Abstract
Strain differences between naive, sucrose- and ethanol-exposed alcohol-preferring (P) and alcohol-nonpreferring (NP) rats were investigated in their consumption of ethanol, sucrose, and NaCl; chorda tympani (CT) nerve responses to sweet and salty stimuli; and gene expression in the anterior tongue of T1R3 and TRPV1/TRPV1t. Preference for 5% ethanol and 10% sucrose, CT responses to sweet stimuli, and T1R3 expression were greater in naive P rats than NP rats. The enhancement of the CT response to 0.5 M sucrose in the presence of varying ethanol concentrations (0.5–40%) in naive P rats was higher and shifted to lower ethanol concentrations than NP rats. Chronic ingestion of 5% sucrose or 5% ethanol decreased T1R3 mRNA in NP and P rats. Naive P rats also demonstrated bigger CT responses to NaCl+benzamil and greater TRPV1/TRPV1t expression. TRPV1t agonists produced biphasic effects on NaCl+benzamil CT responses, enhancing the response at low concentrations and inhibiting it at high concentrations. The concentration of a TRPV1/TRPV1t agonist (Maillard reacted peptides conjugated with galacturonic acid) that produced a maximum enhancement in the NaCl+benzamil CT response induced a decrease in NaCl intake and preference in P rats. In naive P rats and NP rats exposed to 5% ethanol in a no-choice paradigm, the biphasic TRPV1t agonist vs. NaCl+benzamil CT response profiles were higher and shifted to lower agonist concentrations than in naive NP rats. TRPV1/TRPV1t mRNA expression increased in NP rats but not in P rats exposed to 5% ethanol in a no-choice paradigm. We conclude that P and NP rats differ in T1R3 and TRPV1/TRPV1t expression and neural and behavioral responses to sweet and salty stimuli and to chronic sucrose and ethanol exposure.
Keywords: salt taste, sweet taste, chorda tympani, resiniferatoxin, benzamil
ethanol is a gustatory stimulus and elicits responses in chorda tympani (CT) and glossopharyngeal taste nerves and in neurons of the nucleus of the solitary tract (Brasser et al. 2010; Danilova and Hellekant 2000; Hellekant et al. 1997; Lemon et al. 2004; Lyall et al. 2005a, 2005b; Sako and Yamamoto 1999). In conditioned taste aversion studies in inbred animals, C57BL/6J mice generalized taste aversions from sucrose and quinine solutions to 10% ethanol, and, reciprocally, aversions to 10% ethanol were generalized to each of these solutions presented separately. Only conditioned aversions to quinine generalized to ethanol in DBA/2J mice, but an aversion conditioned to ethanol did not generalize reciprocally to quinine. Thus, considering these two gustatory qualities, 10% ethanol tastes both sweet and bitter to C57BL/6J mice but only bitter to DBA/2J mice (Blizard 2007). In contrast, in outbred rats sucrose and quinine aversions did generalize to various alcohol concentrations, but ethanol did not generalize to sucrose and quinine but only to mixtures of these compounds (Blizard 2007; Kiefer and Lawrence 1988; Kiefer and Mahadevan 1993; Lawrence and Kiefer 1987). These studies suggest that in some inbred strains ethanol has both a sweet taste quality and a bitter taste quality and is thus expected to interact with both sweet and bitter taste transduction pathways.
In mixtures, ethanol acutely enhances sweet (Hellekant et al. 1997; Sako and Yamamoto 1999) and salty (Lyall et al. 2005a, 2005b) responses and suppresses sour and bitter responses in the CT nerve (Hellekant et al. 1997; Sako and Yamamoto 1999). Ethanol produces changes in taste nerve responses to salty, sour, sweet, and bitter stimuli by interacting with quality-specific taste receptors or with intracellular signaling effectors in taste receptor cells (TRCs) (Lyall et al. 2005a, 2005b). The sweet taste receptor serves as a receptor for both sucrose and ethanol. Both the tastes of sucrose and ethanol are represented similarly in gustatory regions of the central nervous system (CNS). Gurmarin, a sweet receptor blocker, administered orally specifically inhibited both ethanol and sucrose responses (Brasser et al. 2010; Lemon et al. 2004). In mixtures, ethanol acutely modulates NaCl CT responses by specifically interacting with a putative TRPV1t-dependent benzamil (Bz)-insensitive salt taste receptor (Lyall et al. 2005a, 2005b). Alcohol-preferring (P) and alcohol-nonpreferring (NP) rats (Bice and Kiefer 1990) and alcohol-naive high (HAD)- and low (LAD)-alcohol-drinking rats demonstrated similar taste reactivity responses to a range of alcohol concentrations. However, differences in taste reactivity between two strains became apparent only after rats were chronically exposed to ethanol (Kiefer et al. 1995).
A phenotypic linkage has been demonstrated between ethanol intake and several taste qualities. Differences in sensitivity to or preference for salty taste have been reported in subjects with a paternal history of alcohol dependence relative to control subjects with no paternal history (Scinska et al. 2001). A positive association exists between ethanol intake and sweet taste (Stewart et al. 1994; Woods et al. 2003) involving the gene for T1R3 (Bachmanov et al. 2001, 2002; Blednov and Harris 2007; Blednov et al. 2008; Blizard 2007; Brasser et al. 2010; Inoue et al. 2004; Lu et al. 2005; Nelson et al. 2001), a G protein-coupled receptor (GPCR) that combines with another GPCR (T1R2) to function as the broadly tuned, heterodimeric, sweet taste receptor T1R2+T1R3 (Li et al. 2002; Zhao et al. 2003). Alcohol dependence and use also show significant association with the T2R38 gene, a marker for 6-n-propylthiouracil bitterness, and with hT2R16, a gene encoding a taste receptor for the bitter-tasting β-glucopyranosides (Duffy et al. 2004; Hinrichs et al. 2006).
Accordingly, we hypothesize that neural, behavioral, and molecular correlates of sweet and salty taste quality can be modulated by acute and chronic ethanol exposure and by genetically inducing changes in taste preferences. To test this hypothesis, studies were performed with selectively bred P and NP rats. P rats were chosen because they voluntarily consume ethanol and develop tolerance and dependence through free-choice drinking (Files et al. 1993; Heyman 2000). In P rats, high oral alcohol preference appears to be positively associated with consumption of sweet-tasting solutions and negatively associated with intake of salty solutions (Stewart et al. 1994). We investigated taste behavior differences between P and NP rats by standard two-bottle 24-h preference tests for ethanol, sucrose, and NaCl, in their CT responses to sweet-tasting and salty stimuli, and in their expression levels of T1R3 and TRPV1/TRPV1t mRNA in the anterior lingual epithelium containing fungiform taste papillae before and after chronic oral exposure to ethanol or sucrose. Our results suggest that in P rats the increase in TRPV1t and T1R3 mRNA expression in the anterior tongue correlates with the increased neural and behavioral responsiveness to salty and sweet-tasting stimuli in the absence and presence of ethanol.
MATERIALS AND METHODS
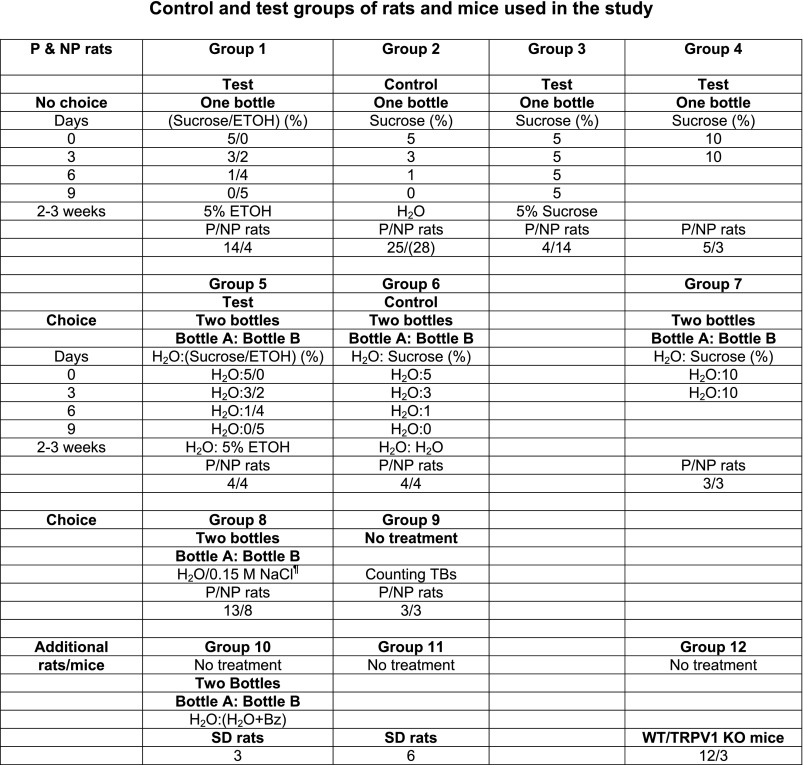
Animals were housed in the Virginia Commonwealth University (VCU) animal facility in accordance with institutional guidelines. All animal protocols were approved by the Institutional Animal Care and Use Committee. Seventy-five 6-wk-old P and seventy-one NP female rats (∼150 g) were obtained from the Indiana University School of Medicine (Indianapolis, IN). In addition to P and NP rats, nine Sprague-Dawley (SD) rats (150–200 g) were obtained from Charles River Laboratories International (Wilmington, MA). We also obtained 12 wild-type (WT; C57BL/6J) and 3 homozygous TRPV1 knockout (KO) (B6.129S4-Trpv1tmijul) mice (30–40 g) from The Jackson Laboratory (Bar Harbor, ME). Upon arrival, P and NP rats were placed in individual plastic cages, given ad libitum access to water and rat chow, and kept in quarantine in the VCU animal facility for 3 wk. The animals were housed in rooms maintained at 22–26°C and 30–70% humidity on a 12-h day and night cycle. At the end of 3 wk the P and NP rats were moved along with SD rats and WT and KO mice to sterile rooms for the duration of the experiment. The animals were divided into 12 groups and used as described in Fig. 1.
Fig. 1.
Control and test groups of rats and mice used in the study. Seventy-five alcohol-preferring (P) and 71 alcohol-nonpreferring (NP) rats were used in this study. Of these, 59 P and 60 NP rats were given 7 different treatments in a no-choice (groups 1–4) or choice (groups 5–7) paradigm. For the no-choice paradigm, group 1 is the test group and group 2 is the control group. For the choice paradigm, group 5 is the test group and group 6 is the control group. P and NP rats in each of groups 1–3, 5, and 6 were either used for chorda tympani (CT) nerve recordings or killed, with their tissues harvested for gene expression and protein analysis. P and NP rats in groups 4 and 7 were used for behavioral studies or for collection of tissues for gene expression studies. ¶In group 8, P and NP rats were used for 2-bottle preference tests for NaCl, NaCl+ benzamil (Bz), and NaCl+Bz+ Maillard reacted peptides conjugated with galacturonic acid (GalA-MRPs). When Bz was used with the salt solution, Bz was also added to the second bottle containing H2O. The fluid intakes were monitored when bottle A and bottle B contained the following solutions (bottle A/bottle B): 1) H2O/0.15 M NaCl; 2) (H2O+5×10−6 M Bz)/(0.15 M NaCl+5×10−6 M Bz); and 3) (H2O+5×10−6 M Bz+0.25% GalA-MRPs)/(0.15 M NaCl+5×10−6 M Bz+0.25% GalA-MRPs). In group 9, lingual epithelium was isolated by collagenase treatment from P and NP rats and used for counting the number of taste buds (TBs). In addition to P and NP rats, we used Sprague-Dawley (SD) rats (groups 10 and 11). Three rats were used to test the effect of Bz on H2O intake. An additional 6 rats were used to test the effect of U73122 and BAPTA on sweet responses. We also used 3 wild-type (WT; group 12) and 3 TRPV1 knockout (KO; group 12) mice to test the effect of TRPV1t agonists on the CT response to 0.15 M NaCl+5×10−6 M Bz. An additional 5 WT mice (group 12) were used to construct a cDNA library from fungiform (FF) TBs, circumvallate (CV) TBs and nongustatory lingual epithelium devoid of TBs (NG Epi) to screen for TRPM5, α-gustducin, and TRPV1.
