Abstract
This study investigated the potential influence of proximal sensory feedback on voluntary distal motor activity in the paretic upper limb of hemiparetic stroke survivors and the potential effect of voluntary distal motor activity on proximal muscle activity. Ten stroke subjects and 10 neurologically intact control subjects performed maximum voluntary isometric flexion and extension, respectively, at the metacarpophalangeal (MCP) joints of the fingers in two static arm postures and under three conditions of electrical stimulation of the arm. The tasks were quantified in terms of maximum MCP torque [MCP flexion (MCPflex) or MCP extension (MCPext)] and activity of targeted (flexor digitorum superficialis or extensor digitorum communis) and nontargeted upper limb muscles. From a previous study on the MCP stretch reflex poststroke, we expected stroke subjects to exhibit a modulation of voluntary MCP torque production by arm posture and electrical stimulation and increased nontargeted muscle activity. Posture 1 (flexed elbow, neutral shoulder) led to greater MCPflex in stroke subjects than posture 2 (extended elbow, flexed shoulder). Electrical stimulation did not influence MCPflex or MCPext in either subject group. In stroke subjects, posture 1 led to greater nontargeted upper limb flexor activity during MCPflex and to greater elbow flexor and extensor activity during MCPext. Stroke subjects exhibited greater elbow flexor activity during MCPflex and greater elbow flexor and extensor activity during MCPext than control subjects. The results suggest that static arm posture can modulate voluntary distal motor activity and accompanying muscle activity in the paretic upper limb poststroke.
Keywords: arm, contraction
stroke survivors frequently experience upper limb hemiparesis, consisting of impaired motor control of the upper limb contralateral to the site of the stroke. Hand function in general and finger extension in particular are strongly affected (Trombly 1989; Trombly et al. 1986). Whereas local impairment mechanisms, such as hand muscle weakness (Kamper et al. 2003, 2006; Kamper and Rymer 2001) and excessive agonist-antagonist coactivation (Kamper et al. 2003; Kamper and Rymer 2001), have been described, other nonlocal mechanisms may also be involved in the impairment of hand function after stroke. Indeed, reflex coupling exists between muscles of the proximal and the distal segments of the upper limb (Alexander and Harrison 2003; Cavallari and Katz 1989; Cavallari et al. 1992; Gracies et al. 1991; Kasai et al. 1992, 1994; McClelland et al. 2001). This heteronymous coupling could influence the activation of muscles throughout the upper limb during voluntary motor activity, and abnormal manifestations of this coupling may play a substantial role in distal motor impairment poststroke. Specifically, sensory feedback from the arm may impact hand function.
In a recently conducted study, we found that static arm posture and surface electrical stimulation of the arm modulated the magnitude of the stretch reflex response of spastic finger flexor muscles in hemiparetic stroke survivors (Hoffmann et al. 2009). The magnitude was greatest in an arm posture in which the elbow was flexed and the shoulder was in a neutral posture, and increased when biceps brachii (BB) was stimulated. These results suggest that proximal sensory feedback can modulate distal reflex activity in the hand poststroke. A similar modulating effect of proximal sensory feedback may exist for voluntary motor activity in the hand poststroke, but to our knowledge, this has not yet been investigated. In neurologically intact individuals, voluntary distal upper limb motor activity has been shown to be modulated by static arm posture (Dominici et al. 2005; Ginanneschi et al. 2005, 2006).
Heteronymous coupling within the upper limb further suggests that distal motor activity may influence the activity of proximal muscles. In that respect, imposed stretch of the spastic finger flexors elicits activity of nonstretched muscles throughout the relaxed upper limb of hemiparetic stroke survivors (Hoffmann et al. 2009), and during voluntary motor activity, abnormal coupling of muscle activities between upper limb joints is commonly observed after stroke, notably between the elbow and the shoulder (Beer et al. 1999; Dewald and Beer 2001; Dewald et al. 1995; Sangani et al. 2009).
The aim of the present study was to investigate whether, in hemiparetic stroke subjects, sensory feedback from the proximal upper limb influences voluntary distal upper limb motor activity, specifically, maximum voluntary isometric force production in the hand. Subjects were asked to generate maximum voluntary isometric flexion and extension torque about the metacarpophalangeal (MCP) joints of the four fingers. Different conditions of proximal sensory feedback were compared by testing two static arm postures (i.e., combinations of static shoulder and elbow angles) and by applying surface electrical stimulation to either BB or triceps brachii (TB). Torque about the MCP joints and patterns of muscle activities throughout the upper limb were investigated. Specific interest was given to coactivation between a primary agonist [flexor digitorum superficialis (FDS) for MCP flexion (MCPflex) and extensor digitorum communis (EDC) for MCP extension (MCPext)] and other muscles. Based on results from our previous study, we expected static arm posture and electrical stimulation of the arm to influence voluntary MCP torque production about the MCP joints in stroke subjects. Specifically, we hypothesized that MCPflex torque would be greater in an arm posture involving a flexed elbow and in the presence of BB stimulation in stroke subjects. Furthermore, we hypothesized that voluntary MCPflex and MCPext would be accompanied by abnormal activity of muscles throughout the upper limb in stroke subjects.
MATERIALS AND METHODS
Subjects.
Ten hemiparetic stroke survivors (six men and four women), exhibiting chronic unilateral motor deficits, volunteered to participate in the present study (see Table 1 for clinical data). Stroke subjects were aged between 48 and 75 yr (mean, 60.2 yr), and all of them were at least 1 yr postincident (range, 13–144 mo). Function of the paretic upper limb was evaluated using the Fugl-Meyer Assessment of Sensorimotor Recovery After Stroke (Fugl-Meyer et al. 1975): upper extremity motor scores ranged from 26 to 62 out of a maximum score of 66. Six of the 10 stroke subjects had right hemiparesis, and four of them had left hemiparesis. Ten neurologically intact individuals (six women and four men) participated in the study as control subjects, who were aged between 26 and 67 years (mean, 42.1 years). We did not match stroke subjects and control subjects in terms of age, because we did not expect changes in the potential influence of sensory feedback from the proximal upper limb with age. In stroke subjects, the paretic upper limb was studied; in control subjects, the dominant upper limb was studied. The paretic upper limb was the dominant upper limb prior to the stroke in six of the 10 stroke subjects. All subjects gave informed consent in accordance with the Helsinki Declaration, and the experimental protocol was approved by the Institutional Review Board of Northwestern University (Chicago, IL).
Table 1.
Demographic and clinical data for the stroke subjects participating in the study
| Subject | Sex | Age (yr) | Time after Stroke (mo) | Side | Clinical Score | Handedness |
|---|---|---|---|---|---|---|
| S1 | M | 75 | 65 | R | 26 | R |
| S2 | F | 48 | 51 | L | 46 | R |
| S3 | M | 68 | 144 | R | 27 | R |
| S4 | M | 52 | 41 | R | 52 | L |
| S5 | M | 59 | 51 | L | 35 | R |
| S6 | M | 72 | 117 | L | 43 | L |
| S7 | F | 60 | 76 | L | 62 | R |
| S8 | F | 64 | 13 | R | 53 | R |
| S9 | F | 48 | 40 | R | 48 | R |
| S10 | M | 56 | 16 | R | 47 | R |
The subject's age is indicated in years. The time at which the experiment was conducted, with respect to the occurrence of the subject's stroke (“Time after Stroke”), is indicated in months. “Side” indicates whether the subject had right (“R”) or left (“L”) hemiparesis and thus which upper limb was studied. “Clinical Score” indicates the subject's Fugl-Meyer upper extremity motor score (out of a maximum score of 66). “Handedness” indicates whether the subject was right-handed or left-handed prior to her/his stroke.
