Abstract
Fragile X syndrome (FXS) is a neurodevelopmental disorder characterized by severe cognitive impairments, sensory hypersensitivity, and comorbidities with autism and epilepsy. Fmr1 knockout (KO) mouse models of FXS exhibit alterations in excitatory and inhibitory neurotransmission, but it is largely unknown how aberrant function of specific neuronal subtypes contributes to these deficits. In this study we show specific inhibitory circuit dysfunction in layer II/III of somatosensory cortex of Fmr1 KO mice. We demonstrate reduced activation of somatostatin-expressing low-threshold-spiking (LTS) interneurons in response to the group I metabotropic glutamate receptor (mGluR) agonist 3,5-dihydroxyphenylglycine (DHPG) in Fmr1 KO mice, resulting in impaired synaptic inhibition. Paired recordings from pyramidal neurons revealed reductions in synchronized synaptic inhibition and coordinated spike synchrony in response to DHPG, indicating a weakened LTS interneuron network in Fmr1 KO mice. Together, these findings reveal a functional defect in a single subtype of cortical interneuron in Fmr1 KO mice. This defect is linked to altered activity of the cortical network in line with the FXS phenotype.
Keywords: interneuron, γ-aminobutyric acid, barrel cortex, developmental disorder
fragile x syndrome (FXS) is the leading known inherited cause of intellectual disability and is caused by a CGG repeat expansion mutation in the Fmr1 gene (Verkerk et al. 1991). This mutation results in loss of expression of FMRP, a key regulator of synaptic protein synthesis, which leads to disruptions in basal and activity-dependent protein expression (Bassell and Warren 2008). In addition to cognitive impairment, affected individuals frequently exhibit characteristics of autism spectrum disorders (ASD; Hagerman et al. 2009), sensory hypersensitivity (Miller et al. 1999), irregular cortical EEG patterns, and seizure syndromes resembling benign focal epilepsy with centrotemporal spikes (Berry-Kravis 2002; Berry-Kravis et al. 2010). Together, these findings provide evidence that altered cortical function is a key component of the FXS phenotype, contributing to abnormal sensory processing and increased circuit excitability.
Normal neural circuit function requires precise and efficient excitatory and inhibitory transmission. A number of studies indicate that aberrant excitatory transmission, particularly unregulated signaling downstream of the group I metabotropic glutamate receptors (mGluRs), contributes to circuit imbalances in FXS (Chuang et al. 2005; Dolen and Bear 2008). Mounting evidence, however, also identifies defective inhibitory neurotransmission as an important contributor to circuit dysfunction and hyperexcitability (Gibson et al. 2008; Olmos-Serrano et al. 2010).
In the cerebral cortex, inhibitory neurotransmission is mediated by a diverse population of interneurons characterized by their distinct morphological, biochemical, and electrophysiological properties (Markram et al. 2004). Different cortical interneuron subtypes exhibit highly specific connectivity and thus form distinct, dynamically different networks, the most prevalent of which are formed by parvalbumin (PV)-expressing fast-spiking (FS) interneurons, which synapse onto the perisomatic region of their targets, and the somatostatin (SOM)-expressing low-threshold-spiking (LTS) interneurons, which are dendrite targeting (Beierlein et al. 2003; Gibson et al. 1999; Kawaguchi and Kubota 1997). These inhibitory networks respond dynamically to cortical activity to control cellular excitability, guide information flow (Porter et al. 2001), and generate neural synchrony and oscillatory activity at behaviorally relevant frequencies (Cardin et al. 2009; Sohal et al. 2009). Neural synchrony is crucial for cognitive processes, and disruptions of cortical synchrony are associated with cognitive impairments in autism and schizophrenia (Uhlhaas and Singer 2006). Furthermore, synchronized neural oscillations are dynamic over development and are proposed to be key contributors to cortical circuit maturation (Uhlhaas et al. 2010). Thus the study of inhibitory neuron subtypes involved in the generation of this synchrony is of great importance in neurodevelopmental disorders such as FXS.
Dendrite-targeting interneurons in cortical layer II/III contribute to the synchronization of cell networks over a range of frequencies, including theta, beta, and gamma (Blatow et al. 2003; Szabadics et al. 2001). Despite this important link between dendrite-targeting interneurons, such as the LTS interneuron, and normal cortical network function, little is known about these interneurons in the context of a neurodevelopmental disorder such as FXS. In the present study, we investigated activity-driven inhibition by LTS interneurons in layer II/III of somatosensory cortex in Fmr1 knockout (KO) mice. Using the group I mGluR agonist 3,5-dihydroxyphenylglycine (DHPG), we demonstrate a deficit in LTS interneuron activation and a weakening of inhibitory control over excitatory neuron output, thus identifying a key component of abnormal cortical function in FXS.
MATERIALS AND METHODS
Animal use.
Wild-type (WT) and Fmr1 KO mice (FVB.129P2 genetic background; Jackson Laboratory) were maintained and all experiments conducted according to protocols approved by Children's National Medical Center and Georgetown University Medical Center.
Brain slice preparation.
Male mice at postnatal days 19–31 (P19–P31; mean WT age: P25 ± 1, mean Fmr1 KO age: P24 ± 1) were deeply anesthetized with carbon dioxide (CO2) and then decapitated. Brains were removed and placed for 2–3 min in ice-cold, oxygenated (95% O2-5% CO2) sucrose slicing solution composed of (in mM) 234 sucrose, 11 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2. Thalamocortical brain slices (Agmon and Connors 1991) were prepared by placing the brain ventral side down in a constructed angle indicator and making a cut 35° from the midline and 10° from the dorsal-ventral axis. The brain was then glued cut side down on a vibratome (Leica) stage, immersed in ice-cold slicing solution. The first 2,100–2,400 μm of the brain were removed, and the four following 300-μm-thick slices were collected and incubated in prewarmed (32°C), oxygenated artificial cerebrospinal fluid (ACSF; in mM): 126 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, and 2 CaCl2 for 45 min before being transferred to the recording chamber, where they were continuously perfused with ACSF (23°C) at a flow rate of 3–4 ml/min.