CT Taste Nerve Recordings
The female rats were anesthetized by intraperitoneal injection of pentobarbital (60 mg/kg body wt), and supplemental pentobarbital (20 mg/kg body wt) was administered as necessary to maintain surgical anesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anesthesia. Body temperatures were maintained at 37°C with a Deltaphase Isothermal PAD (model 39 DP, Braintree Scientific Braintree, MA). The left CT taste nerve was exposed laterally as it exited the tympanic bulla and placed onto a 32G platinum-iridium wire electrode. Stimulus solutions maintained at room temperature were injected into a Lucite chamber (3 ml; 1 ml/s) affixed by vacuum to a 28-mm2 patch of anterior dorsal lingual surface. The CT responses were recorded under zero lingual current clamp and analyzed as described previously (Katsumata et al. 2008; Lyall et al. 2009a, 2010). During surgery P and NP rats in particular demonstrated a propensity to bleed. However, after surgery bleeding stopped within 10–15 min. The wound around the isolated CT nerve was then cleaned with a cotton swab dipped in saline to remove clotted blood. The wound cavity was filled with mineral oil before recording commenced. We were able to record stable and reproducible CT responses from P and NP rats for extended periods (Supplemental Fig. S1).1
CT responses were also monitored in WT and TRPV1 KO mice. Mice (30–40 g) were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg body wt), and supplemental pentobarbital (10 mg/kg body wt) was administered as necessary to maintain surgical anesthesia. The rest of the procedure was the same as in rats. At the end of each experiment animals were humanely killed by an intraperitoneal overdose of pentobarbital (∼195 mg/kg body wt for rats and 150 mg/kg body wt for mice).
The composition of the various stimulating solutions and a list of drugs and their concentrations used in the CT experiments are given in Table 1. The anterior lingual surface was stimulated with the rinse solution (R; pH 6.4) and then with a salt solution (N; pH 6.4) with or without the TRPV1t agonists: ethanol (0–40%), resiniferatoxin (RTX; 0–10 × 10−6 M) and Maillard reacted peptides (MRPs) conjugated with galacturonic acid (GalA-MRPs; 0–1.5%). In previous studies, the TRPV1t agonist-induced increase in the Bz-insensitive NaCl CT response varied with pH. The relationship between pH and the magnitude of the CT response was bell shaped. The maximum increase in the CT response was observed around pH 6.4 (Lyall et al. 2004). Bz (5 × 10−6 M) was used to block Na+ entry via apical epithelial Na+ channels (ENaCs), and N-(3-methoxyphenyl)-4-chlorocinnamide (SB-366791) was used to block TRPV1t activity. In some studies, we topically applied U73122, a nonspecific blocker of phospholipase Cs (PLCs) and its inactive analog, U73343. 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) was used to chelate intracellular Ca2+ (Table 1).
Table 1.
Composition of stimulating solutions and drugs used in CT experiments
| Solution | Composition, M | pH |
|---|---|---|
| Rinse (R) | 0.01 KCl + 0.2 mannitol + 0.01 HEPES | 6.4 |
| Salt stimuli (N) | 0.01 KCl + 0.1 NaCl + 0.01 HEPES | 6.4 |
| R + RTX | R + 0.1×10−6–10×10−6 RTX + 0.01 HEPES | 6.4 |
| N + RTX | N + 0.1×10−6–10×10−6 RTX + 0.01 HEPES | 6.4 |
| R + ETOH* | R + 0-40% ETOH + 0.01 HEPES | 6.4 |
| N+ ETOH* | N + 0-40% ETOH + 0.01 HEPES | 6.4 |
| N + GalA-MRPs | N + 0-1.5% GalA-MRPs + 0.01 HEPES | 6.4 |
| R + GalA-MRPs | R + 0-1.5% GalA-MRPs + 0.01 HEPES | 6.4 |
| Control-1 | 0.3 NH4Cl | |
| Control-2 | 0.3 NaCl | |
| Sucrose | 0.5 | |
| Glycine | 0.25 | |
| SC45647 | 0.005 | |
| Bz | 5 × 10−6 | |
| SB-366791 | 1 × 10−6 | |
| U73122 | 50 × 10−6 or 150 × 10−6 | |
| U73343 | 150 × 10−6 | |
| BAPTA-AM | 33 × 10−3 | |
| Ringer solution | 0.14 NaCl + 0.005 KCl + 0.001 CaCl2 + 0.001 MgCl2 + 0.01 Na-pyruvate + 0.01 glucose + 0.01 HEPES | 7.4 |
ETOH, ethanol (200 proof absolute anhydrous from Pharmco-AAPER); GalA-MRPs, Maillard reacted peptides conjugated with galacturonic acid (Katsumata et al. 2008); Bz, benzamil [blocks Na+ entry through apical epithelial Na+ channel (ENaC)]; SB-366791, 4′-chloro-3-methoxycinnamanilide (blocks Na+ entry through TRPV1t) (Gunthorpe et al. 2004); U73122, 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-y)amino]hexyl]-1H-pyrrole-2,5-dione [a nonspecific blocker of phospholipase Cs (PLCs) and blocks the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol-1,4,5-trisphosphate (IP3) + diacylglycerol (DAG) by PLC] (Lyall et al. 2010); U73343, 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-y)amino]hexyl]-1H-pyrrole-2,5-pyrrolidine-dione (an inactive analog of U73122) (Lyall et al. 2010); HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; RTX, resiniferatoxin. Bz and SB-366791 were added to the salt stimuli and produced their effects on the Bz-insensitive NaCl chorda tympani (CT) responses immediately. However, U73122, U73343, and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM) were dissolved directly in 3 ml of dimethyl sulfoxide(DMSO) and were topically applied to the tongue for at least 30 min. DMSO by itself has no effect on the CT responses to sweet or salty taste stimuli (Lyall et al. 1999). GalA-MRPs were provided by T. Katsumata. All other drugs were purchased from Sigma.
It is important to note that, in contrast to 200 proof alcohol used in this study, beverage-grade alcohol (approximately 190 proof) is consumed by humans and has been used in many previous studies on P and NP rats.
Typically, stimulus solutions remained on the tongue for 1–2 min. Control stimuli consisting of 0.3 M NH4Cl and 0.3 M NaCl (Table 1) applied at the beginning and at the end of experiment were used to assess preparation stability (see Fig. 5B). The preparation was considered stable only if the difference between the magnitude of the control stimuli at the beginning and at the end of the experiment was <10% (Lyall et al. 2009a, 2009b, 2010). The following stimulus series were used in the CT experiments:
The R→(N+Bz+TRPV1t agonist)→R step was repeated for each agonist concentration (Table 1). At the end of the RTX concentration series, the control stimuli were again applied (R→0.3 M NH4Cl→R→0.3 M NaCl→R). At the end of experiment the rats were humanely killed by an intraperitoneal overdose of pentobarbital (∼195 mg/kg body wt).
Fig. 5.
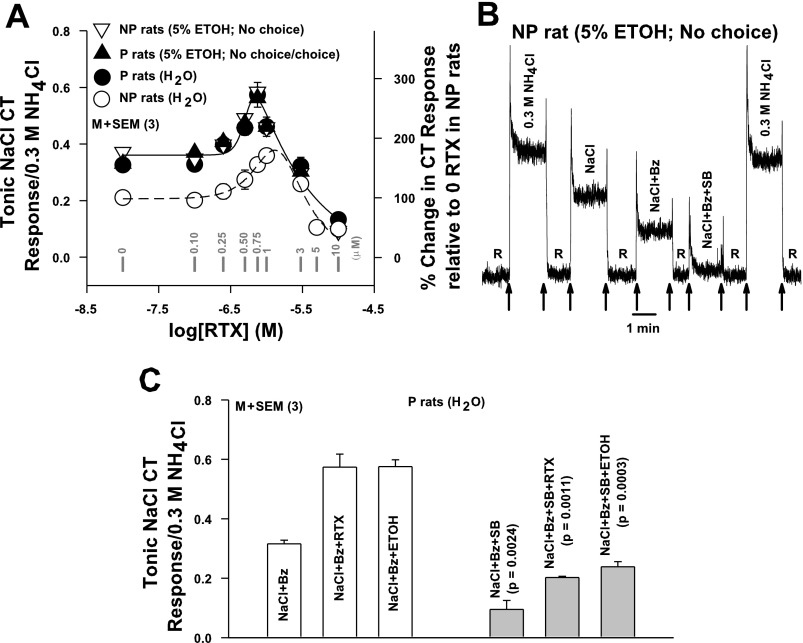
A: effect of chronic ethanol exposure on the RTX vs. NaCl+Bz CT response relationship in P and NP rats. CT responses were measured while the rat tongue was stimulated with R and then with N+Bz+RTX (0–10 × 10−6 M) in P (●) rats maintained on H2O (group 2; Fig. 1), in P rats (▴) given 5% ETOH in a choice (group 5; Fig. 1) or no-choice (group 1; Fig. 1) paradigm, and in NP (▿) rats given 5% ETOH in a no-choice paradigm (group 1; Fig. 1). In each animal tonic CT response was normalized to corresponding tonic CT responses obtained with 0.3 M NH4Cl. Each point represents mean ± SE value of normalized tonic CT response from 3 animals plotted as a function of log RTX concentration. The zero RTX concentration is shown as −8.0 on the x-axis. Gray vertical bars represent points at RTX concentration (in μM). The RTX concentration vs. NaCl+Bz CT response relationship in naive NP rats first shown in Fig. 4B (○) is also plotted for comparison. Right y-axis represents % change in CT response relative to the response at 0 RTX in NP rats. Significant differences were found for RTX concentration (P = 0.0008, Bonferroni corrected) for all 3 (2-way ANOVA), with no significant interaction between effects of strain and concentration. However, no significant difference was observed between RTX-NaCl+Bz CT response relationships in naive P rats, P rats given 5% ETOH in a no-choice or choice paradigm, and NP rats given 5% ETOH in a no-choice paradigm (P > 0.05). All data points are shown connected by a common smooth curve. B and C: effect of SB-366791 on Bz-insensitive NaCl CT responses in P and NP rats. B: representative CT response in a NP rat chronically given 5% ethanol in a no-choice paradigm (group 1; Fig. 1) while its tongue was stimulated with rinse solution R and then with 0.3 M NH4Cl, N, N+Bz, or N+Bz+SB-366791 (1 × 10−6 M; SB). Arrows represent the time period when different solutions were superfused on the rat tongue. C: CT responses were monitored in P rats maintained on H2O (group 2; Fig. 1) while their tongues were stimulated with rinse solution R and then with N+Bz, N+Bz+0.75 × 10−6 M RTX, or N+Bz+40% ETOH in the absence and presence of SB. In each case tonic CT responses were normalized to corresponding tonic CT responses obtained with 0.3 M NH4Cl. Each bar represents mean ± SE value of the normalized tonic CT response from 3 animals. P values (unpaired) for the differences between CT responses in the absence (open bars) and presence (gray bars) of SB are shown above gray bars.
Data and Statistical Analysis
The data were digitized and analyzed off-line. In CT experiments the tonic (steady state) part of the NaCl neural responses was quantified. To quantify the tonic part of a response the area under the response vs. time curve was taken over the final 30 s of the response. To normalize, this area was divided by the area under the 0.3 M NH4Cl response curve over the final 30 s of the tonic response period. The normalized data are reported as means ± SE of n animals. To display a comparison of the relative magnitudes of the responses of NP and P rats to given taste stimuli, the data were normalized by dividing the response to each taste stimulus by the mean tonic response of the rat to 0.3 M NH4Cl. Student's t-test was employed to analyze the differences between sets of data. Since we are comparing the normalized CT responses before and after TRPV1t modulators in the same CT preparation, a paired t-test was used to evaluate statistical significance. When normalized data were compared between P and NP rats, the unpaired t-test was used. In experiments in which repeated measurements to sequential drug applications were obtained, the data were analyzed with two-way ANOVAs with Bonferroni post hoc tests (Oliveira-Maia et al. 2009).
For clarity the points on the graphs of the mean normalized tonic responses versus the logarithm of the agonist concentration were connected respectively by smooth curves. The curves were generated with a fitting function that models the characteristic biphasic property of the agonist concentration versus the magnitude of the CT response. The biphasic property has been observed with every agonist of amiloride- and Bz-insensitive NaCl CT response thus far examined (Katsumata et al. 2008; Lyall et al. 2004, 2005b, 2007, 2009a, 2010). The fitting function used was
| (1) |
where
| (2) |
and
| (3) |
Here R is the response, x is the logarithm of the agonist concentration expressed in moles per liter, and a, b, d, m, n, and r are parameters chosen by least-squares criteria (Katsumata et al. 2008; Lyall et al. 2009a, 2010). For responses plotted against increasing ethanol concentration we used the fitting function
| (4) |
Here R is the response, c is ethanol concentration, and r, a, k, and n are parameters chosen by least-squares criteria.