Protocol.
The potential influence of sensory feedback from the proximal upper limb on distal voluntary motor activity was investigated through the performance of maximum voluntary isometric finger flexion and extension at the MCP joints. The subjects were seated next to an experimental table, and their four fingers were coupled to the shaft of a servomotor (1.4 hp; Kollmorgen, Radford, VA) fit into the table, as described previously (Hoffmann et al. 2009). A fiberglass cast placed around the subject's forearm and wrist maintained the wrist in a posture of neutral flexion/extension and neutral abduction/adduction, with respect to the forearm, and kept the thumb extended and abducted from the palm. The cast was clamped within a jig to prevent arm translation, as well as to ensure that the hand was supported and stabilized without requiring voluntary motor activity by the subjects. The positions of the cast and the jig were adjusted, such that the MCP joints were aligned along a vertical line extending from the shaft of the motor. The subject's forearm was maintained in a posture of neutral pronation/supination.
Experimental trials consisted of producing either maximum voluntary isometric flexion or maximum voluntary isometric extension at the MCP joints, with the servomotor maintaining the MCP joints at 20° of flexion. The subjects produced a single maximum voluntary isometric contraction (MCPflex or MCPext) per trial and were instructed to maintain the maximum contraction for 2–3 s. Between two successive trials, the motor slowly rotated the MCP joints from 20° of flexion to 10° of extension, where they were held for a few seconds before being slowly rotated back to 20° of flexion; this was done to minimize any wind-up effects of finger flexor muscle activity with repeated trials in stroke subjects (Kamper et al. 2003).
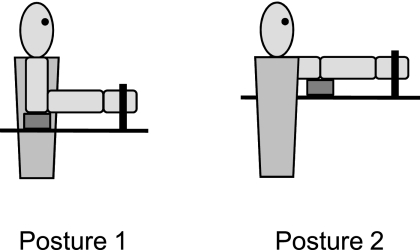
To investigate the potential effect of static proprioceptive feedback from the proximal upper limb, experimental trials were performed in two different static arm postures, which corresponded to two different combinations of shoulder and elbow angles. For posture 1, the goal posture consisted of 90° of elbow flexion, 0° of shoulder flexion, and 0° of shoulder abduction; for posture 2, the goal posture consisted of full elbow extension (0° of elbow flexion), 90° of shoulder flexion, and 0° of horizontal shoulder abduction (Fig. 1). The actual mean values of the shoulder and elbow angles across the 10 stroke subjects were: for posture 1, 74° of elbow flexion, 21° of shoulder flexion, and 30° of shoulder abduction; for posture 2, 19° of elbow flexion, 71° of shoulder flexion, and 34° of horizontal shoulder abduction. Across the 10 control subjects, the actual mean shoulder and elbow angles were: 80° of elbow flexion, 13° of shoulder flexion, and 33° of shoulder abduction for posture 1 and 15° of elbow flexion, 70° of shoulder flexion, and 25° of horizontal shoulder abduction for posture 2. The two arm postures used in the present study had been previously shown to exhibit differences in the magnitude of the stretch reflex response of spastic finger flexor muscles in hemiparetic stroke subjects (Hoffmann et al. 2009). In both arm postures, the subject's arm rested on a cushioned support placed between the elbow and the experimental table. This ensured that the arm was supported without requiring voluntary motor activity by the subjects. Care was taken to make certain that the subjects did not feel any discomfort in either of the two arm postures at any point throughout the experiment.
Fig. 1.
Schematic representation of the 2 arm postures used in the study. The thick black vertical line symbolizes where the subjects' fingers were coupled to the shaft of the servomotor, the thick black horizontal line symbolizes the surface of the experimental table, and the small gray rectangle symbolizes the cushioned support used to support the subjects' arm.
The potential effect of sensory feedback from the proximal upper limb was investigated further through electrical stimulation of either BB or TB. Three stimulation conditions, namely, “no stimulation”, “BB stimulation”, and “TB stimulation”, were tested in each of the two static arm postures. For the BB stimulation and TB stimulation conditions, electrical stimulation was delivered by means of a neuromuscular stimulator (300PV; Empi, St. Paul, MN) and a pair of surface-stimulating electrodes (American Imex, Irvine, CA) placed over the long head of BB or the long head of TB, respectively. Stimulation intensity was set to 120% of motor threshold, which was identified by palpation and visual observation. The duration of the stimulation pulse was 300 μs, and stimulation frequency was 35–40 Hz, depending on comfort. Stimulation was turned on before the beginning of the trial and was maintained until after the end of the maximum voluntary isometric contraction produced by the subject. Electrical stimulation of BB or TB was intended to activate Ia afferents from that muscle but undoubtedly, also produced activation of cutaneous receptors. All subjects perceived the stimulation levels as non-noxious.
Each subject performed three maximum voluntary isometric MCPflex contractions and three maximum voluntary isometric MCPext contractions in both arm postures under all three stimulation conditions. Thus a total of 36 experimental trials [(three MCPflex trials + three MCPext trials) × three stimulation conditions × two arm postures] was performed by each subject. The subjects successively performed all of the 18 trials in a given arm posture and were then moved to the other arm posture. The order in which the two arm postures were tested was not controlled. In effect, all of the subjects but two stroke subjects were tested in posture 1 first. In a given arm posture, the subjects successively performed three trials of a given contraction (MCPflex or MCPext) under a given stimulation condition, and the testing order of contractions and stimulation conditions varied randomly across subjects. There was a short rest period of ∼30–60 s between two successive trials. An auditory cue signaled the beginning of each trial.
Data collection.
Throughout the experimental trials, torque generated about the MCP joints was measured by means of a torque transducer (Transducer Techniques, Temecula, CA). The electromyography (EMG) signals from nine upper limb muscles were recorded by means of pairs of active surface-recording electrodes with differential amplification (Delsys, Boston, MA). Recording electrodes were lightly coated with conductive gel and positioned above the muscle belly of the following nine muscles: FDS, EDC, flexor carpi ulnaris (FCU), brachioradialis (B), BB, TB, pectoralis major, latissimus dorsi, and deltoideus medius. EMG signals were amplified (×1,000 to ×10,000) and band-pass filtered between 20 and 450 Hz (two Bagnoli eight-channel EMG systems; Delsys). At the beginning of the experimental session, the subjects were instructed to perform maximum voluntary contractions (MVCs) for each of the nine muscles; these MVCs were performed for the purpose of normalizing the EMG signals obtained during the experimental trials (cf. Analysis below). The recorded EMG signals from the nine muscles were displayed simultaneously on a computer screen, allowing for online visual inspection of the signals. In particular, if crosstalk was detected, placement of the corresponding recording electrode(s) was changed until the perceived crosstalk was eliminated.
The MCP torque and EMG signals were low-pass filtered at 225 Hz and then sampled at 500 Hz for offline analysis.
Analysis.
The MCP torque data were used to quantify the maximum isometric torque that the subjects produced during the MCPflex and MCPext trials. For each trial, the sampled MCP torque signal was smoothed using a 100-ms sliding window to compute a moving average. The maximum value of the smoothed signal during the trial (maximum MCPflex torque or maximum MCPext torque, respectively) was then located. To account for differences in strength between subjects, the maximum MCP torque value determined for each trial was then normalized according to the following method: for each MCPflex trial, the maximum MCPflex torque value for that trial was divided by the maximum MCPflex torque value across all MCPflex trials from the same subject, yielding MCPflex; for each MCPext trial, the maximum MCPext torque value for that trial was divided by the maximum MCPext torque value across all MCPext trials from the same subject, thereby yielding MCPext. In addition, the instant at which the maximum MCP torque value occurred (tflex or text, respectively) was determined for each trial.
