Abstract
Pain-related hyperactivity in the amygdala leads to deactivation of the medial prefrontal cortex (mPFC) and decision-making deficits. The mechanisms of pain-related inhibition of the mPFC are not yet known. Here, we used extracellular single-unit recordings of prelimbic mPFC neurons to determine the role of GABAA receptors and metabotropic glutamate receptor (mGluR) subtypes, mGluR1 and mGluR5, in pain-related activity changes of mPFC neurons. Background and evoked activity of mPFC neurons decreased after arthritis induction. To determine pain-related changes, the same neuron was recorded continuously before and after induction of arthritis in one knee joint by intra-articular injection of kaolin/carrageenan. Stereotaxic administration of a GABAA receptor antagonist {[R-(R*,S*)]-5-(6,8-dihydro-8-oxofuro[3,4-e]-1,3-benzodioxol-6-yl)-5,6,7,8-tetrahydro-6,6-dimethyl-1,3-dioxolo[4,5-g]isoquinolinium iodide (bicuculline)} into the mPFC by microdialysis reversed pain-related inhibition, whereas offsite injections into the adjacent anterior cingulate cortex had no or opposite effects on prelimbic mPFC neurons. A selective mGluR1/5 agonist [(S)-3,5-dihydroxyphenylglycine (DHPG)] inhibited background and evoked activity under normal conditions through a GABAergic mechanism, because the inhibitory effect was blocked with bicuculline. In the arthritis pain state, DHPG, alone or in the presence of bicuculline, had no effect. Consistent with the involvement of mGluR1 in pain-related inhibition of the mPFC, a selective mGluR1 antagonist [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid] reversed the pain-related decrease of background and evoked activity of mPFC neurons in arthritis, whereas a selective mGluR5 antagonist [2-methyl-6-(phenylethynyl)pyridine hydrochloride] had no effect. The mGluR antagonists had no effect under normal conditions. We interpret our data to suggest that pain-related inhibition of mPFC neurons in the arthritis model depends on mGluR1-mediated endogenous activation of GABAA receptors. Exogenous activation of mGluR1/5 produces GABAergic inhibition under normal conditions. Restoring normal activity in the mPFC may be a therapeutic strategy to improve cognitive deficits associated with persistent pain.
Keywords: mGluR5, GABAergic inhibition of mPFC
the medial prefrontal cortex (mPFC) plays an important role in cognitive functions, such as decision making, avoidance of risky choices, and goal-directed behaviors in animals and humans (Bechara et al. 1999; Kouneiher et al. 2009; Pais-Vieira et al. 2007; Stalnaker et al. 2007; Vertes 2006). Pain has been associated with functional and structural abnormalities in prefrontal cortical areas (Apkarian et al. 2004b; Metz et al. 2009) and can impair emotion-based decision making in humans (Apkarian et al. 2004a) and animals (Ji et al. 2010; Pais-Vieira et al. 2009). A recent study from our laboratory (Ji et al. 2010) showed that hyperactivity in the amygdala, an emotional brain center closely interconnected with the mPFC, leads to the deactivation of the mPFC, but the underlying mechanism remains to be determined.
Here, we tested the hypothesis that group I metabotropic glutamate receptors (mGluRs) activate a GABAergic mechanism to inhibit the activity of mPFC pyramidal cells. Glutamatergic afferents from extracortical areas, such as the amygdala, have been shown to target inhibitory GABAergic interneurons that synapse on pyramidal cells in layer V of the mPFC (Bacon et al. 1996; Gabbott et al. 2006; Kita and Kitai 1990). Group I mGluR1 and mGluR5 subtypes modulate excitatory and inhibitory synaptic transmission in various brain areas and have emerged as therapeutic targets for neuropsychiatric disorders associated with cortical dysfunction (Lesage and Steckler 2010; Niswender and Conn 2010; Olive 2010; Pinheiro and Mulle 2008). mGluR1 can activate glutamate-driven synaptic inhibition in the cerebellum (Karakossian and Otis 2004). A recent study from our laboratory (Sun and Neugebauer 2011) showed that mGluR1 also activates feedforward inhibition of mPFC pyramidal cells.
mGluR1 and mGluR5 subtypes are expressed in the PFC (Cauli et al. 2000; Muly et al. 2003). Activation of group I mGluRs in the mPFC increased GABA release (Segovia and Mora 2005), whereas mGluR1 mediated enhanced glutamate release (Melendez et al. 2005). A mGluR1/5 agonist [(S)-3,5-dihydroxyphenylglycine (DHPG)] increased excitatory transmission (Marek and Zhang 2008) but also inhibitory transmission onto prefrontal pyramidal cells (Chu and Hablitz 1998). Systemic application of a positive allosteric modulator for mGluR5 increased activity of mPFC pyramidal cells in normal animals but reversed hyperexcitability of pyramidal cells in a schizophrenia model (Homayoun and Moghaddam 2010). In our recent study (Sun and Neugebauer 2011), DHPG-activated synaptic inhibition of mPFC pyramidal cells in slices from normal animals was blocked by an antagonist for mGluR1 but not mGluR5.
The diversity of effects suggests that mGluR subtypes are well positioned to fine tune activity of mPFC pyramidal cells, but their role in different conditions and models remains to be determined. The present study was designed to examine the contribution of mGluR1 and mGluR5 to enhanced inhibition of mPFC neurons in a rat model of arthritic pain using electrophysiological single-unit recordings and pharmacology in vivo.
METHODS
Adult male Sprague Dawley rats (250–350 g) were housed in a temperature-controlled room and maintained on a 12-h day/night cycle. Water and food were available without restriction. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The University of Texas Medical Branch and conform to the guidelines of the International Association for the Study of Pain and of the National Institutes of Health.
Animal preparation and anesthesia.
Experimental details have been described in detail in our previous studies (Ji and Neugebauer 2009; Ji et al. 2010). The animal was anesthetized with pentobarbital sodium (50 mg/kg, administered ip). A cannula was inserted into the trachea for artificial respiration and to measure end-tidal CO2 levels. A catheter was placed in the jugular vein for continuous administration of anesthetic and for fluid support (3–4 ml·kg−1·h−1 lactated Ringer solution, administered iv). The animal was kept under anesthesia throughout the experiment. Constant levels of anesthesia were maintained with pentobarbital (15 mg·kg−1·h−1, iv). They were paralyzed with pancuronium (0.3 mg/h, iv) and artificially ventilated (3–3.5 ml; 55–65 strokes/min). End-tidal CO2 levels (kept at 4.0 ± 0.2%), heart rate, and ECG pattern were monitored continuously. Core body temperature was maintained at 37°C by means of a homeothermic blanket system. These measures ensured a constant internal state of body functions. The animal was mounted in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and a small unilateral craniotomy was performed at the sutura frontoparietalis level. The dura mater was opened and reflected; the pia mater was removed over the recording and drug administration sites to allow smooth insertion of the recording electrode and microdialysis probe.
Electrophysiological recording and identification of mPFC neurons.
