Abstract
The creation of several prestin knockout and knockin mouse lines has demonstrated the importance of the intrinsic outer hair cell membrane protein prestin to mammalian hearing. However, the structure of prestin remains largely unknown, with even its major features in dispute. Several studies have suggested that prestin forms homo-oligomers that may be stabilized by disulfide bonds. Our phylogenetic analysis of prestin sequences across chordate classes suggested that the cysteinyl residues could be divided into three groups, depending on the extent of their conservation between prestin orthologs and paralogs or homologs. An alanine scan functional analysis was performed of all nine cysteinyl positions in mammalian prestin. Prestin function was assayed by measurement of prestin-associated nonlinear capacitance. Of the nine cysteine-alanine substitution mutations, all were properly membrane targeted and all demonstrated nonlinear capacitance. Four mutations (C124A, C192A, C260A, and C415A), all in nonconserved cysteinyl residues, significantly differed in their nonlinear capacitance properties compared with wild-type prestin. In the two most severely disrupted mutations, substitution of the polar residue seryl for cysteinyl restored normal function in one (C415S) but not the other (C124S). We assessed the relationship of prestin oligomerization to cysteine position using fluorescence resonance energy transfer. With one exception, cysteine-alanine substitutions did not significantly alter prestin-prestin interactions. The exception was C415A, one of the two nonconserved cysteinyl residues whose mutation to alanine caused the most disruption in function. We suggest that no disulfide bond is essential for prestin function. However, C415 likely participates by hydrogen bonding in both nonlinear capacitance and oligomerization.
Keywords: hair cell, hearing, molecular motor, disulfide bond, fluorescence resonance energy transfer
the cochlear outer hair cell (OHC) motor protein prestin is thought to play a major role in mammalian cochlear amplification (Dallos 2008). Prestin is thought to participate in the generation of mechanical energy in the cochlea by means of receptor potential-driven OHC length change. The creation of several prestin knockout and knockin mouse lines with hearing loss phenotypes has convincingly demonstrated the importance of prestin to mammalian hearing (Dallos et al. 2008; Liberman et al. 2002).
Associated with the length change of OHCs is nonlinear capacitance (NLC), which can also be detected when prestin is expressed in a cell line such as human embryonic kidney (HEK)-293 cells or opossum kidney cells (Ludwig et al. 2001; Zheng et al. 2000). Briefly, NLC refers to the asymmetric charging properties of prestin-containing cellular membranes (Iwasa 1993; Santos-Sacchi 1991). In isolated OHCs, NLC and force generation have proved nearly inseparable. Although deflation of the OHC eliminates length change without also eliminating NLC (Kakehata and Santos-Sacchi 1995), no procedure has yet been shown to block NLC without also blocking OHC length change. Thus NLC serves as a proxy measure of prestin function. NLC is also found in nonplacental mammal prestins in expression systems (Tan et al. 2010), and electromotility has also been observed in marsupial OHCs (Okoruwa et al. 2008 and our own observations).
Prestin is a transmembrane protein of 744 residues in mammals and is a member of an anion transporting protein family, the solute carrier 26, or Slc26a, family (prestin is Slc26a5) (Zheng et al. 2000). The other members of the Slc26 family, where known, are transporters of various species of anion (Dorwart et al. 2008; Ohana et al. 2009). Prestin apparently does not transport anions, although it does require the anion chloride for its conformation change and for NLC (Oliver et al. 2001), which may be a relic of a former transporter identity. Consistent with this hypothesis, the nonmammalian prestin homologs from Gallus gallus and Danio rerio are divalent anion exchangers (Schaechinger and Oliver 2007).
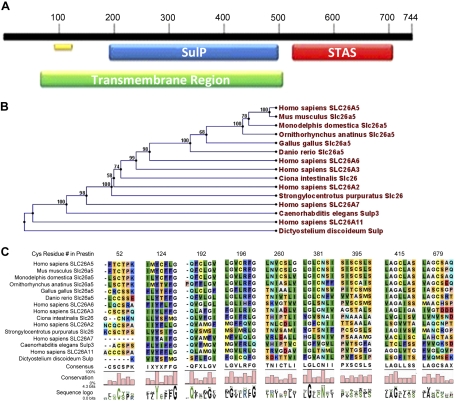
The region, domain, and motif structure of mammalian prestin is shown in Fig. 1A. Of particular importance to our analysis are the sulfate transporter (SulP) and the carboxy-terminal STAS (sulfate transporter and anti-sigma factor antagonist) domain (Ohana et al. 2009). These features are common to nearly all Slc26a5 homologs. Prestin also forms homo-oligomers of unknown stoichiometry, as has been consistently shown by Western blot analysis (Zheng et al. 2006) and fluorescence resonance energy transfer (FRET) analysis (Greeson et al. 2006; Navaratnam et al. 2005; Wu et al. 2007). There is evidence that oligomerization is a common feature of Slc26 family proteins (Detro-Dassen et al. 2008).
Fig. 1.
Homology analysis of prestin. A: region, domain, and motif designation of the human prestin amino acid sequence: green, transmembrane region; blue, sulfate transporter (SulP) domain; red, sulfate transporter and anti-sigma factor antagonist (STAS) domain; and yellow, SulP motif. B: phylogeny of SulP family member proteins. The number at each node indicates the bootstrap number out of 100 repetitions. C: multiple sequence alignment of cysteinyl residues. Residue properties are indicated as follows: gray, small hydrophobic; green, medium-size hydrophobic; cyan, partial positive charge; blue, positive charge; orange, partial negative charge; red, negative charge; pink, proline; light purple, histidine; and yellow, special property.