Chronic Ethanol and Sucrose Exposure
P and NP rats were initially maintained on free access to food and water. The sucrose-fading paradigm (Samson 1986) was used to induce ethanol intake in rats (Fig. 1). Rats were given free access in their cages to 5% sucrose solution as reinforcement. Then, every third day the rats were switched to a sucrose-ethanol mixture containing 3% sucrose-2% ethanol, 1% sucrose-4% ethanol, and finally 0 sucrose-5% ethanol. The 5% ethanol regimen was maintained for 2–3 wk (Fig. 1). In a test choice paradigm group, four P and four NP rats always had two bottles in their cages: one bottle contained H2O and the other the test solution (either a varying sucrose-ethanol mixture or ethanol alone; group 5). In a control choice paradigm group (group 6), another set of four P and four NP rats always had two bottles: one bottle contained H2O and the other a test solution (either varying sucrose concentration or H2O). In group 7 an additional three P and three NP rats were given two bottles, one containing H2O and the other 10% sucrose. In a no-choice paradigm test group, an additional 14 P and 4 NP rats were given one bottle containing the test solution (either a varying sucrose-ethanol mixture or ethanol alone; group 1). In the control no-choice paradigm group (group 2), an additional 25 P and 28 NP rats were given free access in their cages to 5% sucrose solution and then every third day were switched to 3% and 1% sucrose and finally to H2O. Control rats were then maintained for 2–3 wk on H2O. In group 3 4 P and 14 NP rats were given one bottle containing 5% sucrose, and in group 4 5 P and 3 NP rats were given one bottle containing 10% sucrose. P and NP rats were used for CT nerve recordings or were killed, with their tissues collected for gene expression and protein analysis.
Behavioral Studies
Behavioral studies were performed in naive female P and NP rats (150–200 g) with standard two-bottle/24-h tests. In group 8 (Fig. 1), 13 P and 8 NP female rats were given a choice between two bottles, one containing H2O and the other a test solution in the following order: H2O, 0.15 M NaCl, 0.15 M NaCl + 5 × 10−6 M Bz or 0.15 M NaCl + 5 × 10−6 M Bz + 0.25% GalA-MRPs (Tordoff and Bachmanov 2003). When Bz was used with the salt solution, Bz was also added to the second bottle containing H2O. For each 24-h period the volume of H2O versus the volume of the test solution consumed by each rat was measured. The volumes consumed for each solution were converted to grams of fluid consumed by taking into account the density of each solution. The preference ratio for a taste stimulus was calculated as grams of the test solution consumed per 24 h per gram of body weight divided by the total fluid intake (g H2O·24 h−1·g body wt−1 + g test solution·24 h−1·g body wt−1). The bottles containing H2O or the test solution were switched from left to right every day. The same rat was tested for fluid consumption for each solution for 4 days from Monday to Friday of each week. From Friday to the next Monday the animals were maintained on two bottles containing H2O. Before the start of the experiment, rats were given two bottles with H2O for 2 wk. The experiment was started when rats were accustomed to drinking equally from two bottles. At this time point, the preference ratios for two bottles containing water in 13 P rats (0.49 ± 0.03 and 0.50 ± 0.03) and 8 NP rats (0.46 ± 0.03 and 0.49 ± 0.03) were not statistically different.
In some P and NP rats in groups 1, 2, 5, and 6, we monitored intake of 5% sucrose during the start of the sucrose-fading protocol (0–3 days; Fig. 1). At the end of the sucrose-fading protocol, we also monitored their intake of 5% ethanol (10–13 days; Fig. 1). In five naive P and three naive NP rats (group 4; Fig. 1), fluid consumption was measured when rats were given a single bottle containing 10% sucrose over a period of 4 days. In group 7, fluid consumption was measured in three P and three NP rats given a choice between two bottles, one containing H2O and the other 10% sucrose, over a period of 4 days. In a separate experiment, we also tested the effect of Bz on H2O intake in three female SD rats, using a standard two-bottle/24 h test (group 10; Fig. 1). The rats were given a choice between two bottles, one containing H2O and the other H2O or H2O + 5 × 10−6 M Bz. The data were analyzed with one-sample t-tests against 0.5, a reference value meaning indifference of the test solution with respect to the control solution (Oliveira-Maia et al. 2009).
Western Blots for TRPV1/TRPV1t and T1R3
Some P and NP rats in group 2 (Fig. 1) were killed and their tissues collected for protein expression studies. The isolated anterior epithelia containing fungiform taste buds or intestinal mucosal cells were pooled from four P or four NP rats maintained on H2O (group 2; Fig. 1). Rats were anesthetized by exposing them to an inhalation anesthetic, isoflurane (1.5 ml), in a desiccator. When the rats were fully unconscious, a midline incision was made in the chest wall and the aorta was severed. The tongue and small intestine were then rapidly removed and stored in ice-cold Ringer solution (Table 1). The lingual epithelium was isolated by collagenase treatment as described previously (Lyall et al. 2004). The small intestine was cut open and washed with ice-cold Ringer solution (Table 1), and the mucosa was scraped gently with a glass slide. Protein from pooled tissues was isolated in RIPA lysis buffer (Pierce) supplemented with the Complete Mini protease inhibitor pill (Roche). Protein was measured with the Pierce BCA kit, and 40-μg protein samples were loaded into each well of a Criterion 10% polyacrylamide gel (Bio-Rad), separated by PAGE, and transferred to polyvinylidene difluoride (PVDF). The resulting blots were blocked in Odyssey Blocking Buffer (LI-COR Biosciences) and then incubated in α-T1R3 (sc-22459, polyclonal, dilution 1:1,000; Santa Cruz), α-TRPV1 antibody (C-15, sc-12503, polyclonal, dilution 1:1,000; Santa Cruz), β-actin (A-5441, dilution 1:5,000; Sigma), or GAPDH (sc-32233, dilution 1–1,000; Santa Cruz). After washing, the blots were incubated in IRDye donkey α-goat secondary antibody (LI-COR Biosciences), washed and rinsed, and then scanned with the Odyssey Infrared Imaging System (LI-COR Biosciences). The resulting bands were analyzed with AIDA v.3.52 image analysis software (Raytest).
Quantitative RT-PCR for TRPV1/TRPV1t and T1R3
RNA was isolated from lingual epithelium containing fungiform taste buds from a separate group of five P and five NP rats in groups 1, 2, and 3 (Fig. 1) with RNeasy (Qiagen), and cDNA was synthesized with Moloney murine leukemia virus (MMLV; Invitrogen) from 5 μg of RNA in a 50-μl reaction. Primers for quantitative (q)RT-PCR were designed with Micro SEQ ID Analysis Software V2.0 and made by Integrated DNA Technologies (IDT, Coralville, IA) with rat actin used as an internal control. Expression of T1R3 and TRPV1 were analyzed with the TaqMan PCR Master Mix Reagent Kit on the ABI Prism 7300 system. Each sample was tested in triplicate. Cycle threshold (CT) values were obtained graphically for T1R3 and TRPV1/TRPV1t and β-actin. The difference in CT values between β-actin and T1R3 or TRPV1/TRPV1t was represented as ΔCT values. Because the tissue samples were obtained from different animals, there is no means to justify which positive sample is compared with which control sample. Therefore, we did not use the 2−ΔΔCT method to calculate the data (Schmittgen and Livak 2008). The means ± SE for each group of P and NP rats were calculated as individual data points with
| (5) |
An unpaired Student's t-test was used to determine whether the difference in the mean ± SE values of 2−ΔCT between two groups was significant (Schmittgen and Livak 2008). The relative fold change in gene expression was calculated as the ratio of the mean 2−ΔCT value of the test group divided by the mean 2−ΔCT value of the control group. The efficiencies of the amplification for qRT-PCR assays for TRPV1 and T1R3 were 100.2% and 102.0%, respectively.
Primers for qRT-PCR
The primers for qRT-PCR were as follows: rat TRPV1: sense 5′-GACATGCTTCTCGTGGAACCCTTG-3′ nucleotides 1312–1334, antisense 5′ -CCACAGGCCGATAGTAGGCAGC-3′ nucleotides 1452-1431; rat T1R3: sense 5′-CCAGGTGCCAGTCTCCCAGTGC-3′ nucleotides 1491–1512, antisense 5′-GTAGCTCCCTGCCTTGCAGTCCAC-3′ nucleotides 1602–1579.
RT-PCR for TRPM5, α-Gustducin, and TRPV1/TRPV1t
The lingual epithelium was isolated from an additional five WT mice (group 12; Fig. 1) by collagenase treatment (Lyall et al. 2004). The anterior part of the tongue was used to isolate fungiform taste buds and the posterior part of the tongue was used to isolate circumvallate taste buds as described previously (Lyall et al. 2004). We also harvested the nongustatory lingual epithelium without taste buds. RT-PCR was performed to detect the presence of TRPM5, α-gustducin, and TRPV1/TRPV1t in isolated mouse fungiform taste buds, circumvallate taste buds, and nongustatory lingual epithelium. All primers for RT-PCR were made by IDT. The primers were as follows: mouse TRPV1 (Wang et al. 2004): sense 5′-CGGTTTATGTTCGTCTACCTCGTGTTCTTGTTTGG-3′, antisense 5′-GCTCTCTTGTGCAATCTTGTTGACAGTCTCGCC-3′; mouse α-gustducin (DeFazio et al. 2006): sense 5′-GCAACCACCTCCATTGTTCT-3′, antisense: 5′-AGAAGAGCCCACAGTCTTTGAG-3′; mouse TRPM5 (DeFazio et al. 2006): sense 5′-GTCTGGAATCACAGGCCAAC-3′, antisense 5′-GTTGATGTGCCCCAAAAACT-3′.
Quantification of Fungiform Papillae in P and NP Rat Tongues
The lingual epithelium from three P and three NP rats (group 9; Fig. 1) was isolated by collagenase treatment as described previously (Lyall et al. 2004). The lingual epithelium was placed in Ringer solution (Table 1), cut open, and placed on a black rubber stopper with the basolateral membrane facing up and stretched with small stainless steel pins. The fungiform papillae were visualized and counted with a JENA stereomicroscope at 6.3 × 2.5 magnification.
RESULTS
Ethanol, Sucrose, and NaCl Consumption and Preference Ratios in P and NP rats
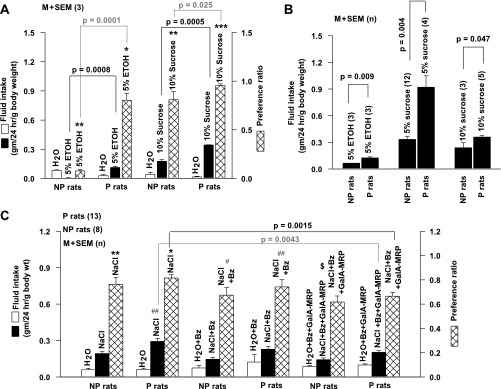
In two-bottle 24-h preference tests, NP rats (group 5; Fig. 1) consumed significantly less 5% ethanol (Fig. 2A) relative to H2O. This indicates that NP rats find ethanol aversive (Fig. 2A; P < 0.001, 1-sample t-test). Consistent with previous studies (Kiianmaa et al. 1991), P rats (group 5; Fig. 1) consumed a significantly greater amount of 5% ethanol relative to H2O and thus demonstrated an innate preference for ethanol (Fig. 2A; P < 0.002, 1-sample t-test). Both naive P and NP rats (group 7; Fig. 1) also consumed a significantly greater amount of 10% sucrose than H2O (Fig. 2A). This indicates that like many species of rodents, NP (P < 0.001) and P (P < 0.0001, 1-sample t-test) rats also demonstrate a preference for sweet-tasting stimuli (Boughter and Bachmanov 2007). P rats consumed a greater amount of sucrose relative to NP rats (Fig. 2A; P = 0.0005, unpaired) and thus demonstrate a greater preference for sucrose relative to NP rats (Fig. 2A; P = 0.025, unpaired).
Fig. 2.