Electrophysiology.
Slices were visualized with a fixed-staged, upright microscope (E600 FN; Nikon) equipped with a ×4 objective and a ×60 insulated objective, infrared (IR) illumination, Nomarski optics, and an IR-sensitive video camera (COHU). Single and dual recordings were collected from neurons in layer II/III of primary somatosensory cortex in the whole cell patch-clamp configuration. Pipettes with an access resistance of 2.5–4.0 MΩ when filled with intracellular recording solution were pulled from borosilicate glass capillaries (King Precision Glass). Data were acquired using a Multiclamp700A patch-clamp amplifier, Digidata1322A digitizer, and pClamp 9.2 acquisition software (Molecular Devices). Data were analyzed off-line, using pClamp and MiniAnalysis 6.0.7 (Synaptosoft).
Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in the voltage-clamp configuration [holding potential (Vhold) = −60 mV] from visually identified layer II/III pyramidal cells using an intracellular solution composed of (in mM) 135 CsCl, 10 EGTA, 2 MgCl2, 10 HEPES, 5 QX-314, 4 MgATP, and 0.3 NaGTP. IPSCs were isolated by including the ionotropic glutamate receptor blockers dl-2-amino-5-phosphonopentanoic acid (APV; 50 μM; Tocris Bioscience) and 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM; Tocris) in the bath. Miniature IPSCS (mIPSCs) were recorded in the added presence of the voltage-gated Na+ channel blocker tetrodotoxin (TTX; 1 μM; Alomone Labs). In all experiments, a baseline period of at least 2 min was recorded before bath application of the group I mGluR agonist DHPG (100 μM; Tocris), to confirm cell health and recording stability. A time window of ∼60 s was analyzed for the control and DHPG conditions.
Spontaneous action potential (AP) firing in response to DHPG was assessed in current-clamp mode, with no adjustment made to resting membrane potential (Vm). These experiments were conducted using a low-Cl− intracellular solution composed of (in mM) 130 K-gluconate, 10 KCl, 10 HEPES, 14 phosphocreatine di(tris), 0.2 EGTA, 2 MgCl2, 4 MgATP, and 0.3 NaGTP. As described above, a baseline period of at least 2 min was recorded before DHPG application, and AP frequency was measured over a period of 2 min. The coefficient of variation (CV) of the inter-AP interval was determined by dividing the standard deviation of inter-AP interval by the mean for each cell. To more closely study DHPG-induced currents in LTS interneurons, we repeated current-clamp experiments in the added presence of TTX. Vm was measured with 10-s samples taken at baseline, peak depolarization, and 2, 5, and 10 min after peak depolarization. A Gaussian distribution was fit to the left side of an all-points histogram from each sample from a point 1–3 pA to the left of the peak, to exclude bias from upward events. The peak of the distribution determined the mean current for that sample. All comparisons were made to baseline Vm.
Recurrent excitation was blocked during dual recordings of AP firing with APV and DNQX, and APs were evoked by delivering long (3 s) simultaneous depolarizing current steps every 30 s such that firing rate was approximately equal in both cells (6.58 ± 0.14 Hz, n = 32; Vhold = −60 mV).
Cell classification.
When the K-gluconate-based intracellular solution was used, layer II/III pyramidal neurons and inhibitory interneurons were electrophysiologically identified by their responses to a series of brief (600 ms) hyperpolarizing and depolarizing current steps (Vhold = −60 mV). This method also allowed differentiation of interneuron subtypes. Whereas FS cells exhibit high AP firing rates with little frequency adaptation, LTS cells were differentiated from these by their lower AP firing rates, greater AP frequency adaptation, higher membrane resistance (Rm), depolarized Vm, and rebound APs after hyperpolarization (Beierlein et al. 2000; Gibson et al. 1999; Kawaguchi 1993). Furthermore, LTS interneurons were distinguished from pyramidal neurons by the difference in the peak afterhyperpolarization (AHP) during a threshold AP train, with the first AHP being more hyperpolarized than the last in LTS cells (Beierlein et al. 2003). Only LTS interneurons with a resting potential less than or equal to −50 mV were included for analysis, and furthermore, only cells with little spontaneous activity (infrequent/no spontaneous APs under control conditions) were used for DHPG experiments. When the Cs+-based intracellular solution was used, pyramidal neurons were identified on the basis of their somatic morphology, prominent apical dendrite, and Rm, measured from a small hyperpolarizing current step.
Rm was measured from a given cell's response to a small hyperpolarizing current step delivered in the current-clamp recording configuration. Membrane constant was determined by fitting a single-exponential function to Vm as it recovered to baseline after a small hyperpolarizing current step. Membrane capacitance (Cm) was calculated on the basis of these Rm and membrane constant measurements. All AP properties reported were calculated by pClamp using templates derived from a series of APs from LTS interneurons.
Cross-correlation analysis.
AP and sIPSC cross-correlations were calculated for individual cell pairs using MiniAnalysis 6.0.7. Bin width was set to 10 ms for sIPSC and 20 ms for AP analysis. In each pair, the cell with the lowest event frequency was assigned as the reference cell, and cross-correlation values represent the average number of events that occurs in each time bin in the nonreference cell for a given event in the reference cell. For sIPSC cross-correlation analysis, a high event threshold (10× root mean square noise) was used, to increase the probability of measuring AP-dependent events. A total time window of ∼60s was analyzed under baseline conditions and in the presence of DHPG for each cell pair. Cross-correlation central bin values were compared between control and DHPG conditions. AP cross-correlation for each cell pair was calculated as the average cross-correlation of five consecutive 3-s current sweeps, both under baseline conditions and in the presence of DHPG. Again, central bin cross-correlation values, i.e., at time 0, were compared. For all cell pairs, baseline sIPSC or AP cross-correlation was zeroed out by calculating the average cross-correlation across bins and then subtracting this value from each bin of the raw cross-correlation.
