy for synapse identification (Brannstrom and Kellerth 1999). VGLUT1-IR synapses did not recover in this situation, either.
Fig. 6.
Synaptic remodeling on motoneurons after TN injuries. A–C: examples of NeuN-IR motoneurons (red, Cy3) surrounded by synaptic contacts (green, FITC) labeled with different synaptic markers [VGLUT2 (A), vesicular GABA/glycine transporter (VGAT, B), vesicular acetylcholine transporter (VAChT, C)] and imaged in the control side (A1, B1, C1) or in the experimental side 2 wk after injury with regeneration prevented (A2, B2, C2) or 12 wk after injury with regeneration allowed (A3, B3, C3). NeuN immunoreactivity decreases at 2 wk but recovers at 12 wk with muscle reinnervation. All 3 synaptic markers are decreased by axotomy at 2 wk after injury, but also all 3 show significant recovery at 12 wk after successful peripheral regeneration. Although bouton densities surrounding the cell bodies were greatly recovered, the synaptic puncta labeled by these vesicular markers frequently appeared smaller than in the control side. D: % depletions for each synaptic marker and comparisons to VGLUT1 at 2 or 4 wk after injury with regeneration prevented (black and light gray bars, respectively) and at 12 wk (dark gray bars) and 6 mo (white bars) after injury with regeneration allowed (and completed by this time). Asterisks indicate significant depletions in experimental side compared with control for each marker and date (*P < 0.05, **P < 0.01, ***P < 0.001, t-test). VGLUT1 contacts were very significantly depleted at all postinjury dates. VGLUT2 terminals recover but only after reinnervation. VGAT and VAChT terminals, in contrast, recover at 4 wk in the absence of peripheral regeneration, and recovery is not significantly different after regeneration in the periphery is completed.
VAChT-IR terminals behaved similarly to inhibitory VGAT-IR terminals. Their density decreased by 41.5% of control 2 wk after axotomy but then recovered even when regeneration was prevented (Fig. 6D). VAChT-IR cholinergic synapses on motoneurons are considered a source of nonglutamatergic excitatory input to the motoneuron (Miles et al. 2007; Zagoraiou et al. 2009); thus excitatory synapse remodeling induced by peripheral nerve injuries is clearly different for inputs of different types and origins.
In conclusion, in our nerve injury and regeneration model most excitatory and inhibitory synapses are stripped and recovered as expected, but VGLUT1-IR synapses behave differently than other excitatory synapses after axotomy, being permanently depleted when regeneration is either allowed or prevented.
Depletions of VGLUT1 Synapses on Dendrites of Reinnervating Motoneurons
The results described above suggest a permanent removal of VGLUT1-IR synapses from LIX and around motoneuron cell somata; however, the vast majority of IA inputs target dendrites, and many of these contacts can occur outside LIX. Therefore we analyzed VGLUT1-IR contacts on the dendritic arbors of retrogradely labeled CTb-555 MG motoneurons to investigate whether this input became redistributed along these dendrites after injury. For this purpose, high-magnification confocal images of the CTb-labeled dendritic arbor were obtained for 10 motoneurons in control, 1 wk, 6 wk, and 6 mo postinjury animals, respectively (2–3 motoneurons analyzed per animal in each group), and analyzed with a Neurolucida cell tracing and reconstruction system (Fig. 7). Only motoneurons dual labeled with Fast Blue and CTb-555 were used in analyses at 6 wk and 6 mo after injury. Comparable numbers and size of CTb-labeled dendrites were sampled in each group (Table 2). The dendritic surface analyzed represents ∼10% of the total estimated for rat triceps surae motoneurons reconstructed in the rostro-caudal orientation after intracellular filling (Chen and Wolpaw 1994). Dendritic surfaces analyzed in our study are more limited because 1) we reconstructed CTb-labeled motoneurons from single 50-μm-thick sections instead of serial sections, 2) fluorescent CTb labeling does not extend into fine branches and higher-order dendrites, and 3) the dendritic arbor was analyzed in the transverse orientation and motoneuron dendrites usually have shorter lengths in the dorso-ventral and medio-lateral directions compared with the rostro-caudal direction. However, our samples include a significant portion of major dendritic branches (we studied on average 6 primary dendrites with a range of 4–10 per motoneuron) and dendritic segments contained within a single transverse section (see Fig. 7, E–H). Several dendrites oriented in dorso/medial-ventro/lateral axes were traced more than 500 μm distal to the cell body (maximum path distance recorded was 672 μm), but the quantitative analysis was restricted to a Sholl distance of 250 μm (Fig. 8), because few neurons contained enough labeled dendritic segments further away to constitute a representative sample. Dendritic segments included in the analyses coursed within the 50-μm-thick section an average 202.3-μm path distance from the cell body. Densities of VGLUT1-IR synapses (i.e., number of VGLUT1-IR contacts per 100 μm2 of dendritic surface) were compared at different distances from the cell body by partitioning the dendritic arbor into 50-μm Sholl bins up to 250 μm. The dendritic surfaces and lengths at the bottom of Table 2 refer to dendrite segments sampled within the 250-μm Sholl sphere around the cell body. Similar amounts of dendrite surface were sampled in control motoneurons and each experimental group at all Sholl bins.
Fig. 7.
VGLUT1 contacts are diminished on the proximal dendrites of MG motoneurons after peripheral nerve injury. A and B: low-magnification confocal images of 2 control motoneurons retrogradely labeled with CTb-555 (white) from the MG muscle and located in a section through the caudal lumbar 5 counterstained with Nissl (blue, 640-Neurotrace). CTb-555 labeling is shown in white to best demonstrate the extent of dendritic labeling within the ventral horn. VGLUT1-IR puncta (green, FITC) are superimposed in B. Inset: 2-dimensional projection of all VGLUT1-IR contacts on the cell body and proximal dendrites of the motoneuron indicated with an asterisk in B. C1 and C2: 2 confocal planes (z depths indicated from the surface of the section) of the cell body and dendrites of the same motoneuron. Arrows indicate positions of VGLUT1 contacts. D: Neurolucida reconstruction of the same cell showing skeleton outlines of dendritic tracings and optical planes through the cell body. VGLUT1 contacts are plotted on the dendrites (circles) and cell body (triangles). The Neurolucida tracing is superimposed in 1 optical plane of the confocal image stack. E: Neurolucida cell reconstruction of the same control motoneuron showing dendritic thicknesses, the positions of dendritic VGLUT1 contacts (black circles, somatic contacts are not shown), and 50-μm Sholl bins used in the analyses. F–H: VGLUT1 contacts plotted on motoneuron reconstructions 1 wk (F), 6 wk (G), and 6 mo (H) after injury. The largest loss of VGLUT1 contacts on dendrites is observed proximally, particularly in the first Sholl bin. Scale bars: in A, 250 μm (B at the same magnification); in inset, C, and D, 30 μm.