The EMG data were used to quantify the patterns of upper limb muscle activities accompanying the production of the maximum isometric MCP torque. Each recorded EMG signal was first notch filtered at 60, 120, and 180 Hz. The signal was subsequently squared and passed through a low-pass filter (10 Hz cutoff frequency) before the square root was taken. This signal was then normalized by the maximum EMG activity value measured for the corresponding muscle across the entire experimental session, i.e., the maximum value recorded across the MVCs performed at the beginning of the experimental session and the experimental trials. This normalized signal (EMGnormalized) was subsequently used to quantify EMG activity of each of the nine upper limb muscles during the MCPflex and MCPext trials. Specifically, the “net EMG activity” (EMGnet) was computed for each muscle. First, a trapezoidal integration of EMGnormalized was performed over a time window defined from 200 ms before tflex or text to 100 ms after tflex or text. This integration yielded the “total EMG activity” (EMGtotal). Baseline EMG activity (EMGbaseline) for each muscle was quantified by integrating EMGnormalized over a baseline time window of 200 ms before the onset of voluntary MCPflex or MCPext. EMGbaseline was multiplied by 1.5 to account for the difference in duration of the time window used to quantify EMGtotal (300 ms) and the baseline time window (200 ms). Two different durations of time windows were used, as 200 ms proved to be the best choice for quantifying EMGbaseline activity without including contaminating artifacts in the baseline time window, whereas 300 ms was preferable for describing muscle activation. After this multiplication, EMGbaseline was subtracted from EMGtotal, and the resulting value was divided by the duration of the time window used to quantify EMGtotal (300 ms), thereby yielding EMGnet.
Additional variables were computed for each experimental trial to investigate coactivation between a primary agonist of the respective contraction (“targeted muscle”: FDS for MCPflex, EDC for MCPext) and the remaining “nontargeted” muscles, using the quantified EMGnet. Specifically, coactivation between the targeted muscle and each nontargeted muscle X was, respectively, quantified by “FDSandX” = Xnet/[net FDS activity (FDSnet) + Xnet] (for the MCPflex trials) or “EDCandX” = Xnet/[net EDC activity (EDCnet) + Xnet] (for the MCPext trials).
The recorded EMG signals were sometimes contaminated by ECG artifacts. If such contamination occurred, the ECG artifacts were removed before the EMG signal was used for analysis. The spikes in the EMG signal that were due to ECG activity were first used to compute a mean ECG spike template, which was then subtracted from the EMG signal at each location where an ECG spike occurred. Furthermore, since proximal electrical stimulation interfered with the recording of the EMG signals, EMG data from the BB stimulation and TB stimulation conditions were not used for analysis. Finally, some EMG data from the no stimulation condition were excluded from the analysis because of contamination by other artifacts.
Statistical analysis.
Statistical analyses were performed using SPSS software (SPSS, Chicago, IL).
Three multivariate ANOVAs (MANOVAs) were performed. A first MANOVA investigated the maximum isometric torque that the subjects produced during the MCPflex and MCPext trials, using “arm posture” (two levels: posture 1 and posture 2), “stimulation condition” (three levels: no stimulation, BB stimulation, and TB stimulation), and “subject group” (two levels: “stroke subjects” and “control subjects”) as fixed factors and MCPflex and MCPext as dependent variables. A second MANOVA investigated the EMGnet activities accompanying the production of the maximum isometric MCP torque, using arm posture, “contraction” (two levels: “MCPflex” and “ MCPext”), and subject group as fixed factors and the nine EMGnet as dependent variables. A third MANOVA investigated the coactivation between the targeted muscle and the nontargeted muscles accompanying the production of the maximum isometric MCP torque, using arm posture and subject group as fixed factors and the eight FDSandX and the eight EDCandX as dependent variables. When a fixed factor proved significant in a MANOVA, post hoc univariate repeated measures ANOVAs or t-tests were performed on the corresponding dependent variables. To account for multiple statistical tests, a Bonferroni correction was used, such that the significance level was set to α = 0.05/3 = 0.017 for each MANOVA and each post hoc univariate repeated measures ANOVA and t-test.
RESULTS
Effects of arm posture and proximal electrical stimulation on MCP torque.
Arm posture influenced maximum voluntary isometric torque production about the MCP joints with differences between stroke subjects and control subjects. The MANOVA performed on MCPflex and MCPext showed a statistically significant dependence on arm posture (P < 0.017), subject group (P < 0.001), and the interaction between arm posture and subject group (P < 0.017) but not on stimulation condition (P = 0.993) or the remaining interactions (arm posture and stimulation condition: P = 0.966; stimulation condition and subject group: P = 0.794; arm posture, stimulation condition, and subject group: P = 0.577).
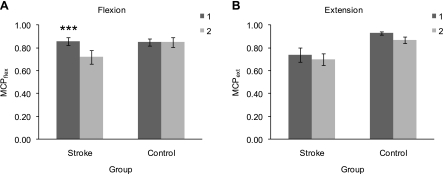
Post hoc univariate repeated measures ANOVAs, using arm posture as the within-subject factor and subject group as the between-subjects factor, were subsequently performed on MCPflex and on MCPext, respectively. Mean maximum normalized MCPflex exhibited significant effects of arm posture (P < 0.001) and subject group (P < 0.017) and a significant interaction between arm posture and subject group (P < 0.001). Mean MCPflex was 0.86 ± 0.04 (mean ± 95% confidence interval) in stroke survivors and 0.85 ± 0.03 in control subjects in posture 1 and 0.72 ± 0.06 in stroke survivors and 0.85 ± 0.04 in control subjects in posture 2 (Fig. 2A). Separate paired-samples t-tests performed for stroke subjects and control subjects, respectively, indicated a significant difference in mean MCPflex between posture 1 and posture 2 in stroke subjects (P < 0.001, two-tailed) but not in control subjects (P = 0.886). Compared with control subjects, the normalized MCPflex torque in stroke subjects exhibited a 15.3% deficit in posture 2 but none in posture 1. Mean maximum normalized MCPext torque exhibited a significant effect of subject group (P < 0.001), but the effect of arm posture did not reach significance (P = 0.039), and there was no significant interaction between arm posture and subject group (P = 0.808). In posture 1, mean MCPext was 0.74 ± 0.06 in stroke subjects and 0.93 ± 0.02 in control subjects, and in posture 2, it was 0.70 ± 0.05 in stroke subjects and 0.87 ± 0.03 in control subjects (Fig. 2B). The mean difference in MCPext between posture 1 and posture 2 was similar for the two subject groups (5.7% in stroke subjects and 6.9% in control subjects). The normalized MCPext torque was reduced greatly in both arm postures in stroke subjects compared with control subjects (20.4% in posture 1 and 19.5% in posture 2). Thus stroke subjects had difficulty repeatedly producing and sustaining maximum MCPext. For the majority of subjects, the maximum MCP torque value used to normalize the MCP torque data was observed in posture 1 for both the MCPflex trials (eight of the 10 stroke subjects and seven of the 10 control subjects) and the MCPext trials (seven of the 10 stroke subjects and nine of the 10 control subjects).
Fig. 2.
Effect of arm posture on maximum normalized metacarpophalangeal flexion (MCPflex; A) and MCP extension (MCPext; B) torque in stroke subjects and control subjects. For each subject group, each box represents the mean value of MCPflex or MCPext, respectively, for the corresponding arm posture (dark gray: posture 1; light gray: posture 2). Bars represent 95% confidence intervals. Asterisks indicate a statistically significant difference between posture 1 and posture 2 (***P < 0.001).