Neuronal morphology.
For post hoc analysis of cellular morphology, biocytin (0.5%; Sigma) was included in the intracellular recording solution. After a given neuron's intrinsic and firing properties were recorded, biocytin was injected by delivering a series of short depolarizing current pulses (1 nA). Slices were removed from the recording chamber and fixed overnight in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS). Cell membranes were permeabilized by placing slices in PBS + Triton X-100 (2%) for 45 min and then incubated for 1 h in PBS containing Texas red-tagged avidin D (10 μl/ml; Vector Labs) and Triton X-100 (0.5%). Three washes in PBS + Triton X-100 were followed by overnight incubation at 4°C in rabbit anti-SOM primary antibody (1:500 dilution; Chemicon International), three more washes, and 1 h in fluorescein-tagged anti-rabbit secondary antibody (1:200 dilution; Vector Labs). After a final wash, slices were mounted with Vectashield mounting medium (Vector Labs) and covered. Filled cells were visualized using a Zeiss Axiovert M200 fluorescent microscope.
Immunohistochemistry.
Mice were transcardially perfused at P21 with 4% PFA in PBS. Brains were fixed overnight, embedded in 4% agar, and sectioned coronally. Incubation of slices in primary antibody [rabbit anti-calretinin (anti-CR; Swant) and rat anti-SOM (Chemicon)] was followed by incubation with secondary antibodies for immunofluorescence [Cy3 at 1:200 dilution or FITC at 1:50 dilution (Jackson ImmunoResearch)] or peroxidase immunohistochemistry [biotinylated anti-IgG (Jackson ImmunoResearch); streptavidin (Sigma Chemical)]. For the latter technique, immunoreactive structures were visualized using nickel-intensified 3,3′-diaminobenzidine-4HCl (Sigma). Sections (50 μm thick) from bregma level −0.70 to −1.70 mm in layer II/III of somatosensory cortex were analyzed. Photomicrographs were obtained with a Zeiss LSM 510 confocal microscope or an Olympus BX51 light microscope. Immunopositive cells were counted using nonstereological count methods. Counts were performed in at least six sections from each brain.
Statistical analysis.
All data are means ± SE, and statistical analyses were performed using Origin software (version 7; OriginLab) and MiniAnalysis (Synaptosoft). The normal distribution of data sets was confirmed using the Shapiro-Wilk test, and statistical comparisons were conducted using independent or paired two-tailed Student's t-tests, as appropriate. The Kolmogorov-Smirnov test was used to compare inter-AP interval distributions.
RESULTS
Group I mGluR-dependent inhibition is dampened in Fmr1 KO mice.
In the rodent cerebral cortex, group I mGluR (mGluRs 1 and 5) activation has been demonstrated to increase inhibitory interneuron output, and thus GABAergic tone (Beierlein et al. 2000; Chu and Hablitz 1998; Fanselow et al. 2008). Given the evidence for impaired inhibition and cortical hyperexcitability in FXS, the effect of the agonist DHPG (100 μM) on synaptic inhibition onto layer II/III excitatory pyramidal cells was examined in Fmr1 KO mice. Under control conditions, sIPSC frequency was not significantly different in wild-types (WT) and Fmr1 KO mice (WT: 8.81 ± 1.00 Hz, n = 9; Fmr1 KO: 7.36 ± 0.95 Hz, n = 10; P = 0.31, independent t-test; Fig. 1, A, B, and E). Bath application of DHPG in WT mice led to a large increase in sIPSC frequency (12.21 ± 1.35 Hz, n = 9; P = 0.012 vs. control, paired t-test; Fig. 1, A and E). DHPG also increased event frequency in Fmr1 KO mice (8.49 ± 1.04 Hz, n = 10; P = 0.039 vs. control, paired t-test; Fig. 1, B and E). Interestingly, however, sIPSC frequency in the presence of DHPG was significantly lower in Fmr1 KO compared with WT mice (P = 0.041, independent t-test; Fig. 1E), indicating that group I mGluR-dependent inhibition is dampened in Fmr1 KO mice. DHPG did not increase mIPSC frequency in either WT or Fmr1 KO mice (WT: 5.41 ± 0.60 vs. 5.08 ± 0.52 Hz, n = 7; P = 0.20, paired t-test; Fmr1 KO: 5.35 ± 0.20 vs. 5.50 ± 0.45 Hz, n = 4; P = 0.62, paired t-test; Fig. 1, C–E), in agreement with previous reports indicating that DHPG exerts its effect on inhibition by increasing the firing of inhibitory interneurons.
Fig. 1.
Increase in synaptic inhibition onto LII/III pyramidal neurons induced by 3,5-dihydroxyphenylglycine (DHPG) is dampened in Fmr1 knockout (KO) mice. A: continuous voltage-clamp traces from a pyramidal neuron under control conditions (i) and in the presence of DHPG (ii), illustrating that DHPG causes an increase in spontaneous inhibitory postsynaptic current (sIPSC) frequency. B: continuous traces from an Fmr1 KO pyramidal neuron, illustrating that sIPSC frequency also increases in response to DHPG in these cells (ii) but that frequency is reduced compared with wild-type (WT) control (i) (see pooled data in E; n = 9 WT and 10 Fmr1 KO neurons). DHPG did not cause an increase in miniature IPSC (mIPSC) frequency in either WT or Fmr1 KO pyramidal neurons (C–E; n = 7 WT and 4 Fmr1 KO neurons). Calibration: 50 pA, 1 s. *P < 0.05.
Cortical LTS interneurons exhibit reduced activation in Fmr1 KO mice.