Ethanol, sucrose, and NaCl consumption and preference ratios in P and NP rats. A: 2-bottle tests. P and NP rats were given a choice between H2O and 5% ethanol (group 5; Fig. 1) or H2O and 10% sucrose (group 7; Fig. 1). The fluid intake for a 24-h period was measured to calculate preference ratios for the above stimuli. Data were expressed as either fluid intake in grams per 24 h per gram of body weight or preference ratio [g test solution consumed·24 h−1·g body wt−1 divided by total fluid intake (g H2O·24 h−1·g body wt−1 + g test solution·24 h−1·g body wt−1)]. Open bars, filled bars, and cross-hatched bars represent means ± SE of H2O intake, 5% ethanol (ETOH) or 10% sucrose intake, and preference ratios, respectively, from 3 P and 3 NP rats in each group. *P < 0.002, **P < 0.001, ***P < 0.0001 (1-sample t-test). B: 1-bottle tests. P and NP rats were presented with a single bottle containing 5% ethanol (group 1; Fig. 1), 5% sucrose (group 3; Fig. 1), or 10% sucrose solutions (group 4; Fig. 1). Their fluid intake was expressed as grams per 24 h per gram of body weight and represented as mean ± SE of n, where n represents the number of rats in each group (in parentheses). C: 2-bottle tests. P and NP rats (group 8; Fig. 1) were given a choice between H2O and 0.15 M NaCl, H2O + 5 × 10−6 M Bz, and 0.15 M NaCl + 5 × 10−6 M Bz, H2O + 5 × 10−6 M Bz + 0.25% GalA-MRPs, or 0.15 M NaCl + 5 × 10−6 M Bz + 0.25% GalA-MRPs. Fluid intake for a 24-h period was measured to calculate preference ratios for the above stimuli. Open bars, filled bars, and cross-hatched bars represent H2O intake, NaCl intake, and preference ratios, respectively. Data are expressed as either fluid intake in grams per 24 h per gram of body weight or preference ratio [g test solution consumed·24 h−1·g body wt−1 divided by total fluid intake (g H2O·24 h−1·g body wt−1 + g test solution·24 h−1·g body wt−1)]. Values are expressed as mean ± SE of n, where n represents the number of animals in each group. Brackets identify the comparisons being made between the 2 treatments by unpaired t-test and their respective P values. *P < 0.002, **P < 0.001, #P < 0.03, ##P < 0.01, $P = 0.045 (1-sample t-test).
When given a single bottle containing 5% ethanol (group 1; Fig. 1), NP rats consumed a greater amount of ethanol (Fig. 2B) relative to NP rats given a choice between H2O and ethanol (Fig. 2A; P = 0.0001, unpaired). P rats (group 1; Fig. 1) still consumed significantly more ethanol than NP rats from a single bottle containing 5% ethanol (Fig. 2B; P = 0.009). However, their consumption of ethanol was the same as in P rats given a choice between H2O and ethanol (Fig. 2A; P > 0.05, unpaired). In a no-choice paradigm, P rats (group 4; Fig. 1) still consumed more 10% sucrose solution than NP rats (Fig. 2B; P = 0.047); however, their consumption of sucrose was not different from P rats given a choice between H2O and sucrose (Fig. 2A; P > 0.05). In a no-choice paradigm, P and NP rats consumed a greater amount of 5% sucrose (group 2; Fig. 1) than 10% sucrose solution (group 4; Fig. 1). P rats also consumed a significantly greater amount of 5% sucrose relative to NP rats (Fig. 2B; P = 0.004). Taken together, the results show that genetically induced alcohol preference in P rats is associated with increased preference for sweet (sucrose) taste stimuli.
In two-bottle 24-h preference tests, both naive P and NP rats (group 8; Fig. 1) consumed a significantly greater amount of 0.15 M NaCl (Fig. 2C) relative to H2O, with preference values significantly greater than 0.5 (P < 0.002 and P < 0.001, respectively; 1-sample t-test). This is consistent with previous observations that rats prefer hypotonic and isotonic NaCl solutions to water (Fregly 1956). No difference was observed in NaCl preference between P and NP rats (P > 0.05). P and NP rats also consumed a significantly greater amount of 0.15 M NaCl + 5 × 10−6 M Bz (Fig. 2C) than H2O + Bz (Fig. 2C), with preference values significantly greater than 0.5 (P < 0.03 and P < 0.01, respectively, 1-sample t-test). In sodium-replete rats, amiloride (an ENaC blocker) did not alter NaCl lick rate (Brot et al. 2000). This suggests that sodium-replete rats retain preference for NaCl in the presence of amiloride. It is likely that in our long-term behavioral studies rats maintained on Bz in two-bottle/24-h preference tests become sodium depleted. In sodium-depleted animals one would predict an increased NaCl intake to maintain sodium homeostasis. In rats made “sodium depleted” by using the diuretic furosemide in short-term licking tests, addition of amiloride to the NaCl solutions reduced lick rates to ∼20–25% of baseline (Brot et al. 2000). These results suggest that even under sodium-depleted conditions, inhibition of ENaC activity by amiloride or Bz decreases sodium intake. However, Bz did not alter NaCl intake or preference in P and NP rats (P > 0.05), suggesting that under the experimental conditions used in our studies, P and NP rats were not sodium depleted. Alternately, it is also possible that the effects of Bz on NaCl intake or preference are more complex. Postabsorptive effects of Bz may cause sodium depletion and sodium appetite, but effects of Bz on taste bud cells suppressed increased preference for NaCl.
Unlike amiloride, rats react to Bz indifferently. In three SD rats (group 10; Fig. 1) given a choice between H2O and H2O + 5 × 10−6 M Bz, the preference ratio for H2O + Bz was 0.55 ± 0.05 (mean ± SE), a value not different from 0.5 (P > 0.05). This indicates that at the concentration used in our experiments Bz is not an aversive stimulus (i.e., bitter) to rats, and thus offers a significant advantage over amiloride in taste behavioral studies. These results suggest that both Bz-sensitive (ENaC dependent) and Bz-insensitive (putative TRPV1t dependent) salt taste receptors in the peripheral taste receptive fields contribute to NaCl preference in P and NP rats.
We hypothesize that modulating the activity of the putative TRPV1t-dependent Bz-insensitive salt taste receptor will produce differential effects on salt taste behavior in P and NP rats. To test this hypothesis, we investigated the effect of 0.25% GalA-MRPs on NaCl and H2O consumption in P and NP rats (group 8; Fig. 1). Consistent with our previous observations in SD rats and WT mice (Katsumata et al. 2008), 0.25% GalA-MRPs produced a maximum enhancement in the Bz-insensitive NaCl CT response in NP rats (Supplemental Fig. S1). Addition of 0.25% GalA-MRPs to NaCl + Bz solutions produced a significant decrease in NaCl intake (Fig. 2C; P = 0.0043) and NaCl preference in P rats (Fig. 2C; P = 0.0015, unpaired; n = 13). In contrast, in NP rats, GalA-MRPs produced a small but significant decrease in NaCl intake (P = 0.047) without a change in NaCl preference (P > 0.05). These results suggest that in P rats further upregulating a putative TRPV1t-dependent salt taste receptor activity by GalA-MRPs decreases NaCl intake and preference. When added to the rinse solution, GalA-MRPs (0.05–1%) do not elicit a CT response (Katsumata et al. 2008). Similarly, in human sensory evaluation, MRPs were also reported to have no taste of their own (Katsumata et al. 2008). Thus it is unlikely that the effect of GalA-MRPs on NaCl intake and preference can be related to its aversive taste. Furthermore, if rats react to GalA-MRPs as an aversive stimulus, it should affect NaCl intake and preference equally in both P and NP rats.
T1R3 and TRPV1/TRPV1t Expression in the Anterior Lingual Epithelium Containing Fungiform Taste Buds and in Intestinal Mucosal Cells in P and NP Rats
In preliminary studies (Supplemental Fig. S2, A–C) in cDNA made from C57BL/6J WT mice (group 12; Fig. 1) or rat (Oliveira-Maia et al. 2009) fungiform taste buds, circumvallate taste buds, and nongustatory lingual epithelium devoid of taste buds, TRPM5 and α-gustducin were detected in taste bud cells and were not detected in the nongustatory lingual epithelium or the CT nerve (Supplemental Fig. S2, A and B). These results are consistent with the notion that the molecular correlates involved in sweet, bitter, and umami taste are expressed only in a subset of taste cells (Zhao et al. 2003). TRPV1 message was detected in fungiform and circumvallate taste bud cells and in testis but not in the nongustatory lingual epithelium (Supplemental Fig. S2C). Therefore, we used the anterior lingual epithelium rather than isolated taste buds to quantitate the expression of T1R3 and TRPV1/TRPV1t mRNA and protein by qRT-PCR and Western blots in P and NP rats.
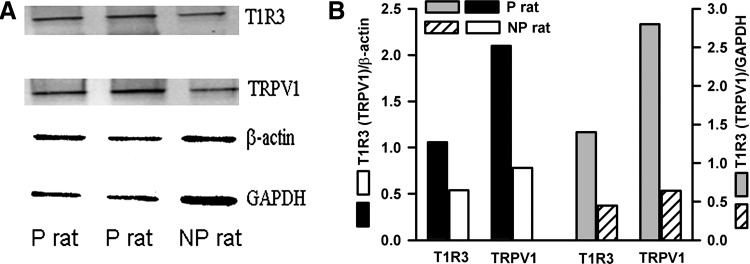
By qRT-PCR from cDNA made from lingual epithelium containing fungiform taste papillae, T1R3 mRNA, normalized to the mRNA for β-actin, was significantly increased in five P rats (Table 2; P = 0.028, unpaired) relative to five NP rats maintained on H2O (group 2; Fig. 1). Western blots of T1R3 with bands for two reference genes, β-actin and GAPDH, from protein samples pooled from isolated lingual epithelia containing fungiform taste papillae from four P or four NP rats (group 2; Fig. 1) are shown in Fig. 3A. The density of the T1R3 bands was computed relative to β-actin or GAPDH bands (Fig. 3B). T1R3 protein was increased in lingual epithelium containing fungiform taste papillae in P rats relative to NP rats (Fig. 3). Thus there is a positive association across P and NP rat strains with increased sucrose preference and levels of T1R3 mRNA and protein in fungiform taste bud cells.
Table 2.
T1R3 and TRPV1/TRPV1t mRNA levels in anterior lingual epithelium containing fungiform taste buds in P and NP rats
| mRNA | NP Rats+H2O (2−ΔCT) | P Rats+H2O (2−ΔCT) | Fold Change (P/NP) | P Value |
|---|---|---|---|---|
| T1R3 | 0.514 ± 0.021 | 0.748 ± 0.031 | 1.45 | 0.028 |
| n | 5 | 5 | ||
| TRPV1/TRPV1t | 0.0075 ± 0.001 | 0.011 ± 0.0008 | 1.47 | 0.034 |
| n | 4 | 3 |
2−ΔCT (where CT is threshold cycle) values are expressed as means ± SE of n, where n represents the number of P and NP rats maintained on H2O (group 2; Fig. 1). P values are for unpaired comparisons of means ± SE of 2−ΔCT values of P and NP rats (Schmittgen and Livak 2008).
Fig. 3.
T1R3 and TRPV1/TRPV1t protein levels in anterior lingual epithelium containing fungiform taste buds in P and NP rats. A: Western blots for T1R3 and TRPV1/TRPV1t with bands for 2 reference genes, β-actin and GAPDH, in anterior lingual epithelium containing fungiform taste buds in P and NP rats maintained on H2O (group 2; Fig. 1). B: density of T1R3 and TRPV1/TRPV1t bands computed relative to β-actin or GAPDH.
With qRT-PCR from cDNA made from lingual epithelium containing fungiform taste papillae, TRPV1/TRPV1t mRNA normalized to the mRNA for β-actin was significantly increased in P rats (Table 2; P = 0.034, unpaired) relative to NP rats maintained on H2O (group 2; Fig. 1). Western blots of TRPV1/TRPV1t with bands for two reference genes, β-actin and GAPDH, from protein samples pooled from isolated lingual epithelia containing fungiform taste papillae from four P or four NP rats (group 2; Fig. 1) are shown in Fig. 3A. The density of the TRPV1/TRPV1t bands was computed relative to β-actin or GAPDH bands (Fig. 3B). Consistent with the qRT-PCR data, the results support the hypothesis that P rats maintained on H2O have a higher expression of TRPV1/TRPV1t protein relative to NP rats maintained on H2O (group 2; Fig. 1). It is interesting to note that in P rats maintained on H2O both T1R3 and TRPV1/TRPV1t mRNA (Supplemental Table S1; P = 0.0076 and 0.022, respectively, unpaired; n = 5) and protein (Supplemental Fig. S3; n = 3) levels in intestinal mucosal cells were also increased relative to NP rats maintained on H2O.
