Abstract
Whole cell patch-clamp recordings were used to investigate the contribution of transient, low-threshold calcium currents (IT) to firing properties of hamster spinal dorsal horn neurons. IT was widely, though not uniformly, expressed by cells in Rexed's laminae I–IV and correlated with the pattern of action potential discharge evoked under current-clamp conditions: IT in neurons responding to constant membrane depolarization with one or two action potentials was nearly threefold larger than IT in cells responding to the same activation with continuous firing. IT was evoked by depolarizing voltage ramps exceeding 46 mV/s and increased with ramp slope (240–2,400 mV/s). Bath application of 200 μM Ni2+ depressed ramp-activated IT. Phasic firing recorded in current clamp could only be activated by membrane depolarizations exceeding ∼43–46 mV/s and was blocked by Ni2+ and mibefradil, suggesting IT as an underlying mechanism. Two components of IT, “fast” and “slow,” were isolated based on a difference in time constant of inactivation (12 ms and 177 ms, respectively). The amplitude of the fast subtype depended on the slope of membrane depolarization and was twice as great in burst-firing cells than in cells having a tonic discharge. Post hoc single-cell RT-PCR analyses suggested that the fast component is associated with the CaV3.1 channel subtype. IT may enhance responses of phasic-firing dorsal horn neurons to rapid membrane depolarizations and contribute to an ability to discriminate between afferent sensory inputs that encode high- and low-frequency stimulus information.
Keywords: single-cell RT-PCR, spinal cord, calcium channels, CaV3 subunits, patch clamp
the dorsal horn of the spinal cord is a structure of major importance for processing sensory information from the body. It receives synaptic terminations from primary sensory neurons that innervate a variety of tissues, including skin, muscle, tendons, joints, and viscera (Willis and Coggeshall 2004). In this region, diverse sensory information is integrated by local neural circuits, which are assembled from synaptic connections between multiple, physiologically distinct types of interneurons (Lu and Perl 2003, 2005; Santos et al. 2007; Schneider 2008) and then relayed to segmental reflex circuitry and ascending sensory pathways transmitted to the brain and brain stem.
Intrinsic electrophysiological properties of neurons can influence the integration of sensory information within dorsal horn circuits. At least three types of dorsal horn neurons have been identified in rodents based on discharge properties to current injection: tonic, phasic, and delayed firing (Graham et al. 2004; Hochman et al. 1997; Prescott and De Koninck 2002; Ruscheweyh and Sandkühler 2002; Schneider 2003; Walsh et al. 2009). Repetitive discharge of tonically firing cells has been reported to be regulated by a combination of voltage-gated Na+ and K+ channels and persistent Ca2+ currents (Melnick et al. 2004; Prescott and De Koninck 2005). Other evidence suggests that a transient, low-voltage-activated (T-type) Ca2+ conductance underlies the rapidly adapting discharge of phasic neurons (Russo and Hounsgaard 1996).
T-type Ca2+ channels are widely expressed in the central nervous system (CNS) (McKay et al. 2006; Nilius et al. 2006; Talley et al. 1999), where they promote burst firing and intrinsic oscillatory activity, and participate in synaptically evoked Ca2+ influx (Huguenard 1996). A Ca2+ current mediated by T-type channels has also been reported in spinal dorsal horn neurons of rat and mouse (Huang 1989; Ryu and Randic 1990; Walsh et al. 2009) and also may contribute to rhythm generation by ventral horn interneurons (Wilson et al. 2005). Dorsal horn T-channels have received renewed focus because of evidence that they enable calcium-dependent long-term potentiation of nociceptive transmission mediating hyperalgesia (Heinke et al. 2004; Ikeda et al. 2003). Our interest in the contribution of T-type Ca2+ channels to spinal somatosensory processing was prompted by two observations. First, fast depolarizing current ramps activate a transient membrane depolarization and discharge in many phasic-firing dorsal horn neurons, with the amplitude of depolarization and firing frequency being graded with the ramp slope (Schneider 2003). Second, phasic cells in the dorsal horn region where mechanically sensitive cutaneous afferents terminate respond selectively to stimuli that produce rapid deformations in the skin (Schneider 2005), suggesting that this class of neurons may be part of specialized spinal circuits for encoding velocity information. A similar observation was made by Prescott and De Koninck (2002), who found that phasic cells in lamina I are driven by trains of high-frequency stimuli and argued that they act as coincidence detectors responding to simultaneous occurrence of separate afferent inputs. In thalamic neurons, T-type currents are selectively activated by fast voltage ramps (Crunelli et al. 1989) and generate large, transient Ca2+ depolarizations that contribute to oscillatory behavior (Gutierrez et al. 2001). Given the importance of T-type channels to rhythmic firing behavior in thalamic neurons, we considered the possibility that these channels also function in dorsal horn sensory integration to shape responsiveness of neurons to dynamic membrane depolarizations induced by primary sensory afferents.
Three pore-forming α1-subunits of T-type Ca2+ channels (CaV3.1, CaV3.2, and CaV3.3) have been cloned (Perez-Reyes 2003), and mRNAs of all three subunits are expressed in the spinal cord (Talley et al. 1999). Moreover, each T-channel subunit exhibits gating properties that may enable them to contribute differentially to neuronal excitability (Chemin et al. 2002). In this study, we investigated the distribution of the T-type Ca2+ current among dorsal horn neurons with the goal of determining whether T-type current expression is related to neuronal firing properties. Experiments were performed in an isolated spinal cord preparation from Syrian hamsters that we have used previously to investigate neuronal properties and connectivity in the spinal dorsal horn (Schneider 2003, 2005, 2008). Our observations indicate that dorsal horn neurons with phasic firing patterns express a higher T-type Ca2+ current density than tonically firing cells and provide evidence that a fast T-type current may help tune responses of phasic-firing neurons to rapid membrane depolarizations and stimulus movement or rate of change (Schneider 2005).
METHODS
All protocols involving the use of live animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Slice preparation.
Spinal cord tissue was obtained from 9- to-14-day-old Syrian hamsters of both sexes under urethane anesthesia (1.5 mg/g ip) followed by exsanguination as described previously (Schneider 2003, 2008). After removal of vertebrae and meninges, a block of lumbosacral spinal cord was isolated and glued to the stage of a vibrating microtome (Vibratome 3000, St. Louis, MO) and 300-μm-thick transverse slices were cut. Dissection and slicing were carried out at 4–8°C in a solution containing (mM) 179 sucrose, 2.5 KCl, 0.2 CaCl2, 10 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 glucose (pH 7.35–7.45, 290–310 mosM) equilibrated with 95% O2-5% CO2. In some experiments, sucrose was replaced by equimolar glycerol in the dissection solution (Ye et al. 2006). (There were no differences in resting potential or amplitude and half-width of action potentials recorded from neurons in slices prepared with sucrose- or glycerol-based dissection solutions.) Slices were incubated in an oxygenated artificial cerebrospinal fluid (ACSF) containing (mM) 120 NaCl, 2.5 KCl, 2.5 CaCl2, 1.5 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose (pH 7.35–7.45, 290–310 mosM) at room temperature for 1 h prior to electrophysiological recording.
Electrophysiology.
For electrophysiological recording, slices were transferred to a chamber (volume 1.5 ml) mounted on a fixed-stage microscope (Olympus BX51WI) and continuously perfused at 5–6 ml/min with ACSF bubbled with 95% O2-5% CO2. Patch pipette recording electrodes (4–7 MΩ) were fabricated from borosilicate glass (N-51A, Drummond Scientific, Broomall, PA). The internal pipette solution contained (mM) 130 K-gluconate, 5 NaCl, 1 CaCl2, 1 MgCl2, 11 EGTA, 10 HEPES, 2 Mg-ATP, and 0.1 Li-GTP (pH 7.3, 280–285 mosM). For isolating T-type Ca2+ currents, we switched to a modified extracellular solution with the following constituents (mM): 107 NaCl, 3 BaCl2, 2 CsCl, 1.3 MgCl2, 26 NaHCO3, 10 glucose, 20 TEA, 0.5 μM TTX, and 10 μM CdCl2 (pH 7.35–7.45, 290–310 mosM). In some experiments, the Ba2+ concentration was lowered to 2 mM and Cd2+ was raised to 20 μM or 40 μM. All recordings took place within 4–5 h after tissue dissection and were performed at 27°C to promote slice viability (Schneider 2003).