As described in detail previously (Ji et al. 2010), extracellular recordings were made from single neurons in the prelimbic part of the mPFC with glass-insulated carbon filament electrodes (4–6 MΩ) using the following stereotaxic coordinates (Paxinos and Watson 1998): 3.0–3.2 mm anterior to bregma; 0.5–1.0 mm lateral to midline; depth, 3.0–4.6 mm. With the use of an electronically remote-controlled microstepping positioner attached to the stereotaxic frame (David Kopf Instruments), the electrode was lowered vertically into the mPFC. Recording electrode and ground electrode were connected with a preamplifier (World Precision Instruments, UK) to a differential amplifier (Warner Instruments, Hamden, CT). The recorded signals were amplified, band-pass filtered (300 Hz–3 kHz), and displayed on analog and digital storage oscilloscopes. Signals were also fed into a window discriminator (World Precision Instruments), whose output was processed by a capacitance electronic disc (CED) interface (1401 Plus, Cambridge Electronic Design, UK) connected to a Pentium 4 personal computer (PC). Spike2 software (version 4; Cambridge Electronic Design) was used to create peristimulus rate histograms online and to store and analyze digital records of single-unit activity offline.
An individual mPFC neuron was identified by the configuration, shape, and height of the recorded action potentials (spikes) that occurred spontaneously (background activity) or in response to mechanical (tissue compression) search stimuli (evoked responses). mPFC pyramidal neurons can be distinguished from interneurons based on their broader action potential waveform and lower baseline discharge rate (Constantinidis and Goldman-Rakic 2002; Homayoun and Moghaddam 2007; Laviolette and Grace 2006). With the use of a combination of criteria established or discussed in these references, we selected neurons with peak-to-valley spike-width >500 μs and a baseline discharge rate <10 Hz (Ji et al. 2010).
Spikes were detected and recorded based on the waveform signal that crossed a trigger level and matched a pre-set shape or template, which was created for the individual neuron at the beginning of the recording period. Spike size and configuration were monitored continuously on the storage oscilloscopes and with the use of Spike2 software. Only those neurons were included in this study whose spike configuration remained constant (matching the template) and could be clearly discriminated from activity in the background throughout the experiment, indicating that the activity of one and the same one neuron was measured.
Experimental protocol.
In each animal, background activity and evoked responses of only one neuron were recorded, as described in detail in our previous studies (Ji and Neugebauer 2007, 2009; Ji et al. 2010). Animals were under continuous anesthesia throughout the experiment, whether or not arthritis was induced (see Arthritis pain model). By the time recordings on an individual neuron started, the animals had been under anesthesia for at least 5 h. Brief (15 s) mechanical test stimuli of innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) intensities were applied to the knee joint by means of a calibrated forceps equipped with a force transducer, whose output signal was amplified, displayed in grams on a liquid-crystal display screen, digitized by the CED interface, and recorded on the Pentium PC for on- and offline analysis. Stimulus intensities of 100 and 500 g/30 mm2, applied to the knee and other deep tissue, are considered innocuous, because they do not evoke hind-limb withdrawal reflexes in awake rats and are not felt to be painful when tested on the experimenters. Pressure stimuli >1,500 g/30 mm2 are noxious, because they evoke hind-limb withdrawal reflexes and vocalizations in awake rats and are distinctly painful when applied to the experimenters (Han et al. 2005; Neugebauer et al. 2007; Neugebauer and Li 2002). For the analysis of net-evoked activity, background activity in the 15-s time period preceding the 15-s stimulus was subtracted from the total activity during stimulation.
To determine changes in the arthritis pain model, one and the same neuron was recorded continuously before and after arthritis induction, allowing paired analysis. For the pharmacological analysis of receptor function, one neuron was recorded before and during drug administration into the mPFC. Before and during drug applications, intervals between test stimuli were 5–10 min; this protocol was the same under normal conditions (no arthritis) and in the arthritis pain state. Sufficiently long control periods were included in each experiment to establish the baseline activity before arthritis induction and/or drug application. The number of stimulations was kept at a minimum to avoid any “sensitization” that might be produced by repeated stimulation. The rationale for testing evoked responses was to link the mPFC neurons to pain processing and pain mechanisms. Throughout the experiment, we carefully monitored several physiological parameters (body temperature, heart rate, ECG, end-tidal CO2 levels) to ensure a stable recording situation.
Arthritis pain model.
Arthritis was induced as described in detail previously (Neugebauer et al. 2007). A kaolin suspension (4%, 100 ml) was injected slowly into the joint cavity through the patellar ligament with the use of a syringe and needle (1 ml, 25 gauge, 5/8 in). After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 100 ml) was injected into the knee-joint cavity, and the leg was flexed and extended for another 5 min. This treatment paradigm reliably leads to a localized inflammation confined to one knee joint within 1–3 h, persists for weeks, and is significantly associated with pain behaviors (Neugebauer et al. 2007).
Drugs and drug application.
The following selective compounds were used. 5-Aminomethyl-3-hydroxyisoxazole (muscimol; GABAA receptor agonist); [R-(R*,S*)]-5-(6,8-dihydro-8-oxofuro[3,4-e]-1,3-benzodioxol-6-yl)-5,6,7,8-tetrahydro-6,6-dimethyl-1,3-dioxolo[4,5-g]isoquinolinium iodide (bicuculline; GABAA receptor antagonist); (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid hydrochloride (CGP55845; GABAB receptor antagonist); group I mGluR1/5 agonist, DHPG; 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP; mGluR5 antagonist); and (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385; mGluR1 antagonist). These were purchased from Tocris Bioscience (Ellisville, MO).
Known concentrations of drugs were administered into the mPFC by microdialysis under normal conditions (before arthritis induction) or 5–6 h postinduction of arthritis. Several hours before the start of the electrophysiological recordings, a microdialysis probe (CMA11, CMA Microdialysis, Sweden; membrane diameter: 250 μm; membrane length: 1 mm) was lowered vertically into the mPFC and positioned stereotaxically within the prelimbic cortex (PL) using the following coordinates: 3.2 mm anterior to bregma; 0.8 mm lateral to midline; depth, 4.3 mm (Ji et al. 2010). The distance between the microdialysis probe and the recording electrode was 0.5–1.0 mm. In some experiments, a microdialysis probe was inserted into the anterior cingulate cortex (ACC) as a placement control, using the following stereotaxic coordinates: 2.7 mm anterior to bregma; 0.8 mm lateral to midline; depth of tip, 3.0 mm. With the use of polyethylene-50 tubing, the microdialysis probe was connected to an infusion pump (Harvard Apparatus, Holliston, MA) and perfused with artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 125.0, KCl 2.6, NaH2PO4 2.5, CaCl2 1.3, MgCl2 0.9, NaHCO3 21.0, and glucose 3.5, oxygenated and equilibrated to pH 7.4. Before each drug application, ACSF was pumped through the fiber for at least 1 h to establish equilibrium in the tissue.
Drugs were dissolved in ACSF on the day of the experiment at a concentration 100 times that predicted to be needed based on our previous microdialysis and in vitro studies (Han and Neugebauer 2005; Ji and Neugebauer 2010; Li and Neugebauer 2004; Li et al. 2011; Ren and Neugebauer 2010) and data in the literature (Lea and Faden 2006; Lesage and Steckler 2010; Niswender and Conn 2010). Drug concentration in the tissue is at least 100 times lower than in the microdialysis probe, due to the concentration gradient across the dialysis membrane and diffusion in the tissue (Han and Neugebauer 2005; Li and Neugebauer 2004). Drugs were administered into the mPFC at a rate of 5 μl/min for at least 15 min to establish equilibrium in the tissue. Drug effects on background and evoked activity were measured every 5–10 min during drug application. The numbers given in this article refer to the drug concentrations in the microdialysis fiber. ACSF served as a vehicle control.
