Abstract
Rewards in the natural environment are rarely predicted with complete certainty. Uncertainty relating to future rewards has typically been defined as the variance of the potential outcomes. However, the asymmetry of predicted reward distributions, known as skewness, constitutes a distinct but neuroscientifically underexplored risk term that may also have an impact on preference. By changing only reward magnitudes, we study skewness processing in equiprobable ternary lotteries involving only gains and constant probabilities, thus excluding probability distortion or loss aversion as mechanisms for skewness preference formation. We show that individual preferences are sensitive to not only the mean and variance but also to the skewness of predicted reward distributions. Using neuroimaging, we show that the insula, a structure previously implicated in the processing of reward-related uncertainty, responds to the skewness of predicted reward distributions. Some insula responses increased in a monotonic fashion with skewness (irrespective of individual skewness preferences), whereas others were similarly elevated to both negative and positive as opposed to no reward skew. These data support the notion that the asymmetry of reward distributions is processed in the brain and, taken together with replicated findings of mean coding in the striatum and variance coding in the cingulate, suggest that the brain codes distinct aspects of reward distributions in a distributed fashion.
Keywords: uncertainty, risk, decision making, neuroeconomics, mean-variance approach
outside the laboratory rewards often occur in an uncertain fashion, and the probability distributions associated with them often exhibit significant asymmetry, or skewness. For the foraging animal, this type of uncertainty can be thought of as the degree to which very large or very small rewards are encountered relative to the overall average expected reward in a given patch. For an organism seeking to maximize its survival over a certain time period (such as the time devoted to foraging each day), seeking out positively skewed food reward distributions maximizes the likelihood that large magnitude rewards are encountered, decreasing the chance of starvation. Indeed, behavioral ecologists have shown positive skewness-seeking preferences in animals (Caraco and Chasin 1984), and economic experiments have shown similar effects in humans [(Astebro et al. 2009); positive skewness roughly refers to the possibility of very large gains]. Skewness preference in humans has been proposed to play a large role in short-term economic decisions, especially with regard to gambling, but may also be a major factor driving entrepreneurship and other long-term investments (Hamilton 2000; Moskowitz and Vissing-Jorgensen 2002). However, research on the neural basis of decision making under uncertainty has primarily focused on the effects of variance (Christopoulos et al. 2009; Tobler et al. 2009), ambiguity (Hsu et al. 2005; Huettel et al. 2006), or expected value (Knutson et al. 2005), with only one paper addressing reward skewness (Wu et al. 2011). We specifically investigated the neural basis of skewness by having human participants learn associations between abstract visual cues and monetary lotteries that differed in their reward skewness, while controlling for and independently varying variance or expected value.
On the basis of previous behavioral findings in animals and humans (Caraco and Chasin 1984; Astebro et al. 2009; Kraus and Litzenberger 1976), we hypothesized that participants would demonstrate preference for stimuli associated with positively skewed rather than negatively skewed lotteries. We also proposed that mean and variance would be the first and second biggest influences on choice. A substantial body of evidence has pointed to the insula as a major candidate structure for the processing of reward-related uncertainty (Singer et al. 2009), suggesting that it codes both general and specific aspects of uncertainty, including risk (Elliott et al. 2000; Huettel et al. 2005), ambiguity (Hsu et al. 2005; Huettel et al. 2006), and risk-prediction errors (Preuschoff et al. 2008). The goal of the present study was to extend this previous research on the neural representation of economic uncertainty by investigating the response of the insula to cues that predicted skewed outcome distributions. We predicted the presence of a skewness signal in this region during the presentation of skewness-predicting stimuli. We also investigated subjective and objective representations of skewness to further assess the exact nature of the skewness signal. A carefully designed conditioning paradigm allowed us to test these behavioral and neural hypotheses while controlling for several alternative explanations from economic theories for skewness preference.
MATERIALS AND METHODS
Participants and prescanning procedures.
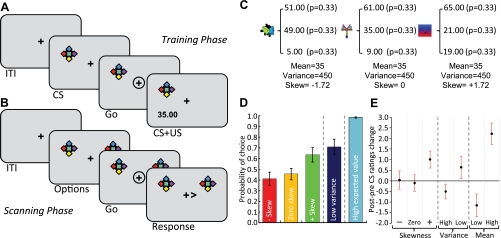
Twenty-five participants (mean age 23.7 yr, range 21–28 yr, 15 women, all right-handed) took part in the experiment. All participants had normal or corrected-to-normal vision and were screened to exclude those with a previous history of neurological or psychiatric disease. All gave informed written consent. The study was approved by the Research Ethics Committee of the Canton of Zurich. After completing a consent form and magnetic resonance safety questionnaire, participants were asked to rate the pleasantness of each of the 7 abstract stimuli used in the experiment on a scale of −5 (very unpleasant) to +5 (very pleasant). After completing preexperiment ratings, participants were instructed that they would be learning to associate each of seven abstract stimuli to seven underlying reward distributions and would subsequently be presented with choices between stimuli to assess their preference. Participants were first shown a sheet of paper containing an image of each of the abstract stimuli. Adjacent to each image were three numbers, indicating the three amounts (in Swiss Francs, CHF) that each stimulus predicted with equal probability.