The observed differences in T1R3 and TRPV1t could be due to up- or downregulation of gene transcription/translation or due to the higher density of fungiform papillae in P rats relative to NP rats. Accordingly, we counted the number of fungiform papillae in isolated lingual epithelia from naive P and NP rats. In three P and three NP rats (group 9; Fig. 1) the number of fungiform papillae in the tongue varied between 170 and 175 (172.7 ± 2.2 papillae/tongue; mean ± SE) and between 172 and 182 (177.5 ± 2.2 papillae/tongue; mean ± SE), respectively, and were not significantly different between the two genotypes (P > 0.05). These values are within the range of fungiform papillae (175–201) found in male SD rat tongue (187 ± 2.9; mean ± SE; n = 10) (Miller and Preslar 1974). These results indicate that the observed differences in T1R3 and TRPV1t expression in P and NP rats are independent of the number of fungiform taste papillae per rat tongue in the two genotypes.
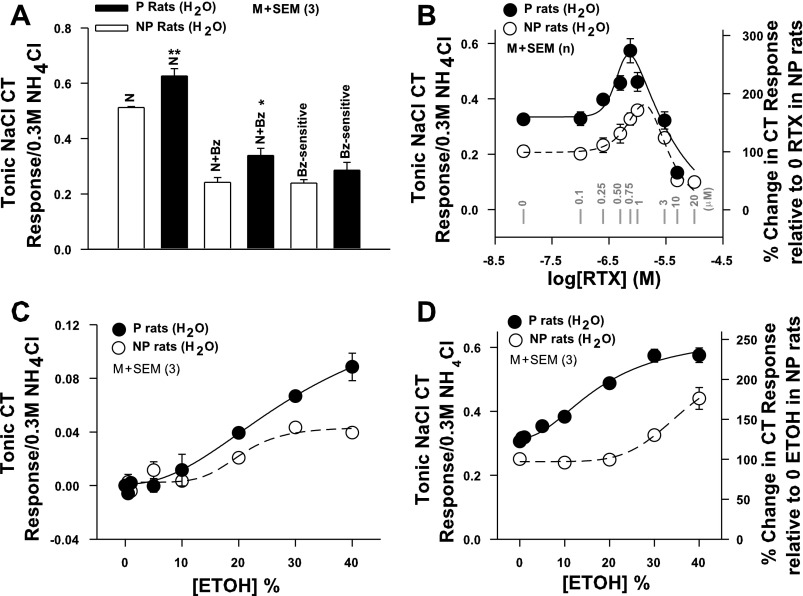
NaCl CT Responses in P and NP Rats
We further investigated the strain differences in the CT responses to salty and sweet-tasting stimuli in P and NP rats in the absence and presence of specific modulators of a putative TRPV1t-dependent salt taste receptor and T1R2+T1R3 sweet taste receptor, respectively. CT responses were measured in P and NP rats maintained on H2O (group 2; Fig. 1) while their tongues were stimulated with a rinse solution (R) and then with NaCl or NaCl + Bz (Table 1). P rats spontaneously elicited a significantly bigger NaCl CT response (Fig. 4A) relative to NP rats (Fig. 4A; P = 0.0133, unpaired; n = 3). The increase in the NaCl CT response was specifically due to the increase in the Bz-insensitive TRPV1t-dependent NaCl CT component (P = 0.0355, unpaired). No statistical difference was observed in the Bz-sensitive ENaC-dependent NaCl CT response between P and NP rats.
Fig. 4.
NaCl CT responses in P and NP rats. A: summary of the magnitudes of the tonic CT responses to NaCl (N) and N+Bz and the Bz-sensitive component of the CT response in P and NP rats maintained on H2O (group 2; Fig. 1). In each case the CT responses were normalized to the corresponding tonic CT responses obtained with 0.3 M NH4Cl and presented as tonic NaCl CT response/0.3 M NH4Cl. Each bar represents mean ± SE value of the normalized tonic CT response from 3 animals. *P = 0.0355, **P = 0.0133 (unpaired). B: CT responses were measured while the tongue was stimulated with rinse solution (R) and then with N+Bz+resiniferatoxin (RTX) (0–10 × 10−6 M) in P (●) and NP (○) rats maintained on H2O (group 2; Fig. 1). In each animal the tonic CT response was normalized to the corresponding tonic CT responses obtained with 0.3 M NH4Cl. In NP rats each point represents mean ± SE value of normalized tonic CT response from 4–9 animals (n) and is plotted as a function of log RTX concentration. In P rats each point represents mean ± SE value of normalized tonic CT response from 3 animals (n) and plotted as a function of log RTX concentration. The zero RTX concentration is shown as −8.0 on the x-axis. Gray vertical bars represent points at RTX concentration (in μM). Right y-axis represents % change in CT response relative to the response at 0 RTX in NP rats. Significant differences were found for RTX concentration (P = 0.04 for both, 2-way ANOVA) and magnitude of the RTX response in P and NP rats (P = 0.008, Bonferroni corrected), with no significant interaction between effects of strain and concentration. In the rinse solution RTX did not increase the CT response above baseline (data not shown). C: CT responses were measured while the tongue was first stimulated with rinse solution R and then with R+ETOH (0–40%) in P (●) and NP (○) rats (group 2; Fig. 1). In each case tonic CT responses were normalized to corresponding tonic CT responses obtained with 0.3 M NH4Cl. Each point represents mean ± SE value of normalized tonic CT response from 3 animals (n) and is plotted as a function of % ETOH concentration. No significant differences were found for ETOH concentration (P > 0.05 for both, 2-way ANOVA, Bonferroni corrected) and magnitude of the ETOH response in P and NP rats (P > 0.05), with no significant interaction between effects of strain and concentration. D: CT responses were measured while the tongue was first stimulated with rinse solution R+ETOH (0–40%) and then with N+Bz+ETOH (0–40%) in P (●) and NP (○) rats (group 2; Fig. 1). In each case tonic CT responses were normalized to corresponding tonic CT responses obtained with 0.3 M NH4Cl. To calculate the magnitude of the N+Bz CT response in the presence of ETOH, the response of ETOH in the rinse was subtracted from the total N+Bz+ETOH response. Each point represents mean ± SE value of normalized tonic CT response from 3 animals and is plotted as a function of % ETOH concentration. Right y-axis represents % change in CT response relative to 0 ETOH in NP rats. Significant differences were found for ETOH concentration (P = 0.027 for both, 2-way ANOVA) and magnitude of the ETOH response in P and NP rats (P = 0.009, Bonferroni corrected), with no significant interaction between effects of strain and concentration.
To investigate the differences between TRPV1t activity in P and NP rats (group 2; Fig. 1), we generated agonist concentration vs. magnitude of Bz-insensitive NaCl CT response relations in P and NP rats using three TRPV1t agonists: RTX, ethanol, and GalA-MRPs (Lyall et al. 2004, 2005a, 2005b, 2007; Katsumata et al. 2008).
Acute effects of RTX on Bz-insensitive NaCl CT responses in P and NP rats.
Consistent with previous studies (Lyall et al. 2004), in the rinse solution (R; Table 1), RTX elicited no CT response above baseline between 0.1 × 10−6 M and 10 × 10−6 M (data not shown). In NP rats, RTX produced a biphasic effect on the Bz-insensitive NaCl CT response (Fig. 4B). Between 0.25 × 10−6 M and 1 × 10−6 M, RTX enhanced the CT response. RTX produced a maximum increase in the Bz-insensitive NaCl CT response at 1 × 10−6 M and inhibited the CT response at 3 × 10−6 and 10 × 10−6 M. In P rats, the Bz-insensitive NaCl CT response in the absence and presence of RTX was greater relative to NP rats. In P rats, RTX produced a maximum enhancement in the CT response at 0.75 × 10−6 M, and at 1 × 10−6, 3 × 10−6, and 10 × 10−6 M RTX inhibited the CT response (Fig. 4B).
Acute effects of ethanol on Bz-insensitive NaCl CT responses.
CT responses were monitored in P and NP rats (group 2; Fig. 1) to NaCl + Bz alone and in mixtures containing 0–40% ethanol. The corresponding rinse solutions were R and R + ethanol (0–40%), respectively. Unlike RTX, stimulating the tongue with rinse solution R containing 10–40% ethanol elicited CT responses in P and NP rats that were not significantly different between the two genotypes (Fig. 4C). In mixtures containing NaCl + ethanol, the tonic CT responses were measured relative to the tonic CT responses to the same concentrations of R + ethanol. In NP rats, ethanol enhanced the Bz-insensitive NaCl CT response starting at ∼20% and the response increased with 30% and 40% ethanol (Fig. 4D). In P rats, ethanol induced an increase in the Bz-insensitive NaCl CT response starting at 10%, and the response further increased with increasing ethanol concentration (Fig. 4D).
Acute effects of GalA-MRPs on Bz-insensitive NaCl CT responses in P and NP rats.
Consistent with previous studies (Katsumata et al. 2008), GalA-MRPs produced biphasic effects on NaCl + Bz CT responses in both P and NP rats (group 2; Fig. 1) (Supplemental Fig. S1). In P rats, the GalA-MRPs concentration vs. magnitude of the Bz-insensitive NaCl response curve was higher and was shifted to the left on the concentration axis.
Effect of chronic ethanol and sucrose treatment on Bz-insensitive NaCl CT responses in P and NP rats.
NP rats given a choice between H2O and 5% ethanol (group 5; Fig. 1) did not show any changes in the RTX vs. magnitude of the Bz-insensitive NaCl CT response relations relative to NP rats maintained on H2O (group 6; Fig. 1). Similarly, P rats given 5% ethanol in a choice (group 5; Fig. 1) or no-choice (group 1; Fig. 1) paradigm did not show any changes in the RTX vs. magnitude of the Bz-insensitive NaCl CT response relations relative to P rats maintained on H2O (groups 2 and 6; Fig. 1). Therefore, the data in P rats from choice and no-choice treatments were combined and are plotted in Fig. 5A versus the data obtained in P rats maintained on H2O. However, in the case of NP rats given 5% ethanol in the no-choice paradigm (group 1; Fig. 1), the RTX dose-response curve was shifted upward and to the left on the RTX concentration axis (Fig. 5A) and was not different from the RTX dose-response relation in P rats maintained on H2O (Fig. 5A). When given a choice between H2O and ethanol, NP rats responded to ethanol as an aversive stimulus. Thus their ethanol consumption per day was negligible (Fig. 2A). In contrast, when given 5% ethanol in the no-choice paradigm, NP rats consumed a significantly greater amount of ethanol per day (Fig. 2B) relative to NP rats given a choice between H2O and ethanol (Fig. 2A). These results suggest that changes in the RTX concentration vs. NaCl + Bz CT response relationship are associated with increased ingestion of ethanol in NP rats in a no-choice paradigm relative to NP rats given a choice between H2O and ethanol.
Since in some inbred strains ethanol has a sweet taste quality, we further tested whether chronic sucrose exposure will also modulate changes in TRPV1/TRPV1t expression and function in the anterior taste receptive field. The NP rats maintained chronically on 5% sucrose in a no-choice paradigm (group 3; Fig. 1) demonstrated a relationship between varying RTX concentration and magnitude of the Bz-insensitive NaCl CT response similar to that of NP rats maintained on H2O (Supplemental Fig. S4A). The maximum increase in the Bz-insensitive NaCl CT response in both cases was observed at 1 × 10−6 M RTX. Similarly, in P rats exposed to 5% sucrose in a no-choice paradigm (group 3; Fig. 1), the varying RTX concentration versus the Bz-insensitive NaCl CT profile was the same as in P rats maintained on H2O (Supplemental Fig. S4B). It is important to note that the maximum increase in the Bz-insensitive NaCl CT response in both cases was observed at 0.75 × 10−6 M RTX. These results suggest that in NP rats the relationship between varying RTX concentration and the magnitude of the NaCl + Bz CT response shown in Fig. 5A is specifically altered by chronic ethanol ingestion in a no-choice paradigm and is not affected by chronic sucrose ingestion. We have previously shown that RTX specifically modulates the Bz-insensitive NaCl CT responses. RTX did not alter CT responses to sweet, bitter, and umami stimuli between 0.1 × 10−6 and 10 × 10−6 M (Lyall et al. 2004). Taken together, these results suggest that TRPV1t activity is modulated by ethanol but not by sucrose.
Effect of SB-366791 on Bz-insensitive NaCl CT responses.
In NP rats given chronic 5% ethanol in a no-choice paradigm (group 1; Fig. 1), the Bz-insensitive NaCl CT response was inhibited in the presence of 1 × 10−6 M SB-366791 (Fig. 5B), a specific TRPV1/TRPV1t blocker (Gunthorpe et al. 2004; Lyall et al. 2004). In P rats maintained on H2O (group 2; Fig. 1), SB-366791 (Fig. 5C) inhibited the Bz-insensitive NaCl CT response and its subsequent enhancement in the presence of TRPV1t agonists RTX (0.75 × 10−6 M) or ethanol (40%) relative to control (Fig. 5C).