Histology.
At the end of each experiment, the recording site in the mPFC was marked by injecting direct current (250 μA for 3 min) through the carbon-filament recording electrode. The brain was removed and submerged in 10% formalin and potassium ferrocyanide. Tissues were stored in 20% sucrose before they were frozen sectioned at 50 μm. Sections were mounted on gel-coated slides, stained with hematoxylin and eosin, and cover slipped. Lesion/recording sites were verified histologically and plotted on standard diagrams (adapted from Paxinos and Watson 1998).
Data analysis and statistics.
Extracellularly recorded single-unit action potentials were analyzed offline from peristimulus rate histograms using Spike2 software (version 4; Cambridge Electronic Design). Responses to mechanical stimuli were measured and expressed as spikes/s (Hz). Background activity was subtracted from the total activity during the stimulus to calculate net-evoked activity. Student's t-test (paired where appropriate) was used to compare two sets of data that have Gaussian distribution and similar variances. For multiple comparisons, one-way ANOVA was used with Bonferroni post-tests (to compare selected data) or Dunnett's post-test (to compare all data with one control set). Statistical analysis was performed on the raw data (firing rate measured as spikes/s). All averaged values are given as the mean ± SE. Statistical significance was accepted at the level P < 0.05. GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA) was used for all statistical analyses.
RESULTS
Extracellular single-unit recordings were made from 69 neurons in the prelimbic region of the mPFC in 69 rats (Fig. 1). Neurons included in this study responded to noxious mechanical stimulation of peripheral tissues, including the knee joint (see methods, Experimental protocol). Receptive fields of prelimbic mPFC neurons were bilateral and symmetrical in the hindlimbs and tail (n = 69), as determined with brief innocuous and noxious mechanical test stimuli (see methods, Experimental protocol). The majority of neurons (n = 41) had additional symmetrical receptive fields in the forepaws and trunk. Action potential duration (582.64 ± 59.08 μs, peak-to-valley) and firing rate (4.31 ± 1.95 spikes/s) were consistent with pyramidal cells rather than fast-spiking interneurons (see methods, Electrophysiological recording and identification of mPFC neurons, and Ji et al. 2010).
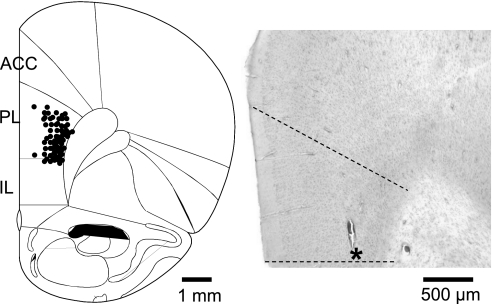
Fig. 1.
Histologically verified recording sites of 46 neurons in the prelimbic cortex (PL). Diagram (adapted from Paxinos and Watson 1998) shows coronal brain section at 3.2 mm anterior to the bregma. Symbols indicate locations of recording electrode tips based on electrolytic lesions. ACC, anterior cingulate cortex; IL, infralimbic cortex. Histology image on the right shows an individual example of a lesion site in the PL (indicated by an asterisk) in 1 brain slice stained with hematoxylin and eosin.
Pain-related deactivation of mPFC neurons involves a GABAergic mechanism.
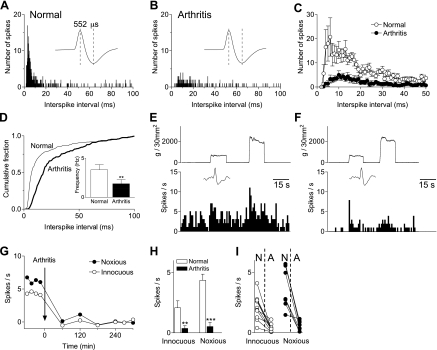
To determine arthritis pain-related changes, background activity and responses to innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) stimuli were measured repeatedly in the same mPFC neuron (n = 10) before and for 5–6 h after induction of an arthritis pain state (Fig. 2). Interspike interval (ISI) analysis of background activity showed a decrease after arthritis induction (Fig. 2C). Cumulative ISI distribution shifted toward higher values (P < 0.05, Kolmogorov-Smirnov test), and mean frequency decreased significantly (n = 10 neurons; P < 0.01, paired t-test; Fig. 2D). Responses to innocuous and noxious compression of the knee also decreased significantly after arthritis induction (n = 10 neurons; P < 0.01 and 0.001, paired t-test; Fig. 2, G and H). The results confirm the pain-related decrease in background and evoked activity of mPFC neurons described in our previous study (Ji et al. 2010). Decreased activity in the pain state was observed in all prelimbic neurons tested (n = 10). Therefore, drugs in the pharmacological studies were tested, not only in neurons that were monitored continuously before and after arthritis induction but also in neurons that were only identified in the arthritis pain state.
Fig. 2.
Deactivation of medial prefrontal cortex (mPFC) neurons in a model of arthritis pain. A–D: interspike interval (ISI) histograms of extracellularly recorded background activity. ISI distribution (10-min sampling period) for an individual prelimbic mPFC neuron before arthritis induction (A) and 5 h postinduction (B). Individual spikes show that size and shape remained constant. Half-width of action potential is indicative of a pyramidal cell. C: averaged ISI data for the sample of neurons tested before and after arthritis induction (n = 10 neurons) show decreased number of spikes and shift toward larger ISIs. D: cumulative ISI distribution shifted toward higher values, and mean frequency decreased significantly after arthritis. E–I: extracellularly recorded, evoked responses of mPFC neurons (spikes/s). Peristimulus time histograms show responses to brief (15 s) innocuous and noxious compression of the knee (see methods) recorded in an individual prelimbic mPFC neuron before (E) and 5 h after arthritis induction (F). G: time course of the decrease in evoked response (background activity was subtracted) after arthritis induction. Each symbol represents 1 measurement of background or evoked activity. H: bar histograms show spikes/s for evoked stimuli (15 s) averaged across the sample of neurons (n = 10). **P < 0.01; ***P < 0.001 (compared with prearthritis control values; paired t-test). I: raw data show the effect of arthritis in individual neurons. N, normal; A, arthritis.