To investigate a potential relationship between maximum normalized MCP torque (MCPflex and MCPext, respectively) and the impairment level of stroke subjects, correlation analyses were performed. Correlation analyses for the MCPflex trials indicated no statistically significant correlation between MCPflex and the Fugl-Meyer score (Pearson correlation coefficient, R = 0.131, P = 0.317, two-tailed), whereas for the MCPext trials, there was a statistically significant positive correlation between MCPext and the Fugl-Meyer score (R = 0.316, P < 0.05). Conversely, the stroke subjects' Fugl-Meyer scores were significantly negatively correlated with the difference between posture 1 and posture 2 in MCPflex (R = −0.385, P < 0.05) but not with the difference between posture 1 and posture 2 in MCPext (R = −0.032, P = 0.867).
Effect of arm posture on upper limb muscle activities.
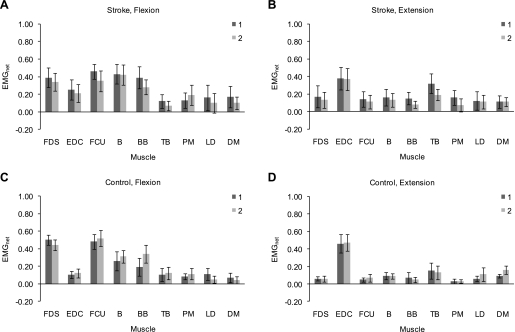
In the MANOVA performed on the nine EMGnet, the effect of arm posture and the interactions between arm posture and contraction or/and subject group were not statistically significant (arm posture: P = 0.361; arm posture and contraction: P = 0.257; arm posture and subject group: P = 0.197; arm posture, contraction, and subject group: P = 0.162). Likewise, in the MANOVA performed on the eight FDSandX and the eight EDCandX, the effect of arm posture (P = 0.690) and the interaction between arm posture and subject group (P = 0.580) were not statistically significant. Based on these results, we investigated potential trends with respect to arm posture for the EMGnet and the FDSandX and EDCandX. Figure 3 shows, for both stroke subjects and control subjects and for both the MCPflex trials and MCPext trials, the mean EMGnet activity of each of the nine upper limb muscles in posture 1 and posture 2. Although the effect of arm posture was not significant, the EMG data appeared to suggest a trend for upper limb muscle activities to be influenced by arm posture during maximum voluntary isometric contraction at the MCP joints.
Fig. 3.
Differences between arm postures in net electromyography activity (EMGnet) of the 9 upper limb muscles during the MCPflex (A and C) and MCPext (B and D) trials in stroke subjects (A and B) and control subjects (C and D). For each subject group, each box represents the mean value of EMGnet for the corresponding muscle and the corresponding arm posture (dark gray: posture 1; light gray: posture 2). Bars represent 95% confidence intervals. FDS, flexor digitorum superficialis; EDC, extensor digitorum communis; FCU, flexor carpi ulnaris; B, brachioradialis; BB, biceps brachii; TB, triceps brachii; PM, pectoralis major; LD, latissimus dorsi; DM, deltoideus medius.
During the MCPflex trials, a trend for the mean EMGnet activity of the targeted muscle FDS (FDSnet) to be greater in posture 1 was observed in control subjects (Fig. 3C) but not in stroke subjects (Fig. 3A). The activity of nontargeted upper limb muscles during the MCPflex trials also appeared to be differentially influenced by arm posture in the two subject groups. In particular, a trend toward greater upper limb flexor activity [mean net FCU activity (FCUnet) and mean net BB activity (BBnet)] in posture 1 was observed in stroke subjects (Fig. 3A), whereas control subjects exhibited a trend toward greater elbow flexor activity in posture 2, in terms of both mean BBnet (Fig. 3C) and the mean coactivation between FDS and BB [(FDSandBB): 0.26 ± 0.08 (mean ± 95% confidence interval) in posture 1 vs. 0.41 ± 0.09 in posture 2].
During the MCPext trials, the mean EMGnet activity of the targeted muscle EDC (EDCnet) appeared not to be different between arm postures in either subject group (Fig. 3, B and D). Similar to the MCPflex trials, arm posture appeared to influence the activity of nontargeted upper limb muscles during the MCPext trials with differences between the two subject groups. A trend toward greater elbow flexor activity (mean BBnet) was observed in posture 1 in stroke subjects (Fig. 3B), as was the case during the MCPflex trials. In addition, elbow extensor activity [mean net TB activity (TBnet)] tended to be greater in posture 1 in stroke subjects (Fig. 3B). Similar trends were observed for the mean coactivation between EDC and BB (EDCandBB; 0.28 ± 0.10 vs. 0.17 ± 0.07) and between EDC and TB (EDCandTB; 0.45 ± 0.08 vs. 0.30 ± 0.09) in stroke subjects. Control subjects appeared not to exhibit differences in elbow flexor or elbow extensor activity.
Correlation analyses were performed to investigate a potential relationship between the EMG data and the impairment level of stroke subjects. For the MCPflex trials, there was no significant correlation between the stroke subjects' Fugl-Meyer scores and any of the nine EMGnet or any of the eight FDSandX. For the MCPext trials, FDSnet, net B activity (Bnet), BBnet, and EDCandBB all exhibited a significant positive correlation with the stroke subjects' Fugl-Meyer scores (FDSnet: R = 0.488, P < 0.05; Bnet: R = 0.506, P < 0.05; BBnet: R = 0.481, P < 0.05; EDCandBB: R = 0.500, P < 0.05). No significant correlation was observed between the stroke subjects' Fugl-Meyer scores and the difference between posture 1 and posture 2 in any of the nine EMGnet or any of the eight FDSandX for the MCPflex trials or the difference between posture 1 and posture 2 in any of the nine EMGnet or any of the eight EDCandX for the MCPext trials. Note that in these correlation analyses for the EMG data, only data from the no stimulation condition could be used, in contrast to the correlation analyses for the MCP torque data, in which data from all three stimulation conditions were used.
Effects of contraction and subject group on upper limb muscle activities.
In the MANOVA performed on the nine EMGnet, the effects of contraction (P < 0.001) and subject group (P < 0.017) and the interaction between contraction and subject group (P < 0.001) were statistically significant. In the MANOVA performed on the eight FDSandX and the eight EDCandX, the effect of subject group (P = 0.150) was not statistically significant. Based on these results, we performed post hoc t-tests to investigate potential statistically significant differences between contractions and between subject groups for the EMGnet, and we investigated potential trends with respect to subject group for the FDSandX and the EDCandX. Paired-samples t-tests were performed to compare each of the nine EMGnet between the MCPflex trials and the MCPext trials, separately for each of the two subject groups. Independent-samples t-tests were performed to compare each of the nine EMGnet between stroke subjects and control subjects, separately for the MCPflex trials and for the MCPext trials. Table 2 shows, for both stroke subjects and control subjects, the mean EMGnet activity of each of the nine upper limb muscles for the MCPflex trials and the MCPext trials, as well as the mean difference between the MCPflex trials and the MCPext trials in EMGnet (ΔEMGnet). Independent-samples t-tests were performed to compare each of the nine ΔEMGnet between stroke subjects and control subjects.
Table 2.