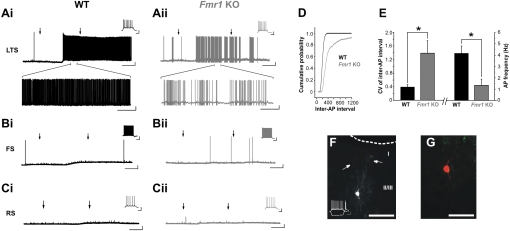
Based on the reduction in AP-dependent synaptic inhibition in Fmr1 KO mice, the responses of inhibitory interneurons to DHPG application were examined. Group I mGluR agonists have been shown to selectively induce AP firing in SOM-expressing LTS interneurons in cortical layer IV (Beierlein et al. 2000), as well as in layer II/III GIN cells, a class of SOM-positive inhibitory interneuron that shares a number of physiological characteristics with the LTS subtype (Fanselow et al. 2008). LTS interneurons were identified in layer II/III by their small soma, depolarized Vm, high Rm, and distinct firing properties (see materials and methods and Fig. 2, A and F, insets). In LTS interneurons from WT mice, DHPG induced depolarization and sustained AP firing (4.17 ± 0.66 Hz, n = 6; Fig. 2Ai). In Fmr1 KO mice, LTS interneurons also depolarized and fired in response to DHPG, but AP frequency was greatly reduced compared with WT mice (1.33 ± 0.57 Hz, n = 4; P = 0.017 vs. WT, independent t-test; Fig. 2, Aii, D, and E). In addition, AP firing in response to DHPG was more intermittent in Fmr1 KO mice, as indicated by an increase in the CV of inter-AP interval (WT: 0.39 ± 0.07, n = 6; Fmr1 KO: 1.39 ± 0.37, n = 4; P = 0.011, independent t-test; Fig. 2E). To understand why LTS interneurons in Fmr1 KO mice fail to fire sustained APs in the presence of DHPG, we examined the depolarizing current induced by DHPG in WT and Fmr1 KO mice in the added presence of TTX. Although peak depolarization from baseline did not differ between WT and Fmr1 KO LTS neurons (WT: 5.88 ± 1.62 mV, n = 4; Fmr1 KO: 3.87 ± 2.02 mV, n = 3; P = 0.47, independent t-test), the difference from baseline was significantly different at both 5 and 10 min after peak depolarization (5 min: WT: 5.24 ± 1.20 mV; Fmr1 KO: 0.23 ± 0.83 mV; P = 0.025, independent t-test; 10 min: WT: 3.49 ± 0.74 mV; Fmr1 KO: −1.44 ± 0.58 mV; P = 0.004, independent t-test), with Fmr1 KO Vm measured in both cases to be closer to baseline Vm. This trend was also visible at 2 min after peak depolarization but did not quite meet statistical significance (WT: 6.47 ± 1.62 mV; Fmr1 KO: 3.87 ± 2.02 mV; P = 0.053, independent t-test). This failure to maintain sustained membrane depolarization is a likely contributor to reduced LTS interneuron activation in Fmr1 KO mice in response to group I mGluR stimulation.
Fig. 2.
Activation of low-threshold-spiking (LTS) interneurons by DHPG is reduced in Fmr1 KO somatosensory cortex. Ai: spontaneous current-clamp recording illustrating that WT LTS interneurons fire action potentials (APs) continuously in response to bath application of DHPG (between arrows, top trace). Inset: AP firing pattern of the cell, identifying it as LTS. Magnified traces (bottom) show AP firing over 1 min in the presence of DHPG. Aii: in Fmr1 KO mice, LTS firing in DHPG is more irregular, as illustrated by the cumulative plot of inter-AP interval for the cells in A (see D; P < 0.001, Kolmogorov-Smirnov test). E: coefficient of variation (CV) of inter-AP interval with DHPG is greater and mean AP frequency is reduced in Fmr1 KO LTS neurons compared with WT (n = 6 WT and 4 Fmr1 KO). *P < 0.05. B and C: FS (Bi and Bii) and regular-spiking (RS) pyramidal neurons (Ci and Cii) do not characteristically respond to DHPG with continuous firing in WT or Fmr1 KO mice. F: biocytin-filled LTS interneuron (see inset current-clamp trace) with projections extending to layer 1 (arrows). Scale bar, 100 μm. G: higher magnification image of the same neuron as in F, with biocytin (red) and somatostatin (SOM; green) overlaid. Scale bar, 50 μm. All (5/5) filled LTS interneurons displayed this combination of morphological and immunohistochemical features. Dotted line in F delineates pial surface. Calibrations: A (top)–C: 10 mV, 2 min; A (bottom): 10 mV, 10 s; insets: 20 mV, 200 ms.
In agreement with previous findings (Fanselow et al. 2008), DHPG did not reliably induce AP firing in FS interneurons or in regular-spiking (RS) pyramidal neurons in either WT or Fmr1 KO mice (5/6 WT and 2/3 Fmr1 KO FS did not fire; 4/4 WT and 7/7 Fmr1 KO RS did not fire; Fig. 2, B and C). This specificity of group I mGluR-mediated effects on LTS interneurons falls in line with evidence that mGluR1, the receptor subtype primarily associated with LTS neuron-specific effects of group I mGluR agonists (Beierlein et al. 2000; Fanselow et al. 2008), is predominantly expressed in SOM-positive inhibitory interneurons in the rodent cortex (Kerner et al. 1997; Stinehelfer et al. 2000). Post hoc morphological examination of biocytin-filled LTS interneurons confirmed SOM expression, as well as the presence of prominent ascending projections to layer I that are ideally positioned to synapse on the dendritic arbors of layer II/III pyramidal cells (n = 5; Fig. 2, F and G), a key feature of SOM-positive “Martinotti” cells (Kawaguchi and Kubota 1997; Wang et al. 2004). Importantly, WT and Fmr1 KO LTS neurons did not differ with respect to a number of fundamental passive and active properties, including resting Vm, Rm, Cm, membrane constant, and AP amplitude and half-width. Fmr1 KO LTS neurons did have a significantly lower AP time to peak, however (Table 1).