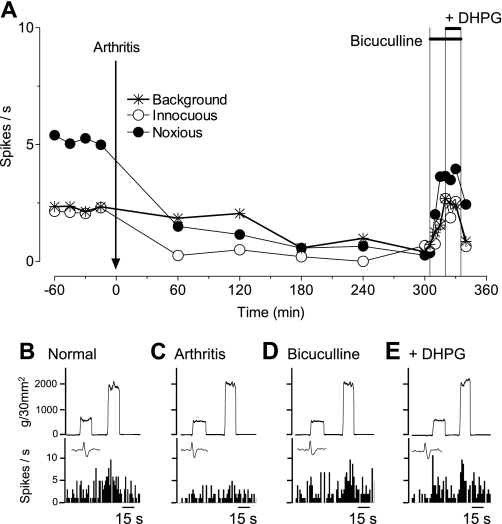
Administration of a GABAA receptor antagonist (bicuculline, 1 mM; concentration in microdialysis probe, 15 min) into the prelimbic mPFC reversed the decrease in background and evoked activity of mPFC neurons in the arthritis pain state (Fig. 3). The facilitatory effects of bicuculline in the pain model (5–6 h postinduction) were significant in the sample of mPFC neurons (n = 6; P < 0.01, ANOVA with Bonferroni post-tests, Fig. 4A; for individual data, see Fig. 4B). Bicuculline (1 mM; n = 6 neurons) increased background activity and responses to innocuous and noxious stimuli to 78 ± 10%, 74 ± 7%, and 87 ± 8% of prearthritis values, respectively. Offsite injection into the ACC (n = 5 neurons) as a control for drug diffusion did not change background activity but had some inhibitory effect on evoked activity of prelimbic neurons, possibly indicating an inverse relationship between anterior cingulate and PLs. A GABAB receptor antagonist (CGP55845, 500 μM; n = 5 neurons) had no effect. The data suggest that GABAA receptors in the prelimbic mPFC mediate deactivation of pyramidal cells in the arthritis pain model.
Fig. 3.
Contribution of a GABAergic mechanism to the deactivation of mPFC neurons. Extracellular recordings of the responses of 1 mPFC neuron to brief (15 s) innocuous and noxious stimulation of the knee and background activity. A: time-course data show the decrease in activity after arthritis induction and the reversal by [R-(R*,S*)]-5-(6,8-dihydro-8-oxofuro[3,4-e]-1,3-benzodioxol-6-yl)-5,6,7,8-tetrahydro-6,6-dimethyl-1,3-dioxolo[4,5-g]isoquinolinium iodide (bicuculline) administered into the mPFC (1 mM; concentration in microdialysis fiber, 15 min). Coapplication of a metabotropic glutamate receptor (mGluR)1/5 agonist [(S)-3,5-dihydroxyphenylglycine (DHPG), 100 μM] had no effect. Artificial cerebrospinal fluid (ACSF) was administered as vehicle control before (predrug) and after bicuculline. B–E: peristimulus time histograms show action potentials (spikes)/s. Insets show individual action potentials. Top traces are recordings of the force (g/30 mm2) applied to the knee joint.
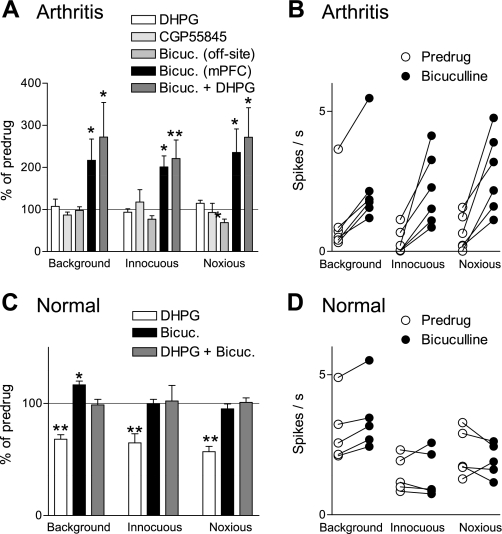
Fig. 4.
Pain-related GABAergic inhibition of mPFC neurons can be activated by mGluR1/5. Summary of the effects of GABA receptor antagonists and mGluR1/5 agonist on mPFC neurons in arthritis (5–6 h postinduction; A) and under normal conditions (B). A: administration of DHPG (mGluR1/5 agonist, 100 μM; n = 5) or 2S(-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl]phenylmethyl)phosphinic acid hydrochloride (CGP55845; GABAB receptor antagonist, 500 μM) into the prelimbic mPFC had no effect (n = 5 neurons). Administration of bicuculline (Bicuc.; GABAA receptor antagonist, 1 mM) into the prelimbic mPFC (n = 6), but not ACC (n = 5), increased background and evoked activity. In the presence of bicuculline, DHPG (100 μM; n = 5) had no effect. B: raw data show the facilitatory effect of bicuculline (1 mM) in individual mPFC neurons in arthritis. Each symbol represents background or evoked activity (spikes/s) before and during drug administration. C: administration of DHPG (100 μM; n = 6) into the prelimbic mPFC inhibited background and evoked activity. Bicuculline alone (1 mM; n = 5) had little or no effect but blocked the inhibition by DHPG (n = 5). D: raw data show the effect of bicuculline (1 mM) in individual mPFC neurons under normal conditions. Each symbol represents background or evoked activity (spikes/s) before and during drug administration. A and C: bar histograms show spikes/s averaged across the sample of neurons (mean ± SE) normalized to predrug controls (set to 100%). *P < 0.05; **P < 0.01 (compared with predrug values, ANOVA with Bonferroni post-tests).
Pain-related deactivation of mPFC neurons involves mGluR1 but not mGluR5.
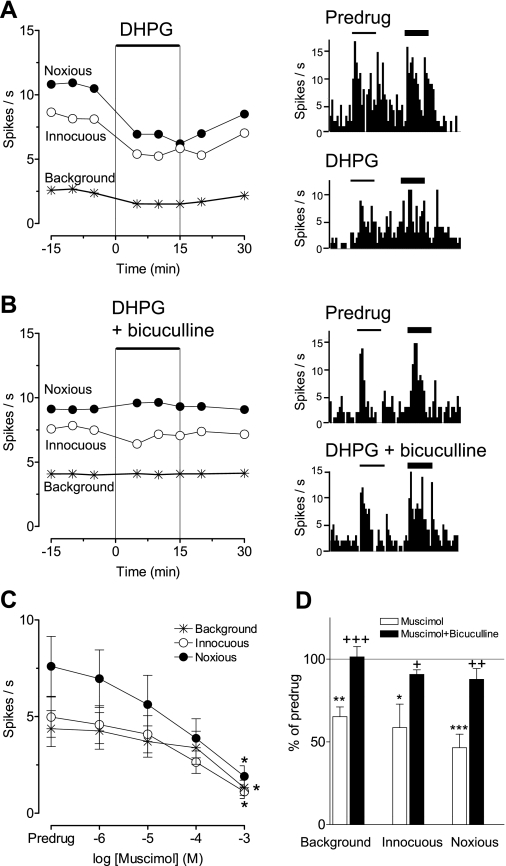
Under normal conditions (Fig. 4C), exogenous activation of mGluR1/5 with a selective agonist (DHPG, 100 μM; n = 6 neurons) inhibited background and evoked activity (P < 0.01, ANOVA with Bonferroni post-tests). The inhibitory effect was blocked by bicuculline (1 mM; n = 5 neurons), suggesting the involvement of GABAA receptors in group I mGluR-activated inhibition of mPFC neurons. Individual examples are shown in Fig. 5, A and B. Bicuculline (1 mM; n = 5 neurons) reversed the inhibitory effect of a GABAA receptor agonist (muscimol, 100 μM; n = 5 neurons; Fig. 5D). Muscimol inhibited background and evoked activity of mPFC neurons in a concentration-dependent fashion (n = 7–8 neurons/concentration; Fig. 5C). The similarity of the effect of bicuculline on the inhibition induced by DHPG and by muscimol suggests the involvement of GABAA receptors.