Stimuli and reward distributions.
The abstract stimuli used in the experiment consisted of equally sized (100 × 100 pixels) bitmap images similar to those previously used in animal-conditioning experiments. Each stimulus was associated with a ternary lottery that on a given trial could pay out one of three equiprobable outcomes (Table 1). The positively skewed (+1.72) lottery had reward outcomes of 19, 21, or 65 CHF, the zero skewed lottery had reward outcomes of 9, 35, or 61 CHF, and the negatively skewed (−1.72) lottery had outcomes of 5, 49, or 51 CHF. Crucially, these distributions had the same mean (35) and variance (450). In addition to these main distributions of interest, we included two ternary lotteries that differed in mean (31 and 39 CHF), but not variance (450) or skewness (zero) (high mean: 13, 39, or 65 CHF; low mean: 5, 31, 57 CHF), and two that differed in variance (150 and 600 CHF), but not mean (35 CHF) or skewness (zero) (low variance: 20, 35, 50 CHF; high variance: 5, 35, 65 CHF). The link between cue and reward distribution was different and randomly assigned for each subject.
Table 1.
Gambles used (outcomes are in CHF)
| Gamble | Outcome 1 | Outcome 2 | Outcome 3 | Mean | Variance | Skewness |
|---|---|---|---|---|---|---|
| Negative skewness | 5 | 49 | 51 | 35 | 450 | −1.72 |
| No skewness | 9 | 35 | 61 | 35 | 450 | 0 |
| Positive skewness | 19 | 21 | 65 | 35 | 450 | 1.72 |
| Low variance | 26 | 35 | 44 | 35 | 54 | 0 |
| High variance | 5 | 35 | 65 | 35 | 600 | 0 |
| Low mean | 1 | 27 | 53 | 27 | 450 | 0 |
| High mean | 13 | 39 | 65 | 39 | 450 | 0 |
Skewness gambles varied in skewness but not variance or mean. All outcomes had the same probability (P = 0.33) of occurrence.
Prescanning task.
Participants were explicitly instructed about the reward probabilities and magnitudes associated with each stimulus. Participants then performed two sessions of a conditioning task where they repeatedly experienced presentations of the stimuli and the associated outcomes. After a fixed intertrial interval (ITI) of 2 s, one of the stimuli was displayed on the left or right side of the computer screen (Fig. 1A). After 2 s, the fixation cross was circled, and participants made a response with their right index or middle finger to indicate which side of the screen the stimulus was displayed on. One of the three outcomes associated with that stimulus was then displayed below it. In the first session, both stimuli and outcomes were blocked to facilitate learning of the reward distributions. As an example, the participant would see the same stimulus for 15 consecutive trials, experiencing each outcome associated with that stimulus for five consecutive trials. In the second session, stimulus types were randomized, with one of the three outcomes associated with each stimulus displayed in an interleaved manner. Participants always experienced each outcome associated with each stimulus an equal number of times, such that the experienced means and probabilities equaled the expected values and probabilities associated with each stimulus. The conditioning task and scanning task were scripted in Cogent (Wellcome Laboratory of Neurobiology, University College, London, UK) using the Matlab programming environment (Mathworks).
Fig. 1.
Experimental design and behavioral results. A: conditioning task. Outside the scanner participants engaged in a conditioning task. Abstract visual stimuli were presented on the left or right side of the screen, and participants were required to indicate the side with left or right key presses to view the associated outcomes. B: scanning task. Inside the scanner the stimuli were presented on the left and right sides of the screen, and participants were required to make a button press to indicate attention. In this example trial, the two stimuli on each side of the screen are the same. Occasionally different stimuli were displayed on each side of the screen, requiring participants to make a choice using left or right button presses (with index and middle finger of the right hand). C: examples of stimuli and reward distributions. The 3 lotteries of interest differed in skewness but had the same variance and mean. Each lottery could generate one of 3 equiprobable outcomes on each trial. Stimuli were randomly assigned to each distribution for each participant. D: behavioral preference on choice trials. On average participants preferred stimuli predicting positively skewed reward distributions over negatively skewed distributions, low variance over high variance, and high mean over low mean. E: pre- and postexperiment stimuli pleasantness ratings. The change in pleasantness ratings for the abstract visual stimuli reflected participants' behavior on choice trials. ITI, intertrial interval. CS, conditioned stimulus; US, unconditioned stimulus.