Table 2.
Somatic and dendritic surfaces and lengths sampled in CTb-labeled motoneurons
| Control | 1 wk | 6 wk | 6 mo | |
|---|---|---|---|---|
| Soma surface, μm2 | 5,508 ± 1,793 | 5,379 ± 1,768 | 5,805 ± 1,464 | 6,941 ± 1,403 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| Dendritic length, μm | 1,103 ± 285 | 1,074 ± 381 | 1,064 ± 348 | 1,096 ± 407 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| Dendritic surface, μm2 | 13,754 ± 4,140 | 12,658 ± 2,099 | 13,339 ± 4,821 | 13,293 ± 4,024 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| Sholl bins, dendritic surfaces analyzed, μm2 | ||||
| 50 μm | 3,675 ± 630 | 3,303 ± 840 | 3,338 ± 709 | 3,699 ± 1393 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| 100 μm | 4,994 ± 998 | 4,695 ± 1,791 | 4,906 ± 1,530 | 4,969 ± 1,214 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| 150 μm | 2,806 ± 1,382 | 3,016 ± 2,217 | 2,806 ± 1,624 | 2,561 ± 1,126 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| 200 μm | 1,471 ± 988 | 1,400 ± 1,628 | 1,835 ± 1,491 | 1,240 ± 715 |
| n = 10 | n = 9 | n = 9 | n = 9 | |
| 250 μm | 687 ± 673 | 778 ± 713 | 886 ± 711 | 626 ± 481 |
| n = 8 | n = 5 | n = 6 | n = 8 |
Values are means ± SD. CTb, cholera toxin b.
Fig. 8.
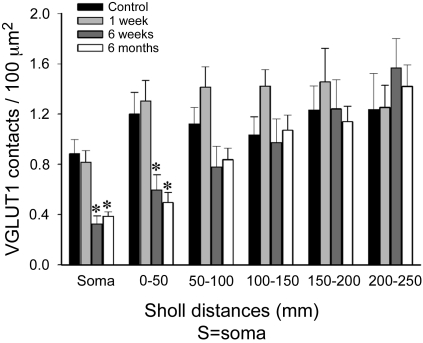
Quantitative analysis of VGLUT1 densities on the dendrites of MG motoneurons after peripheral nerve injury. Average VGLUT1 density was depleted on the soma and first and second 50-μm Sholl bins of motoneurons from 6 wk (dark gray bars) and 6 mo (white bars) animals compared with control (black bars) (n = 10 motoneurons analyzed in each group). However, these depletions were statistically significant only on the soma and most proximal dendritic bin (*P < 0.05, 1-way ANOVA followed by post hoc Bonferroni t-test comparisons of each experimental group with control). No significant depletions were observed 1 wk after injury at any location on the dendritic tree (light gray bars). Depletions in cell body and first 100 μm of dendrite represent ∼50% loss of VGLUT1 synapses, while in the dendritic arbor the overall loss was ∼25% because of the preservation (and even a nonsignificant slight increase) of VGLUT1 synapses at more distal locations.
In control motoneurons we found a remarkably similar VGLUT1-IR contact density along dendrites up to the maximum Sholl bin analyzed (Fig. 8). No statistical significant differences in average density were found between different distance bins (50, 100, 150, 200, and 250; P = 0.951, 1-way ANOVA on ranks). In contrast, the total number of contacts counted in each bin decreased significantly 100 μm distal to the cell body [44.8 ± 7.2 (SE) VGLUT1-IR contacts in bin 50; 52.9 ± 3.4 in bin 100; 27.3 ± 4.6 in bin 150; 14.8 ± 2.5 in bin 200; 5.9 ± 1.7 in bin 250]. Thus the number of VGLUT1 contacts at different distances from the cell body seems to vary in parallel to the amount of available dendritic surface (which depends on the degree of dendrite tapering and branching) for at least the first 250 μm of dendrite. As a result the VGLUT1-IR boutons counted in the cell soma and first 100 μm of dendrite, the regions with the most of the available surface area (Table 2), represent 74.4 ± 3.5% (± SE) of all VGLUT1-IR synapses detected.
VGLUT1-IR synapse density was significantly decreased (50–60% depletion with respect to control values; P < 0.01, post hoc Bonferroni t-test) in the first Sholl bin (0–50 μm) in motoneurons 6 wk and 6 mo after injury compared with control animals (Fig. 8). In the second Sholl bin (50–100 μm) we also detected a decrease (25–30% depletions) at the same postinjury times, but because of the large variability in the density of contacts on different dendrites the difference did not reach statistical significance. No differences were observed in more distal dendritic segments in motoneurons 6 wk and 6 mo after injury or in dendrites at any Sholl distance from motoneurons 7 days after the injury. In conclusion, peripheral injury caused the permanent removal of 45–50% of all VGLUT1-IR contacts on the somata and first 100 μm of dendrite, where they are more numerous, at 6 wk and 6 mo after injury. No changes were observed in the dendritic arbor at Sholl distances from 100 to 250 μm, and no detectable changes were found on dendrites at short postinjury times (1 wk).
MG Motoneurons Receive Monosynaptic EPSPs from Muscle Sensory Afferents
The findings presented above demonstrate extensive loss of VGLUT1-IR synapses from motoneuron soma and proximal dendrites. In the case of TN injury, the losses occur under conditions in which all primary afferent synapses with tibial motoneurons, both homonymous and heteronymous, are presumably directly affected by the injury pre- and postsynaptically. We sought to determine whether these conditions and their failure to reverse with regeneration resulted in a larger failure to recover functional monosynaptic connectivity as tested by electrical stimulation of the peripheral nerve distal to the injury. In previous studies performed in the more restricted situation in which only the MGN was injured, functional connectivity revealed by electrically evoked monosynaptic EPSPs was present but motoneurons failed to respond to stretch (Haftel et al. 2005). Similarly, all 20 tibial motoneurons sampled from five rats ∼6 mo after TN reunion produced EPSPs in response to TN electrical stimulation (Fig. 9). The latencies from stimulus to EPSP onsets were from 1.7 to 3.3 ms (average: 2.6 ± 0.1 ms), within the range obtained in our earlier study of TN EPSPs in control animals (Bichler et al. 2007) consistent with monosynaptic transmission. It is unknown how the amplitudes of these EPSPs compare to control, since stimulus strength was variably adjusted to levels just below antidromic action potential threshold for each recorded motoneuron (see methods), and therefore submaximal for activating all large-diameter afferents. These results suggest that remaining VGLUT1 synapses can sustain functioning monosynaptic connections, although possibly reduced in strength. Of nine motoneurons tested for synaptic response to stretch of the MG muscle 1 yr after TN cut and reunion, only three displayed weak responses (not shown). Because the TN motoneurons were not further differentiated by their motor pool membership, it is possible that some of the motoneurons recorded did not belong to the MG or synergistic motor pools and, therefore, would not normally be expected to receive monosynaptic input evoked by MG muscle stretch. Even so, the proportion of motoneurons that did not respond to stretch is similar to that in previous studies after self-reattachment of the cut MG nerve (Haftel et al. 2005; Bullinger et al. 2011).