Recording sites were first identified at low power (×10) relative to the translucent band corresponding to the substantia gelatinosa (lamina II) visible under transillumination. Individual neurons spanning a range of sizes were then targeted for patch-clamping using a ×40 water immersion objective and infrared differential contrast optics. Whole cell current and membrane voltage were recorded with an Axopatch 1D amplifier (MDS Analytical Technologies, Toronto, ON, Canada). All recordings were initiated in voltage-clamp mode after nulling the input offset voltage and minimizing capacitance transients. After whole cell recording was established, cell capacitance [25 ± 8 pF (SD)] and series resistance [33 ± 9 MΩ (SD)] were compensated. Signals were amplified (bandwidth 0–5 kHz) and saved to disk with a Digidata 1320A data acquisition system running pCLAMP software (MDS Analytical Technologies). Membrane current exceeding −0.05 nA at a holding potential of −60 mV indicated a poor recording condition, and these cells were excluded from analysis. Voltage-activated currents were low-pass filtered at 500 Hz (Gaussian). P/N leak subtraction was used to correct for passive membrane current. In most cases, currents are expressed as current density (pA/pF) by dividing by cell capacitance, as estimated by measuring the transient current in response to 5-mV hyperpolarizing current pulses and observing the value of the whole cell capacitance adjustment on the amplifier. Voltage commands and membrane potentials were corrected post hoc for a −14-mV liquid junction potential calculated with pCLAMP (MDS Analytical Technologies).
Neuronal electrophysiological properties were studied via membrane potential recordings performed in current-clamp mode prior to isolation of T-type Ca2+ currents under voltage clamp. Neurons were stimulated to produce action potentials by application of depolarizing current pulses (3 s) through the recording pipette for analyses of spike-frequency adaptation patterns. Neuronal input resistance (Rin) was measured by recording passive membrane responses to pulses of hyperpolarizing current. The dynamics of spike activation were studied by presenting a series of ramp-hold waveforms (duration 4.5 s) with increasing ramp slopes and determining the minimum rate of depolarization required to activate action potentials (Schneider 2003).
T-type current was activated at −44 mV from a conditioning potential of −104 mV held for 500 ms, and averages of three to five trials were used for subsequent analyses. A small, sustained current at a conditioning potential of −54 mV was subtracted from the current activated from −104 mV prior to analysis. To estimate different kinetic components of the net T-current, a semilogarithmic plot of the current was produced. The slow phase was fitted with a linear function, and the slope of the regression was used to determine the decay time constant. The amplitude was estimated by extrapolating the linear function to the time to peak of the net current. The amplitude and decay time of a fast current were then approximated by subtracting the slow current amplitude from the peak current (Zhuravleva et al. 1999).
Single-cell RT-PCR.
In some experiments, intracellular material was harvested by applying negative pressure to the electrode pipette. The pipette contents (∼5–8 μl) were expelled into a PCR tube containing 5 μl of nuclease-free water (Fisher Scientific, Pittsburgh, PA), 1 μl of dithiothreitol (DTT, 0.1 M; Invitrogen, Carlsbad, CA), 1 μl of RNase inhibitor (40 U/μl; Promega, Madison, WI), and 1 μl of random hexanucleotides (0.5 μg/μl; Promega) and stored at −80°C. Reverse transcription was carried out as previously reported (Han et al. 2005) with minor modification. The mixture was heated to 70°C for 10 min and quickly chilled on ice. After centrifugation, the solution was mixed with 4 μl of 5× First-Strand Buffer (mM: 250 Tris·HCl, 375 KCl, 15 MgCl2; Invitrogen), 1 μl DTT (0.1 M; Invitrogen), 1 μl of mixed deoxynucleotide triphosphates (dNTPs, 10 mM; Invitrogen), and 1 μl of SuperScript II reverse transcriptase (RT; Invitrogen). After 10-min incubation at room temperature, the solution was warmed to 42°C for 50 min for cDNA synthesis. The reaction was terminated by heating at 70°C for 15 min, and RNase H (2 U/μl; Invitrogen) was added to remove RNA from RNA-DNA hybrids. cDNA was stored at −20°C until being used in PCR. PCR conditions were optimized by using total hamster spinal cord RNA. The presence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was examined to confirm successful cell harvesting and reverse transcription. For GAPDH-positive samples, a two-step PCR protocol was carried out to detect the three known CaV3 subunit cDNAs. The first amplification was performed on a TGradient thermocycler (Biometra, Göttingen, Germany). The product was then diluted 10 times and used in subsequent real-time PCR with the Stratagene Mx3000P QPCR system (Agilent Technologies, Santa Clara, CA). For the first-step of CaV3 PCR, one 50-μl reaction mixture was prepared for each sample and contained 25 μl of PCR Master Mix (Promega), 5 μl of single-cell cDNA template, and outer primers: CaV3 forward, 5′-CGCTCCAGCCGGAACAGC-3′; CaV3 reverse, 5′-GCAATGGTGATACAGTTGAGGA-3′. The reaction went through 40 cycles of 95°C for 1 min, 53°C for 1 min, and 72°C for 1 min. Second-step real-time PCR was performed with SYBR GreenER qPCR SuperMix (Invitrogen). Triple 25-μl reactions with 2 μl of single-cell cDNA template or the diluted first PCR product were prepared for each cDNA species of interest. The amplification proceeded with the following profile: 50°C for 2 min and 95°C for 10 min, followed by 45 cycles (50 cycles for GAPDH) of 95°C for 30 s, 60°C for 1 min, and 72°C for 30 s. The sequences of the primers are as follows: GAPDH forward, 5′-AGCAATGCATCCTGCACCACCA-3′; GAPDH reverse, 5′-ACAGCCTTGGCAGCACCAGT-3′; CaV3.1 forward, 5′-GCTTCCTGCCTGTTGCCGAG-3′; CaV3.2 forward, 5′-TGCTCGAACCCTATGCTCCCC-3′; CaV3.3 forward, 5′-GCAGAATGCCCAACATCGCCA-3′. The CaV3 reverse primer was paired with subunit-specific forward primers and used in the second-step semi-nested CaV3 PCR. All primers were designed by Primer3 (Rozen and Skaletsky 2000) with reported cDNA sequences [DDBJ/EMBL/GenBank accession no.: GAPDH, U10983; CaV3.1, AF027984 (rat); CaV3.2, AF290213 (rat); CaV3.3, AF086827 (rat)] and are intron spanning. The sizes of amplicons were (bp) 209 GAPDH, 153 CaV3.1, 166 CaV3.2, 277 CaV3.3. PCR-generated fragments were verified by electrophoresis and sequence analysis, and the melting temperature of the fragments was used for confirmation of specific amplification in real-time PCR. No amplification of GAPDH was detected after reverse transcription in controls in which RT had been omitted. Negative controls without cDNA template and positive controls using total hamster spinal cord cDNA template were included in each PCR. Detection of a transcript was confirmed if the linear phase of the amplification plot crossed the fluorescence intensity threshold before reaching the 40th (or 45th for GAPDH) amplification cycle [threshold cycle (Ct) < 40 or 45].
Drugs and chemicals.
TTX was purchased from MP Biomedicals (Irvine, CA). Mibefradil (Ro 40–5967, dihydrochloride hydrate) was purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were obtained from Sigma-Aldrich or Mallinckrodt Baker (Phillipsburg, NJ).
Statistical analyses.
Numerical data are presented as means ± SE, unless noted otherwise. Statistical analyses were performed with InStat 3 (version 3.06; GraphPad Software). Statistical comparisons between two groups were made with the unpaired t-test with Welch correction or Mann-Whitney test as appropriate. Comparison among three or more groups was conducted with one-way ANOVA with Tukey-Kramer multiple comparisons or Kruskal-Wallis one-way ANOVA with Dunn's multiple comparisons. The criterion for statistical significance was a value of P < 0.05.
RESULTS
Whole cell recordings were obtained from 108 dorsal horn neurons in spinal slices prepared from 71 hamsters. Neurons had resting membrane potentials more negative than −64 mV (corrected for liquid junction potential) under current-clamp conditions. Membrane potential was held at −74 mV during voltage-clamp recordings, unless otherwise noted.
Isolation and characterization of T-type calcium currents in hamster dorsal horn neurons.