Scanning task.
During scanning, participants performed a task with “non”-choice and choice trials. Each trial started with a variable ITI with only the fixation cross visible in the center of the screen (Fig. 1B). The ITI varied in length according to a truncated Poisson distribution with a range of 2–11 s. On nonchoice trials, the ITI was followed by the presentation of the same stimulus on the left and right sides of the screen. The only choice here consisted of selecting the side. After 2 s, the fixation cross was circled, indicating the onset of the response window. Participants were required to make the left or right response within 1 s of the circle appearing, or an error was logged. If the participant made a left or right response during the response window, the side was indicated by a > or < inequality symbol to indicate the direction pressed. Because in nonchoice trials the stimuli on either side of the screen were the same, the direction of the response was inconsequential; the response requirement served to keep participants attentive. The direction was indicated for 1 s (plus remaining time from the response window). The ITI then followed, preceding the next trial. No outcomes were shown during the scanning phase to isolate predictive from outcome signals. Nonchoice trials consisted of seven trial types, depending on the stimuli displayed (each stimulus associated with one of the reward distributions described above and shown in Table 1): negative skewness, zero skewness, positive skewness, high variance, low variance, low mean, and high mean reward distributions (examples of negative, zero, and positive skewness reward distributions and associated stimuli can be seen in Fig. 1C).
Occasional choice trials were used to assess behavioral preferences and proceeded in the same manner as nonchoice trials, except that after the ITI the stimuli displayed differed on each side. Choice trials consisted of five trial types, depending on the choice between stimuli-associated reward distributions: choices between negative and positive skewness, negative and zero skewness, positive and zero skewness, high and low variance, and low and high mean.
During scanning, the task was split into two sessions, each containing 70 test trials (10 for each stimulus), giving 140 test trials for the whole experiment. All trials were randomly interleaved. Choice trials were occasionally and randomly interspersed throughout the experiment to test behavioral preference for the different stimuli. Between sessions, participants were given a break of 1 min, during which they were instructed to relax but keep still. Participants were instructed that seven trials would be randomly selected during the experiment and that their payout would be governed by the lotteries associated with the presented stimuli on those trials. An outcome from a chosen stimulus in each of the seven trials was selected randomly for payment.
Data acquisition.
Images were acquired using a Philips Achieva 3T whole-body scanner with an eight-channel SENSE head coil (Philips Medical Systems, Best, The Netherlands) at the Laboratory for Social and Neural Systems Research, University Hospital Zurich. The task was projected on a display, which participants viewed through a mirror fitted on top of the head coil. We acquired gradient echo T2*-weighted echo-planar images (EPIs) with blood-oxygen-level-dependent (BOLD) contrast (slices/volume, 30; repetition time, 1.55 s). Approximately 280–350 volumes were collected in each session (variation attributable to individual differences in the number of error trials and intertrial intervals) of the experiment, together with five “dummy” volumes at the start and end of each scanning session. Scan onset times varied randomly relative to stimulus onset times. A T1-weighted 3D-TFE (turbo field echo) structural image was also acquired for each participant. Volumes were acquired at a 30° tilt to the anterior commissure-posterior commissure line, rostral > caudal. Imaging parameters were the following: echo time, 30 ms; field of view, 240 mm. The in-plane resolution was 3 × 3 mm, with a slice thickness of 3 mm and an interslice gap of 1 mm. High-resolution T1-weighted structural scans were coregistered to their mean EPIs and averaged together to permit anatomical localization of the functional activations at the group level.
Image analysis.