Differences between MCPflex trials and MCPext trials in net EMG activity of the nine upper limb muscles in stroke subjects (top) and control subjects (bottom)
| Muscle | EMGnet, Flexion | EMGnet, Extension | Pcontraction | ΔEMGnet | Pgroup |
|---|---|---|---|---|---|
| Stroke | |||||
| FDS | 0.37 ± 0.07 | 0.15 ± 0.07 | 0.001 | 0.21 ± 0.11 | 0.001 |
| EDC | 0.23 ± 0.07 | 0.37 ± 0.09 | 0.033 | −0.15 ± 0.14 | 0.007 |
| FCU | 0.41 ± 0.07 | 0.12 ± 0.05 | 0.000 | 0.28 ± 0.07 | 0.000 |
| B | 0.43 ± 0.08 | 0.14 ± 0.06 | 0.000 | 0.28 ± 0.10 | 0.126 |
| BB | 0.34 ± 0.08 | 0.11 ± 0.05 | 0.000 | 0.22 ± 0.09 | 0.762 |
| TB | 0.10 ± 0.05 | 0.26 ± 0.07 | 0.000 | −0.16 ± 0.08 | 0.012 |
| PM | 0.16 ± 0.07 | 0.11 ± 0.06 | 0.172 | 0.05 ± 0.08 | 0.693 |
| LD | 0.10 ± 0.08 | 0.11 ± 0.06 | 0.898 | −0.01 ± 0.10 | 0.845 |
| DM | 0.13 ± 0.07 | 0.11 ± 0.04 | 0.471 | 0.03 ± 0.08 | 0.025 |
| Control | |||||
| FDS | 0.47 ± 0.04 | 0.06 ± 0.02 | 0.000 | 0.41 ± 0.03 | |
| EDC | 0.11 ± 0.03 | 0.46 ± 0.07 | 0.000 | −0.35 ± 0.08 | |
| FCU | 0.50 ± 0.06 | 0.06 ± 0.03 | 0.000 | 0.44 ± 0.06 | |
| B | 0.29 ± 0.06 | 0.09 ± 0.02 | 0.000 | 0.20 ± 0.06 | |
| BB | 0.26 ± 0.08 | 0.06 ± 0.03 | 0.000 | 0.20 ± 0.09 | |
| TB | 0.11 ± 0.05 | 0.14 ± 0.06 | 0.370 | −0.03 ± 0.07 | |
| PM | 0.10 ± 0.04 | 0.03 ± 0.02 | 0.001 | 0.07 ± 0.04 | |
| LD | 0.08 ± 0.04 | 0.08 ± 0.04 | 0.874 | 0.00 ± 0.06 | |
| DM | 0.05 ± 0.04 | 0.13 ± 0.03 | 0.003 | −0.07 ± 0.04 |
For each subject group, “EMGnet, Flexion” and “EMGnet, Extension” show the mean value and the 95% confidence interval for net electromyography activity (EMGnet) for the corresponding muscle and the corresponding contraction, and “ΔEMGnet” shows the mean value and the 95% confidence interval for the difference between the metacarpophalangeal flexion (MCPflex) trials and the MCP extension (MCPext) trials in EMGnet for the corresponding muscle. Pcontraction values (2-tailed) refer to differences between MCPflex trials and MCPext trials in EMGnet. Pgroup values (2-tailed) refer to differences between stroke subjects and control subjects in ΔEMGnet. FDS, flexor digitorum superficialis; EDC, extensor digitorum communis; FCU, flexor carpi ulnaris; B, brachioradialis; BB, biceps brachii; TB, triceps brachii; PM, pectoralis major; LD, latissimus dorsi; DM, deltoideus medius.
Stroke subjects exhibited reduced task specificity in terms of the upper limb muscle activities accompanying maximum voluntary isometric MCPflex or MCPext, respectively. Reduced task specificity in activity was apparent for the targeted muscles FDS and EDC, as the mean difference between the MCPflex trials and the MCPext trials in both FDSnet and EDCnet was significantly smaller in stroke subjects than in control subjects (Table 2). Reduced task specificity in activity was, furthermore, observed for some nontargeted muscles, such as FCU (Table 2). On the other hand, stroke subjects appeared to exhibit a task-specific difference in activity for the nontargeted muscle TB, as the MCPext trials were accompanied by significantly greater mean TBnet than the MCPflex trials in stroke subjects, whereas there was no significant difference in control subjects (Table 2). Accordingly, the mean difference in TBnet was significantly greater in stroke subjects than in control subjects (Table 2).
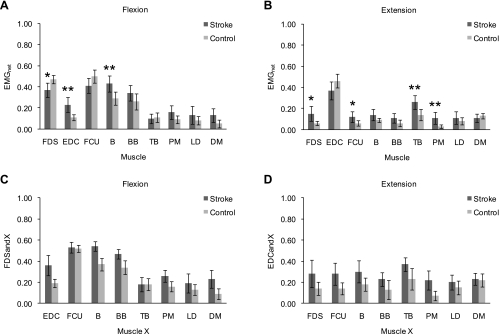
The EMG data exhibited further differences in upper limb muscle activities between stroke subjects and control subjects, for both the MCPflex trials and the MCPext trials. During the MCPflex trials, stroke subjects exhibited a deficit in activating the targeted muscle, as mean FDSnet was significantly smaller in stroke subjects (0.37 ± 0.07) than in control subjects (0.47 ± 0.04; P < 0.017, two-tailed) (Fig. 4A). In addition to the significantly smaller mean activity of the targeted muscle, the MCPflex trials were characterized by significantly greater mean activity of its direct antagonist (EDCnet) in stroke subjects compared with control subjects (0.23 ± 0.07 vs. 0.11 ± 0.03, P < 0.01) (Fig. 4A). A similar trend was observed for the mean coactivation between FDS and EDC (FDSandEDC; 0.36 ± 0.10 vs. 0.19 ± 0.04) (Fig. 4C). Moreover, stroke subjects overall exhibited greater activity of nontargeted upper limb muscles. Notably, greater elbow flexor activity was observed, as mean Bnet was significantly greater in stroke subjects (Fig. 4A), and a similar trend existed for the mean coactivation between FDS and B and mean FDSandBB (Fig. 4C). During the MCPext trials, a deficit in activating the targeted muscle was again observed in stroke subjects, as mean EDCnet was reduced in stroke subjects compared with control subjects, although the difference did not reach significance (0.37 ± 0.09 vs. 0.46 ± 0.07, P = 0.078) (Fig. 4B). Similar to the MCPflex trials, the MCPext trials were also characterized by greater activity of the direct antagonist of the targeted muscle in stroke subjects compared with control subjects. Indeed, mean FDSnet was significantly greater in stroke subjects than in control subjects (0.15 ± 0.07 vs. 0.06 ± 0.02, P < 0.05) (Fig. 4B), and a similar trend was observed for the mean coactivation between EDC and FDS (EDCandFDS; 0.28 ± 0.13 vs. 0.14 ± 0.06) (Fig. 4D). Again, similar to the MCPflex trials, the MCPext trials, furthermore, exhibited greater activity of nontargeted upper limb muscles overall in stroke subjects. In particular, both greater elbow flexor activity and greater elbow extensor activity were observed, as mean TBnet was significantly greater in stroke subjects (Fig. 4B), and mean BBnet (Fig. 4B) and mean coactivation between EDC and B and mean EDCandTB (Fig. 4D) exhibited a similar trend. In contrast to the MCPflex trials, mean FCUnet was significantly greater in stroke subjects (Fig. 4B), and a similar trend existed for mean coactivation between EDC and FCU (Fig. 4D).
Fig. 4.
Differences between stroke subjects and control subjects in net EMG activity (A and B) and coactivation between the targeted muscle and nontargeted muscles (C and D) during the MCPflex (A and C) and MCPext (B and D) trials. Each box represents the mean value for the corresponding subject group (dark gray: stroke subjects, light gray: control subjects) of EMGnet (A and B) for the corresponding muscle or of the coactivation between FDS and each nontargeted muscle X during MCPflex [Xnet/(net FDS activity + Xnet); FDSandX; C] or the coactivation between EDC and each nontargeted muscle X during MCPext [Xnet/(net EDC activity + Xnet); EDCandX; D], respectively, for the corresponding pair of muscles. Bars represent 95% confidence intervals. A and B: asterisks indicate a statistically significant difference between stroke subjects and control subjects (*P < 0.017; **P < 0.01).