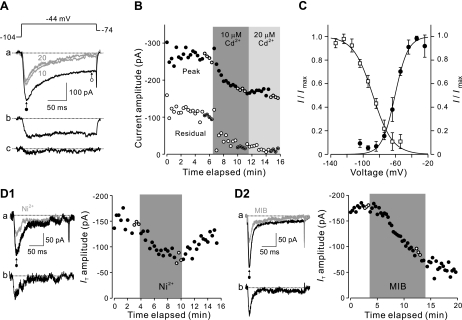
T-type Ca2+ currents (IT) were characterized previously in rat dorsal horn neurons (Huang 1989; Ryu and Randic 1990); however, the expression of T-currents and their properties in hamster dorsal horn is not known. We therefore began our study by first examining IT in hamster dorsal horn neurons under voltage clamp prior to making comparisons with spike discharge patterns. Voltage-clamp recordings were performed in the presence of TEA, TTX, and Ba2+ under ionic conditions appropriate for isolating Ca2+ currents. In the absence of Cd2+, depolarizing the membrane potential to −44 mV after a 500-ms conditioning potential of −104 mV activated an inward current with two components (Fig. 1A), an initial rapidly decaying phase followed by a late sustained phase. Addition of 10 μM Cd2+ reduced the amplitude of the initial phase and almost completely abolished the late sustained current within 3 min of application (Fig. 1B). Increasing the concentration to 20 μM had no additional effect on the current, suggesting that 10 μM Cd2+ was sufficient to block high-voltage-activated Ca2+ channels. Therefore, subsequent experiments were performed with the lower Cd2+ concentration.
Fig. 1.
Isolation and characteristics of low-voltage-activated Ca2+ currents. A: inward currents recorded from a representative dorsal horn neuron were activated every 20 s by a 200-ms voltage step to −44 mV from a conditioning potential of −104 mV held for 500 ms (top). Aa: each of the superimposed traces (black, before application of Cd2+; gray, 10 μM Cd2+ and 20 μM Cd2+) was averaged from 4 consecutive current responses (+ in B). Ab: inward current that was sensitive to 10 μM Cd2+ was obtained by subtraction. Ac: inward current that underwent additional blockade after increase of Cd2+ from 10 μM to 20 μM. Dotted lines indicate the baseline for current measurements. B: amplitude of peak and residual current was plotted against the time elapsed. Cursor positions for measuring peak (●) and residual (○) current are shown in Aa; 10 μM and 20 μM Cd2+ were added sequentially to the bath solution as indicated by shading. C: voltage-dependent inactivation and activation of T-type Ca2+ currents in spinal dorsal horn neurons. The current amplitude was normalized to the maximum level (I/Imax), averaged, and plotted with the holding potential (Vh) for the inactivation curve (□) or the test potential (Vtest) for the activation curve (●). The curves were fitted with the Boltzmann equation. Error bars represent SD (n = 4). D1 and D2: effect of T-type Ca2+ channel blockers Ni2+ (D1) and mibefradil (D2) on IT. IT was activated every 20 s by stepping the membrane potential to −44 mV for 200 ms after a 500-ms conditioning potential of −104 mV. Peak current amplitude is plotted as a function of time elapsed. Cursor position used to measure peak current (●) is shown in a. Shading indicates when the drug was present in the bath. D1a and D2a: the black traces were averaged from 3 consecutive measurements (+) before application of Ni2+ or mibefradil (MIB), and the gray trace was averaged from 3 trials (+) near the end of the drug presentation. D1b and D2b: inward currents that were sensitive to either of the blockers, obtained by subtraction.
We next examined the voltage dependence of steady-state inactivation and activation of IT. Inactivation kinetics were analyzed by stepping the membrane potential to −44 mV every 20 s in 10-mV increments from 500-ms conditioning potentials of −134 mV to −54 mV. IT was largely inactivated at potentials positive to −74 mV, and this inactivation was removed as the membrane potential approached −114 mV. Averaged normalized currents (I/Imax) were plotted as a function of conditioning potential (n = 4) and fitted with the Boltzmann equation (I/Imax = 1/{1 + exp[(V − V1/2)/k]}) having a slope factor (k) of 12 mV and voltage for half-maximal current (V1/2) of −88 mV (Fig. 1C). Activation properties of IT were studied by delivering 200-ms depolarizing voltage pulses after a conditioning potential of −104 mV to −24 mV in 10-mV increments. IT appeared in the low-voltage range with an activation threshold near −74 mV and reached a maximum near −34 mV. Values for I/Imax were plotted against the membrane potential to generate an activation curve yielding k = 8 mV and V1/2 = −61 mV when fitted with the Boltzmann equation. Overall, activation and inactivation parameters were consistent with IT reported previously under similar conditions (Huang 1989; Huguenard and Prince 1992; Ryu and Randic 1990).
Having established that activation and inactivation characteristics of IT were similar in rat and hamster dorsal horn cells, we then investigated the sensitivity of the current to T-channel antagonists. As shown in Fig. 1D1, 200 μM Ni2+ added to the bathing solution depressed IT by 52 ± 6% (n = 6). Reduction in IT was partially reversible, with recovery to 78 ± 10% of control after 4–16 min of washout. The IT antagonist mibefradil (5 μM) resulted in a 47% ± 4% (n = 3) reduction in IT amplitude after 6 to 10 min of perfusion (Fig. 1D2), and the effect persisted after drug washout. The results are consistent with previous pharmacological studies of T-current activation (Martin et al. 2000; Perez-Reyes 2003) but indicated that a portion of the transient inward Ca2+ current was resistant to these two T-channel antagonists.
T-type current in neurons with identified membrane and discharge properties.
Although nearly all hamster dorsal horn neurons expressed IT (n = 41/42, 98%), the current density varied considerably from cell to cell [6 ± 4 pA/pF (SD)]. Despite this, we found no differences in IT between neurons recorded in laminae I/II and III/IV (6 ± 1 pA/pF vs. 7 ± 1 pA/pF; P = 0.46), indicating that expression of IT is similar in the superficial and deep dorsal horn. Previous studies have suggested that burst firing of turtle dorsal horn neurons at a hyperpolarized membrane potential is associated with activation of a low-voltage-activated Ca2+ conductance (Russo and Hounsgaard 1996). Hamster dorsal horn neurons exhibit a range of spike frequency adaptation (Schneider 2003, 2005) and also include a type with a rapidly adapting burst discharge. Thus we examined IT expression in a population of neurons with characterized firing properties. Initial characterization of firing properties was performed in current-clamp mode in standard ACSF solution. Measurements of IT were then made after switching to voltage clamp by depolarizing the membrane potential to −44 mV from −104 mV held for 500 ms and subtracting residual current activated from a conditioning potential of −54 mV to minimize contribution from any unblocked high-voltage-activated Ca2+ current.
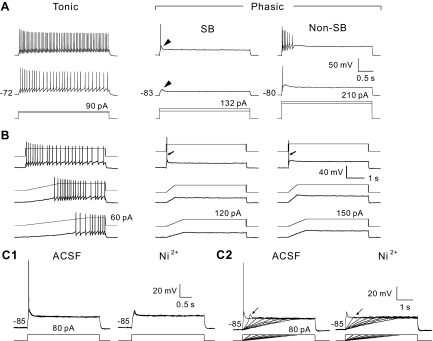
Cells were classified as tonic (n = 16/46, 35%) or phasic (n = 26/46, 57%) according to their firing behavior in response to depolarizing current pulses (Fig. 2, Table 1). [Analyses do not include four neurons that responded to the current pulse with a marked delay to the first action potential (not shown).] Tonic cells fired action potentials continuously during the period of depolarization (Fig. 2A, left). Phasic cells generated a transient, rapidly adapting discharge when activated by a constant level of depolarizing current (Fig. 2A, center and right). Phasic cells had a more hyperpolarized resting membrane potential and lower Rin than tonic cells (Table 1), consistent with previous observations (Schneider 2003). They displayed a fairly wide range of spike frequency adaptation and were further divided into two subgroups (Fig. 2A, Table 1). Short burst (SB) phasic cells responded to depolarizing current injection with only one or two action potentials superimposed on a transient membrane depolarization (Fig. 2A, arrowheads). The remaining phasic cells (non-SB) typically responded to depolarizing current with three or more action potentials at rapidly diminishing frequency, and the number of spikes increased with stimulus amplitude (Fig. 2A, right). When depolarized by ramp-hold current commands, tonic cells discharged primarily during the steady-state depolarization and the discharge frequency was unrelated to the trajectory of the ramp phase (Fig. 2B, left). Typically, SB and non-SB-type phasic cells were activated only by the most rapid membrane depolarizations (Fig. 2B, center and right). The slope of membrane depolarizations needed to activate phasic cells was five times higher than those required for activating tonic-firing neurons (Table 1). The responses of SB cells to step and ramp-hold current injection were blocked by Ni2+ (Fig. 2C).
Fig. 2.