Fig. 9.
Group I excitatory postsynaptic potentials (EPSPs) in motoneurons that regenerated peripherally after cut and reunion of the TN. A: example of a motoneuron antidromic action potential evoked by stimulation of the TN distal to the nerve injury. B: muscle twitch registered in the MG muscle by the same stimulus to the TN and demonstrating successful muscle reinnervation. C: superimposition of compound EPSPs recorded in all 20 motoneurons evoked by stimulation of the TN distal to the injury (arrow indicates stimulation artifact). Each EPSP trace is the average of 10–15 trials elicited at 2 Hz. All recorded motoneurons showed the presence of EPSPs, but their amplitude varied from cell to cell. Stimulation intensity was adjusted such that EPSPs were revealed without contamination from antidromic or orthodromic action potentials. Therefore it was not possible to measure the maximal amplitudes in response to the largest possible axon recruitment by the peripheral stimulus in the tibial nerve. However, 17 of the 20 EPSPs were smaller than 2 mV, while on average TN EPSPs measured in similar conditions are ∼5 mV in amplitude and almost always larger than 2 mV (Bichler et al. 2007).
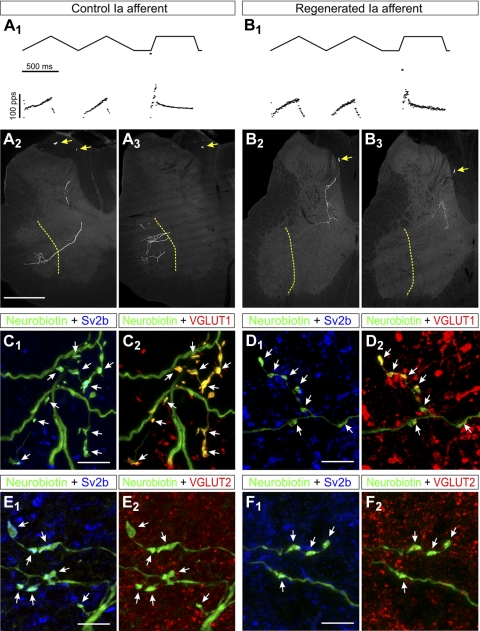
Control and Regenerated IA Afferent Synaptic Varicosities Contain VGLUT1, But the Central Synaptic Varicosities of Regenerated IA Afferents Retract from Lamina IX
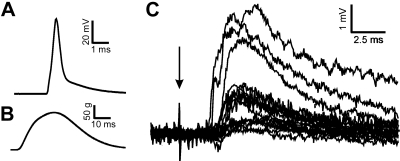
The loss of VGLUT1-IR contacts on motoneuron cell bodies and dendrites could be explained by the removal of IA afferent synapses or, alternatively, downregulation of VGLUT1 expression in the synaptic boutons of IA afferents, or perhaps a phenotypic switch from VGLUT1 to VGLUT2 in injured IA fibers. To test these possibilities we intra-axonally impaled MG sensory fibers in the dorsal roots and recorded their responses to stretch in control animals and in animals that regenerated their peripheral afferent axons after MGN transection and self-reattachment. IA afferents with normal stretch responses (Fig. 10, A 1 and B1) were filled intra-axonally with neurobiotin (Fig. 10, A2,3 and B2,3), and their central varicosities were tested for expression of VGLUT1 (Fig. 10, C and D) or VGLUT2 (Fig. 10, E and F) in their presynaptic vesicle clusters identified by immunohistochemistry against the synaptic vesicle protein SV2 (Buckley and Kelly 1985).
Fig. 10.
Trajectories and VGLUT immunoreactivities of IA afferent fibers in control animals or after peripheral nerve injury and regeneration. A1 and B1: responses of control (A1) and regenerated (B1) sensory afferents to triangular and ramp and hold muscle stretches. The muscle stretch stimulus is shown in the top traces, and the recorded responses depicted as firing frequency time plots are shown in the bottom traces. Both fibers faithfully encode muscle stretch parameters with dynamic responses typical of IA sensory afferents, similar levels of static response during hold phases, and similar history dependence of the initial burst in triangular stretches. Stretch responses were indistinguishable in control and regenerated afferents. A2 and A3: low-magnification epifluorescence images of 2 semiserial spinal cord sections containing labeled collaterals of the sensory axon with stretch responses shown in A1. Segments of the parent axons are visible in the dorsal columns (arrows) and collaterals with terminal branches in laminae V, VII, and IX are easily observed (border between laminae IX and VII is labeled with a dashed yellow line). The arborization in lamina IX was consistently very profuse in all control afferents. B2 and B3: images similar to A2 and A3 but for labeled collaterals from a regenerated axon. Note the parent axon in the dorsal column (arrows) and similar dorsomedial to ventrolateral trajectories of the central collaterals innervating the spinal cord, but these stop before entering lamina IX. C: high-magnification confocal microscopy of a varicose collateral from a neurobiotin-filled IA afferent (FITC-streptavidin, green) with several boutons (arrows) containing immunoreactivity for the synaptic protein SV2b (C1, blue, Cy5) and VGLUT1 (C2, red, Cy3). There was always a perfect correspondence between SV2b-containing varicosities and VGLUT1. D: similar high-magnification images of varicosities (arrows) from a regenerated afferent. All varicosities contained Sv2b (D1) and VGLUT1 (D2); however, neurobiotin-filled varicosities (FTIC, green in D1 and D2) appear of smaller size than in control uninjured afferents. E and F: similar sequence of images but for sections immunolabeled with SV2b and VGLUT2. SV2b-IR neurobiotin-filled varicosities (arrows) in control (E1) and regenerated (F1) afferents lack visible VGLUT2 immunoreactivity (E2 and F2). Scale bars, 500 μm in A1 (A2, B1, and B2 are at the same magnification); 10 μm in C1, D1, E1, and F1 (C2, D2, E2, and F2 are at the same magnification).