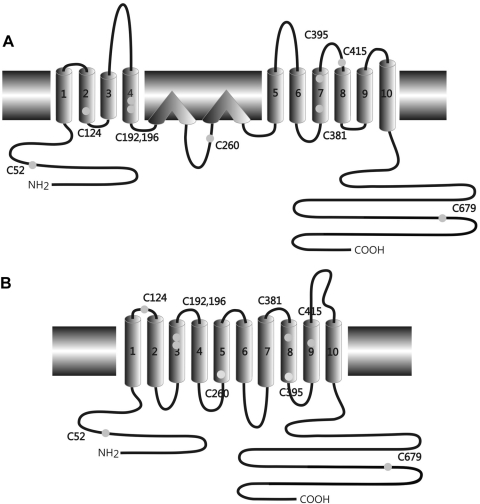
A cysteinyl residue in a polypeptide may contribute to tertiary structure by forming disulfide bonds, either within the polypeptide or between polypeptide strands in oligomers. Cysteinyl residues may also contribute hydrogen bonding to tertiary structure by virtue of their polar character. The mammalian prestin sequence includes nine cysteinyl residues. Their positions in the two published structures are shown in Fig. 2. Two of the positions are consensus intracellular (C52 and C679) and are therefore readily accessible for inter- or intramolecular sulfhydryl linkages. A third, C260, may or may not be included in the membrane. The other six are thought to be in membrane-spanning regions, although this would not completely preclude their participation in sulfhydryl linkages.
Fig. 2.
Diagram showing the positions of the 9 cysteinyl residues in prestin, represented by small gray disks, in the 2 proposed membrane topologies (A, based on Deak et al. 2005; and B, based on Navaratnam et al. 2005).
We first examined the evolutionary conservation of cysteinyl residues in prestin and related proteins in mammals and nonmammals. We determined which cysteinyl residues were conserved, either in identity, which may signify the requirement for a sulfhydryl linkage, or in similarity, which may indicate that the polar character of the residue is important.
We then performed an alanine scan functional analysis of the nine cysteinyl residues in gerbil prestin. NLC was measured in HEK-293 cells transfected with plasmids expressing mutated prestins and wild type. Cysteinyl residues were singly mutated to alanyl residues by site-directed mutagenesis. Each sequence was conjugated at its carboxy terminus with enhanced green fluorescent protein (eGFP) to indicate which cells were synthesizing prestin. Substitution of alanine at a position occupied by a cysteine eliminated both sulfhydryl bonding and any hydrogen bonding essential to function. For those mutations in which a large functional effect was observed, we then back-substituted the similarly sized polar residue seryl to distinguish between polar and sulfhydryl contributions of the original cysteinyl.
We next determined the importance of each residue to prestin oligomerization by performing FRET analysis. In FRET, the energy conferred to a donor fluorophore by a photon in its excitation wavelength range is partly transferred by nonradiative mechanisms to an acceptor fluorophore, from which a photon is emitted in the acceptor's emission wavelength range. FRET occurs only if the donor and acceptor fluorophores are within molecular dimensions of each other, and thus the presence of FRET may be taken as an indication of association. In previous studies, prestin was coupled at its carboxy terminus to the cyan (CFP) or Venus yellow fluorescent protein (vYFP) (Greeson et al. 2006; Navaratnam et al. 2005; Wu et al. 2007). In this study, we used the monomeric teal blue fluorescent protein variant (mTFP), which was chosen because it does not bind to itself or other fluorescent proteins (Day et al. 2008). We measured FRET using the acceptor photobleach technique and fluorescence lifetime imaging, which have been demonstrated to yield more reliable results than intensity-based methods (Suhling et al. 2005).
MATERIALS AND METHODS
Sequence comparison.
The Homo sapiens prestin sequence (NP_945350.1) was used to retrieve prestin homologs from evolutionarily relevant sequences using the Basic Local Alignment Search Tool (BLAST) through the National Center for Biotechnology Information (www.blast.ncbi.nlm.nih.gov). Retrieved sequences were aligned using the CLC Main Workbench custom alignment algorithm (CLC Bio, Cambridge, MA) with default parameters (Feng and Doolittle 1987). Aligned sequences with large gaps or insertions (>50 residues) were rejected. Phylogeny analysis was also performed with CLC Main Workbench using the unweighted pair group method with arithmetic mean with 100 bootstrap replicates.
Plasmid constructs.
For the NLC studies, a plasmid containing gerbil prestin cDNA, ligated in-frame to eGFP cDNA (referred to as pgPG) was obtained from Dr. Peter Dallos (Northwestern University, Evanston, IL). Cysteine-substitution mutations were performed using the QuickChange II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer's instructions. Correct sequence was confirmed by analysis of the insert performed at the Creighton University Molecular Biology Core Laboratory.
For the FRET studies, the pmTFP1-C construct containing the cDNA for the donor mTFP was obtained from Allele Biotechnology (San Diego, CA). The Venus construct, which contained the cDNA for the acceptor vYFP, was obtained from Dr. Atsushi Miyawaki (RIKEN Brain Science Institute, Saitama, Japan) (Nagai et al. 2002). With the use of PCR cloning, the mTFP and vYFP cDNA were cloned into the pAcGFP-N1 plasmid construct, replacing the cDNA for Aequorea coerulescens GFP, to create the pmTFP-N1 and pvYFP-N1 constructs. Gerbil prestin cDNA, without the stop codon, was PCR-cloned in-frame to the pmTFP-N1 and pvPYFP-N1 constructs 5′ to the fluorescent protein open reading frame. Cysteine-substitution mutations for all constructs were performed using the QuickChange II site-directed mutagenesis kit (Agilent Technologies) according to the manufacturer's instructions. Correct sequence was confirmed by sequence analysis of the insert performed at the Creighton University Molecular Biology Core Facility.
Two other constructs, as FRET positive and negative controls, were obtained as plasmids from Dr. Jian Zuo. The positive control consisted of a plasmid expressing a construct of Cerulean and Venus fluorescent proteins linked by a short amino acid sequence (referred to as pLink). The negative control consisted of a construct of the unrelated SLC family protein SLC38A2, linked by carboxy terminus to Venus fluorescent protein (referred to as p38Y). Both plasmids have previously been used as controls in this laboratory (Wu et al. 2007).
Cell culture.
HEK-293 cells were obtained from the American Type Culture Collection and were grown in flasks or on 35-mm glass-bottom dishes by using standard methods, without antibiotics.
Transfection.
HEK-293 cells were transfected with plasmid(s) when plated cells reached between 80 and 100% confluency using Lipofectamine 2000 by following the manufacturer's protocol (Invitrogen, Carlsbad, CA). For NLC measurements, cells were examined 24–48 h after transfection. In one experimental series (C415S), 10 μM salicylate was added to the medium to promote translocation to the plasma membrane (Kumano et al. 2010). Salicylate blocks prestin NLC, so the salicylate was removed at least 1 h before electrophysiological measures (Kakehata and Santos-Sacchi 1996; Tunstall et al. 1995).
