Abstract
Layer 6 (L6) of primary sensory cortices is distinct from other layers in that it provides a major cortical input to primary sensory thalamic nuclei. L6 pyramidal neurons in the primary visual cortex (V1) send projections to the lateral geniculate nucleus (LGN), as well as to the thalamic reticular nucleus and higher order thalamic nuclei. Although L6 neurons are proposed to modulate the activity of thalamic relay neurons, how sensory experience regulates L6 neurons is largely unknown. Several days of visual deprivation homeostatically adjusts excitatory synapses in L4 and L2/3 of V1 depending on the developmental age. For instance, L4 exhibits an early critical period during which visual deprivation homeostatically scales up excitatory synaptic transmission. On the other hand, homeostatic changes in L2/3 excitatory synapses are delayed and persist into adulthood. In the present study we examined how visual deprivation affects excitatory synapses on L6 pyramidal neurons. We found that L6 pyramidal neurons homeostatically increase the strength of excitatory synapses following 2 days of dark exposure (DE), which was readily reversed by 1 day of light exposure. This effect was restricted to an early critical period, similar to that reported for L4 neurons. However, at a later developmental age, a longer duration of DE (1 wk) decreased the strength of excitatory synapses, which reversed to normal levels with light exposure. These changes are opposite to what is predicted from the homeostatic plasticity theory. Our results suggest that L6 neurons differentially adjust their excitatory synaptic strength to visual deprivation depending on the age of the animals.
Keywords: dark exposure, development, homeostatic plasticity, synaptic scaling, visual deprivation
experience-dependent synaptic plasticity in sensory cortices is widely accepted to be essential for developmental fine-tuning and adaptation of cortical circuits to ongoing changes in the sensory environment. Whereas input-specific synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), rapidly adjusts the synapses in response to ongoing neural activity, homeostatic mechanisms are thought to provide stability to the neural network by acting on global cell-wide variables. Experience-induced homeostatic synaptic changes are considered especially important during postnatal development, where they provide stability to the developing neural circuit that is constantly being adjusted to the environment. One form of homeostatic plasticity is global homeostatic synaptic scaling (Turrigiano et al. 1998; Turrigiano and Nelson 2004), in which a period of inactivity results in scaling up of excitatory synaptic strength, whereas increased activity scales it down. Several studies showed that visual deprivation scales up excitatory synapses in primary visual cortex (V1), but such changes happen at distinct periods during postnatal development depending on the lamina. For instance, layer 4 (L4) neurons show an early critical period for synaptic scaling, which starts a few days after eye opening at around age postnatal day 16 (P16) and ends within a few days (by P21) (Desai et al. 2002). On the other hand, in L2/3 neurons, homeostatic synaptic scaling starts later at around P21 (Desai et al. 2002) and persists into adulthood (Goel and Lee 2007). A recent study showed that in L5, visual deprivation suppresses intrinsic excitability of pyramidal neurons and promotes high-frequency firing-induced LTP of intrinsic excitability (Nataraj et al. 2010). This contrasts a lack of change in intrinsic excitability of L4 neurons (Maffei et al. 2004). Collectively, these results suggest that principal neurons in different layers of V1 undergo distinct homeostatic regulation with visual deprivation. In this study, we examined whether and how visual experience alters excitatory synapses on L6 pyramidal neurons.
In a canonical circuit of V1, L6 is similar to L4 in that it receives direct thalamocortical inputs as well as processed intracortical inputs (Binzegger et al. 2004; Burkhalter 1989; da Costa and Martin 2009; LeVay and Gilbert 1976; Ribak and Peters 1975; Zarrinpar and Callaway 2006). However, L6 differs from L4 in that one of its outputs targets the dorsal lateral geniculate nucleus (dLGN), often with collaterals innervating the thalamic reticular complex, and a subset of neurons targets higher order thalamic nuclei (Bourassa and Deschenes 1995). There is evidence that corticogeniculate inputs originating from L6 modulate sensory processing of LGN neurons in diverse species (Briggs and Usrey 2009; de Labra et al. 2007; Marrocco et al. 1996; McClurkin and Marrocco 1984). Despite their proposed role in shaping visual processing in LGN (Thomson 2010), there is little information as to how L6 neurons alter their synapses following alterations in visual experience. Here we report that the changes in visual experience lead to differential regulation of excitatory synapses of L6 pyramidal neurons depending on the developmental age of the animal.
MATERIALS AND METHODS
Animals.