DISCUSSION
Effect of arm posture on voluntary MCPflex and MCPext.
The production of maximum voluntary isometric torque about the MCP joints was influenced by static arm posture in stroke subjects, but only in the direction of flexion, and appeared not to be influenced in control subjects. Stroke subjects produced significantly greater mean maximum normalized MCPflex torque when the elbow was flexed, and the shoulder was in a neutral posture (posture 1) than when the elbow was extended, and the shoulder was flexed (posture 2). Arm posture did not have an effect on MCPflex in control subjects and did not have an effect on mean maximum normalized MCPext torque in either subject group. Compared with control subjects, mean maximum normalized MCP torque in stroke subjects was reduced in posture 2 for MCPflex and in both arm postures for MCPext.
Several studies have investigated the effect of static arm posture on force or strength in the hand or fingers in neurologically intact subjects with various and contradictory results (Balogun et al. 1991; Desrosiers et al. 1995; Kuzala and Vargo 1992; Mathiowetz et al. 1985; Oxford 2000; Roman-Liu 2003; Stegink Jansen et al. 2003; Su et al. 1993, 1994). Notably, whereas some investigators have documented greater grip strength in an extended elbow posture (Kuzala and Vargo 1992; Oxford 2000; Su et al. 1993, 1994), others have found it to be greater in a flexed elbow posture (Mathiowetz et al. 1985) or to be unaffected by elbow posture (Desrosiers et al. 1995). Our results suggest no significant effect of static arm posture on either voluntary MCPflex torque or voluntary MCPext torque in neurologically intact subjects, although voluntary MCPext torque exhibited a trend to be greater in posture 1 by a relatively modest amount (6.9% increase with respect to posture 2) (Fig. 2B). In stroke subjects, on the other hand, we observed significantly greater voluntary MCPflex torque in posture 1 (19.4% increase with respect to posture 2). This suggests a fundamental change in the effect of static proximal upper limb posture on distal voluntary motor activity after stroke.
We propose that the observed effects of static arm posture cannot be attributed merely to the biomechanics of the finger muscles. Both FDS and EDC cross the elbow: the humeroulnar head of FDS originates from the medial epicondyle of the humerus, and EDC originates from the lateral epicondyle of the humerus. As a consequence, changes in elbow angle could potentially influence the length of FDS or/and EDC, respectively, and thus influence the force and the torque that the muscle(s) can generate. In a previous paper (Hoffmann et al. 2009), however, we have argued that the variation in FDS length with elbow angle is minimal, based on an estimation using a musculoskeletal model developed with the SIMM software (MusculoGraphics, Santa Rosa, CA). We obtained similar results for EDC, as the model estimated the difference in EDC musculotendon length between 0° and 90° of elbow flexion to be on the order of 1% of the minimum estimated EDC musculotendon length. From these estimations, we propose that the differences between posture 1 and posture 2 in the present study cannot be attributed merely to differences in FDS length or EDC length between the two arm postures. The differences between arm postures could, furthermore, potentially be attributed to fatigue of the subjects, given that all of the subjects, but two stroke subjects and one control subject, were tested in posture 1 first. However, if fatigue occurred between posture 1 and posture 2, one would expect it to affect both the MCPflex trials and the MCPext trials, whereas this was not observed (in stroke subjects, only mean MCPflex was affected by arm posture). Furthermore, the two stroke subjects who were tested in posture 2 first exhibited greater mean MCPflex in posture 1 than in posture 2, contrary to what would be expected if fatigue occurred.
Rather, the results of the present study suggest a modulation of distal motor output by static posture of the proximal upper limb in hemiparetic stroke subjects. In neurologically intact subjects, it has been shown that the corticospinal activation of distal upper limb muscles in response to transcranial magnetic stimulation under resting conditions can be modulated by static arm posture (Dominici et al. 2005; Ginanneschi et al. 2005, 2006). A similar modulating influence of static arm posture on distal motor output was observed in response to voluntary muscle activation, suggesting that static arm posture can influence the accessibility and recruitment of the corticospinal pathways during voluntary activation (Dominici et al. 2005). Weakness, which in stroke subjects, can affect both finger flexors and extensors (Cruz et al. 2005; Kamper et al. 2006), likely results from a direct reduction in the corticospinal drive from the affected hemisphere. It is possible that the significantly greater mean MCPflex observed in posture 1 in stroke subjects during the MCPflex trials in the present study reflects a greater ability to voluntarily activate finger flexor muscles when the arm is placed in posture 1 compared with posture 2 or in other words, a greater impairment in voluntary finger flexion in posture 2. Indeed, the mean value of MCPflex in stroke subjects was similar to the one in control subjects in posture 1, whereas it was smaller than the one in control subjects in posture 2. However, arm posture did not appear to affect the mean EMGnet activity of the targeted muscle FDS (FDSnet) in stroke subjects. Other, nonrecorded muscles, such as flexor digitorum profundus and dorsal and palmar interossei, may be involved.
Static arm posture may also modulate the activity of spinal circuits and thus indirectly modulate the motor output of a muscle or muscle groups in response to descending drive. In the studies by Dominici et al. (2005) and Ginanneschi et al. (2005) mentioned above, static arm posture had the same effect on the motor output of a distal upper limb muscle in response to transcranial magnetic stimulation and on the excitability of the Hoffmann's reflex response of that muscle. The results observed for the MCPflex trials in stroke subjects of the present study are comparable with those of a recent study (Hoffmann et al. 2009), which showed that the magnitude of the stretch reflex response of the spastic finger flexors in relaxed stroke subjects was greater in posture 1, both in terms of reflex MCPflex torque and in terms of reflex FDS activity. Taken together, our two studies suggest that the spinal excitability of finger flexors poststroke is increased in posture 1. Descending pathways influence the activity of spinal circuits, and an alteration in tonic descending synaptic input to motoneuron pools, potentially due to baseline changes in cortical excitation or inhibition after stroke, is thought to be involved in spasticity after stroke (Katz and Rymer 1989; Powers et al. 1988). Possibly altered descending influence on spinal activity could be involved in the modulation of both reflex activity and voluntary motor activity of finger flexors poststroke by static arm posture.
Stroke subjects and control subjects exhibited a similar mean difference in MCPext between posture 1 and posture 2, although a trend for mean MCPext to be greater in posture 1 was observed that was more pronounced in control subjects than in stroke subjects. However, with respect to control subjects, mean MCPext was reduced in stroke subjects in both arm postures, as opposed to the posture-dependent reduction observed for MCPflex. Increased coactivation between finger extensors and finger flexors may have limited MCPext torque in stroke subjects (Kamper et al. 2006; Kamper and Rymer 2001). In that respect, the mean activity of the direct antagonist FDS (FDSnet) of the targeted muscle EDC and the mean coactivation between EDC and FDS (EDCandFDS) were greater in stroke subjects than in control subjects and were not influenced by arm posture during the MCPext trials in stroke subjects in the present study (FDSnet: 0.17 ± 0.13 in posture 1 vs. 0.13 ± 0.09 in posture 2; EDCandFDS: 0.29 ± 0.21 vs. 0.27 ± 0.20), suggesting generalized exaggerated coactivation between finger extensors and finger flexors, i.e., independent of arm posture. MCPext was significantly positively correlated with the Fugl-Meyer scores of stroke subjects, indicating greater ability to voluntarily extend the fingers for less severely impaired stroke survivors. The observation of a trend, in control subjects compared with stroke subjects, for mean MCPext to be greater in posture 1, may suggest a more limited modulating influence of arm posture on voluntary MCPext than on voluntary MCPflex and a reduction of this influence after stroke. This reduction may be due to an intrinsic limit in the residual ability of stroke subjects to voluntarily activate finger extensor muscles. The observation that contrary to MCPext, MCPflex was not significantly positively correlated with the stroke subjects' Fugl-Meyer scores further suggests that the ability to voluntarily extend the fingers may be more dependent on impairment level than the ability to voluntarily flex the fingers, in accordance with previous studies reporting preferential impairment of voluntary finger extension (Cruz et al. 2005; Kamper et al. 2006). Taken together with the significantly smaller mean MCPflex observed in posture 2 compared with posture 1 in stroke subjects, the significant negative correlation between the Fugl-Meyer score and the difference between posture 1 and posture 2 in MCPflex suggests that more severely impaired stroke survivors may exhibit a posture-dependent impairment in voluntary finger flexion, namely reduced voluntary finger flexion with the elbow extended and the shoulder flexed. Less severely impaired individuals may tend toward being able to generate the same amount of voluntary finger flexion, regardless of elbow and shoulder posture, as appears to be the case in neurologically intact individuals.