We attempted to inject from one to three IA afferents with normal IA-type stretch responses (see Bullinger et al. 2011) in four control and four experimental animals with regenerated afferents, but in all cases one afferent was preferentially labeled based on the time and current intensities of the injections. Twelve hundred and nine SV2-IR neurobiotin-labeled synaptic boutons were analyzed (112–192 boutons per animal; see Table 3). Synaptic varicosities in all three main projection regions of IA afferents in the spinal cord (laminae IV–V, VII, and IX; Fyffe 1992) were recovered in all four control animals used in the VGLUT study. Projections to all three laminar locations were confirmed in IA afferents injected in three more animals in which synaptic varicosities were not identified with SV2. In contrast, only one of the four animals containing regenerated afferents with IA-like responses displayed a few collaterals entering LIX, but their synaptic nature could not be confirmed. In one experimental animal, afferent collaterals with synaptic varicosities were recovered only from lamina V (LV). Lack of collaterals entering LIX in regenerated afferents was confirmed in another fiber from a different animal in which immunodetection of synaptic markers was not performed (not shown). The animals were analyzed 8–16 mo after MGN surgeries, thus long after reinnervation of the MG muscle by motor and sensory axons. The lack of collaterals from stretch-sensitive afferents entering LIX (only 1 fiber of 5 injected) compared with controls (7 of 7) agrees well with the reduction in VGLUT1 varicosities demonstrated earlier.
The average intensity of VGLUT1 and VGLUT2 fluorescence and bouton size were measured in afferent synaptic varicosities (i.e., neurobiotin filled varicosities with clear SV2 immunoreactivity; Fig. 10, C–F) that were then grouped according to laminar location (laminae IV–V, VII, and IX). The average immunofluorescence intensity measured in each varicosity was normalized against background density and compared between control and regenerated fibers (see methods). All afferent synaptic varicosities contained VGLUT1 immunoreactivity 4 standard deviations (SD) above the background fluorescence level in both IA fiber controls and regenerated fibers with IA-like responses to stretch (Fig. 11A). Average VGLUT1 immunofluorescence inside IA afferent varicosities was 10.9 ± 0.7 (±SE) times the background in controls and 11.1 ± 1.1 in regenerated afferent fibers (n = average of 4 estimates in control animals and 4 experimental; P = 0.9, t-test) (Fig. 11B). Moreover, the distribution of VGLUT1 immunofluorescence intensities inside all the boutons analyzed was similar in control and regenerated fibers (Fig. 11C; P = 0.146, Kolmogorov-Smirnov). No significant differences were noted in VGLUT1 immunofluorescence in boutons located in different laminae from control or regenerated fibers.
Fig. 11.
VGLUT1 immunoreactivity is retained in the central synaptic boutons of regenerated IA fibers, but the boutons become smaller. A: average immunofluorescence intensities for VGLUT1 and VGLUT2 in the central terminals of IA afferents recovered in control (gray bars, n = 335 boutons) and experimental (black bars, n = 331 boutons) animals. White dots indicate the average background fluorescence in all images (confocal microscope offsets were always 0). Background fluorescence was distributed very narrowly, as indicated by the lines indicating the upper and lower confidence limit intervals containing 99.9% of the distribution of background values. B: average normalized VGLUT1 (gray bars) and VGLUT2 (black bars) immunofluorescence intensity in each of the 4 control and experimental animals (the immunofluorescence of each varicosity was divided by the local background fluorescence calculated from 3 adjacent regions of equal size to the varicosity, see methods). No values for VGLUT2 were obtained in experimental animal 3 because in this animal the immunoreaction was abnormally weak and did not penetrate enough into the tissue. C: cumulative probability functions of the distributions of immunofluorescence densities for VGLUT1 and VGLUT2 normalized against background (bck). Kolmogorov-Smirnov tests showed no differences between the distribution of intensities in control (circles) and experimental (diamonds) values for VGLUT1 (gray plots) or VGLUT2 (black plots). D: maximum projection of the cross-sectional area of neurobiotin-labeled synaptic boutons (aka SV2b-IR) in the 4 control and 4 experimental animals. The average cross-sectional areas of all IA afferent synaptic boutons in experimental animals were significantly smaller than any of the control animals (P < 0.001, ANOVA on ranks, followed by post hoc Dunn's test pairwise comparisons, *P < 0.05 in all cases). E: probability distributions of bouton sizes in each individual animal (dotted lines) and average distributions for control and experimental groups shown in thicker line plots (gray, control; black, experimental). Bouton sizes show a significant skew toward relatively large boutons in the control distribution. In regenerated afferents most of the boutons are small and many of the larger boutons are not present. Thus the bouton population in regenerated afferents does not scale back in size proportionally from control IA boutons, but rather regenerated afferents generally lack large boutons, increasing the probability of smaller boutons in the population. F: cross-sectional bouton areas in the 4 control and 4 experimental animals distributed by laminar location. N.D. indicates that no boutons were detected in lamina IX from any of the regenerated IA afferents. In 1 experimental animal no boutons were detected in lamina VII, either. Remaining boutons in laminae V and VII were on average smaller in the 4 experimental animals compared with the control populations (P < 0.001, ANOVA on ranks, followed by post hoc Dunn's test pairwise comparisons, *P < 0.05 in all cases). Error bars indicate SE in all histograms.
Nevertheless, the average size of synaptic varicosities (measured in the largest cross-sectional area of their neurobiotin labeling) was significantly smaller in regenerated afferents compared with controls [regenerated boutons: 2.8 ± 0.1 μm2 (±SE), 137 ± 30 (±SD) boutons analyzed per animal/fiber; control boutons: 5.3 ± 0.4 μm2, 165 ± 29 boutons per animal/fiber; P < 0.001, t-test]. The decrease in bouton size was consistent in all four animals with regenerated fibers (Fig. 11D). Smaller boutons contain smaller VGLUT1-IR vesicle clusters (as noted previously) but maintain similar average fluorescence intensities. Control IA afferent bouton size distributions were heavily skewed, with the majority of boutons being of relatively small size and a smaller proportion distributed through a long tail of larger sizes. In regenerated fibers, large-size boutons are greatly reduced in number such that size distributions become more focused around the median (2.1–2.6 μm2 in area in regenerated afferents; Fig. 11E). Bouton sizes were similarly reduced in all laminae (Fig. 11F).