C57BL/6 mice (Jackson Laboratories) were raised in a normal light condition (12:12-h light-dark cycle). Dark exposure (DE) was initiated at P14 or P21 for durations of 2 or 7 days. Age-matched control normal-reared (NR) animals remained in the normal light condition. Animals in the dark were cared for using infrared vision goggles under dim infrared (IR) light. A select group of DE mice were reexposed to normal light condition for 1 day to study the effect of reexposure to light (LE). All experiments were approved by the University of Maryland Institutional Animal Care and Use Committee and followed the guidelines of the Animal Welfare Act.
Visual cortical slice preparation.
Mice were deeply anesthetized using isoflurane vapors. The brain was then quickly removed and immersed in ice-cold dissection buffer (in mM: 212.7 sucrose, 10 dextrose, 3 MgCl2, 1 CaCl2, 2.6 KCl, 1.23 NaH2PO4, and 26 NaHCO3) bubbled with 95% O2-5% CO2 mixture. Blocks containing primary visual cortices were dissected and coronally sectioned into 300-μm-thick slices using a Vibratome 3000 Plus microslicer (Ted Pella, Redding, CA). The slices were then transferred to a holding chamber with artificial cerebrospinal fluid (ACSF; composition in mM: 124 NaCl, 5 KCl, 1.23 NaH2PO4·H20, 26 NaHCO3, 10 dextrose, 1.5 MgCl2, and 2.5 CaCl2; saturated with 95% O2-5% CO2). The slices were then allowed to recover for 1 h at room temperature before use for recording.
Electrophysiology.
Slices were transferred to a submersion-type recording chamber mounted on a fixed stage of an upright microscope (E600 FN; Nikon, Tokyo, Japan) with IR oblique illumination. AMPA receptor (AMPAR)-mediated miniature excitatory postsynaptic currents (mEPSCs) were pharmacologically isolated by adding 1 μM tetrodotoxin, 20 μM bicuculline, and 100 μM dl-2-amino-5-phosphonopentanoic acid to ACSF (30 ± 1°C, saturated with 95% O2-5% CO2), which was continually perfused at a rate of 2 ml/min. Target cells in L6 were identified by the pyramid-shaped soma with the apical dendrite pointing toward the pia. These neurons were patched using a whole cell patch pipette (tip resistance 3–5 MΩ), which was filled with internal solution (in mM: 130 Cs-gluconate, 8 KCl, 1 EGTA, 10 HEPES, 4 ATP, and 5 QX-314; pH 7.4, 285–295 mosmol/l). Recording was initiated 2–3 min after break-in, and each cell was recorded for 8–10 min to collect enough mEPSCs for analysis. Biocytin (1 mg/ml) was included in the internal solution to confirm the morphology and location of a subset of the recorded neurons. All of the reconstructed neurons (n = 14) were identified as pyramidal based on their soma morphology and prominent apical dendrites with spines. Only one of these was excluded from analysis, because it was identified as a L5 pyramidal neuron. The Axon patch-clamp amplifier 700B (Molecular Devices, Union City, CA) was used for voltage-clamp recordings. Cells were held at −80 mV, and the recorded mEPSC data were digitized at 10 kHz with a data acquisition board (National Instruments, Austin, TX) and acquired through custom-made programs using the Igor Pro software (WaveMetrics, Lake Oswego, OR). The MiniAnalysis program (Synaptosoft, Decatur, GA) was used to analyze the acquired mEPSCs. The threshold for detecting mEPSCs was set at three times the root mean square (RMS) noise. There was no significant difference in the RMS noise across the groups [P16: NR = 1.5 ± 0.08 (n = 10), DE = 1.6 ± 0.09 (n = 10), LE = 1.5 ± 0.08 (n = 9); ANOVA, F(2,26) = 0.56, P > 0.58; P24: NR = 1.7 ± 0.07 (n = 10), DE = 1.6 ± 0.06 (n = 10); t-test, P > 0.11; P28: NR = 1.5 ± 0.04 (n = 12), DE = 1.6 ± 0.07 (n = 14), LE = 1.5 ± 0.04 (n = 13); ANOVA, F(2,36) = 0.48, P > 0.63]. Recordings were excluded from analysis if the RMS noise was >2, the series resistance was >25 MΩ, and input resistance was <100 MΩ. To minimize the impact of dendritic filtering, we adopted the standard approach of excluding mEPSCs with rise time >3 ms, as well as cells showing a negative correlation between mEPSC amplitude and rise time (Rall 1969). Only about 4% of the total recorded cells (at most 1 cell per experimental group) were excluded due to negative correlation between mEPSC rise and amplitude. Two hundred consecutive mEPSCs that met the rise time criteria were analyzed from each cell. However, removing the rise time cutoff criteria did not alter the average mEPSC amplitude values (data not shown). Data are means ± SE. One-factor analysis of variance (ANOVA) was used for statistical comparison of data across multiple groups, Student's t-test was used for two-group comparisons, and the Kolmogorov-Smirnov test was used for comparison of cumulative probabilities. For all statistical tests, P < 0.05 was considered statistically significant.