Coupled activities of upper limb muscles.
Differences existed between stroke subjects and control subjects in terms of the patterns of upper limb muscle activities that accompanied the MCPflex trials and the MCPext trials. Stroke subjects appeared to exhibit excessive coactivation between proximal and distal upper limb muscles during both voluntary finger flexion and voluntary finger extension. This excessive proximal-distal coactivation appeared to be at least partly modulated by arm posture. In particular, posture 1 appeared to elicit elbow flexor activity in stroke subjects. Furthermore, reduced task specificity appeared to exist in stroke subjects, both for the targeted muscles (FDS and EDC, respectively) and for nontargeted muscles. The results of the present study suggest an alteration in the effect of descending drive associated with distal voluntary motor activity on upper limb muscle activity and in the modulation of voluntary upper limb motor activity by static arm posture in stroke subjects.
The patterns of upper limb muscle activities that accompanied the MCPflex trials and the MCPext trials in stroke subjects in the present study could be associated with the abnormal coupling of the activities of specific upper limb muscle groups during voluntary motor activity often observed after stroke, in particular, between the shoulder and the elbow (Beer et al. 1999; Dewald and Beer 2001; Dewald et al. 1995; Sangani et al. 2009). Stereotypical muscle activation patterns of “flexor synergy”, characterized notably by shoulder abduction and external rotation and elbow flexion, or “extensor synergy”, characterized notably by shoulder adduction and internal rotation and elbow extension (Brunnstrom 1970), could be involved in the greater activity of nontargeted upper limb muscles during voluntary motor activity at the MCP joints in stroke subjects in the present study. The differences in patterns of upper limb muscle activities observed between arm postures could then reflect a modulation of abnormal coupling by static arm posture. Such a modulation has been reported previously between the shoulder and the elbow (Ellis et al. 2007). The MCPext trials exhibited a significant positive correlation between the stroke subjects' Fugl-Meyer scores and the activity of upper limb flexors (FDS, B, and BB). It is possible that stroke survivors with a higher Fugl-Meyer score are more able to voluntarily extend their fingers, but that this greater ability comes at the cost of an increase in unwanted activation of muscles throughout the upper limb and of upper limb flexors in particular, possibly as an inability to “move out of synergy” and individuate muscle activations.
Whereas the present study did not directly investigate neural pathways, it is informative to consider prior studies that may be relevant to our findings regarding the coupled activities of upper limb muscles in stroke subjects. Stroke may result in alterations in regulatory mechanisms at the cortical level, leading to abnormal coupling of muscle activities (Gerachshenko et al. 2008; Lum et al. 2003). For instance, it has been suggested that disruption of precontraction suppression of antagonist activity is involved in abnormal BB activity during voluntary forearm pronation poststroke (Gerachshenko et al. 2008). As a consequence of the loss of corticospinal pathways, voluntary motor activity in stroke subjects may involve increased reliance on alternative, residual descending pathways. Increased reliance on brainstem pathways has been suggested to underlie the emergence of abnormal coupling between upper limb muscles or muscle groups after stroke (Schwerin et al. 2008). Ellis and coworkers (2007) observed a modulating effect of static shoulder posture on the abnormal coupling between shoulder adduction and elbow extension after stroke and suggested that static arm posture can modulate the balance between descending influence from reticulospinal pathways, potentially favoring upper limb flexion, and from vestibulospinal pathways, potentially favoring upper limb extension. In the macaque monkey, the reticulospinal tract has been shown to facilitate ipsilateral flexor muscles of the shoulder, the elbow, and the wrist (Davidson and Buford 2004, 2006) and to make excitatory ipsilateral connections to motoneurons projecting to distal upper limb muscles, including hand muscles (Riddle et al. 2009). Increased use of reticulospinal pathways after stroke and modulation of their descending influence by static arm posture could potentially be involved in some of the observations of the present study and specifically, the greater activity of nontargeted elbow flexors and possibly, the greater mean MCPflex in posture 1 in stroke subjects. There is evidence that in parallel with its transmission via the monosynaptic corticospinal pathways, the descending corticospinal drive to upper limb motoneurons in humans is in part transmitted via a system of propriospinal interneurons located at the cervical level of the spinal cord (Pierrot-Deseilligny 1996, 2002). These interneurons are thought to have divergent projections onto motoneurons of multiple upper limb muscles (Mazevet and Pierrot-Deseilligny 1994) and may therefore be involved in coupling of muscles throughout the upper limb. The part of the corticospinal drive that is supposed to be transmitted via this propriospinal system, has been shown to be increased after stroke (Mazevet et al. 2003; Pierrot-Deseilligny 1996; Stinear and Byblow 2004), possibly resulting in increased coupling of upper limb muscle activities (Mazevet et al. 2003; Pierrot-Deseilligny 2002).
Absence of effect of proximal electrical stimulation on voluntary MCPflex and MCPext.
In the present study, proximal electrical stimulation had no effect on the production of maximum voluntary isometric torque about the MCP joints in stroke subjects or in control subjects, neither for MCPflex nor for MCPext.
Conversely, in a previous study (Hoffmann et al. 2009), proximal electrical stimulation modulated the magnitude of the stretch reflex response of the spastic finger flexors in relaxed hemiparetic stroke survivors. Specifically, fast imposed extension of the MCP joints elicited greater reflex MCPflex torque during stimulation of BB than when no stimulation was applied or during stimulation of TB. No effect of proximal electrical stimulation was observed for neurologically intact control subjects preactivating their finger flexors (unpublished observations). The combined results of the previous study and the present one suggest that in the upper limb poststroke, proximal electrical stimulation can influence distal reflex activity but may not influence distal voluntary motor activity. One potential explanation for this discrepancy may be a difference in finger flexor motoneuron recruitment. In the previous study, stroke subjects were relaxed, such that motoneurons were presumably not recruited before the onset of the imposed MCPext. It is possible that in stroke subjects, BB stimulation increases the excitability of motoneurons at rest by lowering their recruitment threshold and that this results in additional recruitment of motoneurons in response to imposed MCPext. In the present study, subjects produced maximum voluntary isometric contraction, and it is possible that BB stimulation increased the excitability of motoneurons already being voluntarily recruited by stroke subjects without BB stimulation and thus did not increase muscle activation. The motoneurons involved could be motoneurons with lower recruitment threshold (Calancie and Bawa 1984). In control subjects, BB stimulation may not influence the excitability of motoneurons, or the influence may exist but have no effect, because the motoneurons that are influenced are already voluntarily recruited, both in the situation of preactivation in the previous study and in the situation of maximum voluntary isometric contraction in the present study. An alternative explanation for the discrepancy between the two studies is based on the evidence that peripheral afferents do not exert presynaptic inhibition on descending motor pathways (Berardelli et al. 1987; Jackson et al. 2006; Nielsen and Petersen 1994) and that the influence of peripheral afferent input on spinal motor circuits is reduced during voluntary motor activity (Seki et al. 2003). This may prevent an influence of proximal electrical stimulation on voluntary distal upper limb motor activity in the present study.