For cotransfection and FRET experiments, if donor and acceptor fluorescence intensity were not approximately equal, plasmid volumes were adjusted to compensate. Cells were fixed between 24 and 48 h after transfection using 4% paraformaldehyde in phosphate-buffered saline (PBS). After 30 min of fixation, cells were washed with PBS and mounting medium was applied (1:1 vol/vol PBS-glycerol). Coverslips (no. 1½ 30-mm round; Warner Instruments, Hamden, CT) were sealed over the cells using rubber cement (Elmer's Products, Columbus, OH). Plates were stored at 4°C in the dark before experimentation.
Determination of membrane targeting.
The incubation medium surrounding transfected HEK cells was removed and replaced with PBS containing 2 μg/ml wheat germ agglutinin coupled to the red fluorophore AlexaFluor 568 (WGA-568; Invitrogen). After 10 min of exposure to WGA-568, the cells were rinsed three times with PBS alone and fixed using 4% paraformaldehyde in PBS (30 min). The fixed cells were rinsed in PBS, mounted in mounting medium, and coverslipped and sealed as described above. Cells were examined using the LSM 510 META NLO confocal microscope (Carl Zeiss, Thornwood, NY) of the Creighton University Integrated Biomedical Imaging Facility (IBIF). Images were analyzed using ImageJ (http://rsbweb.nih.gov/ij/).
Measurement of nonlinear capacitance.
Prestin NLC in HEK cells was measured as a function of membrane potential 24–48 h after transfection. Cells were bathed in a medium designed to block voltage-dependent ionic currents. The medium consisted (in mM) of 120.0 sodium chloride, 2.0 magnesium chloride, 20.0 tetraethylammonium chloride, 2.0 cobalt chloride, 10.0 dextrose, and 10.0 HEPES, buffered to pH 7.25 and adjusted to 300 mosmol/l. Cells with clearly membrane-resident fluorescent label were identified on the stage of an Olympus IX-70 inverted microscope (Olympus America, Center Valley, PA) using a ×100 1.4-numerical aperture (NA) objective. All electrophysiological measurements were performed at room temperature.
The whole cell patch-clamp method was used to measure membrane currents evoked by voltage commands. Patch pipettes were pulled from 8250 glass capillaries (A-M Systems, Carlsborg, WA) on a Sutter P-97 electrode puller (Novato, CA) and polished on a Narashige MF-830 polisher (East Meadow, NY). The pipette solution consisted (in mM) of 140.0 cesium chloride, 10.0 EGTA, 10.0 HEPES, 2.0 magnesium chloride, and 2.0 potassium adenosine triphosphate. Filled pipette resistances were between 1.5 and 5.0 MΩ. Membrane currents in response to voltage commands were recorded at the output of a Warner Instruments PC-501A patch-clamp amplifier (Hamden, CT). Voltage commands were generated and currents digitized using custom software written in TestPoint (C.E.C., Burlington, MA) and a Keithley Instruments (KCPI 3801; Cleveland, OH) analog-to-digital/digital-to-analog board in a personal computer. Currents were low-pass filtered at 5 kHz before digitization.
The two-sinusoid method (Kakehata and Santos-Sacchi 1996) was used to measure membrane capacitance. This method is superior to other methods, including single-sinusoid methods, because it enables independent calculation of series resistance, which often varies during a stimulus protocol. Simultaneous sinusoidal voltage commands of frequencies 195.3 and 390.6 Hz at amplitudes of 10 mV were superimposed on membrane potential bias commands that were stepped in 32 intervals of 5 mV, starting at −100 mV. The membrane holding potential in the absence of voltage commands was −70 mV. Membrane potentials were corrected off-line for series resistance error but were not corrected for the change in liquid junction potential on breaking into the cell (measured as −4.7 mV). Series resistances were generally less than 20 MΩ. Results were discarded if the cell input resistance were less than 500 MΩ.
Corrected capacitance-membrane potential functions were fitted by the Levenberg-Marquardt algorithm using the program Origin (Origin Lab, Northampton, MA) to the following equation (Kakehata and Santos-Sacchi 1996):
where Cl is the linear capacitance, V is the membrane potential in volts, Vpk is the membrane potential at peak NLC (also in volts), e is the charge on the electron (coulombs), z is the number of elementary charges transferred by each molecule between states, Q is the total number of elementary charges transferred between states in a cell, and k and T are Boltzmann's constant and absolute temperature, respectively.
The results of the curve fits were accepted only if the R2 goodness of fit value were greater than 0.9 (with one exception, see results) and the error in any single parameter estimate did not exceed 20% of the estimate. The NLC values were normalized for comparison by dividing by the peak NLC value predicted from the curve fit.
Statistical analysis of NLC results.
To prevent potential data discrepancies due to daily fluctuations in equipment and cell passage, we always compared mutant NLC measurements with a similar number of measurements obtained from wild-type transfected cells of the same or similar passage number. Differences in cysteine-mutant and wild-type NLC measurements were analyzed using independent sample t-tests (SPSS, Chicago, IL). The t-tests were corrected for unequal variance when a Levene's test for equality of variance indicated unequal variances between the test and control (wild type) groups.
Lifetime imaging of donor mTFP fluorescence.
Fixed cells were imaged using a ×40 1.4-NA oil-immersion objective on the IBIF confocal microscope. Cells containing donor alone and/or acceptor fluorophore were imaged using a 512 × 512-pixel field with ×4 digital magnification. Prebleach lifetime images were captured using two-photon excitation at wavelengths of 820 (for cCFP) or 870 nm (for mTFP) with a titanium-sapphire laser (Chameleon Ultra; Coherent, Santa Clara, CA) and time-correlated single photon counting (SPC-830; Becker & Hickl, Nahmitzer Damm, Berlin, Germany). Donor lifetime was calculated using SPCImaging (Becker & Hickl) with either 1 (mTFP) or 2 (cCFP) decay components (lifetime data range set with minima and maxima of 500 and 3,000 ps and a threshold of 25 photon counts per pixel). The decay matrix distribution over the region of interest was exported. A Gaussian fit of the histogram was calculated using Origin. Cell lifetimes were rejected if the Gaussian fit parameter had a correlation coefficient R2 < 0.9.