Firing patterns of hamster dorsal horn neurons. A: responses of a representative cell from each category (top 2 traces) to depolarizing current pulses applied through the recording pipette (bottom trace) (categories: SB, short burst phasic; non-SB, non-short burst phasic; see text for details). Resting membrane potential (Vm) for each neuron is indicated to left of the first response. Arrowheads indicate transient membrane depolarizations characteristic of SB neuron activation. B: responses of the same neurons to ramp-hold current commands that depolarized Vm at different rates (waveforms are superimposed on individual traces). Slope of membrane depolarization (dV/dt) for lower and middle traces: 5 mV/s and 10 mV/s for tonic cell; 18 mV/s and 37 mV/s for SB; 25 mV/s and 55 mV/s for non-SB. Top trace: rectangular current step to the same level of depolarization. Action potential responses of phasic-firing cells in B are indicated by arrows. C: effect of Ni2+ on SB-type phasic neurons. Top traces show current commands, and bottom traces show voltage responses. Ni2+ (200 μM) blocked the spike discharges generated by current steps (C1) and ramp-activated depolarization (C2). In C2, blockade of the transient depolarization response is indicated by arrows. The series of ramp commands depolarized Vm at rates of 6 mV/s to 48 mV/s, and a transient depolarization was activated by a voltage ramp of 48 mV/s. Records showing responses to current steps (C1, top traces) consist of 5 consecutive traces. Resting Vm for each condition is indicated to left of the traces. ACSF, artificial cerebrospinal fluid.
Table 1.
Comparison of IT and intrinsic membrane properties of spinal dorsal horn neurons with different firing properties
| Phasic |
|||
|---|---|---|---|
| Tonic (n = 16) | Short burst (n = 7) | Non-short burst (n = 19) | |
| Resting membrane potential, mV | −69 ± 2 (14) | −81 ± 1*† | −76 ± 2* |
| Input resistance, MΩ | 654 ± 84 | 261 ± 66* | 377 ± 63* (18) |
| Ramp slope threshold, mV/s | 9 ± 3 (12) | 43 ± 3* | 46 ± 7* (11) |
| IT, pA/pF | 5 ± 1 | 14 ± 4*† | 5 ± 1 |
All values are listed as means ± SE. The number of measurements used to calculate each value is given in parentheses if different from the number of cells for the group. IT, transient calcium current.
Significantly different from tonic cells;
significantly different from non-short burst cells.
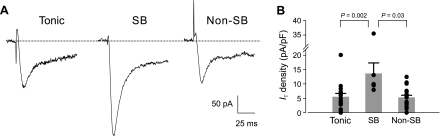
As expected, IT was found in the majority of neurons in this sample (n = 43/46, 93%) and showed considerable variation in amplitude [7 ± 6 pA/pF (SD); 1–36 pA/pF], consistent with the results of our initial voltage-clamp experiments. Figure 3A shows examples of IT from tonic, SB-type phasic, and non-SB-type phasic cells, and data from all cells in the sample, expressed as current density, are summarized in Table 1 and Fig. 3B. Notably, IT in SB-type phasic cells was almost threefold larger than that recorded from tonic-firing neurons. Thus IT expression was greatest in dorsal horn neurons with signature rapidly adapting burst firing that are selectively activated by rapid membrane depolarizations.
Fig. 3.
IT in neurons with characterized firing patterns. A: dorsal horn neurons were voltage clamped at a Vh of −104 mV, and IT was evoked by applying depolarizing voltage steps to −44 mV. External bathing solution for isolating T-type Ca2+ currents contained 3 mM Ba2+ and 10 μM Cd2+ and was applied after firing pattern characterization under current clamp in normal ACSF. Currents for each firing pattern classification are computed from averages of 5 traces. IT density: 6 pA/pF (tonic); 19 pA/pF (SB); 7 pA/pF (non-SB). Dotted line indicates the baseline for inward current measurements. B: quantitation of IT current density for tonic (n = 16), SB (n = 7), and non-SB (n = 19) phasic cells. Each symbol represents the measurement from 1 neuron. Height of histogram bars reflects the mean of measured values for each group, and error bars signify SE.
Characterization of T-current components.
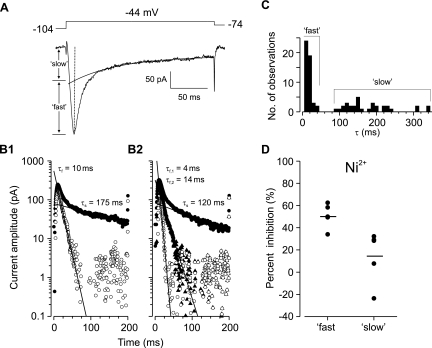
Previous studies in thalamic neurons (Shcheglovitov et al. 2005; Tarasenko et al. 1997; Zhuravleva et al. 1999, 2001) suggested that multiple channel subtypes may contribute to the net T-current in dorsal horn neurons when the high-voltage-activated Ca2+ current is blocked. We found that for the majority of cells (67%, 29/43), IT could be dissected into two components based on time constants of decay (Fig. 4, A and B1). A rapidly inactivating component (“fast”) had a time constant (τf) of 12.0 ± 1.1 ms (n = 48, Fig. 4C), and a slower component (“slow”) inactivated with a significantly longer time constant (τs) of 177 ± 12 ms (n = 32; vs. τf, P < 0.0001; Fig. 4C). The amplitudes of fast and slow currents were similar across cells (4.6 ± 0.7 vs. 3.5 ± 0.4 pA/pF, P = 0.2823). However, 10–90% rise time for the fast subtype was shorter than for the slow current (5.2 ± 0.8 vs. 14.3 ± 3.0 ms, P = 0.0167), suggesting a difference in activation kinetics. Two fast components, τf,1 and τf,2 (Fig. 4B2), having significantly different decay τ (5.7 ± 0.9 vs. 21.1 ± 3.9 ms, P = 0.0018) but similar current densities (3.2 ± 0.5 vs. 3.4 ± 0.5 pA/pF, P = 0.7374) could be distinguished in eight neurons (19%, 8/43). Therefore, the statistical sample size reflects the number of current components, rather than that of recorded neurons. Bath application of Ni2+ produced greater reduction of the fast current than the slow component (51 ± 5% vs. 15 ± 11%, P = 0.0144; Fig. 4D). Furthermore, the fast current in SB-type cells was two- and threefold larger than in tonic and non-SB neurons, respectively, while the slow subtype was uniformly expressed across the different firing pattern groups (Table 2).
Fig. 4.
Estimating subtypes of T-type Ca2+ currents in dorsal horn neurons. A: an inward current (lower) was activated from a representative dorsal horn neuron by a voltage step (upper) under conditions used for isolating T-current described in methods. A single exponential was fitted to the slow decay phase and extended to the beginning of the current trace. The amplitude of a “slow” current component is estimated by extrapolating the fitted exponential to the time of the peak current, indicated by the vertical dotted line. The amplitude of a “fast” component is obtained by subtracting the slow component from the peak current. B1: semilogarithmic plot of the current trace in A (●) with the slow phase fitted with a linear function, the slope of which provides an estimate of the time constant for exponential decay (τs = 175 ms), and semilogarithmic plot of a “fast” current component (○) resulting after subtraction of the “slow” component and fitted with a line having a slope indicating the decay time constant (τf = 10 ms). B2: example from a cell in which two “fast” components were isolated; one with τf,1 = 4 ms (○) and another with τf,2 = 14 ms (▲). C: histogram showing the distribution of decay time constants estimated for fast and slow current subtypes (n = 80) from 43 dorsal horn neurons. D: effect of 200 μM Ni2+ on the fast and slow T-current subtypes in spinal dorsal horn neurons (n = 5 cells). Horizontal bars indicate mean % inhibition of the peak current. The difference in inhibition is significant (P = 0.0144).
Table 2.
Comparison of IT subtypes in spinal dorsal horn neurons with different firing properties
| Phasic |
|||
|---|---|---|---|
| Tonic (n = 14) | Short burst (n = 7) | Non-short burst (n = 16) | |
| “Fast” current, pA/pF | 4.2 ± 0.9 (16) | 9.0 ± 2.7 (9)*† | 3.0 ± 0.3 (17) |
| τf, ms | 15.3 ± 2.6 (16) | 9.3 ± 1.2 (9) | 10.3 ± 1.0 (17) |
| “Slow” current, pA/pF | 3.8 ± 0.9 (8) | 3.3 ± 0.8 (7) | 3.6 ± 0.8 (13) |
| τs, ms | 174 ± 18 (8) | 167 ± 26 (7) | 187 ± 25 (13) |
Data are given as means ± SE. τf, time constant of fast component; τs, time constant of slow component. The number of measurements used to calculate each value is given in parentheses if different from the number of cells for the group.