Fig. 5.
mGluR1/5 and GABAA receptor activation inhibits mPFC neurons under normal conditions. Extracellular recordings of the responses of 1 mPFC neuron to brief (15 s) innocuous and noxious stimulation of the knee and background activity under normal conditions (no arthritis). A: administration of DHPG (100 μM) into the prelimbic mPFC inhibited background and evoked activity. Line graph shows time-course data. Peristimulus time histograms on the right show responses of the neuron to brief (15 s) innocuous (thin bars) and noxious (thick bars) stimuli (compression of the knee) before (Predrug) and during administration of DHPG. B: in the presence of bicuculline (1 mM), DHPG had no effect. Time-course data are shown in the graph on the left. Peristimulus time histograms on the right show individual responses before and during coapplication of DHPG and bicuculline. C: 5-aminomethyl-3-hydroxyisoxazole (muscimol; 100 μM) inhibited background and evoked activity of mPFC neurons in a concentration-dependent fashion (n = 7–8 neurons/concentration). Each symbol represents the activity (spikes/s) during 1 concentration averaged across the sample of neurons (mean ± SE). Two to 3 different concentrations were tested in each neuron in a cumulative fashion. *P < 0.05 (compared with predrug values, ANOVA with Dunnett's post-tests). D: bicuculline (1 mM; n = 5 neurons) reversed the inhibitory effect of a GABAA receptor agonist (muscimol, 1 mM; n = 5 neurons). Bar histograms show spikes/s averaged across the sample of neurons (mean ± SE) normalized to predrug controls (set to 100%). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with predrug values); +P < 0.05, ++P < 0.01, +++P < 0.001 (compared with muscimol alone, ANOVA with Bonferroni post-tests).
Bicuculline alone had no effect on evoked activity but increased background activity (n = 5 neurons; P < 0.05, ANOVA with Bonferroni post-tests, Fig. 4C; for individual data, see Fig. 4D), which would be consistent with a GABAergic tone in the mPFC. In the arthritis pain state (Fig. 4A), DHPG (100 μM) alone had no effect on the suppressed activity of mPFC neurons (n = 5 neurons). DHPG also had no effect when the activity was restored to prearthritis levels by blocking GABAA receptors with bicuculline (n = 5 neurons). We interpret our data to suggest that the inhibitory GABAA receptor-mediated effect of DHPG seen under normal conditions is occluded in the arthritis pain model by the endogenous activation of GABAergic inhibition.
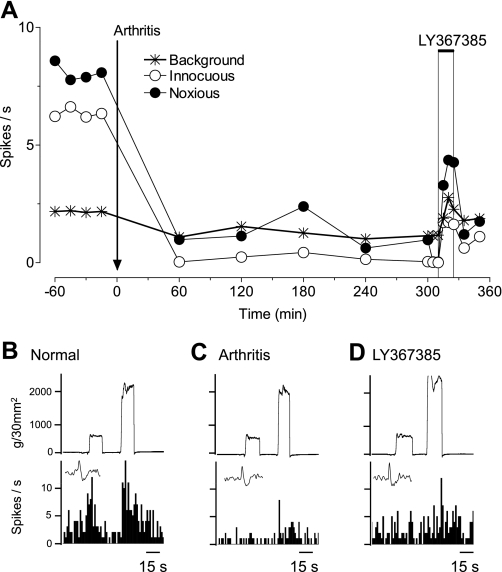
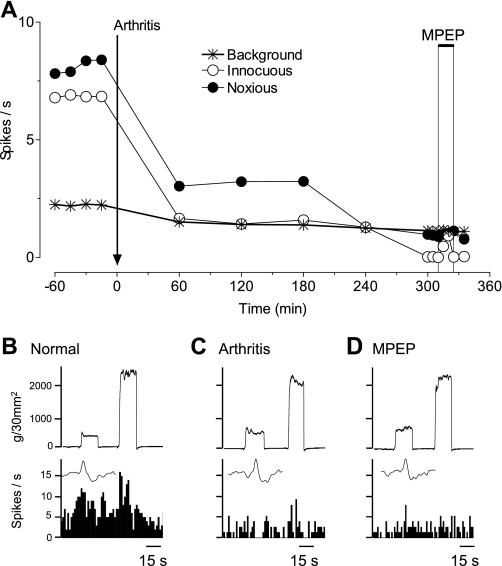
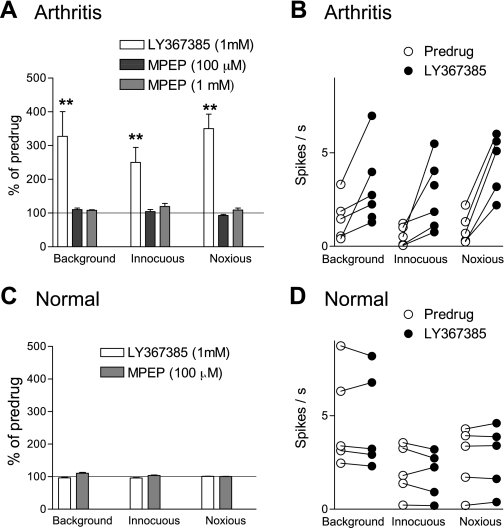
Since DHPG inhibited mPFC activity through a GABAergic mechanism under normal conditions (Figs. 4C and 5), and GABAA receptors mediate pain-related inhibition of mPFC neurons in the arthritis model (Fig. 4A), we hypothesized that mGluR1 or mGluR5 may be activated endogenously in the pain state to depress mPFC activity. Administration of a selective antagonist for mGluR1 (LY367385, 1 mM; Fig. 6), 5 h postinduction of arthritis, partially reversed the depressed responses of a mPFC neuron. In contrast, a selective mGluR5 antagonist (MPEP, 100 μM) had no effect (Fig. 7). The data for the sample of neurons are summarized in Fig. 8. LY367385 (1 mM; n = 5 neurons), but not MPEP (100 μM, n = 8; 1 mM, n = 6 neurons), partially reversed the inhibition of background and evoked activity in the arthritis pain model (5–6 h postinduction; P < 0.01, compared with predrug, ANOVA with Bonferroni post-tests, Fig. 8A; for individual data, see Fig. 8B). In those neurons that were recorded before and after arthritis induction, LY367385 (1 mM; n = 3 neurons) increased background activity and responses to innocuous and noxious stimuli to 73 ± 15%, 68 ± 13%, and 65 ± 13% of prearthritis values, respectively. The values for MPEP (1 mM; n = 3 neurons) were 34 ± 3%, 45 ± 6%, and 32 ± 7% of prearthritis levels. LY367385 (1 mM; n = 5 neurons) and MPEP (100 μM; n = 4 neurons) had no effect under normal conditions (n = 4 neurons each, Fig. 8C; for individual data, see Fig. 8D). The results suggest that arthritis pain increases the GABAergic tone in the mPFC through a mechanism that involves mGluR1 and GABAA receptor activation.
Fig. 6.
mGluR1 antagonist partially reverses inhibition of a mPFC neuron in arthritis pain. Extracellular recordings of the responses of 1 mPFC neuron to brief (15 s) innocuous and noxious stimulation of the knee and background activity. A: time-course data show the decrease in activity after arthritis induction and the partial reversal by a selective mGluR1 antagonist [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), 1 mM; concentration in microdialysis fiber, 15 min] administered into the mPFC. ACSF was administered as vehicle control before (predrug) and after LY367385. B–E: peristimulus time histograms show action potentials (spikes)/s. Insets show individual action potentials. Top traces are recordings of the force (g/30 mm2) applied to the knee joint.