Table 1.
Passive and active membrane properties
| WT | Fmr1 KO | P Value | |
|---|---|---|---|
| n | 16 | 9 | |
| Rm, MΩ | 398 ± 23 | 496 ± 54 | 0.067 |
| Vm, mV | −54.5 ± 0.8 | −54.4 ± 1.4 | 0.91 |
| Membrane constant, ms | 36.4 ± 3.7 | 38.6 ± 4.1 | 0.71 |
| Cm, pF | 92.1 ± 8.4 | 80.8 ± 8.6 | 0.40 |
| AP half-width, ms | 1.98 ± 0.13 | 2.25 ± 0.17 | 0.21 |
| AP amplitude, mV | 64.0 ± 2.5 | 59.3 ± 2.4 | 0.23 |
| AP time to peak, ms | 1.37 ± 0.02 | 1.28 ± 0.02 | 0.013* |
| Threshold current, pA | 16.9 ± 2.4 | 18.9 ± 2.6 | 0.60 |
Values are means ± SE for passive and active properties of layer II/III low-threshold-spiking interneurons from wild-type (WT) and Fmr1 knockout (KO) mice; n = no. of neurons. Rm, membrane resistance; Vm, resting membrane potential; Cm, membrane capacitance; AP, action potential.
P < 0.05.
Counts of layer II/III SOM-positive interneurons did not reveal any differences between WT and Fmr1 KO mice (WT: 199 ± 23 cells, n = 3 brains; Fmr1 KO: 194 ± 28 cells, n = 3; P = 0.91, independent t-test; Fig. 3, A–C). Because SOM-expressing interneurons in layer II/III exhibit different physiological features concurrent with CR expression (Xu et al. 2006), we also investigated SOM/CR coexpression. Like total SOM-positive counts, the proportion of layer II/III SOM/CR-positive interneurons did not differ between WT and Fmr1 KO mice (WT: 63.2 ± 1.5%, n = 3; Fmr1 KO: 61.2 ± 0.4%, n = 3; P = 0.34, independent t-test; Fig. 3, D–F). Together, these findings indicate that weakened LTS interneuron activation in Fmr1 KO mice is not related to significant changes in basic intrinsic properties or in numbers of SOM-positive cells in layer II/III.
Fig. 3.
A–C: immunostaining for SOM in WT (A) and Fmr1 KO mice (B) revealed no difference in the number of SOM-positive cells in layer II/III (C; n = 3 WT brains and 3 Fmr1 KO brains). D–F: immunostaining for SOM (red) and calretinin (CR; green) in layer II/III of WT (D) and Fmr1 KO mice (E) revealed no difference in the proportion of double-positive cells (arrowheads) (F; n = 3 WT and 3 Fmr1 KO). Scale bars, 250 μm.
Synchronization of synaptic inhibition is impaired in Fmr1 KO mice.
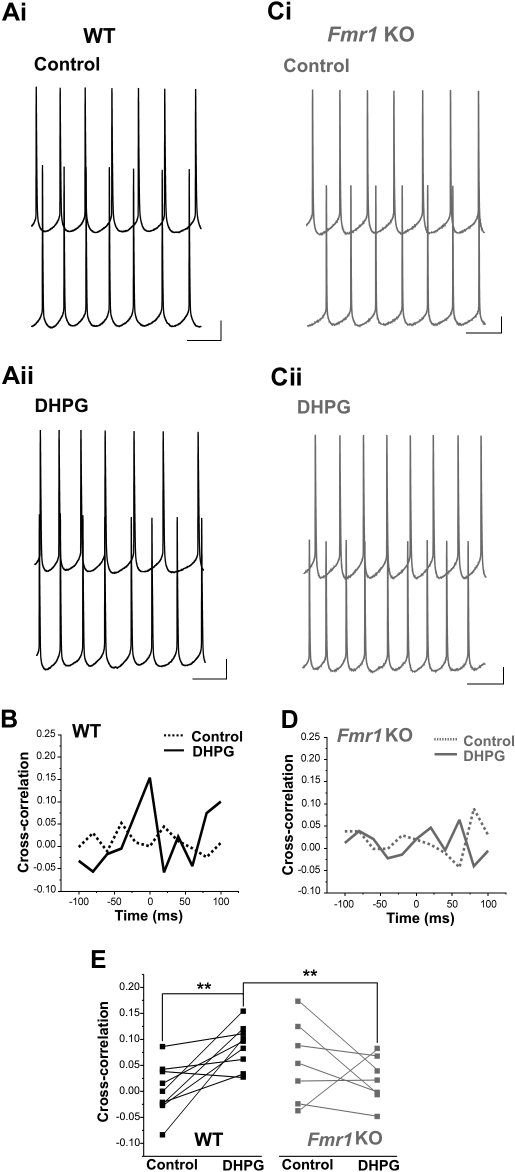
A key characteristic of LTS interneurons is that they form electrically coupled networks and are capable of generating synchronized inhibitory output onto their synaptic targets (Beierlein et al. 2000; Long et al. 2005). Given that LTS interneuron activation in response to group I mGluR stimulation is weakened in Fmr1 KO mice, we hypothesized that synchronous inhibition onto pyramidal neurons might likewise be impaired. This possibility was investigated by collecting dual voltage-clamp recordings from pairs of pyramidal neurons located <125 μm apart. In all WT cell pairs tested, DHPG robustly induced increases in synchronous sIPSCs (Fig. 4A). Cross-correlation analysis of sIPSCs in DHPG revealed a sharp central peak (Fig. 4B), whereas under control conditions, sIPSCs were largely unsynchronized, with cross-correlation analysis generating no discernible peaks (control: 0.05 ± 0.01; DHPG: 0.27 ± 0.02, n = 5; P = 0.00007, paired t-test; Fig. 4, Ai and B). Similarly to WT mice, DHPG induced an increase in sIPSC synchronization in Fmr1 KO mice, but this effect was dampened; central cross-correlation values in the presence of DHPG were significantly lower in Fmr1 KO mice (WT: 0.27 ± 0.02, n = 5; Fmr1 KO: 0.17 ± 0.03, n = 5; P = 0.013, independent t-test; Fig. 4, B–E). This finding indicates that the reduction in group I mGluR-dependent activation of LTS interneurons in Fmr1 KO mice results not only in a reduced frequency of inhibitory synaptic events (Fig. 1) but also in reduced inhibitory synchrony onto pyramidal neurons.