We used a standard rapid-event-related fMRI approach in which evoked hemodynamic responses to each event type are estimated separately by convolving a canonical hemodynamic response function with the onsets for each event and regressing these against the measured fMRI signal. Statistical parametric mapping (SPM5; Functional Imaging Laboratory, University College London, UK) served to spatially realign functional data, normalize them to a standard EPI template, and smooth them using an isometric Gaussian kernel with a full-width at half-maximum of 8 mm. Onsets of stimuli and participant responses were modeled as separate delta functions and convolved with a canonical hemodynamic response function. Each condition (negative skew, zero skew, positive skew, high variance, low variance, low mean, high mean) was modeled with a separate regressor at stimulus onset. In addition, subject responses (i.e., button press) were modeled with a regressor that included direction (left/right button) and response time as parametric modulators. Choice trials were modeled at stimulus onset (option presentation) with five different regressors for the five different combinations used (high skew-low skew, high skew-no skew, no skew-low skew, high variance-low variance, high mean-low mean). Participant-specific movement parameters (3 regressors for rotation and 3 for translation) were modeled as regressors of no interest. Linear contrasts of regression coefficients such as positive skewness-negative skewness were computed at the individual subject level and then taken to group-level t-tests. Small-volume correction was used to control for multiple comparisons in our predefined regions of interest (ROIs). Anatomically defined small-volume correction in the insula was performed within the full extent of this region according to the boundaries defined in the AAL atlas. All reported imaging results pertain to stimulus-related activity time-locked to stimulus presentation.
RESULTS
Participants first engaged in a classical conditioning task outside the scanner where they learned to associate abstract visual stimuli with lotteries (Fig. 1A). We used lotteries with three equiprobable outcomes. Three of the lotteries differed in the skewness of their reward distributions (while holding mean and variance constant, Fig. 1C). In addition to these three main lotteries of interest, we also used two lotteries with different variance (but with equal mean and skewness) and two with different mean (but with equal variance and skewness, Table 1). After repeated stimulus-outcome pairings during this training phase, participants proceeded to the scanner, where they performed a second task requiring them to respond to the presentation of the previously learned stimuli (Fig. 1B). Outcomes were not shown to the participant during this phase.
Behavior.
To assess behavioral preference, participants occasionally faced five types of decision trials, consisting of choices between stimuli associated with the different reward distributions. These consisted of three trial types with choices between the skewed stimuli (positive/negative, positive/zero, and negative/zero), one between high-variance and low-variance options, and one between low- and high-mean options (Fig. 1D). Linear ordinary least-squares regression analysis showed that skewness significantly predicted choice (b = 7.88, P < 0.01) for stimuli that differed in terms of reward distribution asymmetry (but not in terms of mean or variance). Further investigation revealed that on average participants preferred positive skew more than zero skew (P < 0.05, paired t-test), but there was no significant difference between the probabilities of choosing negatively skewed and nonskewed distributions (P = 0.16, paired t-test). At the individual level, 15 out of 25 participants chose the positively skewed distribution more often than the negatively skewed distribution. For choice trials involving decisions between low-variance and high-variance stimuli, participants exhibited variance aversion by choosing low-variance options significantly more often than high-variance options (P < 0.05, t-test). Preference for skewness did not correlate with preference for variance (R2 = 0.110; P = 0.105, linear regression). As expected, participants almost always chose the option with the highest mean (P < 0.001, t-test), demonstrating that participants had well learned the distributions during the classical conditioning task outside the scanner. Given that stimuli and reward distributions were randomly assigned for each subject, these effects cannot be explained by simple visual properties of the specific stimuli. These choice-based results were largely mirrored in the pre- and postexperiment changes in pleasantness ratings of the various stimuli, with 10 participants increasing their ratings more for positively skewed than negatively skewed stimuli, 4 showing no change, and 11 increasing their ratings for negatively over positively skewed stimuli. At the group level, rating increases for positively skewed stimuli were significantly greater than zero (P < 0.05, one tailed t-test), whereas rating increases for negatively skewed stimuli were not significantly different from zero (P = 0.70, one-tailed t-test), mirroring preference for positive skewness, low variance, and high-mean options in the choice data (Fig. 1E). Thus humans show distinct preferences for distinct risk terms, with overall (positive) skewness seeking and variance avoidance.
BOLD responses: skewness processing in the insula.