VGLUT2 immunofluorescence was minimal in control IA afferent varicosities and did not increase in regenerated afferents (Fig. 11, A–C). We noted, however, that in our measurements VGLUT2 fluorescence was consistently higher than background (on average controls were 1.70 ± 0.06 times background, experimental 1.79 ± 0.01; P = 0.32, t-test). This low fluorescence was, however, usually not noticeable to the experimenters in the images but only resolved quantitatively, in part because of the extremely low background of our preparations (usually <10% of the intensity of immunofluorescence signals present in well-labeled varicosities). The low VGLUT2 signal in neurobiotin synaptic varicosities could arise either because of extremely low levels of VGLUT2 (we described previously that low levels of VGLUT2 can be reliably detected with quantitative colloidal gold electron microscopy immunocytochemistry in some synapses from cutaneous mechanoreceptors also containing strong VGLUT1; Alvarez et al. 2004) or because of a small crossover of neurobiotin or SV2 fluorescence in the VGLUT2 channel. Because of the very low levels and the lack of differences between control and regenerated fibers, we did not pursue this issue further.
We conclude that VGLUT2 is not upregulated in IA afferents after injury and that the density of VGLUT1-IR vesicles is similar in IA boutons in control and experimental animals independent of their size.
DISCUSSION
The major finding of this study is the progressive elimination of VGLUT1-IA afferent synapses from LIX and proximal somatodendritic regions of the motoneuron surface as a consequence of peripheral nerve injury. This process proceeds with independence of ongoing regeneration and muscle reinnervation in the periphery and results in a permanent reduction of the number of sensory synapses on the motoneuron. These observations cannot be explained by VGLUT1 downregulation. Intracellular labeling of regenerating afferent axons with IA-like responses to stretch combined with the observed decreases in VGLUT1 labeling suggest a significant retraction of IA afferent axon collaterals and synapses from LIX as a consequence of nerve injury. These are not recovered even long after regeneration in the periphery has been completed. The anatomical data therefore suggest that, after peripheral nerve injury, motoneurons permanently lose a significant number of IA afferent synapses. Therefore the recovery of relatively normal electrically evoked afferent monosynaptic EPSPs in motoneurons after muscle reinnervation cannot be explained by reestablishment of lost IA afferent synapses. In fact, the lack of recovery of lost VGLUT1 IA afferent synapses best correlates with a significant functional disconnection between sensory afferents and motoneurons demonstrated in the companion article (Bullinger et al. 2011).
IA Afferent Synapses Are Unique in Being Permanently Lost After Peripheral Nerve Injuries
The loss of VGLUT1-IA afferent synapses resembles the well-known and well-studied phenomenon of synaptic stripping on axotomized motoneurons (Blinzinger and Kreutzberg 1968; Brannstrom and Kellerth 1998, 1999; Chen 1978; de la Cruz et al. 1994; Linda et al. 1992, 2000; Novikov et al. 2000; Sumner 1975, 1976; Sumner and Sutherland 1973); however, there are also important differences: 1) losses of VGLUT1 synapses on the motoneuron cell soma were disproportionally higher than other synapses; 2) the time course of VGLUT1 synaptic loss was prolonged compared with other synaptic inputs and continued after the first week postinjury independent of whether sensory and motor axons were in the process of regeneration and muscle reinnervation; 3) VGLUT1 synaptic loss was accompanied by a significant retraction of the preteminal axonal arbor; and 4) VGLUT1 synapses did not recover to any significant extent after the peripheral sensory apparatus encoding muscle stretch was recovered. In fact, it is possible that in this study we underestimated VGLUT1 synaptic losses. The largest loss of VGLUT1 puncta occurs in LIX regions occupied by motor pools projecting axons in the TN; however, because of the transverse cut of our sections and the fact that we studied the dendrites of retrogradely CTb-labeled motoneurons in single sections, we undersampled rostrocaudal dendrites traveling mainly in this LIX region. Because of the section orientation, the analyses were biased toward transversely oriented dendrites. These dendrites usually course into lamina VII (LVII) after relatively short trajectories in LIX. We always observed a lesser amount of VGLUT1 synaptic losses in LVII compared with LIX. This caveat, however, does not prevent us from concluding that a large number of VGLUT1-IA afferent synapses (perhaps larger than estimated) are permanently lost from the motoneuron somatodendritic surface. In contrast, VGLUT2, VAChT, and VGAT synapses were restored.
These conclusions were further supported by the trajectories of intracellularly filled regenerated afferents with IA-like muscle stretch sensitivity. A common interpretation problem in analyses of regenerated afferents is the difficulty in assessing the nature before injury of the afferents recorded after regeneration. Primary afferents are rather unspecific when choosing regeneration pathways in the periphery, and it is common for an afferent to innervate the wrong tissue or peripheral sensory receptor. For example, it is well known that IB afferents (and other afferents), which do not project to LIX, can innervate muscle spindles during regeneration after nerve injury (Banks and Barker 1989). Moreover, IA afferents and proprioceptors can easily innervate skin and cutaneous receptors (Koerber et al. 1994; Lewin and McMahon 1991). Therefore it is possible that some of the stretch-sensitive afferents filled in this study were originally IB or type II proprioceptive afferents that do not extend projections to LIX. However, a significant deletion of central synapses is demonstrated by the loss of VGLUT1 synapses and is also in agreement with the conclusions of Koerber et al. (1994), who demonstrated major modifications of the central boutons of intra-axonally filled sensory afferents after axotomy and regeneration in the cat. In that study most proprioceptive axons regenerated to skin territories after a TN injury. Centrally, only two of six proprioceptive afferents displayed boutons in LIX after regeneration. LIX-projecting afferents were classified as putative preinjury IA afferents and non-LIX-projecting afferents assumed to be IB or type II afferents before the injury. However, Koerber et al. (1994) also reported that the central projections appeared abnormal (in fact, few collaterals in LIX are illustrated), and, interestingly, cord dorsum potentials were recorded from activity in regenerated proprioceptors (which is not the case in normal animals), suggesting an anomalous enhancement of the more dorsal synaptic connections from these fibers. The large depletions of VGLUT1 synapses from LIX that we report here strongly suggest major modifications of the central connections of regenerated IA proprioceptors. Therefore, intra-axonally filled IA-like stretch-responsive afferents lacking LIX projections after peripheral regeneration could represent fibers that before the injury were IB, II, or IA afferents, with their distal collaterals now retracted in the latter case. Moreover, in our companion electrophysiological study (Bullinger et al. 2011) we found that muscle stretch and single stretch-responsive afferent fibers connected to some motoneurons produced synaptic potentials in many fewer homonymous motoneurons than normal, suggesting a significant functional retraction of connections. Taken together, our data in the rat and the data of Koerber et al. in the cat can now be best interpreted by suggesting a significant anatomical retraction (with resulting loss of function) of the more distal synaptic collaterals of many IA afferents that extensively projected to LIX before the nerve injury.