Significantly different from tonic cells;
significantly different from non-short burst cells.
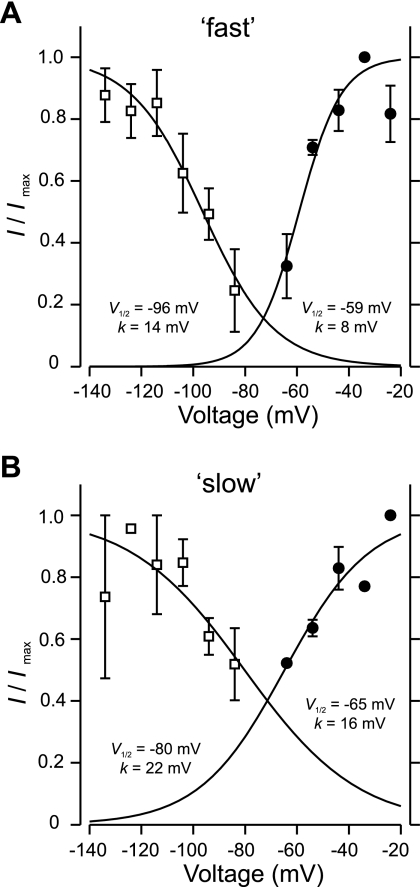
We next examined the voltage dependence of steady-state inactivation and activation of the fast and slow T-currents. Inactivation kinetics were assessed by stepping the membrane potential to −44 mV every 20 s in 10-mV increments from conditioning potentials of −134 mV to −84 mV held for 500 ms. Activation properties were studied by delivering 200-ms depolarizing voltage steps in 10-mV increments from −64 mV to −24 mV after a conditioning potential of −104 mV. Currents generated by voltage steps to values below −64 mV were too small to be reliably analyzed by the stripping procedure, perhaps because of the small size of hamster dorsal horn neurons. Average normalized currents (I/Imax) were plotted as a function of conditioning potentials for inactivation or testing steps for activation (n = 3, “fast”; n = 2, “slow”) and fitted with the Boltzmann equation (I/Imax = 1/{1 + exp[(V − V1/2)/k]}) (Fig. 5). The half-inactivation potentials for the fast and slow components were −96 mV and −80 mV, respectively, while the voltage required for half-maximal activation was −59 mV for the fast subtype and −65 mV for the slow subtype. The overlap region between the activation and inactivation curves (“window” current) is greater for the slow current component.
Fig. 5.
Steady-state activation and inactivation of fast and slow T-currents in spinal dorsal horn neurons. A and B: inactivation (□) and activation (●) curves for the fast (A) and slow (B) subtypes were fitted with the Boltzmann equation (I/Imax = 1/{1 + exp[(V − V1/2)/k]}). V1/2 and k derived from the fits are shown next to each curve. Error bars represent SE (n = 3, A and n = 2, B). r = 0.99 for all fits in A and B.
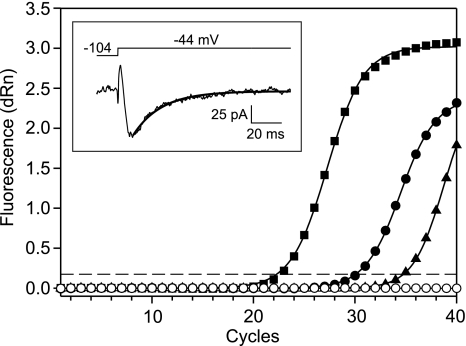
To find out what channel subtypes might contribute to IT in hamster dorsal horn neurons, we performed single-cell RT-PCR (scRT-PCR) following patch-clamp recordings. Among 29 cells in which GAPDH mRNA was detected in the pipette aspirates, 6 were in LI/LII, 23 were from LIII–IV, and nearly all of them (93%, 27/29) evidenced IT (7 ± 1 pA/pF). CaV3 mRNAs were detected in 12 neurons (Table 3), all except 1 recorded from LIII–IV. The majority (64%, 7/11) had phasic firing patterns, with the remainder (35%, 4/11) being tonic firers, not different from non-CaV3-expressing neurons. Similarly, CaV3-expressing cells evidenced IT similar to their non-CaV3-expressing counterparts (8 ± 3 pA/pF vs. 6 ± 1 pA/pF). Analyses showed a higher occurrence of the CaV3.1 subunit (67%, 8/12) than CaV3.2 (8%, 1/12) and CaV3.3 (25%, 3/12). An example of a CaV3.1 amplification plot resulting from a second-step real-time PCR, along with a voltage-clamp recording of IT from the same cell, is shown in Fig. 6. In contrast to our electrophysiological analyses, there was no evidence from scRT-PCR that single neurons express multiple T-channel subtypes.
Table 3.
Summary of T-type calcium channel subtype expression in characterized hamster dorsal horn neurons
| CaV Isotype |
T-Current Component |
|||||
|---|---|---|---|---|---|---|
| Cell | Firing pattern | CaV3.1 | CaV3.2 | CaV3.3 | “Fast” | “Slow” |
| 080707-1 | P | + | − | − | + | − |
| 080708-1 | T | + | − | − | + | − |
| 080709-2 | T | + | − | − | + | − |
| 080523-1 | T | + | − | − | + | + |
| 080530-1 | P | + | − | − | + | + |
| 080619-1 | P | + | − | − | + | + |
| 080620-2 | P | + | − | − | + | + |
| 080718-1 | T | + | − | − | + | + |
| 080716-1 | P | − | + | − | + | + |
| 080605-3 | P | − | − | + | + | + |
| 080709-1 | na | − | − | + | + | + |
| 090212-4 | P | − | − | + | + | + |
Cell 080620-2 was located in Rexed's lamina (L) II; all other cells were in LIII–V. Expression of CaV3 subunit mRNAs was examined by single-cell RT-PCR (scRT-PCR). IT was recorded under voltage clamp. “Fast” and “slow” current components were identified post hoc by fitting the net T-current with exponential functions (see methods). +, Detection of a channel isotype or current component; −, channel isotype or current absent; P, phasic; T, tonic; na, not analyzed.
Fig. 6.
Detection of CaV3.1 subunit mRNA from a dorsal horn neuron with a “fast” IT component (see text). Amplification plots of CaV3.1 subunit fragments were generated from a real-time PCR experiment. Each measurement was averaged from 3 replicate reactions. The fluorescence signal was normalized to an internal passive reference dye (dRn), and plots were base-lined by Stratagene software. Amplified product from cell 080707-1 (●) and hamster spinal cord cDNA with (■, positive control 1) and without (▲, positive control 2) a first-step CaV3 PCR amplification are shown. For a negative control, cDNA template was replaced with water (○). The threshold fluorescence value (indicated by dashed line) was determined by the software using an amplification-based algorithm, resulting in threshold cycles (Ct) of 23, 31, and 35 for positive control 1, amplified cell product, and positive control 2, respectively. Inset: the decay of IT (bottom) recorded from the same dorsal horn neuron can be fitted with a single exponential (τ = 20 ms). The voltage command used to activate the current is shown above the trace.
Activation of T-type calcium currents in dorsal horn neurons by voltage ramps.
T-type calcium currents in dorsal horn neurons could have important functional consequences for spinal sensory processing. IT in thalamic neurons exhibits a strong rate dependence; activation requires membrane depolarization exceeding 30 mV/s (Crunelli et al. 1989). Therefore, nonuniformity of IT expression by dorsal horn neurons could contribute to their differential responses to rate of membrane depolarization and time-varying sensory stimuli (Schneider 2003, 2005). Hence, we investigated whether IT in dorsal horn cells is related to the slope of membrane depolarization (dV/dt), similar to the characteristic reported for thalamic neurons (Crunelli et al. 1989; Gutierrez et al. 2001).