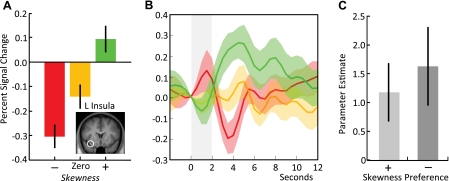
To assess brain activity uncontaminated by the presence of choice options with different meaning, we scanned trials in which both choice options had the same meaning. In a first-pass analysis, we investigated the BOLD response in the insula to the presentation of skewness-predicting stimuli, comparing high vs. low skewness trials. In agreement with our original hypothesis, we found that the left insula was active for this contrast (positive skewness − negative skewness), (Fig. 2A; peak at −33, 14, −17; Z = 3.17; P < 0.05 corrected for insula) and for a contrast involving all three skewness levels [(positive skewness > zero skewness > negative skewness), peak at −30, 8, −17; Z = 3.26, P < 0.05 insula corrected]. No insula activity was present for the opposite contrasts (negative skewness − positive skewness and negative skewness > zero skewness > positive skewness). The left insula was also responsive to the average skewness of available options during choice trials [(positive skew vs. zero skew choice trials > positive skew vs. negative skew choice trials > negative skew vs. zero skew choice trials), peak at 30, 8, 16, Z = 2.98, P < 0.001 uncorrected]. However, a comparison between choice and nonchoice trials revealed that the insula was significantly more responsive to skewness during nonchoice trials (P = 0.028, two-tailed t-test). Note that the mean presented skewness varied more in nonchoice situations (i.e., between +1.72 for 2 positively skewed options and −1.72 for 2 negatively skewed options) than in choice situations (variation between +0.86 for 1 positively skewed and 1 nonskewed option and −0.86 for 1 negatively skewed and 1 nonskewed option). One potential interpretation of the finding is therefore that the insula activation scales with the average skew of the two available options in both the choice and the nonchoice condition. Time-course analysis revealed that the BOLD response in the left insula was maximally differentiated for positively and negatively skewed stimuli at the expected peak of the hemodynamic response function ∼4 s after stimulus presentation, with the nonskewed (although possessing equal variance and mean) stimulus eliciting a response in between the two (Fig. 2B). Thus activation in the insula increased with increasing skewness (from negative to zero to positive).
Fig. 2.
Blood-oxygen-level-dependent (BOLD) response in the left ventral insula to the presentation of skewness-predicting stimuli. A: neural activity in the left insula showed a monotonic increase in response to skewness for the (negative skewness > zero skewness > positive skewness) contrast (peak at 30, 8, −17; Z = 3.26, P < 0.05 insula corrected). B: time courses of activity for the left insula reflected the same pattern with increasing response to increasing skewness at the expected peak of the hemodynamic response function ∼4 s after stimulus onset (the red, orange, and green lines represent the average response to negatively, zero, and positively skewed stimuli, respectively). C: skewness responses in the insula did not simply reflect behavioral preference. There was no significant difference in the monotonic response of the ventral insula to increasing skewness between positive and negative skewness-preferring participants (P = 0.878, two-tailed t-test).
Given that objective skewness and average behavioral skewness preference are somewhat related (Fig. 1D), one potential explanation for the pattern of insula activity observed here is that it reflects behavioral preference for the skewness stimuli rather than objective skewness. We tested for this possibility by splitting the participants into positive and negative skewness preferring groups according to individual participant's preference on choice trials. We then tested the (positive skewness − negative skewness) contrast in the two groups separately at the location of the previous peak. A conjunction analysis revealed significant left insula activation in both groups for the (positive skewness − negative skewness) contrast, and there was no significant difference in this activity between the groups (P = 0.878, two-tailed t-test, Fig. 2C). Thus the data suggest that the insula processes skewness in an objective fashion.
To further corroborate that the pattern of activity observed here reflected objective skewness processing, we searched for common activation in the insula ROI for the (positive skewness − negative skewness) contrast in the positive skewness-preferring group and the (negative skewness − positive skewness) contrast in the negative skewness-preferring group. No insula activity was observed for this conjunction, even at the liberal threshold of P < 0.05 uncorrected, providing no evidence that subjective behavioral preference is the main driver of the differential BOLD responses across the skewed stimuli in the insula.
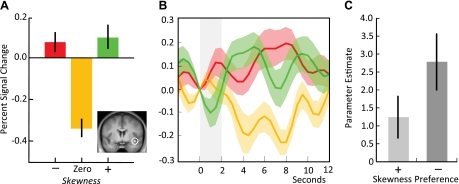
We next evaluated the response of the insula to any reward distribution asymmetry [i.e., the contrast (positive skew + negative skew) > zero skew]. Stimuli predicting both positively and negatively skewed reward distributions elicited higher activation in the right insula (Fig. 3, A and B; peak at 39, 2, −14; Z = 3.73, P < 0.01, corrected for insula). No insula activity was observed for the opposite contrast (i.e., nonskewed stimuli eliciting higher activation than skewed stimuli). To determine whether this activation depended on skewness preferences, we tested the contrast in both positive skewness-preferring and negative skewness-preferring groups. In a similar manner to the objective skewness signal in left insula, the right insula showed a significant response to any type of skewness in both groups, and this response was not significantly different between groups (P = 0.103, two-tailed t-test, Fig. 3C).
Fig. 3.