Fig. 7.
mGluR5 antagonist does not change inhibition of a mPFC neuron in arthritis pain. Extracellular recordings of the responses of 1 mPFC neuron to brief (15 s) innocuous and noxious stimulation of the knee and background activity. A: time-course data show the decrease in activity after arthritis induction and the lack of effect of a selective mGluR5 antagonist [2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), 100 μM; concentration in microdialysis fiber, 15 min] administered into the mPFC. ACSF was administered as vehicle control before (predrug) and after MPEP. B–E: peristimulus time histograms show action potentials (spikes)/s. Insets show individual action potentials. Top traces are recordings of the force (g/30 mm2) applied to the knee joint.
Fig. 8.
Differential effects of mGluR1 and mGluR5 antagonists on pain-related inhibition of mPFC neurons. Summary of the effects of antagonists for mGluR1 (LY367385) and mGluR5 (MPEP) on background and evoked activity of mPFC neurons in arthritis (A) and under normal conditions (C). A: administration of LY367385 (1 mM; 15 min) into the prelimbic mPFC increased the suppressed responses of mPFC neurons (n = 5), 5–6 h postinduction of arthritis. Administration of MPEP (100 μM, n = 8; 1 mM, n = 6 neurons) had no significant effect. B: raw data show the facilitatory effect of LY367385 (1 mM) in individual mPFC neurons in arthritis. Each symbol represents background or evoked activity (spikes/s), before and during drug administration. C: administration of LY367385 (1 mM; n = 5 neurons) or MPEP (100 μM; n = 4) under normal conditions had no effect. Bar histograms show spikes/s, averaged across the sample of neurons (mean ± SE), normalized to predrug controls (set to 100%). D: raw data show the effect of LY367385 (1 mM) in individual mPFC neurons under normal conditions. Each symbol represents background or evoked activity (spikes/s) before and during drug administration. A and C: bar histograms show spikes/s averaged across the sample of neurons (mean ± SE) normalized to predrug controls (set to 100%). **P < 0.01 (compared with predrug values, ANOVA with Bonferroni post-tests).
DISCUSSION
This study tested the hypothesis that the endogenous activation of group I mGluRs contributes to enhanced GABAergic inhibition of mPFC neurons in an arthritis pain model described in our previous study (Ji et al. 2010). Key findings are as follows. Our data show a pain-related decrease in background and evoked activity of mPFC neurons that process nociceptive information. Selective antagonists for GABAA receptors (bicuculline) and mGluR1 (LY367385), but not mGluR5 (MPEP), reverse the enhanced inhibition of mPFC neurons in the arthritis pain state. The results suggest that endogenous activation of GABAA receptors and mGluR1 contributes to pain-related, decreased mPFC activity. Our data further show that exogenous activation of mGluR1/5 with a selective agonist (DHPG) inhibits mPFC neurons under normal conditions, and this effect is blocked with bicuculline, suggesting that mGluRs can regulate mPFC output (action-potential firing) through a GABAergic mechanism. In the arthritis pain state, when mGluR1 and GABAA receptors are endogenously activated to inhibit mPFC activity, exogenous activation of group I mGluRs with DHPG has no effect. DHPG also has no effect when activity is restored to prearthritis levels with bicuculline. The results are consistent with the concept that pain-related mPFC deactivation is mediated by the endogenous activation of mGluR1 and GABA. It should be emphasized that the direct link between mGluR1 and GABAA receptors was only shown under normal conditions but not in the arthritis pain state, where both receptor types are endogenously activated. The definitive demonstration of the dependence of mGluR1 function on GABA receptors in the arthritis pain model requires the detailed analysis at the synaptic level.
Pain-related mPFC dysfunction.
The PFC generally serves executive functions, and its medial and orbital regions play a key role in value-based decision making, which guides advantageous, goal-directed behaviors (Bechara et al. 1999; Kouneiher et al. 2009; Pais-Vieira et al. 2007; Stalnaker et al. 2007; Vertes 2006). Loss or impairment of PFC function is associated with well-documented cognitive deficits in a number of neuropsychiatric disorders (Bowie and Harvey 2006; Clarke et al. 2004; Goto et al. 2010; Gu et al. 2008; Stalnaker et al. 2009). More recent studies have linked dysfunction of PFC areas to pain-related cognitive deficits, such as impaired emotion-based decision making (Apkarian et al. 2004b; Ji et al. 2010; Pais-Vieira et al. 2009). Structural and functional changes of pyramidal cells in the mPFC were observed in a model of neuropathic pain (Metz et al. 2009). Pain patients (Apkarian et al. 2004a) and patients with mPFC lesions (Bechara et al. 1999) show similar decision-making deficits, which result in disadvantageous choices because of their inability to switch strategies.
The mechanisms of pain-related mPFC dysfunction remain to be determined. The amygdala is a major source of input to the mPFC (Bacon et al. 1996; Gabbott et al. 2006; Kita and Kitai 1990; McDonald 1991). A brain center for emotional processes (Ehrlich et al. 2009; Maren and Quirk 2004; Phelps and Ledoux 2005; Seymour and Dolan 2008), the amygdala plays a critical role in emotion-driven and reward-based decision making (Bechara et al. 1999, 2003). Functional interactions between amygdala and PFC are important for emotional learning and behavior (Herry et al. 2008; Holland and Gallagher 2004; Laviolette and Grace 2006; McGaugh 2004; Roozendaal et al. 2009; Stalnaker et al. 2007). Since the amygdala has emerged as an important center for emotional-affective aspects of pain (Neugebauer et al. 2004, 2009), and pain can impair cognitive functions (Apkarian et al. 2004a, 2009), we hypothesized that pain-related amygdala plasticity would impair mPFC function. Indeed, our previous study showed increased synaptic inhibition and decreased output of layer V pyramidal cells as the consequence of hyperactivity of amygdala neurons in a model of arthritis pain (Ji et al. 2010).
Mechanisms of mPFC deactivation—role of mGluRs.
Extracortical inputs to the mPFC from the amygdala, hippocampus, and thalamus are generally glutamatergic. How then would increased amygdala output lead to the deactivation of mPFC neurons? Anatomical studies showed that glutamatergic afferents from the amygdala monosynaptically innervate GABAergic interneurons in the mPFC, which synapse on layer V pyramidal cells (Bacon et al. 1996; Gabbott et al. 2006; Kita and Kitai 1990) and control pyramidal cell output (Markram et al. 2004). This arrangement allows for so-called “feedforward inhibition”, which has been shown in several brain areas to regulate neuronal output (Doyle and Andresen 2001; Ferrante et al. 2009; Lawrence and McBain 2003; Ling and Benardo 1995; Silberberg and Markram 2007). Evidence from our previous studies suggests that amygdala-driven feedforward inhibition of pyramidal cells regulates mPFC output (Sun and Neugebauer 2011) and contributes to pain-related mPFC deactivation (Ji et al. 2010).