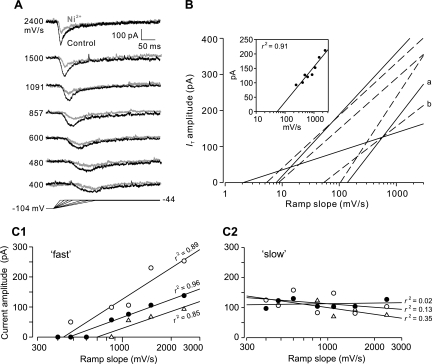
IT activation dynamics were investigated by using voltage ramps to depolarize neurons to −44 mV at different rates after a 500-ms hyperpolarizing prepulse to −104 mV. As can be seen in the example in Fig. 7A, the amplitude of IT increased and time to peak decreased along with dV/dt between ramp slopes of 400 mV/s and 2,400 mV/s. Bath application of Ni2+ depressed the ramp-activated currents (Fig. 7A, gray) in a manner similar to those activated by step depolarizations. Best-fit regression lines calculated from plots of IT amplitude versus ramp slope for seven cells are shown in Fig. 7B. Both the x-intercept and slope varied widely within our sample. The threshold for IT activation, as estimated from the x-intercept values, averaged 46 ± 21 mV/s (2–138 mV/s). The sensitivity of IT to ramp dV/dt (defined as slope of the regression lines, pA·s/mV) varied nearly fivefold (51–242 pA·s/mV) for the cells studied. Taken together, the data suggest that dorsal horn neurons are heterogeneous with respect to activation by voltage ramps. Four additional neurons were examined with a similar protocol ramping the membrane voltage from a conditioning potential of −114 mV to −104 mV to a potential of −44 mV to −34 mV, but with the Ba2+ concentration lowered to 2 mM Ba2+ and Cd2+ increased to 40 μM. The maximum amplitude, threshold, and rate sensitivity of IT under these circumstances were not significantly different from data obtained with the standard conditions described above.
Fig. 7.
Activation of IT in dorsal horn neurons by ramp depolarizations. A: IT in a representative cell was activated by using voltage ramps (bottom) to depolarize the membrane potential to −44 mV from a 500-ms conditioning potential of −104 mV at different rates (indicated to left of each current trace). IT before (control, black traces) and during (Ni2+, gray traces) presentation of 200 μM Ni2+ is shown. B: linear regression analysis between IT amplitude and ramp slope is shown for 7 dorsal horn neurons recorded in media containing 2 or 3 mM Ba2+. Individual responses to ramp depolarizations for regression line a are shown in A. Inset: representative linear fit to data points for regression line b. Solid lines denote tonic cells, and dashed lines indicate phasic cells (without regard to burst duration). The coefficients of determination (r2) from regression analyses (0.72–0.99) indicate that IT amplitude and ramp slope are highly correlated. C: relationship between the amplitude of IT current components and the ramp slope is described by a linear function for 3 dorsal horn neurons. C1: rate sensitivity of the fast component. C2: rate sensitivity of the slow component. The coefficient of determination (r2) from linear regression analysis indicates that the amplitude of the fast subtype and the slope are highly correlated, but the amplitude of the slow component does not parallel changes in ramp slope.
Despite the cell-to-cell variability, regression analyses showed that 87 ± 4% of the current amplitude (72–99%) was determined by dV/dt. The question then became whether the fast and slow T-channel subtypes contribute to the ability of phasic-firing neurons to signal rapid membrane depolarization. IT of three dorsal horn neurons possessing both subtypes were activated by ramping the membrane potential from a 500-ms hyperpolarizing prepulse of −104 mV to −44 mV at different rates ranging from 400 to 2,400 mV/s. After isolation of the T-current components, the fast subtype showed rate-dependent activation while the amplitude of the slow component appeared independent of dV/dt over the range of voltage ramps used (Fig. 7, C1 and C2). Linear regression analysis indicated that 90 ± 3% of the fast current amplitude was determined by dV/dt, and the activation threshold estimated from the x-intercepts was 533 ± 83 mV/s, significantly greater than the value observed for the net T-current (46 ± 21 mV/s).
DISCUSSION
The present results supplement earlier studies showing that rodent dorsal horn neurons possess a low-threshold “T-type” Ca2+ current (Huang 1989; Russo and Hounsgaard 1996; Ryu and Randic 1990; Walsh et al. 2009). Our new findings point out that, although IT appears to be prevalent among spinal LI–IV cells, expression is nonuniform, being highest in neurons responding to depolarizing current pulses with a rapidly adapting discharge of one or two action potentials. Here, we show that IT in the dorsal horn is dependent on the rate of membrane depolarization. In light of our observations that both IT and action potential discharges of phasic-firing dorsal horn neurons are activated by similar rates of membrane depolarization and are blocked by T-type Ca2+ channel antagonists, IT appears to contribute to selective excitation of these cells by rapid membrane depolarizations (Schneider 2003, 2005).
IT expressed in hamster dorsal horn resembles a low-voltage-activated, transient Ca2+ current reported elsewhere in the CNS. Steady-state activation and inactivation kinetics are broadly similar to T-type Ca2+ currents in thalamus (Crunelli et al. 1989), hypothalamus (Niespodziany et al. 1999), suprachiasmatic nucleus (Kim et al. 2005), and cerebellum (Mouginot et al. 1997). As in these studies, our internal pipette solution contained the Ca2+ buffer EGTA. EGTA has been reported to slow the time course of recombinant CaV3.1 channel activation and inactivation but have no effect on the current density or voltage dependence of steady-state inactivation (Lacinová et al. 2006). Therefore, as with similar studies using a Ca2+ buffer in the internal recording solution, we cannot rule out possible effects of EGTA on T-channel function and on neuronal firing properties. We found that Ni2+ was an incomplete antagonist of IT when applied at a concentration of 200 μM. This might be explained by an observation that 100 μM Ni2+ blocked only 30–50% of the current through CaV3.1 and CaV 3.3 T-type currents recorded from cloned channels in oocytes and HEK-293 cells (Lee et al. 1999). We also found that mibefradil potency was lower than what has been reported in other studies (Kim et al. 2005; Lee et al. 2002; McDonough and Bean 1998; Viana et al. 1997). This may be due to the fact that mibefradil is a less effective T-channel antagonist when Ba2+ replaces Ca2+ as the charge carrier (Martin et al. 2000). As discussed below, incomplete antagonism of IT by Ni2+ and mibefradil may also indicate that other ionic conductances contributed to the inward current in our experiments.
Our experiments were performed on hamster spinal cord at an age when organization and electrical properties of dorsal horn neurons undergo developmental changes in rodents (Beal et al. 1988; Bicknell and Beal 1984; Walsh et al. 2009). For example, it has been reported that A-type potassium current in mouse spinal LI–II neurons decreases during the first 3 wk of life (Walsh et al. 2009). Although the discharge properties of tonic and phasic firing neurons in 9- to 14-day-old hamsters were broadly similar to those recorded from animals at 3–4 wk of age (Schneider 2003), the extent to which dorsal horn T-type Ca2+ channel expression changes postnatally and how these changes influence discharge properties is not known. In the present study, we found that nearly all dorsal horn neurons expressed IT, with no difference between neurons recorded in the superficial and deep laminae. However, Walsh et al. (2009) reported that a T-current was present in only 25% of superficial dorsal horn neurons in mouse at ages comparable to the hamsters used our study. Therefore, it is possible that T-type current expression in dorsal horn neurons may exhibit species, as well as developmental, differences.
Ionic basis of responses to time-varying membrane depolarizations.
The activation of IT in dorsal horn neurons by rapid ramp depolarizations resembles a property of T-type Ca2+ currents described in thalamic neurons (Crunelli et al. 1989), adrenal glomerulosa cells (Várnai et al. 1995), and olfactory receptor neurons (Kawai and Miyachi 2001). In particular, depolarizations exceeding 30 mV/s are required to activate an IT in cat lateral geniculate neurons (Crunelli et al. 1989). In thalamic cells, voltage ramps of lower velocity appear to reverse the removal of T-channel inactivation by a prior hyperpolarization, so that there are fewer channels available for activation, resulting in diminishing current amplitude at slower membrane depolarizations (Crunelli et al. 1989; Gutierrez et al. 2001). It should be noted that cell-to-cell variability in the threshold for ramp activation for IT in hamster dorsal horn neurons (46 ± 21 mV/s) is greater than that reported by Crunelli et al. (1989) for thalamic cells (30 ± 2 mV/s). Furthermore, the sensitivity of dorsal horn neurons to rate of depolarization varied considerably between cells (Fig. 7B). These differences could reflect expression of T-channel subunits differing in activation kinetics (Chemin et al. 2002; Talley et al. 1999).