BOLD response to any skewness in the right insula. A: activity in the right ventral insula increased during the presentation of both negatively skewed and positively skewed stimuli relative to the zero-skew predicting stimulus (peak at 39, 2, −14; Z = 3.73, P < 0.01, corrected for insula). B: time course of data extracted from the right insula reflects the same pattern, with higher activity during the anticipation of negatively skewed (red line) and positively skewed (green line) reward distributions compared with the zero-skew distribution (orange line). C: response of the right insula to any skewness did not differ between positive and negative skewness-preferring participants.
In addition to objective representations of skewness, we also investigated whether insula activity correlated with subjective preferences for skewness. We took the difference between the probabilities of choosing the positively skewed and negatively skewed stimuli to generate a preference metric for positive skewness. We found a significant correlation of the BOLD response in the dorsal and posterior insula with this subjective measure of skewness preference for the contrast of (positive skewness > negative skewness) (Fig. 4; peak at 33, −25, 19; R2 = 0.238; P = 0.013, ordinary least-squares linear regression). At the employed thresholds, we find no evidence for subjective variance coding in the insula. Thus differential activity during anticipation of skewed reward distributions was highest for positive skewness in the most positive skewness-preferring participants in this subregion.
Fig. 4.
Subjective coding of skewness preference in the dorsal insula. The BOLD response in the dorsal and posterior insula correlated with subjective preference for positive skewness during the presentation of positively vs. negatively skewed stimuli (peak at 33, −25, 19; R2 = 0.238; P = 0.013, linear regression).
Mean and variance coding in striatum and cingulate.
In addition to the three stimuli that differed in skewness, our task also included two stimuli predicting different means and two that predicted different variance of the reward distribution. This allowed us to test for mean (or expected value) coding and variance coding in two a priori ROIs, the ventral striatum (for mean) and the anterior cingulate (for variance).
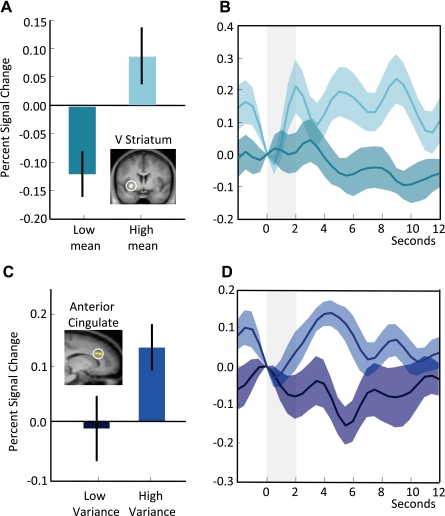
The low-mean stimulus predicted outcomes of 5, 31, or 57 CHF, whereas the high-mean stimulus predicted outcomes of 13, 39, or 65 CHF. Variance and skewness did not differ between the two distributions. For the contrast of (high mean − low mean) we found the ventral striatum to be active (peak at −24, 5, −5; P < 0.05, small volume corrected for ventral striatum; Fig. 5, A and B). Some voxels of this cluster extended into the left insula. By contrast, the high-variance (5, 35, and 65 CHF) and low-variance (20, 35, and 50 CHF) reward distributions did not differ in mean and skewness. As hypothesized, we found activation of the anterior cingulate during the contrast of (high variance − low variance) stimulus presentation (Fig. 5, C and D; peak at 15, 23, 28; P < 0.05, small volume corrected for 5-mm sphere around peak coordinates for variance reported in Christopoulos et al. 2009).
Fig. 5.
Neural activity corresponding to mean and variance. A: ventral (V) striatum showed an increased response during the presentation of stimuli that predicted high-mean reward distributions (peak at −24, 5, −5; P < 0.05, small volume corrected for ventral striatum). B: time course of ventral striatum response to high- (light blue) and low- (dark blue) mean predicting stimuli. C: anterior cingulate showed increased BOLD response during the presentation of stimuli that predicted high-variance rewards (peak at 15, 23, 28; P < 0.05, small volume corrected for 5-mm sphere around peak coordinates for variance reported in Christopoulos et al. 2009). D: time course of anterior cingulate response to high- (light blue) and low- (dark blue) variance predicting stimuli.
DISCUSSION
This study provides evidence for the neural representation of skewness in the insula during the anticipation of uncertain outcomes, extending previous research on the processing of outcome variance in this region. Our behavioral results show that participants learned to associate abstract stimuli with different outcomes and are consistent with the idea that people prefer high-mean (expected value), low-variance, and positively skewed reward distributions (Kraus and Litzenberger 1976). In addition to this finding, the impact of skewness on choice appeared to be less than that of variance, which in turn had a smaller effect than the mean (at least with the gambles used in this study). BOLD responses in the insula to skewness-predicting cues correlated with both signed and unsigned skewness in addition to spatially separated signals corresponding to subjective skewness preferences.