To directly demonstrate that RTX, ethanol, and GalA-MRPs produce their effects on the NaCl + Bz CT responses by acting on TRPV1/TRPV1t, further studies were performed on WT and TRPV1 KO mice (group 12; Fig. 1). In WT mice GalA-MRPs (0.25%), RTX (1 × 10−6 M), and ethanol (30%) enhanced the CT response to NaCl + Bz (Supplemental Fig. S5). Consistent with previous studies (Katsumata et al. 2008; Lyall et al. 2004, 2005a, 2005b, 2007, 2009a, 2010), TRPV1 KO mice elicited no spontaneous CT response to NaCl + Bz above the rinse baseline, and no increase in the NaCl + Bz CT response was observed in the presence of GalA-MRPs, RTX, or ethanol above the baseline rinse level (Supplemental Fig. S5). Taken together, the above results provide direct evidence that RTX, ethanol, and GalA-MRPs produce their effects on the Bz-insensitive NaCl CT responses by interacting with TRPV1t. RTX, capsaicin, and H+ modulate TRPV1 activity by binding to different sites on the protein molecule (Petrocellis and Marzo 2005).
Effect of chronic ethanol and sucrose treatment on TRPV1/TRPV1t mRNA levels in P and NP rats.
As shown in Table 3, in five NP rats chronically exposed to 5% sucrose for 2 wk in a no-choice paradigm (group 3; Fig. 1) the TRPV1/TRPV1t mRNA level was not different from its corresponding value in five NP rats maintained on H2O (group 2; Fig. 1). These results suggest that chronic sucrose exposure does not affect TRPV1/TRPV1t mRNA levels in NP rats. In contrast, three NP rats chronically exposed to 5% ethanol in a no-choice paradigm demonstrated a significant increase in TRPV1/TRPV1t mRNA level relative to four NP rats maintained on H2O (Table 3). In five P rats chronically exposed to 5% ethanol in a no-choice paradigm (group 1; Fig. 1), the ratio (mean 2−ΔCT P ratETOH/mean 2−ΔCT P ratH2O) of TRPV1/TRPV1t mRNA relative to five P rats maintained on H2O (group 2; Fig. 1) was 0.97 (data not shown). These results suggest that the effect of chronic ethanol exposure in a no-choice paradigm in NP rats is related to an increase in TRPV1t mRNA in the taste receptive field in the anterior tongue.
Table 3.
TRPV1/TRPV1t mRNA levels in anterior lingual epithelium containing fungiform taste buds in NP rats chronically maintained on H2O, 5% sucrose, or 5% ethanol
| mRNA | NP Rats + H2O (2−ΔCT) | NP Rats + 5% sucrose (2−ΔCT) | Fold Change (P/NP) | P Value | NP Rats+H2O (2−ΔCT) | NP rats +5% Ethanol (2−ΔCT) | Fold Change (P/NP) | P Value |
|---|---|---|---|---|---|---|---|---|
| TRPV1/TRPV1t | 0.00508 ± 0.0008 | 0.00503 ± 0.001214 | 0.99 | NS | 0.0087 ± 0.0013 | 0.0149 ± 0.001214 | 1.78 | 0.046 |
| n | 5 | 5 | 4 | 4 |
2−ΔCT values are expressed as means ± SE of n, where n represents the number of NP rats maintained on H2O (group 2; Fig. 1) or NP rats chronically exposed to 5% sucrose or NP rats exposed to 5% ethanol in a no-choice paradigm. P values are for unpaired comparisons of means ± SE of 2−ΔCT values of NP rats maintained on H2O or chronically exposed to ethanol or sucrose (Schmittgen and Livak 2008). NS, not significant.
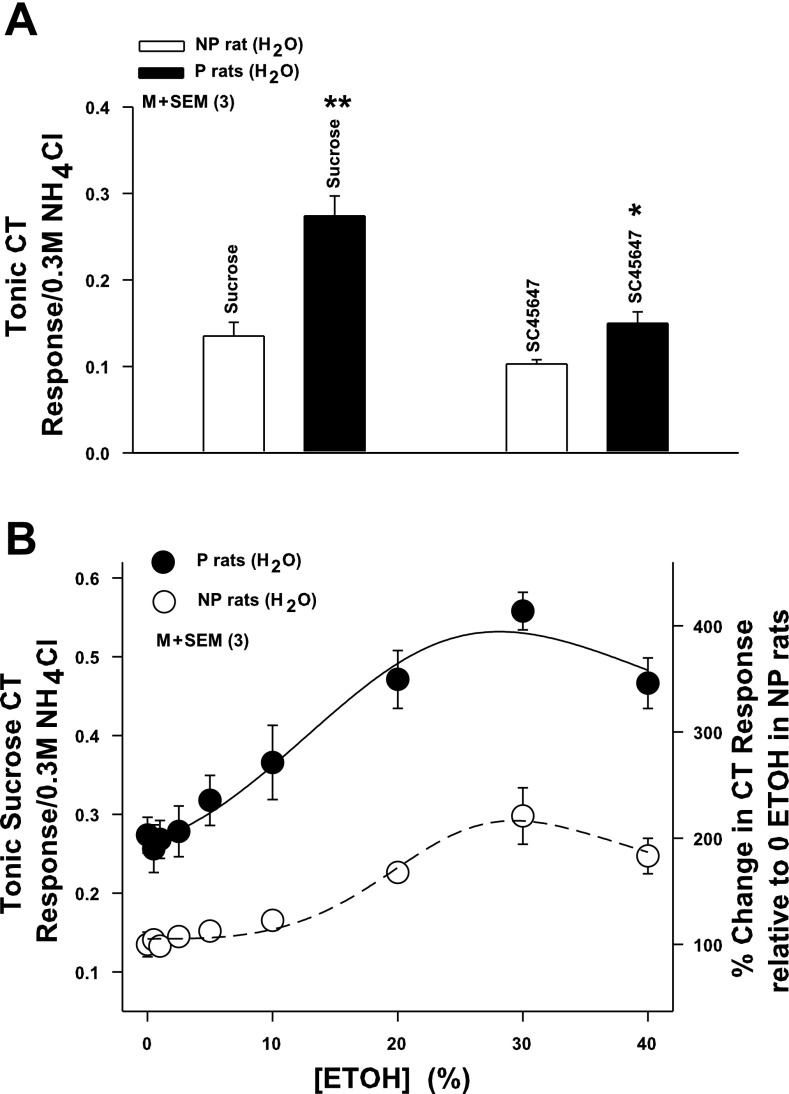
CT Responses to Sweet-Tasting Stimuli in P and NP Rats
P rats maintained on H2O (group 2; Fig. 1) (P rats + H2O; Fig. 6A; n = 3) spontaneously elicited a bigger CT response to 0.5 M sucrose (P = 0.0013, unpaired) and 0.008 M SC45647 (P = 0.0289, unpaired) relative to NP rats (NP + H2O; Fig. 6A; n = 3; group 2; Fig. 1). In mixtures containing a fixed concentration of sucrose (0.5 M) and varying ethanol concentration (0–40%), ethanol enhanced the CT response to sucrose in both NP and P rats (Fig. 6B). In P rats, ethanol increased the CT response to sucrose, starting at ∼10%. The response further increased at 20% and 30%. At 40% ethanol the CT response was less than its maximum value (Fig. 6B). In NP rats, in the absence of ethanol the sucrose CT response was lower than in P rats. Ethanol enhanced the CT response of sucrose starting around 20%, and thereafter the response increased with increasing ethanol concentration to 30% and 40% (Fig. 6B).
Fig. 6.
CT responses to sweet taste stimuli in P and NP rats. A: CT responses were monitored while P and NP rat tongues (group 2; Fig. 1) were first stimulated with rinse solution R and then with 0.5 M sucrose or 0.0008 M SC45647. In each case CT responses were normalized to corresponding CT responses obtained with 0.3 M NH4Cl. Each bar represents mean ± SE value of normalized tonic CT response from 3 animals. *P = 0.0289, **P = 0.0013 (unpaired). B: CT responses were measured while the tongue was first stimulated with rinse solution R and then with R+0.5 M sucrose in the absence and presence of ETOH (0–40%) in P (●) and NP (○) rats (group 2; Fig. 1). To calculate the magnitude of the CT response to sucrose in the presence of ETOH, the response of ETOH in the rinse was subtracted from the total ETOH+sucrose response. In each case tonic CT responses were normalized to corresponding tonic CT responses obtained with 0.3 M NH4Cl. Each point represents mean ± SE value of the normalized sucrose tonic CT response from 3 animals plotted as a function of % ETOH concentration. Right y-axis represents % change in CT response relative to 0 ETOH in NP rats. Significant differences were found for ETOH concentration (P = 0.014 for both, 2-way ANOVA) and magnitude of the ethanol+sucrose response in P and NP rats (P = 0.0006, Bonferroni corrected), with no significant interaction between effects of strain and concentration.
Effect of chronic exposure to sucrose and ethanol on CT responses to sweet stimuli and T1R3 mRNA levels in P and NP rats.
Chronic exposure to 5% sucrose in NP rats (group 3; Fig. 1) in a no-choice paradigm produced a 27.6% decrease in T1R3 mRNA relative to NP rats maintained on H2O (Table 4). However, the magnitude of the CT response to 500 mM sucrose in NP rats chronically exposed to 5% sucrose was not different from that in NP rats maintained on H2O (Table 4). In contrast, chronic exposure to 5% ethanol in P rats (group 1; Fig. 1) in a no-choice paradigm decreased T1R3 levels by 49% relative to P and NP rats maintained on H2O (group 2; Fig. 1). In addition, the magnitude of the CT response to 500 mM sucrose in P rats chronically exposed to 5% ethanol was significantly decreased (P = 0.0007, unpaired) from that in P rats maintained on H2O (Table 4). These results suggest that chronic sucrose or ethanol exposure can alter neural responses to sweet stimuli by modulating T1R3 mRNA levels in type II TRCs.
Table 4.
T1R3 mRNA levels in anterior lingual epithelium containing fungiform taste buds and CT response to sucrose in P and NP rats chronically maintained on H2O, 5% sucrose, or 5% ethanol
| NP Rats + H2O (2−ΔCT) | NP Rats + 5% Sucrose (2−ΔCT) | Fold Change (P/NP) | P Value | P Rats+H2O (2−ΔCT) | P Rats + 5% Ethanol (2−ΔCT) | Fold Change (P/NP) | P Value | |
|---|---|---|---|---|---|---|---|---|
| T1R3 mRNA | 0.513 ± 0.0447 | 0.372 ± 0.0273 | 0.724 | 0.0397 | 0.748 ± 0.0674 | 0.381 ± 0.0393 | 0.51 | 0.0015 |
| n | 5 | 5 | 5 | 5 | ||||
| 0.5 M sucrose CT response | 0.135 ± 0.016 | 0.150 ± 0.016 | NS | 0.274 ± 0.023 | 0.032 ± 0.011 | 0.0007 | ||
| n | 3 | 3 | 3 | 3 |
2−ΔCT values are expressed as means ± SE of n, where n represents the number of P and NP rats maintained on H2O (group 2; Fig. 1) or chronically exposed to 5% ethanol or 5% sucrose in a no-choice paradigm. P values are for unpaired comparisons of means ± SE of 2−ΔCT values of P and NP rats maintained on H2O or chronically exposed to ethanol or sucrose (Schmittgen and Livak 2008). In each rat the CT response was normalized to the corresponding CT response obtained with 0.3 M NH4Cl and is presented as mean ± SE value of the normalized tonic CT response from 3 rats in each group (n). P values are for unpaired comparisons of means ± SE of the normalized tonic CT response between P and NP rats maintained on H2O or after chronic exposure to sucrose or ethanol.
Effect of U73122 on CT responses to sweet-tasting stimuli in the absence and presence of ethanol.
In type II TRCs, the enzyme PLCβ2 is an essential downstream intracellular signaling effector that is involved in the transduction of sweet, bitter, and umami taste (Chandrashekar et al. 2006). To test whether alcohol increases CT responses to sweet taste stimuli by also targeting the sweet taste transduction mechanism involving PLCβ2, we topically applied the nonspecific PLC blocker U73122 to the tongue at 150 × 10−6 M (Table 1). CT responses in SD rats (group 11; Fig. 1) were recorded while the tongue was stimulated with 0.008 M SC45647 in the presence and absence of 30% ethanol, before and after U73122 exposure. U73122 inhibited the tonic CT response to SC45647 to near baseline and also inhibited the alcohol-induced increase in SC45647 CT response (Supplemental Fig. S6A). U73122 inhibited the tonic CT response to 0.5 M sucrose to near baseline (Lyall et al. 2010). In addition, U73122 also inhibited the ethanol-induced increase in the CT response to sucrose (data not shown). The inactive analog U73343 had no effect on the sweet taste responses in the absence (Lyall et al. 2010) or presence (data not shown) of ethanol. These results suggest that ethanol modulates sweet taste responses by targeting (T1R2+T1R3)-PLCβ2-TRPM5 sensing pathways for sweet taste transduction (Chandrashekar et al. 2006).