The concept of feedforward inhibition can explain why mPFC pyramidal cells are excited by brief nociceptive signals under normal conditions but show decreased activation in persistent pain (arthritis model). Physiological nociceptive signals that normally activate mPFC output cells (Ji et al. 2010) likely serve protective functions, such as attention, awareness, and appraisal (Apkarian et al. 2005; Lorenz and Casey 2005; Ohara et al. 2006). In persistent pain, maladaptive plasticity in emotional brain centers, such as the amygdala (Neugebauer 2006; Neugebauer et al. 2004, 2009), drives excessive activation of cortical inhibitory networks (feedforward inhibition) to override excitatory drives to mPFC pyramidal cells (Ji et al. 2010). The relative dominance of inhibitory over excitatory influences on mPFC pyramidal cells results in decreased mPFC output (“deactivation”) and impaired cognitive functions, such as decision making (Apkarian et al. 2011; Ji et al. 2010; Moriarty et al. 2011; Sun and Neugebauer 2011).
The mechanism by which feedforward inhibition in the mPFC is activated in pain remains to be determined. Group I mGluR1 and mGluR5 subtypes can modulate excitatory and inhibitory transmission and play important roles in synaptic plasticity (Anwyl 1999; Cartmell and Schoepp 2000; Lesage and Steckler 2010; Neugebauer 2001; Nicoletti et al. 2010; Niswender and Conn 2010; Swanson et al. 2005). Importantly, mGluR1 has been implicated in feedforward inhibition in the cerebellum, where mGluR1 activated inhibitory interneurons and increased inhibitory transmission onto Purkinje cells (Karakossian and Otis 2004). mGluR1 also mediated the depolarization of inhibitory interneurons in the hippocampus, resulting in increased inhibitory transmission (Mannaioni et al. 2001). mGluR5 acted predominantly postsynaptically on pyramidal cells in the hippocampus (Mannaioni et al. 2001) and somatosensory cortex (Ballester-Rosado et al. 2010) but also mediated a variety of effects on inhibitory interneurons in the somatosensory cortex (Sun et al. 2009).
mGluR1 and mGluR5 subtypes are expressed in the mPFC (Cauli et al. 2000; Muly et al. 2003). Activation of group I mGluRs in the mPFC increased GABA release (Segovia and Mora 2005); mGluR1 mediated enhanced glutamate release (Melendez et al. 2005). A mGluR1/5 agonist (DHPG) increased excitatory transmission (Marek and Zhang 2008) but also inhibitory transmission onto prefrontal pyramidal cells (Chu and Hablitz 1998). Importantly, mGluR1 (Lesage and Steckler 2010) and mGluR5 (Niswender and Conn 2010) have emerged as novel targets for the treatment of cognitive deficits associated with mPFC dysfunction. Their ability to modulate synaptic transmission and feedforward inhibition in the cortex and their potential role as “cognitive enhancers” led us to focus on mGluR1 and mGluR5 as contributors to enhanced inhibition of mPFC pyramidal cells in pain.
The data of the present study suggest that mGluR1 rather than mGluR5 mediates the increased inhibition of mPFC pyramidal cells in a model of arthritis pain. A selective antagonist for mGluR1 (LY367385), but not mGluR5 (MPEP), partially reversed the inhibition of background and evoked activity of mPFC cells. A selective antagonist for GABAA (bicuculline), but not GABAB (CGP55845), receptors largely restored prearthritis control levels of mPFC activity, emphasizing the GABAergic nature of pain-related mPFC deactivation. The fact that blockade of mGluR1 only partially restored normal activity may suggest that other receptors or mechanisms contribute to the activation of feedforward inhibition or that inhibitory interneurons have undergone plasticity that is not easily reversed by blocking their mGluR1-mediated activation. Non-GABAergic (for example, peptidergic) inhibitory mechanisms could also be involved in mPFC deactivation. Linking mGluR1 directly to GABAergic inhibition in the pain model is difficult to accomplish with the in vivo approach of the present study, because our hypothesis implies that activation of mGluR1 or GABAA receptors would produce similar results. We chose two strategies to address this issue. Under normal conditions, DHPG produced inhibition that was reversed by bicuculline. This finding is consistent with our recent patch-clamp study, which showed that mGluR1 activates feedforward inhibition to decrease output function of mPFC pyramidal cells (Sun and Neugebauer 2011). In arthritis, DHPG alone or coapplication of DHPG with bicuculline did not produce inhibition of activity. These data also argue against a direct effect of mGluR1 on pyramidal cells but suggest the involvement of a GABAergic mechanism. The definitive link between mGluR1 and feedforward inhibition of mPFC pyramidal cells through activation of GABAergic interneurons would require analysis at the synaptic levels using a brain-slice preparation. On the other hand, the whole animal preparation in this study allowed the analysis of specific inputs, such as those related to noxious stimuli.
Methodological considerations and caveats.
The conclusions of this electrophysiological study rely on the results of pharmacological manipulations using selective agents and microdialysis for drug delivery. Both mGluR antagonists are subtype selective at the concentrations used in this study (Lea and Faden 2006; Lesage and Steckler 2010; Niswender and Conn 2010; Ren and Neugebauer 2010), and their differential effects argue against nonselective drug actions. LY367385 antagonizes mGluR1 in the low micromolar range and does not interact with other mGluR subtypes up to 100 μM (Kingston et al. 2002). Concentration of LY367385 in the microdialysis fiber was 1 mM, confirming the appropriateness of the factor 100 to estimate tissue concentration (see methods, Drugs and drug application). At concentrations of <10 μM, MPEP is selective for mGluR5 (Battaglia et al. 2002; Lea and Faden 2006), although offsite effects on mGluR1 and N-methyl-d-aspartate receptor subunits have been reported for concentrations of >10 μM and >18 μM, respectively (Cosford et al. 2003; Loane et al. 2009). The concentration of MPEP of 100 μM in the microdialysis fiber that we used is expected to result in a tissue concentration of 1 μM based on the comparison of MPEP effects in microdialysis experiments (Han and Neugebauer 2005; Ji and Neugebauer 2010; Li and Neugebauer 2004) with slice studies (Li et al. 2011; Neugebauer et al. 2003; Ren and Neugebauer 2010). The lack of effect of MPEP up to 1 mM in this study and differential effects of mGuR1 and mGluR5 antagonists in our previous studies (Han and Neugebauer 2005; Ji and Neugebauer 2010; Li and Neugebauer 2004; Li et al. 2011; Neugebauer et al. 2003; Ren and Neugebauer 2010) argue against offsite effects of MPEP as confounding factors.
Bicuculline methiodide, which was used in this study, can have off-target effects on calcium-dependent potassium channels at concentrations as low as 10 μM (Seutin and Johnson 1999). Because of the concentration gradient across the dialysis membrane and diffusion in the tissue, the predicted concentration of bicuculline in the tissue is at least 100 times lower (<10 μM) than in the microdialysis probe (1 mM), which is at the very low end of nonspecific concentrations. More importantly, if nonspecific effects (not involving GABAA receptors) were to account for the facilitatory effects of bicuculline in the arthritis pain state, these effects would also be expected to be present under normal conditions, but they were not. Furthermore, in a previous patch-clamp study, we showed that picrotoxin and bicuculline had similar effects on synaptically evoked spiking (Sun and Neugebauer 2011). Finally, bicuculline blocked the inhibitory effects of a GABAA receptor agonist (muscimol).