FRET analysis of oligomerization.
The acceptor photobleach fluorescence lifetime imaging version of FRET analysis (apFRET) required five steps: 1) a prebleach spectrum determination to determine the relative levels of donor and acceptor fluorescence, 2) a prebleach fluorescence lifetime measurement of the acceptor (mTFP or CFP), 3) photobleaching of the acceptor (YFP), 4) a postbleach fluorescence lifetime measurement of the acceptor, and 5) a postbleach spectrum determination to determine the effectiveness of the acceptor photobleach. Prebleach spectral images were determined using excitation with the 453-nm line of the argon laser of the confocal microscope and were captured using the META detector in lambda mode. Spectral images were linearly unmixed into either donor or acceptor based on previously acquired donor and acceptor spectra. If the cell image did not contain approximately equal intensities of donor and acceptor fluorescence, then the image was rejected. Acceptor photobleaching was performed by 30 repetitions of excitation of the region of interest with the 545-nm laser line of the confocal microscope and band-pass filtered from 565 to 615 nm. Postbleach lifetime and spectral images were captured as described above. FRET efficiency was calculated with the following equation:
where EFRET is FRET efficiency, τD is the lifetime of donor alone (after photobleaching), and τDA is the lifetime of the donor in the presence of an acceptor before photobleaching.
RESULTS
Determination of conserved cysteinyl residues.
More than 110 prestin homologs were identified on the basis of a filtered BLAST database search. Sequences that did not contain both a SulP domain and a STAS domain were not included in the analysis. Homologous sequences were obtained from species in five kingdoms (animals, plants, fungi, amoeba, and bacteria).
We analyzed cysteine conservation by aligning prestin homologs in a subset of sequences (Fig. 1B). Bias toward any phylogeny class was avoided by selecting single Slc26a5 representatives from each chordate class in which a published Slc26a5 homolog exists. The analysis data set included Slc26 sequences from Caenorhabditis elegans, Strongylocentrotus purpuratus, Ciona intestinalis, and Dictyostelium discoideum, as well as nonmammalian and mammalian Slc26a5 ortholog sequences and mammalian (human) sequences of SLC26 paralogs. The phylogenetic relationships between the selected sequences are shown in Fig. 1B, as determined using CLC Main Workbench (see materials and methods). Particularly noteworthy is the large evolutionary distance between H. sapiens SLC26A5 and its paralog, SLC26A11, greater even than the distance between mammalian prestin and its C. intestinalis and S. purpuratus homologs.
We then performed a multiple sequence alignment focused on the cysteinyl residues, also using CLC Main Workbench (Fig. 1C), and organized the results into three groups representing different levels of conservation. The amino-terminal residue C52, which we allocated to group 1, was identically conserved in all prestin orthologs and in nearly all paralogs and homologs. In the only exceptions, human SLC26A7 and D. discoideum SulP, there were gaps at that position, and we also noted poor overall conservation in their amino-terminal sequences. This high degree of identity conservation suggested a role for C52 in a function common to the entire Slc26 family, such as an ion transport-related function or binding to some membrane-localization element.
The other cysteinyl residues showed mixed patterns of conservation that could be organized in two further groups: those residues that were replaced in paralogs and homologs by mainly polar residues (group 2) and those that were replaced by mainly nonpolar residues (group 3). Group 2 consisted of C196, C381, and C679. For example, the group 2 carboxy-terminal residue C679 was identically conserved in all Slc26a5 orthologs and in some closely related paralogs, as determined by the phylogenetic relationships depicted in Fig. 2B. Some paralogs also had a cysteinyl residue in that position (for example, H. sapiens SLC26A2), but other polar residues were also found. In the other members of group 2, the SulP domain residues C196 and C381, the cysteinyl residue was identically conserved in mammalian orthologs (except for the monotreme in C196). In the nonmammalian orthologs, paralogs, and homologs, the residue was replaced by one of several small polar residues. The results suggested that group 2 cysteinyl residues contribute polar character rather than sulfhydryl linkages to structure in the Slc26 family proteins.
The group 3 cysteinyl residues C124, C192, C415, and C395 (also in the SulP domain) were conserved only in mammalian prestin sequences and were replaced in nonmammalian orthologs, and in paralogs and homologs, with residues of various properties, including polar residues, nonpolar aliphatic residues, charged residues, and even prolyl in C260. The most striking of these was C415, which was found to be identically conserved only in the mammalian prestin orthologs, which are the ones that have been shown to exhibit large NLC. In the nonmammal orthologs, the paralogs, and the homologs, this cysteinyl residue was replaced by various medium-size and small hydrophobic residues. This position may therefore be important in some functional aspect unique to mammalian Slc26a5, such as its membrane potential-dependent conformation change or the associated NLC. The results of replacement of cysteinyl residues in other paralogs and homologs in group 3 suggest that neither their polar character nor their ability to form sulfhydryl linkages contribute to conserved, Slc26-related aspects of prestin structure or function.
We concluded that the cysteinyl residues in groups 1 and 2 are related to function across the entire Slc26 family, whereas the cysteinyl residues in group 3 more likely contribute to some mammalian prestin-specific function, such as NLC.
All cysteine-alanine substitution mutations were membrane targeted.