Fig. 4.
DHPG-induced sIPSC synchronization is reduced in Fmr1 KO pyramidal neurons. A: simultaneous voltage-clamp recordings from 2 WT pyramidal RS neurons before (i) and after (ii) DHPG application, illustrating that sIPSC synchronization increases with DHPG (see magnified traces, bottom). B: cross-correlation analysis (bin width = 10 ms) revealed a strong central peak in DHPG, but not under control conditions. C: representative pyramidal neuron pair from an Fmr1 KO mouse (i). DHPG induced some sIPSC synchronization, but this was reduced compared with WT (ii). D: cross-correlogram for the cell pair in C is shown. E: DHPG increased cross-correlogram peak value in both WT and Fmr1 KO mice, but this effect was significantly greater in WT (n = 5 WT pairs and 5 Fmr1 KO pairs). *P < 0.05; **P < 0.01; ***P < 0.005. Calibrations: A and C (top traces): 50 pA, 2 s; A and C (bottom traces): 50 pA, 500 ms.
The LTS interneuron network in Fmr1 KO mice exhibits weakened inhibitory control over pyramidal neuron output.
Even though layer II/III inhibitory interneurons are outnumbered by their excitatory counterparts, they exert a powerful influence over the flow of excitatory neurotransmission. Indeed, it has been shown that specific activation of LTS neurons leads not only to synchronization of synaptic inhibition onto excitatory neurons but also to the generation of synchronized output of these excitatory targets. Specifically, targeted LTS neuron activation using the mGluR agonist 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) can induce synchronization of evoked APs in target excitatory neurons (Long et al. 2005).
Given that the reduced sIPSC synchronization onto pairs of pyramidal neurons in the presence of the group I mGluR agonist DHPG in Fmr1 KO mice is related to a specific deficit in LTS interneuron output (Fig. 2), we hypothesized that LTS neuron-driven control of pyramidal neuron output would also be altered under this condition. To test this, pairs of pyramidal neurons were induced to fire continuous AP trains with long (3 s) sweeps of depolarizing current. In WT pyramidal neurons, baseline AP synchronization was low (central cross-correlation value: 0.003 ± 0.017, n = 9). As expected, DHPG application reliably increased the incidence of synchronous APs, as indicated by a significant increase in central cross-correlation peak value (0.087 ± 0.014, n = 9; P = 0.0059 vs. control, paired t-test; Fig. 5, A and B). In contrast, DHPG application did not exert a significant effect on AP synchronization in Fmr1 KO pyramidal neuron pairs (control: 0.056 ± 0.029; DHPG: 0.022 ± 0.017, n = 7; P = 0.33, paired t-test; Fig. 5, C and D), in agreement with the observed reduction in group I mGluR-dependent inhibition in Fmr1 KO mice. Furthermore, whereas baseline AP synchronization in WT and Fmr1 KO mice was not significantly different (P = 0.12, independent t-test), AP synchronization in the presence of DHPG was significantly lower in Fmr1 KO compared with WT mice (P = 0.0092, independent t-test; Fig. 5E). These findings indicate that a net result of impaired LTS interneuron firing and dampened inhibitory synchrony in Fmr1 KO mice in response to group I mGluR stimulation is a reduction in inhibitory control over pyramidal neuron output, a potential indicator of altered network dynamics and output.
Fig. 5.
DHPG-induced AP synchronization is reduced in Fmr1 KO pyramidal neurons. A: simultaneous recordings of evoked AP firing in 2 WT pyramidal neurons. AP synchronization was low under control conditions (i) and then increased with DHPG application (ii), as illustrated by the increase in cross-correlogram central peak value (B; bin width = 20 ms). C: example of a pair of Fmr1 KO pyramidal neurons that similarly exhibited low AP synchronization under control conditions (i) but did not show an increase with DHPG application (ii). D: the cross-correlogram for the cell pair in C lacks a defined central peak under both conditions, indicating a lack of AP synchronization. E: DHPG induced a significant increase in the synchronization of evoked APs in WT but not Fmr1 KO mice. AP cross-correlation in DHPG was also significantly greater in WT than in Fmr1 KO mice (n = 9 WT pairs and 7 Fmr1 KO pairs). **P < 0.01. Calibrations: 10 mV, 200 ms.
DISCUSSION
In the present study, we report that group I mGluR-dependent activation of a specific subtype of cortical interneuron, the SOM-expressing, dendrite-targeting LTS interneuron, is impaired in Fmr1 KO mice. Consequently, these interneurons exert dampened inhibitory control over their excitatory targets, illustrated by a reduction in synchronized synaptic inhibition onto layer II/III pyramidal neurons. AP synchronization in pyramidal neurons in response to DHPG is likewise reduced in Fmr1 KO mice, revealing that normal patterns of cortical excitation governed by the LTS interneuron network are altered in these animals. These findings are the first to identify LTS interneuron dysfunction in a mouse model of FXS and implicate a weakened LTS network as an important contributor to impaired cortical function in this disorder.
Role of LTS interneurons in cortical circuit dysfunction.