Effect of chelating TRC intracellular Ca2+ on the CT response to sweet stimuli in the absence and presence of ethanol.
To test whether alcohol also increases CT responses to sweet stimuli by modulating TRC intracellular Ca2+ levels ([Ca2+]i) we loaded TRCs in vivo with BAPTA-AM. CT responses in SD rats (group 11; Fig. 1) were recorded in the presence of 0.5 M sucrose solutions containing 0 or 30% ethanol before and after topical lingual application of 33 × 10−3 M BAPTA-AM (Table 1). Chelating TRC [Ca2+]i did not affect the magnitude of the CT response to sucrose (Supplemental Fig. S6B) relative to control. However, it inhibited the ethanol-induced increase in CT response to sucrose observed under control conditions. This suggests that the synergistic effect of ethanol on the CT response to sweet taste stimuli is Ca2+ dependent.
DISCUSSION
The results presented in this article support the hypothesis that in P rats genetically induced alcohol preference is associated with 1) increased preference for sweet taste stimuli; 2) increased expression of T1R3 and TRPV1/TRPV1t mRNA and protein in the fungiform taste receptive field; 3) spontaneously bigger CT responses to sweet and salty taste stimuli; and 4) increased sensitivity of the CT responses to mixtures containing ethanol plus sweet or salty taste stimuli. Below we discuss the physiological significance of increased alcohol preference and its potential role in sweet and salty taste during acute and chronic ethanol consumption.
Alcohol Preference and Salt Taste
The Bz-insensitive component of the NaCl CT response is spontaneously enhanced in P rats relative to NP rats maintained on H2O (Fig. 4, A and B). Several lines of evidence suggest that the Na+ entry via a putative nonspecific cation channel, TRPV1t, contributes to the Bz-insensitive NaCl CT response. 1) In P and NP rats (Fig. 4, B and D, Fig. 5A, and Supplemental Fig. S1), SD rats, and WT mice, TRPV1t agonists (e.g., RTX, ethanol, and GalA-MRPs) modulate the Bz-insensitive NaCl CT responses in a biphasic manner. 2) A specific TRPV1/TRPV1t blocker, SB-366791, inhibited the spontaneous NaCl + Bz CT response (Fig. 5B) and the subsequent increase in the NaCl + Bz CT response in the presence of TRPV1t agonists (Fig. 5C). 3) TRPV1t KO mice demonstrate no spontaneous CT response to NaCl + Bz and no increase in the NaCl + Bz CT response above baseline in the presence of NaCl + Bz + TRPV1t agonists (Supplemental Fig. S5) (Lyall et al. 2004, 2005a, 2005b, 2007, 2009a, 2010; Katsumata et al. 2008). Thus our studies have identified the P rat as the first animal model in which a putative TRPV1t-dependent salt taste receptor activity is spontaneously enhanced.
Our qRT-PCR data (Table 2) and Western blot data (Fig. 3) suggest that the increased activity and sensitivity of the Bz-insensitive NaCl CT responses to TRPV1t agonists in P rats (Fig. 4, B and D, and Supplemental Fig. S1) are associated with increased expression of TRPV1/TRPV1t mRNA and protein in the fungiform taste receptive field. The increase in TRPV1t activity in P rats cannot be explained simply by differences in the regulation of TRPV1t by intracellular effectors in P and NP rats. We have previously shown that increasing the phosphorylation state of TRPV1t by activating PKC or inhibiting its dephosphorylation by blocking the phosphatase calcineurin enhanced channel activity without a leftward shift in the RTX concentration vs. magnitude of the NaCl + Bz CT response relationship (Lyall et al. 2009a). Similarly, relieving the channel from phosphatidylinositol 4,5-bisphosphate (PIP2) inhibition increased TRPV1t activity without a shift in the RTX concentration vs. magnitude of the NaCl + Bz CT response relationship (Lyall et al. 2010).
While P rats demonstrated a spontaneous increase in TRPV1t-dependent NaCl CT responses, NP rats, surprisingly, displayed the same relationship between RTX concentration and magnitude of the Bz-insensitive NaCl CT response as P rats when given chronic 5% ethanol in a no-choice paradigm (Fig. 5A). In a no-choice paradigm (Fig. 2B), NP rats consumed more ethanol relative to NP rats given a choice between H2O and ethanol (Fig. 2A). The increase in ethanol consumption was associated with an increase in the expression of TRPV1/TRPV1t mRNA in the fungiform taste receptive field (Table 3). These results suggest that a shift in the agonist concentration vs. magnitude of the Bz-insensitive NaCl CT response relationship is associated with increased expression of the TRPV1t channel protein in the fungiform taste receptive field. However, at present it is not clear whether the induction of TRPV1t in NP rats is also related to the amount of ethanol consumed and the length of time of chronic ethanol consumption. It is also presently not known whether the NP rat regains its phenotypically smaller and right-shifted CT response to NaCl + Bz + RTX after it is allowed to choose water again.
It is important to note that, similar to P and NP rats (Bice and Kiefer 1990), naive HAD and LAD rats demonstrated similar taste reactivity responses to a range of alcohol concentrations. Differences in taste reactivity between two strains became apparent only after rats had continuous access to alcohol, during which time HAD rats consumed significantly more alcohol than LAD rats (Kiefer et al. 1995). After alcohol exposure, HAD rats displayed a general increase in ingestive responses and a decrease in aversive responses relative to LAD rats. These results suggest that experience with ethanol produces an increase in ingestive and a decrease in aversive responses. In the no-choice paradigm, P and NP rats were forced to consume ethanol over a period of 2–3 wk. The alcohol consumption of P rats remained the same as in the choice paradigm. However, NP rats consumed a significantly greater amount of ethanol relative to rats in a choice paradigm (Fig. 2, A and B). Thus it is likely that the long-term experience with ethanol induces changes in gene expression of TRPV1t in the fungiform taste receptive field and in the TRPV1t-dependent NaCl CT response profile.
Conditioned taste aversion studies suggest that in some inbred strains ethanol has both a sweet taste quality and a bitter taste quality (Blizard 2007). At low concentrations some humans perceive ethanol as sweet, and at higher concentrations it is perceived as bitter (Scinska et al. 2000). However, besides its taste, additional factors contribute to ethanol preference and oral avoidance. Recent studies suggest that TRPV1 KO mice display elevated preference and consumption of ethanol and reduced oral avoidance responses to ethanol regardless of concentration, insensitivity to capsaicin, and little to no difference in sweet or bitter taste responses relative to WT mice (Blednov and Harris 2007; Ellingson et al. 2009). TRPV1 KO mice not only lack TRPV1 in the trigeminal nerve endings but also do not display Bz-insensitive NaCl CT responses, suggesting that they also do not have a functional TRPV1t, a TRPV1 variant cation channel, in their fungiform taste receptive field. (Lyall et al. 2004). These data suggest that elevated alcohol preference in TRPV1 KO mice is not related to the absence of a putative TRPV1t-dependent salt taste receptor per se but is rather related to the absence of trigeminal irritation caused by ethanol. Thus, while increased alcohol preference in P rats is associated with increased TRPV1/TRPV1t expression in the fungiform taste receptive field, the elevated preference for ethanol in TRPV1 KO mice is independent of a putative TRPV1t-dependent salt taste receptor but is associated with decreased trigeminal irritation induced by ethanol.
Our results further suggest that chronic ethanol usage and changes in the diet can have important consequences on salt taste receptor expression and function and changes in NaCl preference in animal models. Ethanol drinking and the conditions under which ethanol is consumed have been shown to differentially affect protein expression levels in the nucleus accumbens and amygdala (Bell et al. 2006). Chronic oral ethanol consumption increased the expression of TRPV1/TRPV1t mRNA (Table 3) in NP rats, increased neural responses to salty stimuli, and increased their sensitivity to TRPV1t agonists (Fig. 5A). In a previous study (Stewart et al. 1994), in P rats high oral alcohol preference appeared to be negatively associated with the intake of salty solutions varying in concentration between 0.004 and 0.55 M. In the study by Stewart et al. (1994) the NaCl solutions were presented continuously with water and food always available, and the concentrations of NaCl were doubled every 48 h. However, in our studies we only used one NaCl concentration. There was no significant difference in NaCl preference between P and NP rats when presented with isotonic NaCl solutions (Fig. 2C). Since the relationship between NaCl concentration and the NaCl preference is a bell-shaped curve, we also expect that at NaCl concentrations above 0.15 M the NaCl consumption and the NaCl preference will decrease in both P and NP rats. In P rats, further increasing TRPV1t activity with GalA-MRPs induced a significant decrease in NaCl intake and NaCl preference (Fig. 2C). We have previously shown that in C57BL/6J mice (Rhyu et al. 2009) NaCl preference for 0.1 M NaCl + 10 × 10−6 M amiloride is modulated by the addition of MRPs in a biphasic manner. At the concentration (0.25%) at which MRPs were shown to maximally enhance the Bz-insensitive NaCl CT response (Supplemental Fig. S1), MRPs decreased NaCl intake and preference. Alternately, at the concentration at which MRPs inhibited the CT response (0.5%), MRPs increased NaCl preference. These studies support our hypothesis that modulating TRPV1t activity by the addition of MRPs in the diet can modulate NaCl taste. However, additional behavioral studies need to be performed over a wide range of NaCl concentrations in P and NP rats to determine whether NaCl preference curves are significantly different between P and NP rats in the absence and presence of MRPs.
During stimulation of the tongue with salt solutions containing agonists, the contribution of TRPV1t to the total NaCl CT response can be increased by 100% or more (DeSimone and Lyall 2006; Lyall et al. 2009a, 2010). In humans, salt taste is predominantly amiloride insensitive and may contribute as much as 80% to human salt taste perception (Feldman et al. 2003; Ossebaard and Smith 1995). Recent studies suggest that the activation of TRPV1t, especially with nonpungent agonists such as MRPs, produces increased salt taste sensitivity in humans (Katsumata et al. 2008) and in animal models (Rhyu et al. 2009). Human subjects demonstrated significant increase in salt taste perception when NaCl was presented in mixtures containing low concentrations of MRPs. At high MRP concentrations, the same human subjects reported a significant decrease in salt taste perception (Katsumata et al. 2008). This suggests that TRPV1t may also play a role in human salt taste perception.
Alcohol Preference and Sweet Taste
In our studies, genetically induced alcohol preference in P rats was associated with increased T1R3 mRNA (Table 2) and protein expression in the fungiform taste receptive field (Fig. 3) for the detection of sweet taste stimuli and also in the gut enteroendocrine cells (Supplemental Table S1 and Supplemental Fig. S3) that regulate nutrient-responsive secretion of gut hormones (Kokrashvili et al. 2009a, 2009b). Chronic sucrose exposure in NP rats and chronic ethanol exposure in P rats resulted in a decrease in T1R3 mRNA (Table 4). These results suggest that changes in the sweet taste receptor can be induced by chronic ethanol usage and/or diets containing high sugar.
In P rats the neural responses to sweet taste stimuli demonstrated a significantly greater sensitivity to ethanol relative to NP rats (Fig. 6B). This increased magnitude of CT response to sucrose as a function of ethanol concentration is most likely due to increased T1R3 levels in type II TRCs (Table 2). In mixtures with sweet stimuli, acute oral ethanol application enhanced CT taste nerve responses to sweet taste stimuli (Fig. 6B and Supplemental Fig. S6). Inhibiting sweet taste transduction by blocking PLCβ2 with U73122 not only inhibited the sweet taste response but also eliminated the enhancement in the sweet taste response in the presence of ethanol (Supplemental Fig. S6A). While ethanol modulates sweet taste responses primarily by interacting with the sweet taste receptor (T1R3) (Bachmanov et al. 2001, 2002; Inoue et al. 2004; Lu et al. 2005; Nelson et al. 2001), other intracellular effectors, such as cell Ca2+, also play a role in mixture interactions. Chelating TRC [Ca2+]i with BAPTA loading did not affect the CT responses to sucrose but inhibited the subsequent increase in the neural response in the presence of ethanol (Supplemental Fig. S6B). These results suggest that the modulation of the CT nerve responses to sweet stimuli by ethanol is dependent upon an increase in TRC Ca2+. We hypothesize that the synergistic effects of alcohol on sucrose taste involve the Ca2+-activated nonselective cation channel, TRPM5 (Talavera et al. 2005).