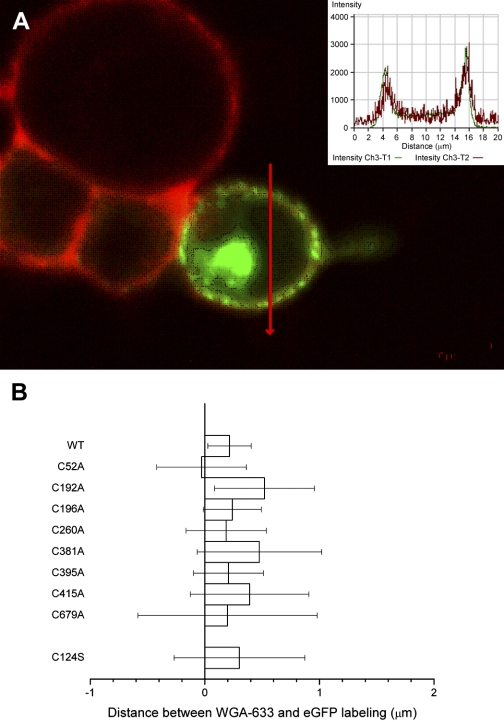
For electrophysiological analysis, we transfected HEK-293 cells with plasmids expressing wild-type prestin or cysteine-substitution prestin mutations as constructs coupled to eGFP. The protein products of wild-type and alanine-substituted prestin-eGFP constructs were synthesized at useful levels within 24 h of transfection. All appeared to be membrane targeted. Membrane targeting was confirmed by labeling the membrane of living intact cells with WGA-568, as described in materials and methods. Confocal microscopic observation of labeled cells revealed distinct and nearly overlapping WGA-568 label and eGFP fluorescence (Fig. 3A). Measurements of label found no significant difference in the relative positions of eGFP and WGA-568 label in all alanine-substitution constructs, indicating incorporation of eGFP-coupled prestin into the plasma membrane (Fig. 3A).
Fig. 3.
Determination of successful membrane targeting of prestin mutations. A: typical human embryonic kidney (HEK) cell expressing prestin-enhanced green fluorescent protein (eGFP) (green) and labeled with WGA-633 (red). Inset: fluorescence intensity profiles of the line in the main image. B: average (+SE) of separation of WGA-633 and prestin in profiles, as in A, for wild-type (WT) and mutated prestins as indicated.
All cysteine-alanine substitution mutants were functional by NLC analysis.
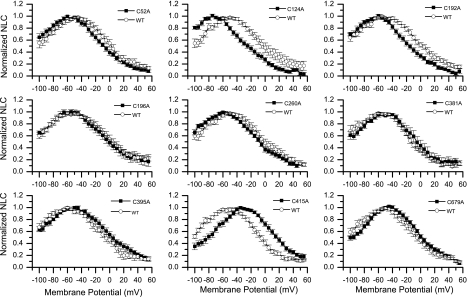
We obtained NLC measurements from transfected and expressing HEK cells using the methods described. All constructs demonstrated NLC, which confirmed our confocal microscopic analysis of prestin incorporation. NLC results were fitted to Boltzmann-derived functions to determine the NLC parameters, as described in materials and methods. Plots of averaged normalized NLC values for each of the cysteine-alanine substitution constructs, as a function of membrane potential, are shown in Fig. 4. Functions in Fig. 4 were compared with similarly averaged normalized NLC measurements from cells transfected at about the same time with wild-type prestin.
Fig. 4.
Normalized nonlinear capacitance (NLC) as a function of membrane potential for cysteine-alanine substitutions (solid squares) compared with wild-type prestin (open circles). Functions are not corrected for series resistance membrane potential error, which, although small (0.4% or less), means that the plots are shown for illustrative purposes only.
Four cysteine-alanine substitution mutations exhibited significantly different NLC from wild-type prestin.
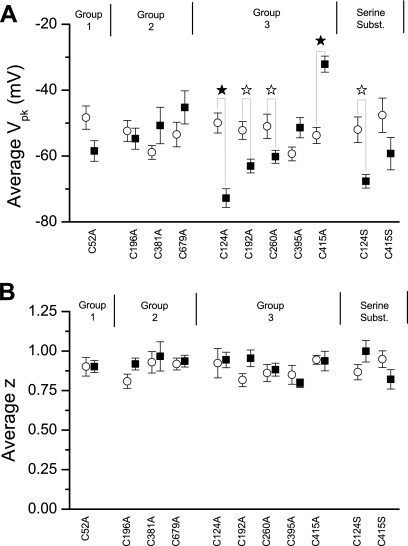
Our statistical analysis of the data is depicted in Fig. 5 and the results are listed in Table 1. The analysis showed that the NLC parameters Vpk and z were insignificantly different from wild-type NLC in the group 1 and group 2 alanine substitutions. However, all of the group 3 alanine substitutions (with 1 exception) exhibited significant positive or negative changes in Vpk, without changes in z.
Fig. 5.
Summary of NLC properties of cysteine-alanine substitutions (solid squares) compared with wild-type prestin (open circles), plotted as mean + SE. A: peak membrane potential (Vpk). B: charge transfer (z). ☆P < 0.05; ★P < 0.001, different from WT.
Table 1.
Averaged Vpk and z values of prestin NLC of wild-type prestin and prestin mutations, their statistically significant differences from wild type in the same batch, and their conservation group status
| Conserved | Mutation | Vpk, mV | P Value | z | P Value |
|---|---|---|---|---|---|
| Group 1 | C52A | ||||
| Control | −48.33 | 0.901 | |||
| Test | −58.45 | 0.051 | 0.902 | 0.986 | |
| Group 2 | C196A | ||||
| Control | −52.38 | 0.809 | |||
| Test | −54.71 | 0.615 | 0.918 | 0.082 | |
| C381A | |||||
| Control | −50.25 | 0.913 | |||
| Test | −59.34 | 0.211 | 0.931 | 0.907 | |
| C679A | |||||
| Control | −53.48 | 0.918 | |||
| Test | −45.25 | 0.055 | 0.936 | 0.740 | |
| Group 3 | C124A | ||||
| Control | −49.94 | 0.923 | |||
| Test | −72.78 | <0.001 | 0.944 | 0.849 | |
| C192A | |||||
| Control | −52.23 | 0.817 | |||
| Test | −63.05 | 0.006 | 0.954 | 0.077 | |
| C260A | |||||
| Control | −50.98 | 0.861 | |||
| Test | −60.22 | 0.025 | 0.882 | 0.766 | |
| C395A | |||||
| Control | −59.34 | 0.850 | |||
| Test | −51.41 | 0.053 | 0.797 | 0.438 | |
| C415A | |||||
| Control | −53.74 | 0.944 | |||
| Test | −32.11 | <0.001 | 0.937 | 0.928 | |
| Group 3 | C124S | ||||
| Control | −52.03 | 0.866 | |||
| Test | −67.65 | 0.007 | 0.997 | 0.124 | |
| C415S | |||||
| Control | −47.62 | 0.949 | |||
| Test | −59.26 | 0.132 | 0.821 | 0.139 |
Values are averaged peak membrane potential (Vpk) and charge transfer (z) for prestin nonlinear capacitance (NLC) of wild-type prestin (control) and prestin mutations. See text for descriptions of conservation groups 1–3.