In economics and finance, skewness preference can be accommodated by several formal models. Two prominent examples are prospect theory (Kahneman and Tversky 1979) and an extension of the mean-variance approach of Markowitz (1952) that includes the third central moment (Preuschoff et al. 2008). Prospect theory models are able to produce skewness preference in two distinct ways. The first is through the under- or overweighting of large and small probabilities associated with different outcomes in reward distributions (probability distortion; in binary distributions for example, probabilities smaller than 0.5 are positively skewed, probabilities larger than 0.5 are negatively skewed). Second, loss aversion can generate positive skewness preference when probabilities are kept constant but potential losses are introduced. Crucially, these prospect theory-based approaches propose that skewness preference can arise without the individual decision maker processing the skewness of a reward distribution per se but through probability distortion and loss aversion, mechanisms previously demonstrated at both the behavioral (Kahneman and Tversky 1979; Prelec 1998) and neural level (Tom et al. 2007; Tobler et al. 2008; Hsu et al. 2009). However, a major limitation of prospect theory is that it can only explain skewness preferences when the probabilities attached to the outcomes in a lottery are asymmetric, as is typically the case in previous experiments (Caraco and Chasin 1984; Astebro et al. 2009), or when losses are involved. In our task, we used equiprobable three-outcome distributions with no potential losses, reducing the possibility that prospect theory can explain the effect of skewness on both behavior and neural activity.
Prospect theory might still be able to explain preferences in the present task by invoking the reference-dependent rather than absolute nature in which outcomes are processed (Kahneman and Tversky 1979). According to this view, a gain that is low relative to some reference point might be experienced as a loss. Loss aversion and differential weighting of positive and negative prediction errors (Frank et al. 2007) may thereby contribute to the formation of preferences. Although this possibility is conceivable in principle, much depends on the choice of the reference point.
By contrast, an extension of a mean-variance model (Markowitz 1952) to include skewness may be more straightforward. This approach proposes that the utility of risky prospects depends on the moments of outcome distributions, with skewness being the third, variance the second, and mean the first moment. Such decomposition of risky prospects into their constituent moments allows options to be considered and compared in a computationally efficient manner and facilitates learning in volatile environments (Preuschoff et al. 2008). This approach naturally posits the existence of a skewness signal in the brain, coded in a similar manner to other reward-related moments such as mean (Tobler et al. 2009; Knutson et al. 2005) and variance (Christopoulos et al. 2009; McCoy and Platt 2005; Preuschoff et al. 2006; O'Neill and Schultz 2010). The objective skewness signals and behavior observed in the present study are compatible with the mean-variance-skewness approach.
Our procedure differed somewhat from tasks usually used by behavioral economists studying the effect of uncertainty on behavior. We chose to use a conditioning approach with abstract visual stimuli, allowing us to avoid the use of alphanumeric representations of options or visual depictions of probability (for example through the use of pie charts). The main reason for taking this approach was to minimize the conceptual distance between this study and previous studies on value, risk, and probability in animals. The use of alphanumeric stimuli and graphical representation of probability introduces language or complex visual representations of mathematical concepts, faculties that most animals do not possess. A second reason for using the nonchoice conditioning procedure was to allow each participant to experience every stimulus-outcome pairing exactly the same number of times before they entered the scanner and thus to ensure that the experienced means and probabilities equaled the expected values and probabilities associated with each stimulus.
A recent study by Wu et al. (2009) examined the affective impact of financial skewness using fMRI. In that study, participants passively viewed different pie charts that explicitly represented the probabilities of gains and losses. After a delay, the gambles were resolved. All gambles had a mean of zero. Both studies report insula activation for unsigned skewness. In addition we found a signal that increases with increasing skewness, irrespective of individual preferences. Unlike Wu et al. (2009), we did not find any skewness-related signals in the ventral striatum. A possible reason for this difference is that in their task negative and positive outcomes are used to manipulate skewness, whereas the reward distributions used in our task were all in the positive domain. The BOLD response in the ventral striatum has been previously shown to correlate with the subjective value of mixed gambles, reflecting loss aversion (Tom et al. 2007). Future research may wish to compare and contrast the effects of gains, losses, and mixed gambles on skewness processing (Lovallo and Kahneman 2000) to assess the role of losses in generating skewness preference. Although both our behavioral and neural results are in line with the mean-variance-skewness hypothesis (while excluding prospect theory), the possibility remains that participants rely on alternative decision-making strategies when it comes to processing skewness (Caraco 1980; Green 1980). One such possibility that we cannot exclude from our results here is that participants use a “maxi-min” strategy (Caraco and Chasin 1984). Participants may prefer positive skewness if the minimum reward provided by the positively skewed distribution exceeds the minimum reward of the negatively skewed distribution (as is the case in our experiment).