The site of drug action and spread of drugs also need to be considered. In this study, offsite injections into the ACC as a control for drug diffusion did not change background activity but had some inhibitory effect on evoked activity (particularly by noxious stimuli) of prelimbic neurons. Opposite effects of drug injections into PL and ACC argue for site-specific drug actions and may indicate an inverse relationship between these cortical areas. In fact, pain-related synaptic plasticity was found in the ACC, which correlated positively with pain behavior (Li et al. 2010; Wu et al. 2005), whereas activity in the PL decreased in this and our previous study (Ji et al. 2010), resulting in cognitive deficits.
The deactivation of mPFC neurons after arthritis induction is unlikely the result of nonspecific processes, such as fatigue or desensitization. Changes of evoked responses and background activity in the pain model were reversible with specific antagonists for mGluR1, but not mGluR5, and GABAA receptors. Reversibility and dependency on specific receptors argue against nonspecific factors. Evoked and background activity remained constant in the control period before arthritis induction in this and our previous study (Ji et al. 2010), suggesting that the decrease in neuronal activity reflects a (mal)adaptive process in response to persistent pain. The pain-related activity change is the consequence of receptor-dependent activation of inhibitory cortical mechanisms.
Significance.
Dysfunction of the PFC is associated with cognitive decision-making deficits (Bechara et al. 1998, 1999) observed in neuropsychiatric disorders (Beasley et al. 2002; Bechara 2005; Bechara et al. 2001; Bowie and Harvey 2006; Clarke et al. 2004; Goto et al. 2010; Gu et al. 2008; Stalnaker et al. 2009). Functional and structural abnormalities in the PFC were also observed in pain patients (Apkarian et al. 2004b, 2009) and in animal pain models (Ji et al. 2010; Metz et al. 2009; Sun and Neugebauer 2011). Pain can lead to impaired decision making and other cognitive deficits associated with PFC dysfunction in humans (Apkarian et al. 2004a; Borsook et al. 2007; Legrain et al. 2009; Moriarty et al. 2011) and animals (Galhardo and Pais-Vieira 2005; Ji et al. 2010; Moriarty et al. 2011; Ren et al. 2011). The results of this study suggest that mGluR1 blockade may be a useful strategy to restore normal activity in the mPFC in pain to overcome cognitive deficits.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke, Grants NS-38261 and NS-11255.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83–120, 1999 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 87: 81–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152: S49–S64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 108: 129–136, 2004a [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24: 10410–10415, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res 720: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J, Lee LJ, Lu HC. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci 30: 16896–16909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci 22: 2135–2141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 52: 708–715, 2002 [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8: 1458–1463, 2005 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci 985: 356–369, 2003 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19: 5473–5481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 18: 428–437, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39: 376–389, 2001 [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11: 7–20, 2007 [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat 2: 531–536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907, 2000 [DOI] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA 97: 6144–6149, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ. Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol 80: 621–627, 1998 [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878–880, 2004 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002 [DOI] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem 46: 204–206, 2003 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771, 2009 [DOI] [PubMed] [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA. Feed-forward inhibition as a buffer of the neuronal input-output relation. Proc Natl Acad Sci USA 106: 18004–18009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139: 1039–1048, 2006 [DOI] [PubMed] [Google Scholar]
- Galhardo V, Pais-Vieira M. Decision-making cognitive deficits in a rat model of chronic pain. Soc Neurosci Abstracts 50.3, vol 35, 2005 [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry 67: 199–207, 2010 [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain 131: 155–164, 2008 [DOI] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods 141: 261–269, 2005 [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 454: 600–606, 2008 [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol 14: 148–155, 2004 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol 639: 33–39, 2010 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol 97: 3893–3904, 2007 [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 102: 2253–2264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons. J Neurophysiol 104: 218–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30: 5451–5464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakossian MH, Otis TS. Excitation of cerebellar interneurons by group I metabotropic glutamate receptors. J Neurophysiol 92: 1558–1565, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Griffey K, Johnson MP, Chamberlain MJ, Kelly G, Tomlinson R, Wright RA, Johnson BG, Schoepp DD, Harris JR, Clark BP, Baker RS, Tizzano JT. Inhibition of group I metabotropic glutamate receptor responses in vivo in rats by a new generation of carboxyphenylglycine-like amino acid antagonists. Neurosci Lett 330: 127–130, 2002 [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 298: 40–49, 1990 [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12: 939–945, 2009 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26: 6458–6468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci 26: 631–640, 2003 [DOI] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev 12: 149–166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain 144: 230–232, 2009 [DOI] [PubMed] [Google Scholar]
- Lesage A, Steckler T. Metabotropic glutamate mGlu1 receptor stimulation and blockade: therapeutic opportunities in psychiatric illness. Eur J Pharmacol 639: 2–16, 2010 [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91: 13–24, 2004 [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330: 1400–1404, 2010 [DOI] [PubMed] [Google Scholar]
- Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 31: 1114–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS. Recruitment of GABAA inhibition in rat neocortex is limited and not NMDA dependent. J Neurophysiol 74: 2329–2335, 1995 [DOI] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284: 15629–15639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Casey KL. Imaging of acute versus pathological pain in humans. Eur J Pain 9: 163–165, 2005 [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci 21: 5925–5934, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Zhang C. Activation of metabotropic glutamate 5 (mGlu5) receptors induces spontaneous excitatory synaptic currents in layer V pyramidal cells of the rat prefrontal cortex. Neurosci Lett 442: 239–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852, 2004 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44: 1–14, 1991 [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28, 2004 [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314: 139–147, 2005 [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA 106, 2423–2428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 93: 385–404, 2011 [DOI] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol 467: 521–535, 2003 [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Metabotropic glutamate receptors: novel targets for pain relief. Expert Rev Neurother 1: 207–224, 2001 [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Subcortical processing of nociceptive information: basal ganglia and amygdala. In: Pain, edited by Cervero F, Jensen TS. Amsterdam: Elsevier, 2006, p. 141–158 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 60: 226–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain 3: 8–20, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 87: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23: 52–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 10: 221–234, 2004 [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60: 1017–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates “pain networks” defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain 123: 244–253, 2006 [DOI] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol 639: 47–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience 145: 225–231, 2007 [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 161: 671–679, 2009 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ledoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187, 2005 [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 9: 423–436, 2008 [DOI] [PubMed] [Google Scholar]
- Ren W, Neugebauer V. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol Pain 6: 93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology 36: 979–992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433, 2009 [DOI] [PubMed] [Google Scholar]
- Segovia G, Mora F. Effects of the metabotropic glutamate receptor agonist, ACPD, on the extracellular concentrations of GABA and acetylcholine in the prefrontal cortex of the rat during the normal process of aging. Brain Res Bull 65: 11–16, 2005 [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci 20: 268–270, 1999 [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron 58: 662–671, 2008 [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53: 735–746, 2007 [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron 54: 51–58, 2007 [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology 56, Suppl 1: 63–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol 106: 960–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Zhang Z, Jiao Y, Zhang C, Szabo G, Erdelyi F. Differential metabotropic glutamate receptor expression and modulation in two neocortical inhibitory networks. J Neurophysiol 101: 2679–2692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4: 131–144, 2005 [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142: 1–20, 2006 [DOI] [PubMed] [Google Scholar]
- Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FWF, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 25: 11107–11116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]