The loss of IA afferent synaptic innervation in LIX suggests involvement of unique mechanisms, different from those affecting synaptic stripping of other excitatory and inhibitory synapses around the cell body of axotomized motoneurons. The fact that these differences were overlooked despite extensive investigation of the mechanisms of synaptic reorganization on the surfaces of motoneurons following peripheral nerve injury is surprising. The most likely explanation is failure to positively identify IA afferent synapses from other excitatory synapses on motoneuron cell bodies and dendrites with methods used in previous studies (ultrastructural identification of excitatory synapses or glutamate immunoreactivity). Moreover, IA afferent synapses constitute a very small percentage (∼2%; Burke and Glenn 1996; Fyffe 2001) of all the excitatory inputs to motoneurons, particularly on cell bodies (where most studies on “synaptic stripping” have been carried out). Thus a large depletion of IA synapses would contribute little to the overall population of excitatory synapses, and for this reason it might have gone unnoticed in previous anatomical studies. The use of a marker for these synapses (VGLUT1) allowed us to overcome these difficulties.
In addition, the results suggest there are important differences in synaptic plasticity of excitatory synapses on motoneurons after nerve injuries that are dependent on the source and type of input. VGLUT2 inputs on motoneurons arising from a diversity of excitatory interneurons (Bannatyne et al. 2006, 2009; Jankowska et al. 2009; Todd et al. 2003) recover well, but only after peripheral muscle reinnervation. In contrast, cholinergic synapses controlling motoneuron excitability (Miles et al. 2007; Zagoraiou et al. 2009) recover after peripheral nerve injury independent of successful peripheral regeneration and muscle reinnervation and behave similar to VGAT synapses, with origins most likely in premotor inhibitory interneurons. These differences necessarily must result in changes in the relative weights among excitatory inputs from different sources during firing modulation of motoneurons and should be considered in addition to the known differences between inhibitory and excitatory synapses after peripheral injuries (Brannstrom and Kellerth 1998, 1999; Davis-López de Carrizosa et al. 2009; Linda et al. 1992, 2000; Novikov et al. 2000). Changes in the composition of excitatory drive and in the balance between excitation and inhibition both have the potential of significantly altering the properties of synaptic integration in motoneurons after nerve injury and regeneration.
IA afferent inputs differ from other inputs to motoneurons in that they themselves are also injured in the periphery. Given that there is no evidence for cell death of proprioceptive neurons after peripheral nerve injuries (Tandrup et al. 2000) the strong retraction of their central collaterals might represent a response to peripheral axotomy. Whether this is due to a reaction to injury or to the loss of neurotrophic signals from the periphery is unknown. A firm neurotrophic candidate is muscle spindle-derived neurotrophin-3 (NT3), which is known to exert important actions for maturing and maintaining the strength of IA afferent central synapses during development and also after injury (Arvanian et al. 2003; Arvanov et al. 2000; Chen et al. 2002; Mendell et al. 1999; Munson et al. 1997; Shneider et al. 2009; Wang et al. 2007; reviewed in Mentis et al. 2010 and Munson et al. 1999). Chronic supply of NT3 after injury prevents synaptic stripping of phasic inputs in oculomotor motoneurons (Davis-López de Carrizosa et al. 2009) and the normal decay of the peak amplitude of IA-motoneuron EPSPs in lumbar spinal motoneurons after peripheral nerve injuries (Mendell et al. 1999; Munson et al. 1997). It is thus possible that stripping of IA afferent synapses and retraction of LIX-directed IA afferent axon collaterals occur as a consequence of disconnection with muscle and downregulation of NT3 trophism. However, reinnervation of intrafusal muscle fibers restores NT3 expression by muscle spindles (Copray and Brouwer 1997), but this does not reestablish central connectivity. Therefore, muscle spindle NT3 might not be sufficient to induce regrowth of IA afferent collaterals into adult LIX. The adult spinal cord, similar to other central nervous system regions, might constitute an environment that disfacilitates IA afferent axon growth (Huebner and Strittmatter 2009). In this situation, application of compounds that block endogenous axon growth inhibitors or overcome their effects might be necessary to promote central IA afferent axonal regrowth.
Functional Implications of Lost IA Afferent/VGLUT1 Input and the Nature of Remaining Distal VGLUT1 Synapses
Electrically evoked IA afferent monosynaptic EPSPs on motoneurons exhibit changes after peripheral nerve injury that parallel the general behavior and time course of synaptic stripping and recovery of excitatory synapses with muscle reinnervation (Eccles et al. 1960, 1962; Goldring et al. 1980; Haftel et al. 2005; Kuno and Llinas 1970; Mendell and Scott 1975; Mendell et al. 1995). Logically, changes in IA-motoneuron EPSP amplitude and kinetics were argued to parallel changes in synaptic stripping and recovery of synapses, including IA afferent synapses, in axotomized neurons (Mendell 1988; Titmus and Faber 1990). However, it has now become clear that the recovery of electrically evoked monosynaptic EPSPs from sensory afferents onto motoneurons following peripheral reinnervation is not synonymous with the recovery of function in the stretch reflex, the recovery of stretch-evoked synaptic potentials on motoneurons, or even stretch-evoked modulation of motoneuron firing (Bullinger et al. 2011; Cope et al. 1994; Haftel et al. 2005). None of these shows significant recovery after peripheral regeneration, similar to the permanent changes on IA afferent/VGLUT1 synapses.
These results raise questions not only about proposed mechanisms for the recovery of electrically evoked EPSPs in regenerated motoneurons but also about their functional significance. Obviously, the recovery of electrically evoked sensory afferent EPSPs in motoneurons does not necessarily imply the recovery of useful muscle stretch signals reaching the motoneuron cell body. Recuperation of sensory afferent EPSPs might be related to strengthening or enhanced transmission toward the cell body of synaptic currents generated in remaining, mostly distal, synapses and not to recovery of lost synapses. In addition, it appears that a normal density of proximal synapses is necessary for conveying muscle stretch information to the motoneuron. The simplest explanation for this surprising result is that remaining VGLUT1 synapses are either too weak or too inhibited (for example by presynaptic inhibition) to transmit stretch information. Alternatively, they might originate from axons not connected peripherally to muscle spindles.