Several observations suggest that IT contributes to an ability of phasic-firing dorsal horn neurons to selectively signal rapid membrane depolarizations and rapidly moving cutaneous stimuli (Schneider 2003, 2005). First, IT activated when dV/dt exceeded ∼46 mV/s and increased with the rate of depolarization. Second, phasic-firing cells expressed the highest IT density in the dorsal horn. Third, Ni2+ blocked rate-sensitive activation of IT under voltage-clamp conditions and also a transient depolarization with associated action potential discharge under current clamp. Although these findings support an involvement of IT in shaping the ability of certain dorsal horn neurons to signal dynamic membrane depolarizations, the present experiments do not rule out the participation of other ionic mechanisms. For example, we found that the rate of membrane depolarization for activating SB and non-SB type phasic cells was significantly higher than for activating tonic cells. However, IT was elevated only in SB cells, suggesting that another mechanism may underlie rate sensitivity in non-SB cells (Table 1). Safronov et al. (1997) described a slowly inactivating, TTX-sensitive Na+ current accounting for ∼5–20% of total Na+ current in rat dorsal horn neurons that broadly resembles a persistent Na+ current (INaP) activated near −65 mV (Magistretti et al. 2006; Magistretti and Alonso 1999; Parri and Crunelli 1998). Recent studies have also reported that INaP in spinal lamina I neurons and ventral horn motoneurons is activated by voltage ramps (Kuo et al. 2006; Prescott and De Koninck 2005). This current has a slower threshold (∼10 mV/s) for ramp activation but could still contribute to neuronal excitation over the range of ramp depolarizations used in the present study.
Expression of multiple T-channel subtypes in dorsal horn neurons.
We used an indirect method in the present study to distinguish subtypes of T-currents in dorsal horn neurons based on differences in kinetics of inactivation. The results suggest that the net T-current is composed of “fast” and “slow” components, having decay time constants similar to two subtypes of low-voltage-activated Ca2+ currents reported for thalamic neurons (Shcheglovitov et al. 2005; Zhuravleva et al. 1999, 2001). The current densities for the dorsal horn subtypes (∼3–4 pA/pF) are similar to neurons recorded in thalamic slices (Tarasenko et al. 1997), and the two subtypes appear to be present in approximately equal ratios in the spinal cord and thalamus. Besides kinetics, the two subtypes also appear to have other distinguishing features. First, the activation and inactivation curves for the slow component exhibit a larger overlap region (“window” current) than the fast subtype, similar to the slowly inactivating low-voltage Ca2+ current in isolated thalamic neurons (Zhuravleva et al. 2001). Second, the fast subtype was more effectively blocked by Ni2+ than the slow subtype, consistent with thalamic neurons recorded in transverse brain slices (Joksovic et al. 2005; Shcheglovitov et al. 2005). Third, and perhaps most interesting from a functional standpoint, we found that the amplitude of the fast current component was correlated to the slope of depolarizing voltage ramps, whereas the slow subtype appeared to be independent of depolarization rate over the testing range. Although rate sensitivity of the T-current subtypes was determined in only a few neurons, it is nonetheless tempting to speculate that the responses of phasic cells in the spinal dorsal horn to rapid membrane depolarizations as reported here and elsewhere (Schneider 2003, 2005) may partly arise from expression of the fast channel subtype. A result that bears mentioning is that the average threshold for activation of the fast current by voltage ramps was >10-fold higher that the activation threshold of IT. One possible explanation for this difference is that activation of the slow channels during ramp depolarizations might cause partial inactivation of the rate-sensitive fast channels, thereby shifting the activation threshold of the net IT to slower voltage rates.
Our results showing that a subpopulation of spinal LI–IV neurons express CaV3 mRNA are consistent with a previous report of T-type channel transcripts in the spinal cord. The finding that CaV3.1 was the most abundant of the three known T-channel subtypes is in agreement with Talley et al. (1999), who reported relatively higher signal for α1G (CaV3.1) mRNA than for α1H (CaV3.2) and α1I (CaV3.3) mRNA in the rat spinal cord. We found that the proportion of dorsal horn neurons expressing CaV3 mRNA (44%) was much lower than the proportion of cells exhibiting a macroscopic T-current (>90%). In performing analyses only on samples in which GAPDH mRNA was detected, we hoped to reduce the impact of incompletely harvesting cellular mRNA in the patch pipette. Still, we may not have collected mRNA from dendrites, where some CaV3 gene products may function (Shcheglovitov et al. 2005; Tarasenko et al. 1997; Zhuravleva et al. 2001). We also took measures to limit recording time and protect the targeted CaV3 transcripts from degradation. However, it is possible that our procedures were not optimal for the detection of small amounts of CaV3 mRNA. In fact, other studies using scRT-PCR have also reported that only a fraction of cells with functional neurotransmitter receptors express the corresponding mRNA (e.g., Férézou et al. 2006; Gallopin et al. 2005). It is therefore likely that the proportion of dorsal horn neurons expressing CaV3 isotypes is higher than what is indicated by our scRT-PCR results. Nonetheless, our results suggest that CaV3.1 is the most common subtype in the hamster dorsal horn and is expressed by neurons having a wide range of firing properties.
Determining the identity of CaV3 subunits that underlie kinetically distinct fast and slow T-currents in dorsal horn cells was beyond the scope of the present study. However, comparisons of the properties of endogenous T-current components with studies on recombinant T-type channel expression may offer clues about what molecular subtypes contribute to neuronal excitability in the dorsal horn. First, the decay τ we estimated for the slow current is similar to the inactivation τ reported for recombinant CaV3.3 subunits (Chemin et al. 2002; Klöckner et al. 1999; McRory et al. 2001). Second, decay τ of the fast current is in the range reported for inactivation of cloned CaV3.1 and CaV3.2 subunits (Chemin et al. 2002; McRory et al. 2001). Finally, our isolation of two fast currents having different decay τ also imply contributions from CaV3.1 and CaV3.2 subunits. These findings suggest that CaV3.1, and possibly CaV3.2, underlie the fast T-current in dorsal horn cells and that CaV3.3 contributes to the slow component, in general agreement with conclusions reached for thalamic neurons (Shcheglovitov et al. 2005).
Functional significance of dorsal horn T-current.
Our results suggest a role for T-type Ca2+ channels in spinal sensory function besides spinal nociception (Heinke et al. 2004; Ikeda et al. 2003). IT is largest in phasic-firing dorsal horn neurons that are selectively activated by a steep voltage trajectory. The dependence of IT on high rates of membrane depolarization may boost responses to fast excitatory postsynaptic potentials (EPSPs), relative to slow EPSPs, generated by nociceptive and tactile inputs terminating in laminae I–II and III–IV, respectively. Almost all phasic cells in the dorsal horn are interneurons that form connections with neighboring neurons (Schneider 2003, 2008), suggesting that IT contributes to signaling related to rapid stimulus movement or rate of change within local networks (Schneider 2003, 2005). Other mechanisms may selectively influence responses to sustained or slowly changing inputs. A persistent Na+ current (INaP) appears to enhance responses of spinal neurons to sustained excitatory inputs (Kuo et al. 2006). INaP is strongly expressed in tonic-firing dorsal horn cells and prolongs responses to transient inputs, leading to the proposal that it facilitates encoding of stimulus intensity (Prescott and De Koninck 2005). Thus differential expression of transient and persistent inward currents may contribute to processing of stimulus intensity and velocity information in functionally defined spinal circuits. T-channels may further tune responses to afferent inputs by modulating hyperpolarizing K+ currents. Calcium influx through T-channels modulates gating of small-conductance Ca2+-activated potassium (SK) channels (Cueni et al. 2009), which are strongly expressed in the dorsal horn (Mongan et al. 2005). Activation of SK channels decreases neuronal excitability by increasing firing adaptation to membrane depolarization (Stoker 2004) and may represent another means for sharpening dorsal horn neuronal responses to transient stimuli. Moreover, calcium entry from T-channels can also regulate the function of low-voltage-activated A-type K+ channels (Anderson et al. 2010). A-type K+ channels are also widely expressed in dorsal horn neurons (Grudt and Perl 2002; Hu and Gereau 2011; Huang et al. 2005; Walsh et al. 2009), suggesting that IT may control firing frequency and timing by this mechanism as well. The influence of T-type channels on neuronal firing via modulation of K+ channel function would appear to be strongest for SB-type phasic cells, which express the largest IT. Taken as a whole, we believe our results expand a role for low-voltage, T-type Ca2+ channels in spinal sensory processing and suggest that these channels are particularly important for sharpening responses of dorsal horn neurons to afferent sensory stimuli that vary rapidly in the time domain.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant NS-25771 (S. P. Schneider).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JDT, Hameed S, Zamponi GW, Turner RW. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 13: 333–337, 2010 [DOI] [PubMed] [Google Scholar]