Our results implicate the insula in processing skewness-related uncertainty in both an objective and subjective fashion. The anticipatory activity in the insula to objective skewness reported in this study primarily occurs in the middle and ventral regions of the insula, with activity correlating with subjective skewness preference in a more dorsal region. These areas have typically been implicated in the processing of interoceptive input, and uncertainty-related signals in these regions are frequently used to support the somatic marker hypothesis (Yu et al. 2010). It remains to be seen whether skewness-related uncertainty has an effect on somatic markers such as skin-conductance response. Interestingly, the differences we observe with respect to monotonic (positive > negative) and any skewness may also be related to differential interoceptive inputs to the left and right insula, with left insula preferentially innervated by parasympathetic autonomic activity and right insula preferentially innervated by sympathetic autonomic activity (Craig 2009). This suggests that left insula may be more involved in approach behavior (given the activity of this region correlated with skewness-induced behavioral preference), whereas right insula may signal departures from reward symmetry to increase attention.
GRANTS
This work was supported with funding from the Swiss National Science Foundation (PP00P1_128574) and the Swiss National Centre of Competence in Research (NCCR) in Affective Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Juri Fujiwara and Ernst Fehr for helpful discussions. We also thank the Neuroscience Center Zurich and the Zurich Center for Integrative Human Physiology.
REFERENCES
- Astebro T, Mata J, Santos-Pinto L. Preference for Skew in Lotteries: Evidence from the Laboratory. MPRA Paper 17165. Munich, Germany: University Library of Munich, 2009 [Google Scholar]
- Caraco T, Chasin M. Foraging preferences: response to reward skew. Anim Behav 32: 76–85, 1984 [Google Scholar]
- Caraco T. On foraging time allocation in a stochastic environment. Ecology 61: 119–128, 1980 [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci 29: 12574–12583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009 [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci 20: 6159–6165, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA 104: 16311–16316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RF. Bayesian birds: a simple example of Oaten's stochastic model of optimal foraging. Theor Popul Biol 18: 244–256, 1980 [Google Scholar]
- Hamilton BH. Does entrepreneurship pay? An empirical analysis of the returns to self-employment. J Polit Econ 108: 604–631, 2000 [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision making. Science 310: 1680–1683, 2005 [DOI] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer C. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci 29: 2231–2237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel S, Song A, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci 25: 3304–3311, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron 49: 765–775, 2006 [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica 4: 263–291, 1979 [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci 25: 4806–4812, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Litzenberger R. Skewness preference and the valuation of risk assets. J Finance 31: 1085–1100, 1976 [Google Scholar]
- Lovallo D, Kahneman D. Living with uncertainty: attractiveness and resolution timing. J Behav Decis Making 13: 179–190, 2000 [Google Scholar]
- Markowitz H. Portfolio selection. J Finance 7: 77–91, 1952 [Google Scholar]
- McCoy AN, Platt ML. Risk-sensitive neurons in macaque posterior cingulate cortex. Nat Neurosci 8: 1220–1227, 2005 [DOI] [PubMed] [Google Scholar]
- Moskowitz T, Vissing-Jorgensen A. The returns to entrepreneurial investment: a private equity premium puzzle? Am Econ Rev 92: 745–778, 2002 [Google Scholar]
- O'Neill M, Schultz W. Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron 68: 789–800, 2010 [DOI] [PubMed] [Google Scholar]
- Prelec D. The probability weighting function. Econometrica 66: 497–527, 1998 [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron 51: 381–390, 2006 [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28: 2745–2752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Markowitz in the brain? Rev Econ Polit 118: 75–95, 2008 [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13: 334–340, 2009 [DOI] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Neuronal distortions of reward probability without choice. J Neurosci 28: 11703–11711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci USA 106: 7185–7190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision making under risk. Science 315: 515–518, 2007 [DOI] [PubMed] [Google Scholar]
- Wu CC, Bossaerts P, Knutson B. The affective impact of financial skewness on neural activity and choice. PLos One 6: e16838, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Mobbs D, Seymour B, Calder A. Insula and striatum mediate the default bias. J Neurosci 30: 14702–14707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]