Biocytin processing.
Visual cortex slices (300 μm thick) were fixed in 4% paraformaldehyde overnight at 4°C. Slices were then rinsed two times for 10 min each in 0.1 M phosphate buffer (PB: 19 mM NaH2PO4·H2O, and 81 mM Na2HPO4) at room temperature and permeabilized in 2% Triton X-100 in 0.1 M PB for 1 h. Slices were incubated in the dark overnight at 4°C in avidin-AlexaFluor 488 conjugate diluted 1:2,000 in 1% Triton X-100–0.1 M PB. Slices were kept in the dark as they were rinsed two times for 10 min each in 0.1 M PB. Slices were then mounted on precleaned glass slides and allowed to dry overnight, again in the dark, before they were coverslipped with mounting solution (ProLong antifade; Invitrogen) and sealed with nail polish. Fluorescence signals were detected using a Leica SP5X confocal laser scanning microscope under a ×20 multi-immersion objective lens (NA = 0.7, free working distance = 260 μm). Z-stacked images were taken at 3-μm intervals and analyzed using the image analysis program Volocity (Improvision) to visualize the location and morphology of the labeled cell.
RESULTS
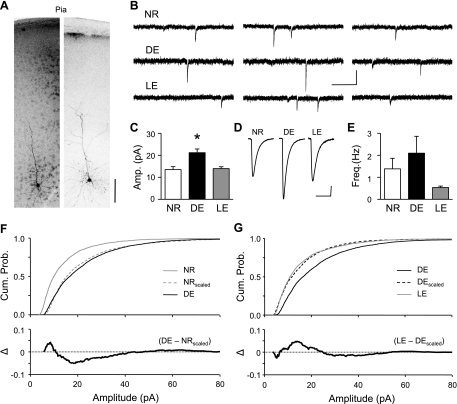
Visual deprivation scales up excitatory synapses in L2/3 and L4 of V1, but with distinct critical periods. Synaptic scaling in L4 has an early critical period that closes by P21 (Desai et al. 2002), whereas in L2/3 it starts by P21 (Desai et al. 2002) and persists into adulthood (Goel and Lee 2007). To determine whether L6 neurons undergo homeostatic synaptic changes during a defined critical period, we dark-exposed (DE) mice for a few days (2 or 7 days) starting at different ages (P16 or P21) and measured AMPAR-mediated mEPSCs in visually identified L6 pyramidal neurons. A subset (∼15%) of the cells was filled with biocytin to confirm their location in L6 and identify the pyramid-shaped morphology of their soma (Fig. 1A). On average, the soma of the reconstructed L6 pyramidal neurons were located 775 ± 54 μm away from the pia (n = 13), and all had a prominent apical dendrite with visible dendritic spines.
Fig. 1.
Brief manipulations of visual experience homeostatically regulate miniature excitatory postsynaptic currents (mEPSCs) of L6 neurons of postnatal day 16 (P16) mice. A: examples of biocytin-filled L6 pyramidal neurons in V1. Fluorescent images of processed V1 slices are shown in inverted grayscale for better visualization of the filled neurons. The images are projected images of z-stacks (40 stacks at 3-μm intervals). Scale bar: 100 μm. B: representative mEPSC traces from a normal-reared (NR; 3 traces at top), a dark-exposed (DE; 3 traces at middle), and a light-exposed cell (LE; 3 traces at bottom). Each trace is 1 s in duration. Scale bars: 20 pA, 250 ms. C: average mEPSC amplitude increased with 2 days of DE and reversed after 1 day of LE. *P < 0.001 [1-way ANOVA followed by Fisher's protected least significant difference (PLSD) post hoc test, P < 0.002]. D: average mEPSC traces. Scale bars: 3 pA, 15 ms. E: average mEPSC frequency was not significantly changed. F, top: cumulative probability graph showing mEPSCs of NR (solid gray line) are smaller than those of DE (solid black line). NRscaled (dashed gray line) represents NR mEPSCs scaled up by a scaling factor of 1.54 to match the average mEPSC amplitude to that of DE. Note that the cumulative probability curves of NRscaled and DE are significantly different (Kolmogrov-Smirnov test, P < 0.005), which suggests that the change is not multiplicative. Bottom: subtraction of cumulative probability graphs of DE and NRscaled to illustrate the nonmultiplicative change (Δ = DE − NRscaled). G, top: cumulative probability graph demonstrating that 1 day of LE (gray line) decreased mEPSC amplitudes compared with DE (black line) levels. Scaling factor is 0.71 for the DEscaled (dashed line). The cumulative probability curves of DEscaled and LE are significantly different (Kolmogrov-Smirnov test, P < 0.02). Bottom: subtraction of cumulative probability graphs of LE and DEscaled (Δ = LE − DEscaled). Amp., amplitude; Freq., frequency; Cum. Prob., cumulative probability.