- Beal JA, Russell CT, Knight DS. Morphological and developmental characterization of local-circuit neurons in lamina III of the rat spinal cord. Neurosci Lett 86: 1–5, 1988 [DOI] [PubMed] [Google Scholar]
- Bicknell HR, Beal JA. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol 226: 508–522, 1984 [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (α1G, α1H and α1I) to neuronal excitability. J Physiol 540: 3–14, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium 40: 121–134, 2006 [DOI] [PubMed] [Google Scholar]
- Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol 413: 543–561, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Adelman JP, Lüthi A. Ca2+ signaling by T-type Ca2+ channels in neurons. Pflügers Arch 457: 1161–1172, 2009 [DOI] [PubMed] [Google Scholar]
- Férézou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex 17: 1948–1957, 2006 [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB and CCK and their effects on pyramidal cells in the neocortex. Cereb Cortex 16: 1440–1452, 2005 [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. In vivo responses of mouse superficial dorsal horn neurones to both current injection and peripheral cutaneous stimulation. J Physiol 561: 749–763, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, van den Pol AN, Perl ER. Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. J Physiol 538: 527–525, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C, Cox CL, Rinzel J, Sherman SM. Dynamics of low-threshold spike activation in relay neurons of the cat lateral geniculate nucleus. J Neurosci 21: 1022–1032, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Murchison D, Griffith WH. Low voltage-activated calcium and fast tetrodotoxin-resistant sodium currents define subtypes of cholinergic and noncholinergic neurons in rat basal forebrain. Brain Res Mol Brain Res 134: 226–238, 2005 [DOI] [PubMed] [Google Scholar]
- Heinke B, Balzer E, Sandkühler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci 19: 103–111, 2004 [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Pockett S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Res 767: 214–219, 1997 [DOI] [PubMed] [Google Scholar]
- Hu H, Gereau RW., IV Metabotropic glutamate receptor 5 regulates excitability and Kv4.2-containing K+ channels primarily in excitatory neurons of the spinal dorsal horn. J Neurophysiol 105: 3010–3021, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Cheng JK, Shih YH, Chen PH, Wang CL, Tsaur ML. Expression of A-type K+ channel α subunits Kv4.2 and Kv4.3 in rat spinal lamina II excitatory interneurons and colocalization with pain-modulating molecules. Eur J Neurosci 22: 1149–1157, 2005 [DOI] [PubMed] [Google Scholar]
- Huang LYM. Calcium channels in isolated rat dorsal horn neurones, including labelled spinothalamic and trigeminothalamic cells. J Physiol 411: 161–177, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 58: 329–348, 1996 [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca2+-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci 12: 3804–3817, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299: 1237–1240, 2003 [DOI] [PubMed] [Google Scholar]
- Joksovic PM, Bayliss DA, Todorovic SM. Different kinetic properties of two T-type Ca2+ currents of rat reticular thalamic neurones and their modulation by enflurane. J Physiol 566: 125–142, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Miyachi E. Enhancement by T-type Ca2+ currents of odor sensitivity in olfactory receptor cells. J Neurosci 21: RC144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Choi HJ, Kim JS, Kim YS, Jeong DU, Shin HC, Kim MJ, Han HC, Hong SK, Kim YI. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci 21: 1215–1222, 2005 [DOI] [PubMed] [Google Scholar]
- Klöckner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci 11: 4171–4178, 1999 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinová L, Kurejová M, Klugbauer N, Hofmann F. Gating of the expressed T-type CaV3.1 calcium channels is modulated by Ca2+. Acta Physiol (Oxf) 86: 249–260, 2006 [DOI] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J 77: 3034–3042, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim EG, Park BG, Kim KH, Cha SK, Kong ID, Lee JW, Jeong SW. Identification of T-type α1H Ca2+ channels (Cav3.2) in major pelvic ganglion neurons. J Neurophysiol 87: 2844–2850, 2002 [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci 23: 8752–8758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci 25: 3900–3907, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study. J Gen Physiol 114: 491–509, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Castelli L, Forti L, D'Angelo E. Kinetic and functional analysis of transient, persistent and resurgent sodium currents in rat cerebellar granule cells in situ: an electrophysiological and modelling study. J Physiol 573: 83–106, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RL, Lee JH, Cribbs LL, Perez-Reyes E, Hanck DA. Mibefradil block of cloned T-type calcium channels. J Pharmacol Exp Ther 295: 302–308, 2000 [PubMed] [Google Scholar]
- McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 54: 1080–1087, 1998 [DOI] [PubMed] [Google Scholar]
- McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. CaV3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci 24: 2581–2594, 2006 [DOI] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem 276: 3999–4011, 2001 [DOI] [PubMed] [Google Scholar]
- Melnick IV, Santos SFA, Szokol K, Szucs P, Safronov BV. Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J Neurophysiol 91: 646–655, 2004 [DOI] [PubMed] [Google Scholar]
- Mongan LC, Hill MJ, Chen MX, Tate SN, Collins SD, Buckby L, Grubb BD. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience 131: 161–175, 2005 [DOI] [PubMed] [Google Scholar]
- Mouginot D, Bossu JL, Gähwiler BH. Low-threshold Ca2+ currents in dendritic recordings from Purkinje cells in rat cerebellar slice cultures. J Neurosci 17: 160–170, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niespodziany I, Derambure P, Poulain P. Properties of T-type calcium current in enkephalinergic neurones in guinea-pig hypothalamic slices. Pflügers Arch 437: 871–880, 1999 [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Verkhratsky A. T-type calcium channels: the never ending story. Cell Calcium 40: 81–88, 2006 [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci 18: 854–86, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003 [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol 539: 817–836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci 25: 4743–4754, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by Krawetz S, Misener S. Totowa, NJ: Humana, 2000, p. 365–386 [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol 541: 231–244, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Burst-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol 493: 55–66, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu PD, Randic M. Low- and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J Neurophysiol 63: 273–285, 1990 [DOI] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Functional distribution of three types of Na+ channel on soma and processes of dorsal horn neurones of rat spinal cord. J Physiol 503: 371–385, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SFA, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol 581: 241–254, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP. Spike frequency adaptation and signaling properties of identified neurons in rodent deep spinal dorsal horn. J Neurophysiol 90: 245–258, 2003 [DOI] [PubMed] [Google Scholar]
- Schneider SP. Mechanosensory afferent input and neuronal firing properties in rodent spinal laminae III-V: re-examination of relationships with analysis of responses to static and time-varying stimuli. Brain Res 1034: 71–89, 2005 [DOI] [PubMed] [Google Scholar]
- Schneider SP. Local circuit connections between hamster laminae III and IV dorsal horn neurons. J Neurophysiol 99: 1306–1318, 2008 [DOI] [PubMed] [Google Scholar]
- Shcheglovitov A, Zhelay T, Vitko Y, Osipenko V, Perez-Reyes E, Kostyuk P, Shuba Y. Contrasting the effects of nifedipine on subtypes of endogenous and recombinant T-type Ca2+ channels. Biochem Pharmacol 69: 841–854, 2005 [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19: 1895–1911, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko AN, Kostyuk PG, Eremen AV, Isaev DS. Two types of low-voltage-activated Ca2+ channels in neurones of rat laterodorsal thalamic nucleus. J Physiol 499: 77–86, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várnai P, Osipenko ON, Vizi ES, Spät A. Activation of calcium current in voltage-clamped rat glomerulosa cells by potassium ions. J Physiol 483: 67–78, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Van Den Bosch L, Missiaen L, Vandenberghe W, Droogmans G, Nilius B, Robberecht W. Mibefradil (Ro 40–5967) blocks multiple types of voltage-gated calcium channels in cultured rat spinal motoneurones. Cell Calcium 22: 299–311, 1997 [DOI] [PubMed] [Google Scholar]
- Walsh MA, Graham BA, Brichta AM, Callister RJ. Evidence for a critical period in the development of excitability and potassium currents in mouse lumbar superficial dorsal horn neurons. J Neurophysiol 101: 1800–1812, 2009 [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Primary afferent neurons and the spinal dorsal horn. In: Sensory Mechanisms of the Spinal Cord. New York: Kluwer Academic/Plenum, 2004, vol. 1, p. 155–186 [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25: 5710–5719, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhang J, Xiao C, Kong J. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods 158: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- Zhuravleva SO, Kostyuk PG, Shuba YM. Divalent cation selectivity of the subtypes of low voltage-activated Ca2+ channels in thalamic neurons. Neuroreport 10: 651–657, 1999 [DOI] [PubMed] [Google Scholar]
- Zhuravleva SO, Kostyuk PG, Shuba YM. Subtypes of low-voltage-activated Ca2+ channels in laterodorsal thalamic neurons: possible localization and physiological roles. Pflügers Arch 441: 832–839, 2001 [DOI] [PubMed] [Google Scholar]