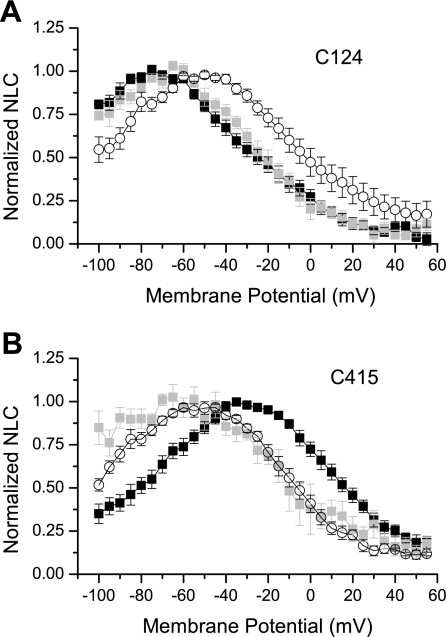
Group 3 cysteinyl residues were replaced in nonmammalian orthologs, paralogs, and homologs by nonpolar residues, for the most part. Introducing a nonpolar residue (alanine) in place of a cysteinyl had therefore disrupted a function specific to mammalian prestin. We then attempted to determine whether the polar nature of the cysteinyl residue, rather than its capacity to form covalent bonds, was functionally significant in NLC. The amino acid serine is closest to cysteine in molecular weight and polar nature. We therefore created cysteinyl-seryl substitutions of prestin-eGFP for the two group 3 positions with the largest changes in Vpk (C124 and C415, >10 mV). Our reasoning for selecting these two positions was that we would have the best chance to detect restoration of normal NLC with recordings from a practical number of cells. We then measured the resulting NLC and compared it with wild-type prestin (Fig. 5 and Table 1). If the polar character of the residue were important, we would expect that the NLC parameters would be restore to wild-type or near wild-type values.
For the C124A substitution, Vpk was hyperpolarized compared with wild type (−72.78 mV), and z was not significantly different. For the C124S substitution, Vpk was also significantly hyperpolarized compared with wild type, although less so than for C124A (−67.65 mV), and z was again not significantly different (Fig. 6A and Table 1). In contrast, for the C415A substitution, Vpk was depolarized compared with wild type (−32.11 mV), and z was not significantly different. For the C415S substitution, the substitution of serine essentially corrected the depolarizing effect of alanine substitution at that position (−59.26 mV) (Fig. 6B and Table 1). Thus we conclude that the polar character of the cysteine at position 415 contributes to prestin function.
Fig. 6.
A: normalized NLC of C124A (solid squares), C124S (shaded squares), and WT prestin (open circles) as a function of membrane potential. B: normalized NLC of C415A (solid squares), C415S (shaded squares), and WT prestin (open circles) as a function of membrane potential. Functions are not corrected for series resistance membrane potential error.
It should be noted that membrane incorporation levels of C415S prestin were remarkably low, although synthesis levels appeared comparable to those of wild-type prestin. Thus, for this construct, we resorted to promoting membrane incorporation using incubation with salicylate (see materials and methods). Even with this additional step, the fit criterion had to be relaxed to R2 > 0.8 to obtain enough measurements.
FRET efficiency.
As explained in the Introduction, oligomerization is a feature of mammalian prestin and at least some paralogs and homologs. We reasoned that if particular cysteinyl residues participate in oligomerization, then wild-type subunits would interact less efficiently with cotransfected alanyl-substituted subunits than with wild-type subunits. We therefore measured FRET efficiency in HEK-293 cells transfected with wild-type prestin coupled to the donor (mTFP) and alanine-substituted prestins coupled to the acceptor (vYFP).
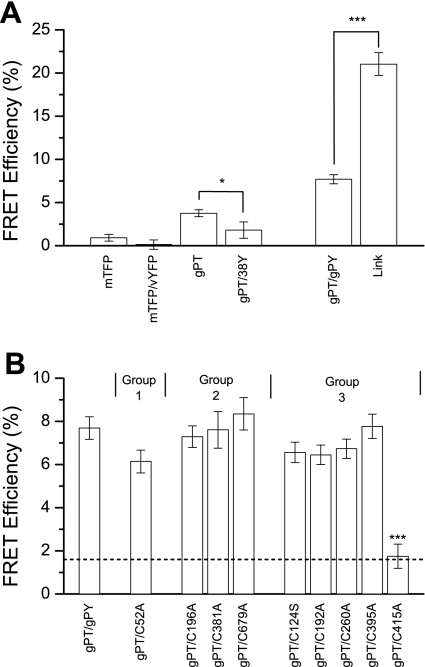
First, to establish the limits of our ability to detect FRET using apFRET, we examined several positive and negative controls (Fig. 6A). Two positive controls were used: the pLink construct, in which cCFP is ligated in-frame to vYFP, and the pgPT/pgPY combination, in which gerbil prestin donor and acceptor constructs were cotransfected. FRET was detectable with both positive controls, although pLink-transfected cells had significantly greater average FRET efficiencies than cells transfected with the pgPT/pgPY pair (Fig. 6A; Student's t-test, P < 0.01).
Several negative controls were also used to establish the minimum detectable FRET efficiency. These included cells transfected with donor alone (pT and pgPT) and cells transfected with a pair not expected to oligomerize (pT/pY and pgPT/p38Y). The average FRET efficiencies obtained for these tests are also shown in Fig. 6A. The negative control transfected cells all had significantly smaller average FRET efficiencies than the positive controls (Student's t-test, P < 0.001), which demonstrated our ability to distinguish between interacting and noninteracting fluorophores. Cells transfected with pgPT had significantly greater FRET efficiencies than the other negative controls (Student's t-test, P < 0.05, P < 0.001). The other three negative controls were indistinguishable. For the remainder of the FRET experiments, the FRET efficiency of pgPT/pA38Y cotransfected cells was considered the lower level detection of FRET (EFRET = 1.70%) and the FRET efficiency of pgPT/pgPY transfected cells was considered the normal FRET efficiency of gerbil prestin subunits (EFRET = 7.64%).