Conclusion.
The present study provides evidence for a modulating effect of static arm posture on voluntary distal upper limb motor activity in hemiparetic stroke subjects. Static arm posture also modulated the activities of upper limb muscles that accompanied voluntary distal upper limb motor activity, with differences between stroke subjects and neurologically intact control subjects in both the coupling patterns of muscle activities and the effect of arm posture on these patterns. The results of the present study could potentially open possibilities for upper limb rehabilitation strategies after stroke, involving manipulation of static posture of upper limb joints. In that respect, further study is warranted to investigate how effects such as the ones observed in the present study, may impact the ability of hemiparetic stroke survivors to perform functional movements of the fingers, the hand, and the arm.
GRANTS
Support for this work was provided by the National Institute of Neurological Disorders and Stroke (Grant R01-NS052509 to B. D. Schmit) and the National Institute on Disability and Rehabilitation Research (Grant H133P040007 to Zev Rymer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Bridget Iwamuro for her help and technical assistance with the experiments and Hua Chen and Kristen Triandafilou for their assistance with data interpretation.
Present address of J. H. Kahn: Department of Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611.
REFERENCES
- Alexander CM, Harrison PJ. Reflex connections from forearm and hand afferents to shoulder girdle muscles in humans. Exp Brain Res 148: 277–282, 2003 [DOI] [PubMed] [Google Scholar]
- Balogun JA, Akomolafe CT, Amusa LO. Grip strength: effects of testing posture and elbow position. Arch Phys Med Rehabil 72: 280–283, 1991 [PubMed] [Google Scholar]
- Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil 80: 766–772, 1999 [DOI] [PubMed] [Google Scholar]
- Berardelli A, Day BL, Marsden CD, Rothwell JC. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol 391: 71–83, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnstrom S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. New York: Harper & Row, 1970 [Google Scholar]
- Calancie B, Bawa P. Recruitment order of motor units during the stretch reflex in man. Brain Res 292: 176–178, 1984 [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res 78: 465–478, 1989 [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R, Pénicaud A. Pattern of projections of group I afferents from elbow muscles to motoneurones supplying wrist muscles in man. Exp Brain Res 91: 311–319, 1992 [DOI] [PubMed] [Google Scholar]
- Cruz EG, Waldinger HC, Kamper DG. Kinetic and kinematic workspaces of the index finger following stroke. Brain 128: 1112–1121, 2005 [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006 [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers J, Bravo G, Hebert R, Mercier L. Impact of elbow position on grip strength of elderly men. J Hand Ther 8: 27–30, 1995 [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 24: 273–283, 2001 [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995 [DOI] [PubMed] [Google Scholar]
- Dominici F, Popa T, Ginanneschi F, Mazzocchio R, Rossi A. Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp Brain Res 164: 500–504, 2005 [DOI] [PubMed] [Google Scholar]
- Ellis MD, Acosta AM, Yao J, Dewald JP. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res 176: 594–602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Gerachshenko T, Rymer WZ, Stinear JW. Abnormal corticomotor excitability assessed in biceps brachii preceding pronator contraction post-stroke. Clin Neurophysiol 119: 683–692, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res 161: 374–382, 2005 [DOI] [PubMed] [Google Scholar]
- Ginanneschi F, Dominici F, Biasella A, Gelli F, Rossi A. Changes in corticomotor excitability of forearm muscles in relation to static shoulder positions. Brain Res 1073–1074: 332–338, 2006 [DOI] [PubMed] [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper limb motoneurones. J Physiol 434: 151–167, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G, Kamper DG, Kahn JH, Rymer WZ, Schmit BD. Modulation of stretch reflexes of the finger flexors by sensory feedback from the proximal upper limb poststroke. J Neurophysiol 102: 1420–1429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Baker SN, Fetz EE. Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. J Physiol 573: 107–120, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper DG, Fischer HC, Cruz EG, Rymer WZ. Weakness is the primary contributor to finger impairment in chronic stroke. Arch Phys Med Rehabil 87: 1262–1269, 2006 [DOI] [PubMed] [Google Scholar]
- Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve 28: 309–318, 2003 [DOI] [PubMed] [Google Scholar]
- Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve 24: 673–681, 2001 [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawanishi M, Yahagi S. Effects of upper limb muscle vibration on human voluntary wrist flexion-extension movements. Percept Mot Skills 78: 43–47, 1994 [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawanishi M, Yahagi S. The effects of wrist muscle vibration on human voluntary elbow flexion-extension movements. Exp Brain Res 90: 217–220, 1992 [DOI] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989 [PubMed] [Google Scholar]
- Kuzala EA, Vargo MC. The relationship between elbow position and grip strength. Am J Occup Ther 46: 509–512, 1992 [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve 27: 211–221, 2003 [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg Am 10: 694–697, 1985 [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain 126: 988–1000, 2003 [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in humans. Acta Physiol Scand 150: 27–38, 1994 [DOI] [PubMed] [Google Scholar]
- McClelland VM, Miller S, Eyre JA. Short latency heteronymous excitatory and inhibitory reflexes between antagonist and heteronymous muscles of the human shoulder and upper limb. Brain Res 899: 82–93, 2001 [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol 477: 47–58, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford KL. Elbow positioning for maximum grip performance. J Hand Ther 13: 33–36, 2000 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve 26: 155–172, 2002 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol 48: 489–517, 1996 [DOI] [PubMed] [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Liu D. Maximum handgrip force in relation to upper limb posture—a meta-analysis. AIHA J (Fairfax, Va) 64: 609–617, 2003 [DOI] [PubMed] [Google Scholar]
- Sangani SG, Starsky AJ, McGuire JR, Schmit BD. Multijoint reflex responses to constant-velocity volitional movements of the stroke elbow. J Neurophysiol 102: 1398–1410, 2009 [DOI] [PubMed] [Google Scholar]
- Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185: 509–519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003 [DOI] [PubMed] [Google Scholar]
- Stegink Jansen CW, Simper VK, Stuart HG, Jr, Pinkerton HM. Measurement of maximum voluntary pinch strength: effects of forearm position and outcome score. J Hand Ther 16: 326–336, 2003 [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol 21: 426–434, 2004 [DOI] [PubMed] [Google Scholar]
- Su CY, Lin JH, Chien TH, Cheng KF, Sung YT. Grip strength: relationship to shoulder position in normal subjects. Gaoxiong Yi Xue Ke Xue Za Zhi 9: 385–391, 1993 [PubMed] [Google Scholar]
- Su CY, Lin JH, Chien TH, Cheng KF, Sung YT. Grip strength in different positions of elbow and shoulder. Arch Phys Med Rehabil 75: 812–815, 1994 [PubMed] [Google Scholar]
- Trombly CA. Stroke. In: Occupational Therapy for Physical Dysfunction, edited by Trombly CA. Baltimore, MD: Williams & Wilkins, 1989 [Google Scholar]
- Trombly CA, Thayer-Nason L, Bliss G, Girard CA, Lyrist LA, Brexa-Hooson A. The effectiveness of therapy in improving finger extension in stroke patients. Am J Occup Ther 40: 612–617, 1986 [DOI] [PubMed] [Google Scholar]