Inhibitory interneurons are key determinants of neural circuit function, and loss or dysfunction of specific interneuron subtypes has been identified in animal models of a number of disease states involving excitatory/inhibitory imbalances, such as autism and epilepsy (Buckmaster and Jongen-Rêlo 1999; Gogolla et al. 2009). In addition, impaired FS interneuron function has been implicated in cortical hyperexcitability in FXS (Gibson et al. 2008). We now identify LTS interneuron dysfunction as a central contributor to altered cortical function in the Fmr1 KO model.
The role of LTS interneurons in the cortical network is determined by their unique physiology and connectivity, and altered function of these particular interneurons may contribute to a number of behavioral phenotypes observed in FXS individuals. In contrast to their FS counterparts, LTS interneurons receive facilitating excitatory inputs (Beierlein et al. 2003; Gibson et al. 1999, 2008). As a consequence of this, the inhibitory output of these cells increases with network activation (Kapfer et al. 2007; Silberberg and Markram 2007). LTS interneurons are therefore well positioned to dynamically control cortical network function during periods of elevated activity, such as when the individual is subject to continuous sensory input. In Fmr1 KO mice, we observed a failure of LTS interneuron activation in response to group I mGluR stimulation, suggesting that activity-dependent increases in inhibition may be compromised in vivo. Indeed, the sensory hypersensitivity and tactile defensiveness frequently exhibited by FXS patients, in addition to a number of characteristics of ASD, might reflect a deficit in sensory state-dependent shifts in cortical function, which rely on the ability of the network to provide dynamic functional inhibition (Moore et al. 2010). In this respect, it is noteworthy that Fmr1 KO mice exhibit sensory-driven seizures (Musumeci et al. 2000), which, similarly, could be related to a failure of the LTS network to sufficiently increase its output in the face of high network activity. Remarkably, FXS patients are characterized by a high incidence of hyperexcitable EEG patterns, cortically derived seizures and epilepsies characterized by centrotemporal “rolandic” spikes (Berry-Kravis 2002; Berry-Kravis et al. 2010). The prevalence of this comorbidity suggests a potential shared cellular mechanism between FXS and epileptiform activity. We propose LTS interneuron dysfunction as a potential contributor to the comorbid condition.
Dendrite-targeting inhibitory connections from LTS interneurons are well suited to control the integration of excitatory inputs along the dendritic tree. This form of inhibition is crucial for the control of cellular excitability, as evidenced by models of temporal lobe epilepsy, in which the selective loss of dendritic inhibition, due to a loss of SOM-expressing interneurons, contributes to hyperexcitability in the hippocampus (Buckmaster and Jongen-Rêlo 1999; Cossart et al. 2001). Although we did not observe a loss of SOM-expressing interneurons in the cortex of Fmr1 KO mice, we did reveal impaired function of dendrite-targeting inhibitory interneurons that might contribute to cortical hyperexcitability. Interestingly, GIN cells (which include LTS interneurons) recently have been proposed to control cortical excitability by contributing to the termination of up states in layer II/III (Fanselow and Connors 2010).
Alongside selectively reduced activation of LTS interneurons in Fmr1 KO mice, we observed reduced DHPG-induced synchronization of synaptic inhibition in layer II/III pyramidal neurons. Whereas impaired firing of individual cells primarily implies cell-intrinsic defects, reduced inhibitory synchrony suggests the possibility of a larger LTS network defect. LTS interneurons are tightly coupled by gap junctions, electrical connections that enable synchronous firing of interneuron networks (Deans et al. 2001) and, thus, synchronization of inhibition onto multiple postsynaptic target neurons and generation of coordinated neuronal oscillations. Network synchronization is crucial for normal cognitive function, and disrupted synchrony is proposed to underlie cognitive dysfunction in ASD (Perez Velazquez et al. 2009; Uhlhaas and Singer 2006). In this study, we observed a failure of LTS interneuron activation and synchronization of pyramidal cell output in Fmr1 KO mice, suggesting that defective generation of important cortical rhythms may contribute to impaired cortical function in FXS. In agreement with a previous report (Fanselow et al. 2008), our results indicate that layer II/III LTS interneurons fire in the theta frequency range in response to DHPG application and thus might contribute to the generation of this rhythm in vivo. This rhythm is reported to be important for learning and memory in rodents, nonhuman primates, and humans (Kahana et al. 2001; Lee et al. 2005; Nyhus and Curran 2010; Raghavachari et al. 2006; Sederberg et al. 2003).
A clear link between LTS interneuron output and synchronization of excitatory neuron output in response to mGluR signaling was established in studies in layer IV of the somatosensory cortex by Long et al. (2005). Importantly, AP synchronization in excitatory neurons in the presence of ACPD was established to have a developmental onset coinciding with the maturation of a number of LTS neuron properties, including increased strength of LTS-to-RS synapses, increased LTS neuron firing frequency in response to ACPD, increased correlation between ACPD-induced rhythmic subthreshold voltage fluctuations in LTS cells and IPSPs in excitatory neurons, and, finally, axonal and dendritic maturation of LTS neurons. Thus LTS neuron function is required to generate group I mGluR-dependent AP synchronization in excitatory neurons, and the reduction in AP synchronization in response to group I mGluR signaling in Fmr1 KO mice reported in the current study is very likely to result from reduced LTS interneuron output.
Beyond their requirement for normal cognitive function, synchronized neuronal oscillations are important contributors to cortical circuit maturation (Uhlhaas et al. 2010). The timeline for the development of inhibitory deficits in FXS is largely unclear, but it is intriguing to consider not only how disruptions in inhibitory circuit development might affect neural synchrony in the developing circuit but also how abnormal synchrony early in development might contribute to inhibitory abnormalities observed later in life.
How does reduced mGluR activation of LTS interneurons relate to the “mGluR hypothesis” in FXS?