Nonspindle sources of VGLUT1 synapses in the ventral horn include IB afferents and the corticospinal tract. The exact trajectories of IB afferents and their connectivity with motoneurons have not been thoroughly studied in the rat, and therefore it is possible that IB afferents express higher connectivity with motoneurons than in the cat (Brown and Fyffe 1979). However, IB tendon-organ terminals are also sensitive to muscle stretch (Haftel et al. 2005), and if they were monosynaptic on rat motoneurons their synaptic activity should have translated into some depolarization of the motoneuron membrane during ramp and hold stretches. Another possibility is that at least some of the retained VGLUT1 synapses belong to corticospinal tract fibers (Alvarez et al. 2004; Betley et al. 2009; Persson et al. 2006), but the presence of a significant number of direct corticospinal connections on rat motoneurons is controversial. Direct corticospinal inputs have been demonstrated on cervical motoneurons involved in fine voluntary movement of hand digits in primates (reviewed in Lawrence et al. 1985). In rat motoneurons very few contacts were revealed by dual labeling of corticospinal axons and cervical motoneuron dendrites (Liang et al. 1991), and these were argued not to establish synaptic contacts when studied with electron microscopy (Yang and Lemon 2003) and not to mediate monosynaptic cortico-motoneuronal EPSPs (Alstermark et al. 2004). To our knowledge there are currently no anatomical data for direct connections on lumbar rat MG motoneurons. In conclusion, direct VGLUT1 corticospinal synapses on lumbar MG motoneurons might be few, if any at all, and thus if a significant number of VGLUT1 synapses on regenerated MG motoneurons belong to corticospinal axons it would imply significant sprouting of this input and formation of new connections as a result of injury. Currently there is no experimental evidence for this phenomenon.
Therefore, the most plausible explanation for VGLUT1 synapses on regenerated MG motoneurons is that a proportion of distal VGLUT1 spindle-afferent synapses are retained after injury. It is known that synaptic stripping following peripheral nerve injury is more intense for synapses in the more proximal somatodendritic regions (Brannstrom and Kellerth 1998); however, it is not clear whether all IA afferents that remain connected centrally are capable of reinnervating muscle spindles peripherally. If many VGLUT1 synapses belong to IA afferents that failed to reconnect with spindles peripherally it would explain why a proportion of the remaining VGLUT1 synapses fail to transmit any stretch information. Moreover, it would imply that retention of some central connectivity between IA afferents and motoneurons in adults is independent of muscle spindle reinnervation. Finally, it is possible that some belong to heteronymous IA afferents that travel in nerves other than the injured TN, but these sources have not yet been characterized. While these possibilities necessarily need to remain speculative until further experimentation, it is clear that the synaptic losses described here are translated into a dramatic reduction in connectivity between spindle-responsive afferents and motoneurons, but not between electrically excitable group I afferents and motoneurons, as demonstrated and discussed further in the companion paper to this article (Bullinger et al. 2011).
In summary, the described retraction of VGLUT1 IA afferent synapses from motoneuron proximal regions likely contributes significantly to the permanent reduction, and in many cases absence, of stretch-evoked synaptic influences on motoneurons that otherwise successfully regenerated in the periphery.
GRANTS
This work was funded by National Institute of Neurological Disorders and Stroke Program Project Grant P01-NS-057228.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Lori Goss for her excellent surgical work in all the animals used in this study, Ricardo Zerda for his histological help with some of the preparations, and Eileen Fitzsimons and N'Koli Upkabi for their invaluable histological help at the beginning of this project. In addition, we thank Drs. Mark Rich, Kathy Engisch, and Robert Fyffe for their very helpful discussions and insights during the development of this project and the preparation of this manuscript.
REFERENCES
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000 [DOI] [PubMed] [Google Scholar]
- Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol 91: 1832–1839, 2004 [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann NY Acad Sci 1198: 231–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, McMillin P, Fyffe RE. Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study. J Physiol 515: 787–797, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Nardelli P, Bullinger KL, Ukpabi N, Crum JM, Zerda R, Kraszpulski M, Cope TC. VGLUT1 content in central synapses of normal and regenerated Ia afferents (Abstract). 2008 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2008, Program No. 74.1 (online) [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004 [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101: 485–498, 2000 [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Horner PJ, Gage FH, Mendell LM. Chronic neurotrophin-3 strengthens synaptic connections to motoneurons in the neonatal rat. J Neurosci 23: 8706–8712, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Seebach BS, Mendell LM. NT-3 evokes an LTP-like facilitation of AMPA/kainate receptor-mediated synaptic transmission in the neonatal rat spinal cord. J Neurophysiol 84: 752–758, 2000 [DOI] [PubMed] [Google Scholar]
- Banks RW, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol 408: 345–372, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci 26: 2871–2880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol 587: 379–399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139: 161–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichler EK, Nakanishi ST, Wang QB, Pinter MJ, Rich MM, Cope TC. Enhanced transmission at a spinal synapse triggered in vivo by an injury signal independent of altered synaptic activity. J Neurosci 27: 12851–12859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat 85: 145–157, 1968 [DOI] [PubMed] [Google Scholar]
- Brannstrom T, Kellerth JO. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp Brain Res 118: 1–13, 1998 [DOI] [PubMed] [Google Scholar]
- Brannstrom T, Kellerth JO. Recovery of synapses in axotomized adult cat spinal motoneurons after reinnervation into muscle. Exp Brain Res 125: 19–27, 1999 [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol 296: 215–226, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Butler RG. Regeneration of afferent and efferent fibres to muscle spindles after nerve injury in adult cats. J Physiol 260: 253–266, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 100: 1284–1294, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol (August 10, 2011). doi:10.1152/jn.01097.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol 372: 465–485, 1996 [DOI] [PubMed] [Google Scholar]
- Chang YH, Scholtz JP, Nichols TR. Hindlimb control during cat locomotion after loss of stretch reflexes. Integr Comp Biol 43: 987, 2003 [Google Scholar]
- Chen DH. Qualitative and quantitative study of synaptic displacement in chromatolyzed spinal motoneurons of the cat. J Comp Neurol 177: 635–664, 1978 [DOI] [PubMed] [Google Scholar]
- Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci 22: 3512–3519, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Triceps surae motoneuron morphology in the rat: a quantitative light microscopic study. J Comp Neurol 343: 143–157, 1994 [DOI] [PubMed] [Google Scholar]
- Collins WF, 3rd, Mendell LM, Munson JB. On the specificity of sensory reinnervation of cat skeletal muscle. J Physiol 375: 587–609, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994 [DOI] [PubMed] [Google Scholar]
- Copray JC, Brouwer N. Neurotrophin-3 mRNA expression in rat intrafusal muscle fibres after denervation and reinnervation. Neurosci Lett 236: 41–44, 1997 [DOI] [PubMed] [Google Scholar]