It is suggested that the ability of ethanol to stimulate neural pathways involved in the transduction of sweet taste (Hellekant et al. 1997; Lemon et al. 2004) plays an important role in its consumption (Blednov et al. 2008). Consistent with this, genetic deletion of T1R3 and its associated G protein subunit (α-gustducin) or the downstream nonspecific cation channel (TRPM5) significantly decreases alcohol preference in KO mice (Ellingson et al. 2009). Recent studies suggest that a reciprocal genetic relationship also exists between the consumption of saccharin and sucrose and self-administration of alcohol and other drugs of abuse (Carroll et al. 2008). Rats selectively bred for high saccharin intake demonstrated significantly higher ethanol consumption relative to rats bred for low saccharin intake (Dess et al. 2005).
The effects of alcohol ingestion on taste preferences arise because of its effects on the CNS and on the peripheral taste system. The development of alcoholism is accompanied by alterations in gene and protein expression levels within the brain's reward neurocircuitry. In P rats, chronic ethanol drinking produced changes in expression levels for 22 of the 27 identified proteins in the amygdala and 6 proteins in the nucleus accumbens compared with control rats. The proteins could be grouped into functional categories of chaperones, cytoskeleton, intracellular communication, membrane transport, metabolism, energy production, and neurotransmission. Thus it appears that ethanol drinking and the conditions under which it is consumed differentially affect protein expression levels between the nucleus accumbens and amygdala (Bell et al. 2006). cAMP-protein kinase A (PKA) signaling has been strongly implicated in the CNS effects of ethanol. Ethanol promotes activation and translocation of the PKA catalytic subunit (Cα) into the cell nucleus. PKA Cα translocation to the nucleus is followed by cAMP response element (CRE) protein phosphorylation (pCREB) and CRE-mediated gene expression (Asyyed et al. 2006). The results presented in this study suggest that, in addition to the above effects in the CNS, the genetic differences in alcohol preference and chronic ethanol exposure are accompanied by alterations in gene expression levels in chemosensory cells in the taste buds involved in sweet and salty taste transduction.
In our acute studies, in naive animals alcohol and other agonists were applied topically to the tongue with the stimulus using a lingual flow chamber and produced their effects on the CT responses instantaneously. Under these conditions, the stimuli applied to the lingual surface do not mix with saliva and are not ingested. Thus it is expected that very little, if any, systemic uptake of alcohol or other drugs occurs under these conditions. Therefore, the acute effects of alcohol, RTX, and GalA-MRPs on the CT responses to taste stimuli are independent of postingestive effects or their effects on the CNS. However, under chronic conditions both of these factors, including additional factors such as stress, may play a role in modulating CT responses via changes in the taste receptors by themselves and/or in the downstream intracellular signaling effectors.
Randomly bred rats (Kampov-Polevoy et al. 1990), inbred and congenic mice (Blizard 2007; Blizard and McClearn 2000), and the F2 progeny derived from crosses of P and NP rats (Foroud et al. 2002) demonstrate positive correlations between alcohol and sweetener consumption. In F2 progeny generated from inbred P and NP rats, a quantitative trait locus (QTL) has been identified on rat chromosome 3 that appears to contribute to both alcohol and saccharin consumption (Foroud et al. 2002). The genetic locus that encodes for the T1R3 protein overlaps with a locus that strongly influences alcohol intake (Bachmanov et al. 2002). The allelic variations in the T1R3 gene are associated with differential sweet preferences in mice (Reed et al. 2004). Recent studies using WT and T1R3 KO mice provide further evidence for the involvement of T1R3 receptor in the sensory detection and transduction of ethanol taste (Brasser et al. 2010). At present, QTL studies with a putative TRPV1t-dependent Bz-insensitive salt taste receptor are lacking.
In summary, our studies suggest that genetically induced alcohol preference in P rats or forced chronic alcohol exposure in NP rats elevates the expression of a putative TRPV1t-dependent Bz-insensitive salt taste receptor in taste cells and increases the responsiveness of the CT nerve to salt taste stimuli in the absence and presence of ethanol. P rats also demonstrated increased expression of T1R3, a sweet taste receptor component, and increased responsiveness of the CT nerve to sweet taste stimuli in the absence and presence of ethanol. Chronic sucrose or ethanol exposure decreased T1R3 mRNA expression that can result in decreased neural responsiveness to sweet stimuli.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-000122 and DC-005981 to V. Lyall.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Gerard L. Heck for technical help with CT recordings.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res 1106: 63–71, 2006. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, Tordoff MG. Voluntary ethanol consumption by mice: genome-wide analysis of quantitative trait loci and their interactions in a C57BL/6ByJ x 129P3/J F2 intercross. Genome Res 12: 1257–1268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, Rodd ZA, Strother WN, Bai F, Peper CL, Mayfield RD, Lumeng L, Crabb DW, McBride WJ, Witzmann FA. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol 40: 3–17, 2006. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Kiefer SW. Taste reactivity in alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res 14: 721–727, 1990. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) increases ethanol consumption; possible role of endocannabinoids (Abstract). Alcohol Clin Exp Res 31 (suppl): 9A, 2007. [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7: 1–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet 37: 146–159, 2007. [DOI] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res 24: 253–258, 2000. [PubMed] [Google Scholar]
- Boughter JD, Bachmanov AA. Behavioral genetics and taste. BMC Neurosci 8, Suppl 3: S3–S21, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics 41: 232–243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot MD, Watson CH, Bernstein IL. Amiloride-sensitive signals and NaCl preference and appetite: a lick-rate analysis. Am J Physiol Regul Integr Comp Physiol 279: R1403–R1411, 2000. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol 19: 435–460, 2008. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. The taste of ethanol in a primate model. II. Glossopharyngeal nerve response in Macaca mulatta. Alcohol 21: 259–269, 2000. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26: 3971–3980, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Taste receptors in the gastrointestinal tract. III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol 291: G1005–G1010, 2006. [DOI] [PubMed] [Google Scholar]
- Dess NK, O'Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol 37: 9–22, 2005. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, Reed DR, Snyder DJ, Bartoshuk LM. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res 28: 1629–1637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39: 62–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman GM, Mogyorósi A, Heck GL, DeSimone JA, Santos CR, Clary RA, Lyall V. Salt-evoked lingual surface potential in humans. J Neurophysiol 90: 2060–2064, 2003. [DOI] [PubMed] [Google Scholar]
- Files FJ, Lewis RS, Samson HH. Ethanol self-administration by alcohol-nonpreferring (NP) rats in a continuous access situation. Alcohol 10: 139–144, 1993. [DOI] [PubMed] [Google Scholar]
- Foroud T, Bice P, Castelluccio P, Bo R, Ritchotte A, Stewart R, Lumeng L, Li TK, Carr L. Mapping of QTL influencing saccharin consumption in the selectively bred alcohol-preferring and -nonpreferring rat lines. Behav Genet 32: 57–67, 2002. [DOI] [PubMed] [Google Scholar]
- Fregly MJ. Effect of renal hypertension on the preference threshold of rats for sodium chloride. Am J Physiol 187: 288–292, 1956. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 46: 133–149, 2004. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model. I. Chorda tympani nerve response in Macaca mulatta. Alcohol 14: 473–484, 1997. [DOI] [PubMed] [Google Scholar]
- Heyman GM. A comparison of the reinforcing efficacy of alcohol in alcohol-preferring (P) and alcohol-nonpreferring (NP) rats. Pharmacol Biochem Behav 66: 455–463, 2000. [DOI] [PubMed] [Google Scholar]
- Hinrichs AL, Wang JC, Bufe B, Kwon JM, Budde J, Allen R, Bertelsen S, Evans W, Dick D, Rice J, Foroud T, Nurnberger J, Tischfield JA, Kuperman S, Crowe R, Hesselbrock V, Schuckit M, Almasy L, Porjesz B, Edenberg HJ, Begleiter H, Meyerhof W, Bierut LJ, Goate AM. Functional variant in a bitter-taste receptor (hTAS2R16) influences risk of alcohol dependence. Am J Hum Genet 78: 103–111, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variations of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 24: 2296–2303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol 7: 83–85, 1990. [DOI] [PubMed] [Google Scholar]
- Katsumata T, Nakakuki H, Tokunaga C, Fujii N, Egi M, Phan THT, Mummalaneni S, DeSimone JA, Lyall V. Effect of Maillard reacted peptides (MRPs) on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t). Chem Senses 33: 665–680, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, Badia-Elder N, Bice PJ. Taste reactivity in high alcohol drinking and low alcohol drinking rats. Alcohol Clin Exp Res 19: 279–284, 1995. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: aversion generalized to various sweet-quinine mixtures in the rat. Chem Senses 13: 633–641, 1988. [Google Scholar]
- Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses 18: 509–522, 1993. [Google Scholar]
- Kiianmaa K, Stenius K, Sinclair JD. Determinants of alcohol preference in the AA and ANA rat lines selected for differential ethanol intake. Alcohol Alcohol Suppl 1: 115–120, 1991. [PubMed] [Google Scholar]
- Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90: 822S-–825S, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann NY Acad Sci 1170: 91–94, 2009b. [DOI] [PubMed] [Google Scholar]
- Lawrence GJ, Kiefer SW. Generalization of specific taste aversions to alcohol in the rat. Chem Senses 12: 591–599, 1987. [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol 92: 536–544, 2004. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, McDaniel AH, Tordoff MG, Li X, Beauchamp GK, Bachmanov AA, VanderWeele DA, Chapman CD, Dess NK, Huang L, Wang H, Reed DR. No relationship between sequence variation in protein coding regions of the Tas1r3 gene and saccharin preference in rats. Chem Senses 30: 231–240, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, DeSimone JA, Feldman GM. Effects of osmolarity on taste receptor cell size and function. Am J Physiol Cell Physiol 277: C800–C813, 1999. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan THT, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+ flux. J Gen Physiol 125: 569–585, 2005a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol 125: 587–600, 2005b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan THT, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol 98: 1662–1674, 2007. [DOI] [PubMed] [Google Scholar]
- Lyall V, Phan THT, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the benzamil-insensitive salt taste receptor by intracellular Ca2+, protein kinase C, and calcineurin. J Neurophysiol 102: 1591–1605, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan THT, Mummalaneni S, Melone P, Coleman J, DeSimone JA. The benzamil (Bz)-insensitive NaCl chorda tympani (CT) taste nerve responses demonstrate increased sensitivity to TRPV1t modulators in alcohol-preferring (P) rats (Abstract). Chem Senses 34: A44, 2009b. [Google Scholar]
- Lyall V, Phan THT, Ren Z, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the putative TRPV1t salt taste receptor by phosphatidylinositol 4,5-bisphosphate. J Neurophysiol 103: 1337–1349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ, Preslar AJ. Spatial distribution of rat fungiform papillae. Anat Rec 181: 679–684, 1974. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zucker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan THT, Mummalaneni S, Melone P, DeSimone JA, Nicolelis MAL, Simon SA. Trpm5-independent activation of taste-responsive neurons by nicotine. Proc Natl Acad Sci USA 106: 1596–1601, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses 20: 37–46, 1995. [DOI] [PubMed] [Google Scholar]
- Petrocellis LD, Marzo VD. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 77: 1651–1666, 2005. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24: 938–946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu M, Song A, Abe K, Lyall V. Effect of kokumi taste active peptides on amiloride-insensitive salt taste preferences in C57BL/6J mice (Abstract). Chem Senses 34: A41-–A42, 2009. [Google Scholar]
- Sako N, Yamamoto T. Electrophysiological and behavioral studies on taste effectiveness of alcohols in rats. Am J Physiol Regul Integr Comp Physiol 276: R388–R396, 1999. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10: 436–442, 1986. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend 60: 199–206, 2000. [DOI] [PubMed] [Google Scholar]
- Scinska A, Bogucka-Bonikowska A, Koros E, Polanowska E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Taste responses in sons of male alcoholics. Alcohol Alcohol 36: 79–84, 2001. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-non-preferring rats. Alcohol Clin Exp Res 18: 375–381, 1994. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses 28: 315–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem 279: 37423–37430, 2004. [DOI] [PubMed] [Google Scholar]
- Woods JE, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res 27: 926–936, 2003. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.