To test interaction between wild-type and cysteine-substitution mutations, FRET efficiency was measured between wild-type donor subunits and cysteine-mutated acceptor subunit. The average FRET efficiencies are shown in Fig. 7B. All cysteine mutants showed no difference between pgPT/pgPY-transfected cells and gPT/cysteine-alanine mutant acceptor construct, except for the cells transfected with pgPT/pgPY-C415A (Dunnett's 2-way t-test, P < 0.001). The average FRET efficiency of pgPT/pgPY-C415A-transfected cells was indistinguishable from the lower limit of FRET detection (Dunnett's 1-way t-test, P ≥ 0.05). Thus we conclude that only the group 3 residue C415 contributes to prestin oligomerization.
Fig. 7.
Averaged fluorescence resonance energy transfer (FRET) efficiencies of control and prestin construct-transfected cells. A: FRET efficiencies of cells transfected with negative controls (left) or positive controls (right) [ANOVA against negative control (3, 108, P < 0.01) and Student's t-tests]. *P < 0.05; ***P < 0.001. B: comparison of FRET efficiencies of positive (left) or negative control (dashed line) to WT/mutant donor/acceptor-cotransfected cells (right) [ANOVA against positive control (9, 320, P < 0.001), ANOVA against negative control (9, 295, P < 0.001), and Dunnett's 2-sided t-tests]. ***P < 0.001. Error bars indicate SE. See text for description of groups 1–3.
DISCUSSION
Our results point to a significant functional role specifically in mammalian prestin for the cysteine at position 415. Mutation of this position to the nonpolar amino acid alanine depolarized the peak membrane potential of NLC (Vpk) without changing the charge transfer (z). Mutation of the same position to the polar amino acid serine had no effect. Both mutations were functional in that they underwent the conformation change associated with NLC. This finding, and the preservation of normal function with the serine substitution, eliminates the possibility that the cysteine is required for a sulfhydryl linkage. However, it does not eliminate the possibility that one exists. Indeed, the fact that the alanine substitution reduced or eliminated FRET between it and wild-type prestin suggests a dramatic change in the properties of the assembled molecules. FRET could be reduced by uncoupling of the subunits or by a conformation change that sufficiently separates the carboxy-terminal fluorophores from each other. Our results cannot distinguish between these possibilities. We can, however, assert that the cysteine at position 415 is involved in both NLC and oligomerization in mammalian prestin.
The cysteine at position 415 is one of our group 3 cysteinyl residues, which are replaced in paralogs and orthologs by a variety of nonpolar residues. The group 3 cysteine residues were predicted, from their lack of conservation, to be involved in mammalian prestin-specific functions, which proved to be the case. For all but one of the group 3 cysteinyl residues, mutation to alanyl resulted in a change in Vpk of NLC (depolarizing for C415, hyperpolarizing for the others) without changing z. We suggest that these residues contribute to masking (or unmasking) of the voltage sensor governing the prestin conformation change but do not move during that conformation change, at least not orthogonally to the plane of the membrane.
Mutation of the highly conserved group 1 cysteine at position 52 had no effect on NLC. The same applied to the group 2 cysteinyl residues, which are replaced by polar residues in nonmammalian prestin and in paralogs and homologs. The group 2 cysteinyl residues are not apparently required to form sulfhydryl linkages for functionally normal mammalian prestin, although, as before, we cannot dismiss the possibility that they are present. They also are not required for oligomerization. They may participate in hydrogen bonding, as may their polar substitutes in other Slc26 family members, but if so, it may contribute to some family-wide function such as ion transport or binding to membrane-localization elements.
All of the group 3 cysteinyl residues, with the exception of C395, appear to participate in NLC to some degree, since the substitution of alanyl modified Vpk, without, however, modifying z. Modifying Vpk alone suggests an effect on the voltage sensor, without modification of the conformation change, or at least that part of the conformation change that contributes to NLC. The cysteinyl may be masking, or unmasking, the voltage sensor to some extent. Current hypotheses attribute the NLC to either movement of a bound chloride ion (like a transporter) or movement of charged or polar residues, modulated by chloride ions in an allosteric manner (Oliver et al. 2001; Rybalchenko and Santos-Sacchi 2003). If the bound chloride hypothesis applied, we might infer that the affinity of prestin for the chloride ion had been modified by alanyl substitution, but the translocation of chloride was unaltered. If the allosteric modulation hypothesis applied, we might infer that either the chloride binding site, or the voltage sensor, had been modified by alanyl substitution. Without further information, it is not yet possible to distinguish between these hypotheses.
We do not find the apparent hyperpolarized shift in Vpk in wild-type prestin relative to isolated hair cells (Bai et al. 2010; McGuire et al. 2010). Our average Vpk value for wild-type prestin, −51 mV, is much closer to reported Vpk values for OHCs and prestin-transfected HEK cells.
The percentage FRET obtained with pgPT/pgPY, at 7%, is substantial and comparable to recent observations by us and other laboratories (McGuire et al. 2010; Navaratnam et al. 2005; Wu et al. 2007). The higher FRET percentage of the pLink construct (20%) is likely a consequence of the forced 1:1 donor-acceptor ratio and the short distance between the donor and acceptor fluorophores in the construct. The higher FRET efficiency of gPT alone may be due to donor-to-donor FRET (Koushik and Vogel 2008) in the dense puncta of fluorescent label that are normally seen in HEK cells (Rajagopalan et al. 2007 and our own observations).