- Cull RE. Role of nerve-muscle contact in maintaining synaptic connections. Exp Brain Res 20: 307–310, 1974 [DOI] [PubMed] [Google Scholar]
- Davis-López de Carrizosa MA, Morado-Diaz CJ, Tena JJ, Benitez-Temino B, Pecero ML, Morcuende SR, de la Cruz RR, Pastor AM. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci 29: 575–587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz RR, Pastor AM, Delgado-Garcia JM. Effects of target depletion on adult mammalian central neurons: morphological correlates. Neuroscience 58: 59–79, 1994 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Monosynaptic excitatory action on motoneurones regenerated to antagonistic muscles. J Physiol 154: 68–88, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Shealy CN. An investigation into the effect of degenerating primary afferent fibers on the monosynaptic innervation of motoneurons. J Neurophysiol 25: 544–558, 1962 [DOI] [PubMed] [Google Scholar]
- Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc Natl Acad Sci USA 106: 13588–13593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. Laminar organization of primary afferent terminations in the mammalian spinal cord. In: Sensory Neurons, Diversity, Development, and Plasticity, edited by Scott SA. New York: Oxford Univ. Press, 1992, p. 131–139 [Google Scholar]
- Fyffe RE. Spinal motoneurons: synaptic inputs and receptor organization. In: Motor Neurobiology of the Spinal Cord, edited by Cope TC. Boca Raton, FL: CRC, 2001, p. 21–46 [Google Scholar]
- Goldring JM, Kuno M, Nunez R, Snider WD. Reaction of synapses on motoneurones to section and restoration of peripheral sensory connexions in the cat. J Physiol 309: 185–198, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol 91: 2164–2171, 2004 [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci 25: 4733–4742, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 48: 339–351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res 1017: 69–76, 2004 [DOI] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of activation level. J Neurophysiol 90: 1537–1546, 2003 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol 587: 401–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Brown PB, Mendell LM. Central sprouting and functional plasticity of regenerated primary afferents. J Neurosci 14: 3655–3671, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M, Llinas R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol 210: 823–838, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Porter R, Redman SJ. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol 232: 499–510, 1985 [DOI] [PubMed] [Google Scholar]
- Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skeletal muscle in adult rats. J Neurophysiol 66: 1218–1231, 1991 [DOI] [PubMed] [Google Scholar]
- Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J Comp Neurol 311: 356–366, 1991 [DOI] [PubMed] [Google Scholar]
- Linda H, Cullheim S, Risling M. A light and electron microscopic study of intracellularly HRP-labeled lumbar motoneurons after intramedullary axotomy in the adult cat. J Comp Neurol 318: 188–208, 1992 [DOI] [PubMed] [Google Scholar]
- Linda H, Shupliakov O, Ornung G, Ottersen OP, Storm-Mathisen J, Risling M, Cullheim S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J Comp Neurol 425: 10–23, 2000 [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Fenwick NM, Dicarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol 503: 741–767, 2007 [DOI] [PubMed] [Google Scholar]
- Lundborg G. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst 8: 209–226, 2003 [DOI] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 181: 377–393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail LT, McBride CB, McGraw J, Steeves JD, Tetzlaff W. Axotomy abolishes NeuN expression in facial but not rubrospinal neurons. Exp Neurol 185: 182–190, 2004 [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological aspects of synaptic plasticity: the Ia/motoneuron connection as a model. Adv Neurol 47: 337–360, 1988 [PubMed] [Google Scholar]
- Mendell LM, Johnson RD, Munson JB. Neurotrophin modulation of the monosynaptic reflex after peripheral nerve transection. J Neurosci 19: 3162–3170, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Scott JG. The effect of peripheral nerve cross-union on connections of single Ia fibers to motoneurons. Exp Brain Res 22: 221–234, 1975 [DOI] [PubMed] [Google Scholar]
- Mendell LM, Taylor JS, Johnson RD, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-motoneuron synapse. J Neurophysiol 73: 662–673, 1995 [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Shneider NA, Siembab VC, O'Donovan MJ. Mechanisms regulating the specificity and strength of muscle afferent inputs in the spinal cord. Ann NY Acad Sci 1198: 220–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O'Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci 26: 13297–13310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson JB, Johnson RD, Mendell LM. Neurotrophin-3 and maintenance of muscle afferent function. Prog Brain Res 123: 157–163, 1999 [PubMed] [Google Scholar]
- Munson JB, Johnson RD, Mendell LM. NT-3 increases amplitude of EPSPs produced by axotomized group Ia afferents. J Neurophysiol 77: 2209–2212, 1997 [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82: 163–201, 2007 [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol 217: 75–85, 1983 [DOI] [PubMed] [Google Scholar]
- Novikov LN, Novikova LN, Holmberg P, Kellerth J. Exogenous brain-derived neurotrophic factor regulates the synaptic composition of axonally lesioned and normal adult rat motoneurons. Neuroscience 100: 171–181, 2000 [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50: 117–129, 2003 [DOI] [PubMed] [Google Scholar]
- Persson S, Boulland JL, Aspling M, Larsson M, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA, Broman J. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol 497: 683–701, 2006 [DOI] [PubMed] [Google Scholar]
- Puigdellivol-Sanchez A, Valero-Cabre A, Prats-Galino A, Navarro X, Molander C. On the use of fast blue, fluoro-gold and diamidino yellow for retrograde tracing after peripheral nerve injury: uptake, fading, dye interactions, and toxicity. J Neurosci Methods 115: 115–127, 2002 [DOI] [PubMed] [Google Scholar]
- Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev 4: 42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O'Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci 29: 4719–4735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE. A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp Neurol 49: 406–417, 1975 [DOI] [PubMed] [Google Scholar]
- Sumner BE. Quantitative ultrastructural observations on the inhibited recovery of the hypoglossal nucleus from the axotomy response when regeneration of the hypoglossal nerve is prevented. Exp Brain Res 26: 141–150, 1976 [DOI] [PubMed] [Google Scholar]
- Sumner BE, Sutherland FI. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol 2: 315–328, 1973 [DOI] [PubMed] [Google Scholar]
- Svensson M, Tornqvist E, Aldskogius H, Cova JL. Synaptic detachment from hypoglossal neurons after different types of nerve injury in the cat. J Hirnforsch 32: 547–552, 1991 [PubMed] [Google Scholar]
- Swett JE, Wikholm RP, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol 93: 227–252, 1986 [DOI] [PubMed] [Google Scholar]
- Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol 422: 172–180, 2000 [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol 35: 1–51, 1990 [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13–27, 2003 [DOI] [PubMed] [Google Scholar]
- Wang Z, Li LY, Taylor MD, Wright DE, Frank E. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci 27: 3686–3694, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SX, Koshimizu Y, Feng YP, Okamoto K, Fujiyama F, Hioki H, Li YQ, Kaneko T, Mizuno N. Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res 1011: 247–251, 2004 [DOI] [PubMed] [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res 149: 458–469, 2003 [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]