There is a large pool of evidence from studies in Fmr1 KO mice indicating that excessive signaling downstream of group I mGluRs forms the basis for aberrant synaptic function and cellular excitability in FXS (Bianchi et al. 2009; Chuang et al. 2005; Dolen and Bear 2008; Huber et al. 2002). Our findings, in contrast, indicate a reduction in the mGluR response in Fmr1 KO mice that, at first, appears to conflict with the mGluR hypothesis. Could this decrease in mGluR-dependent synaptic inhibition result, however, from an actual increase in mGluR signaling? Recent evidence from the hippocampus and striatum indicate that excessive signaling downstream of group I mGluRs leads to greater suppression of inhibition in Fmr1 KO mice via endocannabinoid-dependent mechanisms (Maccarrone et al. 2010; Zhang and Alger 2010). In addition, mGluR signaling is a modulator of electrical coupling, with receptor stimulation acting to reduce electrical synapse strength in inhibitory interneurons of the thalamic reticular nucleus (Landisman and Connors 2005). Given the importance of electrical coupling for temporal coordination of interneuron output (Gibson et al. 1999), and thus coordinated synaptic inhibition and pyramidal cell output, this presents another intriguing avenue by which excessive signaling via group I mGluRs might contribute to reduced inhibitory synchrony mediated by LTS interneurons in Fmr1 KO mice. The effects of FMRP deletion on interneuron function are likely to be highly complex, however. It is well established that Fmr1 KO mice are characterized by altered expression of a wide array of proteins, including cation channels, neurotransmitter receptors, components of the vesicular release machinery, and important signaling and structural proteins (Liao et al. 2008), and dysregulation of processes mediated by these proteins are likely to underlie disturbances in cellular and circuit development.
In conclusion, we report an interneuron subtype-specific deficit that results in reduced inhibitory control of excitatory neuron output in the Fmr1 KO cortex. Given that the unique anatomical, synaptic, and physiological properties of LTS interneurons position them to control a number of crucial cortical processes, we suggest that LTS interneuron dysfunction could potentially contribute to a number of comorbid conditions in FXS, including cognitive deficits, sensory hypersensitivity, and seizures. Moving forward, a primary point of interest in FXS, as well as other diseases of synaptic development and function such as ASD, will be to gain insight into the link between alterations in synaptic transmission and behavior and cognitive function.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS053719 (to M. M. Huntsman), Autism Speaks (to M. M. Huntsman and J. G. Corbin), FRAXA Research Foundation (M. M. Huntsman and J. G. Corbin), and a Canadian Institutes of Health Research Doctoral Research Award (to S. M. Paluszkiewicz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the members of the Huntsman and Corbin laboratories, Dr. Vittorio Gallo, and Dr. John Huguenard for critical input.
Present address of J. L. Olmos-Serrano: Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA 02118.
REFERENCES
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991 [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60: 201–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3: 904–910, 2000 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol 44: 724–728, 2002 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am J Intellect Dev Disabil 115: 461–472, 2010 [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogenesis. J Neurosci 29: 3497–3507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38: 805–817, 2003 [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci 19: 9519–9529, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ. Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol 80: 621–627, 1998 [DOI] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci 25: 8048–8055, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4: 52–62, 2001 [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron 31: 477–485, 2001 [DOI] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586: 1502–1508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. J Neurophysiol 104: 596–606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100: 2640–2652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol 100: 2615–2626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof T, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 1: 172–181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics 123: 378–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99: 7746–7750, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol 11: 739–744, 2001 [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfield LL, Atallah BV, Scaniziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci 10: 743–753, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69: 416–431, 1993 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486, 1997 [DOI] [PubMed] [Google Scholar]
- Kerner JA, Standaert DG, Penney JBJ, Young AB, Landwehrmeyer GB. Expression of group one metabotropic glutamate receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Mol Brain Res 48: 259–269, 1997 [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science 310: 1809–1813, 2005 [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45: 147–156, 2005 [DOI] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P, Yates JR. Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci USA 105: 15281–15286, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Cruickshank SJ, Jutras MJ, Connors BW. Abrupt maturation of a spike-synchronizing mechanism in neocortex. J Neurosci 25: 7309–7316, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Bernardi G, Bagni C, Centonze D. Abnormal mGlu5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology 35: 1500–1509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet 83: 268–279, 1999 [PubMed] [Google Scholar]
- Moore CI, Carlen M, Knoblich U, Cardin JA. Neocortical interneurons: from diversity, strength. Cell 142: 189–193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizure susceptibility in transgenic mice with fragile X syndrome. Epilepsia 41: 19–23, 2000 [DOI] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 34: 1023–1035, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci 30: 9929–9938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Velazquez JL, Barcelo F, Hung Y, Leschenko Y, Nenadovic V, Belkas J, Raghavan V, Brian J, Garcia Dominguez L. Decreased brain coordinated activity in autism spectrum disorders during executive tasks: reduced long-range synchronization in the fronto-parietal networks. Int J Psychophysiol 73: 341–349, 2009 [DOI] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci 21: 2699–2710, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol 95: 1630–1638, 2006 [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23: 10809–10814, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53: 735–746, 2007 [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinehelfer S, Vruwink M, Burette A. Immunolocalization of mGluR1alpha in specific populations of local circuit neurons in the cerebral cortex. Brain Res 861: 37–44, 2000 [DOI] [PubMed] [Google Scholar]
- Szabadics J, Lorincz A, Tamas G. Beta and gamma frequency synchronization by dendritic GABAergic synapses and gap junctions in a network of cortical interneurons. J Neurosci 21: 5824–5831, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci 14: 72–80, 2010 [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168, 2006 [DOI] [PubMed] [Google Scholar]
- Verkerk A, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, van Ommen GB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914, 1991 [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol 561: 65–90, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499: 144–160, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci 30: 5724–5729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]