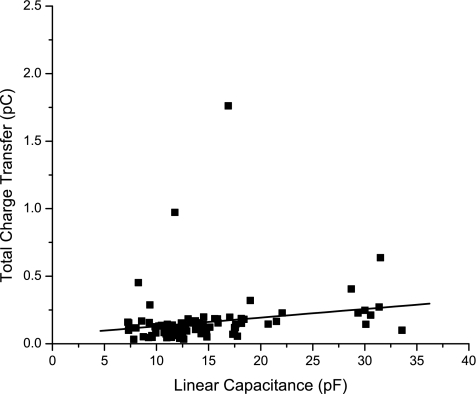
We have chosen not to report Q/Cl results as a measure of membrane incorporation, as others have done (Bai et al. 2010; McGuire et al. 2010), because they do not have the significance ascribed to them. In transfected cell experiments, the cells selected for examination are a vanishingly small subset of those available to the experimenter and are undoubtedly selected to be those with the clearest membrane label. Even then, the experimenter does not sample a fixed number of cells but continues until a satisfactory number of recordings has been achieved. Thus the sample population is far from unbiased. Furthermore, Cl is a poor predictor of Q even for a single construct. In our analysis of 107 cells that were visibly synthesizing wild-type prestin (coupled to eGFP) and incorporating it into their membranes, Q was only weakly correlated with Cl (Fig. 8). We conclude that comparisons of membrane incorporation between mutations using Q/Cl are not reliable. This is not to say that there are no differences among constructs in membrane incorporation. For example, as we described, the C415S construct was robustly synthesized but poorly incorporated into the membrane, so much so that a frustratingly large number of cells had to be examined to provide even the limited data shown.
Fig. 8.
Total nonlinear charge transfer as a function of linear capacitance for 117 cells synthesizing WT prestin (slope = 0.0058 pC/pF, R = 0.118).
GRANTS
This work was supported by National Science Foundation-Nebraska EPSCoR (Experimental Program to Stimulate Corporate Research) Grant EPS-0701892 (to R. Hallworth). Research was conducted in a facility constructed with support from National Center for Research Resources (NCRR) Research Facilities Improvement Program C06 RR17417-01. The Integrated Biomedical Imaging Facility is supported in part by NCRR Grant P20 RR16469.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Atsushi Miyawaki (RIKEN Brain Science Institute, Japan), Dr. Jian Zuo (St. Jude Children's Research Hospital, Memphis, TN), and Dr. Peter Dallos (Northwestern University, Evanston, IL) for gifts of cDNA. We thank Dr. Jing Zheng and Dr. Peter Dallos of Northwestern University, and Dr. Kirk Beisel and Dr. Venkatesh Govindarajan of Creighton University, for valued help in getting these experiments started. We acknowledge the use of the confocal microscope facility of the Integrated Biomedical Imaging Facility of Creighton University.
Present address of B. Currall: Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
REFERENCES
- Bai JP, Surguchev A, Bian S, Song L, Santos-Sacchi J, Navaratnam D. Combinatorial cysteine mutagenesis reveals a critical intramonomer role for cysteines in prestin voltage sensing. Biophys J 99: 85–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18: 370–376, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RN, Booker CF, Periasamy A. Characterization of an improved donor fluorescent protein for Forster resonance energy transfer microscopy. J Biomed Opt 13: 031203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak L, Zheng J, Orem A, Du GG, Aguinaga S, Matsuda K, Dallos P. Effects of cyclic nucleotides on the function of prestin. J Physiol 563: 483–496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem 283: 4177–4188, 2008 [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 23: 104–114, 2008 [DOI] [PubMed] [Google Scholar]
- Feng DF, Doolittle RF. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol 25: 351–360, 1987 [DOI] [PubMed] [Google Scholar]
- Greeson JN, Organ LE, Pereira FA, Raphael RM. Assessment of prestin self-association using fluorescence resonance energy transfer. Brain Res 109: 140–150, 2006 [DOI] [PubMed] [Google Scholar]
- Iwasa KH. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J 65: 492–498, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci 16: 4881–4889, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys J 68: 2190–2197, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Vogel SS. Energy migration alters the fluorescence lifetime of Cerulean: implications for fluorescence lifetime imaging Forster resonance energy transfer measurements. J Biomed Opt 13: 031204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano S, Iida K, Ishihara K, Murakoshi M, Tsumoto K, Ikeda K, Kumagai I, Kobayashi T, Wada H. Salicylate-induced translocation of prestin having mutation in the GTSRH sequence to the plasma membrane. FEBS Lett 584: 2327–2332, 2010 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419: 300–304, 2002 [DOI] [PubMed] [Google Scholar]
- Ludwig J, Oliver D, Frank G, Klocker N, Gummer AW, Fakler B. Reciprocal electromechanical properties of rat prestin: the motor molecule from rat outer hair cells. Proc Natl Acad Sci USA 98: 4178–4183, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire RM, Liu H, Pereira FA, Raphael RM. Cysteine mutagenesis reveals transmembrane residues associated with charge translocation in prestin. J Biol Chem 285: 3103–3113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90, 2002 [DOI] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89: 3345–3352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoruwa OE, Weston MD, Sanjeevi DC, Millemon AR, Fritzsch B, Hallworth R, Beisel KW. Evolutionary insights into the unique electromotility motor of mammalian outer hair cells. Evol Dev 10: 300–315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292: 2340–2343, 2001 [DOI] [PubMed] [Google Scholar]
- Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A, Raphael RM, Davidson AL, Oghalai JS, Pereira FA, Brownell WE. Tuning of the outer hair cell motor by membrane cholesterol. J Biol Chem 282: 36659–36670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. Cl− flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol 547: 873–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci 11: 3096–3110, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci USA 104: 7693–7698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhling K, French PM, Phillips D. Time-resolved fluorescence microscopy. Photochem Photobiol Sci 4: 13–22, 2005 [DOI] [PubMed] [Google Scholar]
- Tan X, Pecka JL, Tang J, Okoruwa OE, Zhang Q, Beisel KW, He DZ. From zebrafish to mammal: functional evolution of prestin, the motor protein of cochlear outer hair cells. J Neurophysiol 105: 36–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Gale JE, Ashmore JF. Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. J Physiol 485: 739–752, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Currall B, Yamashita T, Parker LL, Hallworth R, Zuo J. Prestin-prestin and prestin-GLUT5 interactions in HEK293T cells. Dev Neurobiol 67: 483–497, 2007 [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham M. Analysis of the oligomeric structure of the motor protein prestin. J Biol Chem 281: 19916–19924, 2006 [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155, 2000 [DOI] [PubMed] [Google Scholar]