Dark exposure increases AMPA receptor-mediated mEPSC amplitude in L6 of P16 mice.
DE for 2 days starting at P16 significantly increased the average mEPSC amplitude in L6 pyramidal neurons, which was reversed with 1 day of LE [P16: NR, 13.5 ± 1.4 pA, n = 10 cells from 5 mice; DE, 21 ± 1.7 pA, n = 10 cells from 7 mice; LE, 13.9 ± 0.8 pA, n = 9 cells from 5 mice; ANOVA, F(2,26) = 9.98, P < 0.001] (Fig. 1, B–D). The decrease in mEPSC amplitude in the 1-day LE group was accompanied by a significant increase in mEPSC decay kinetics (Table 1), which suggests changes in AMPAR function (Mosbacher et al. 1994). On the other hand, there was no significant difference in either the average mEPSC frequency [P16: NR, 1.4 ± 0.5 Hz, n = 10; DE, 2.1 ± 0.8 Hz, n = 10; LE, 0.5 ± 0.07 Hz, n = 9; ANOVA, F(2,26) = 2.04, P > 0.15] (Fig. 1E) or the general cell properties (Table 1) among the three groups.
Table 1.
Neuronal properties
| Age, postnatal day | Conditions | n | Rise Time, ms | τ, ms | Rin, MΩ | Rser, MΩ |
|---|---|---|---|---|---|---|
| P16 | NR | 10 | 1.7 ± 0.08 | 3.9 ± 0.3 | 439 ± 70 | 22.6 ± 1.1 |
| 2d-DE | 10 | 1.4 ± 0.11 | 3.5 ± 0.4 | 487 ± 93 | 23.5 ± 0.6 | |
| 1d-LE | 9 | 2.0 ± 0.06† | 4.8 ± 0.2‡ | 518 ± 72 | 23.8 ± 0.7 | |
| P23 | NR | 10 | 1.8 ± 0.07 | 3.7 ± 0.3 | 362 ± 31 | 20.5 ± 0.9 |
| 2d-DE | 10 | 1.8 ± 0.02 | 4.5 ± 0.3* | 395 ± 80 | 21.9 ± 1.3 | |
| P28 | NR | 12 | 1.6 ± 0.07 | 3.5 ± 0.2 | 405 ± 62 | 24.1 ± 0.5 |
| 7d-DE | 14 | 1.7 ± 0.07 | 4.2 ± 0.3§ | 394 ± 58 | 22.7 ± 0.9 | |
| 1d-LE | 13 | 1.7 ± 0.08 | 3.1 ± 0.2 | 264 ± 47 | 23.7 ± 0.4 |
Data are means ± SE for neuronal properties determined at the age of recording (n = no.of neurons). τ, Decay time constant; Rin, input resistance; Rser, series resistance. Statistics:
P < 0.05, t-test.
P < 0.002, 1-way ANOVA; Fisher's protected least significant difference (PLSD) post hoc test: P < 0.03 between NR and 1d-LE, P < 0.001 between 2d-DE and 1d-LE.
P < 0.03, 1-way ANOVA; Fisher's PLSD post hoc test: P < 0.01 between 2d-DE and 1d-LE.
P < 0.02, 1-way ANOVA; Fisher's PLSD post hoc test: P < 0.01 between 7d-DE and 1d-LE.
Previously, we reported that in L2/3 neurons, the DE-induced scaling up of mEPSCs follows the rules of multiplicative synaptic scaling (Gao et al. 2010; Goel et al. 2006; Goel and Lee 2007). The interesting property of multiplicative synaptic scaling is that it allows preservation of relative differences in synaptic strength across synapses despite global changes across all synapses (Turrigiano et al. 1998; Turrigiano and Nelson 2004). To test whether the DE-induced scaling in L6 is multiplicative, we compared the cumulative probability curve of mEPSC amplitude of DE with that of NR mEPSCs scaled up by multiplication with a factor of 1.54 (NRscaled) to match the average mEPSC to that of DE. We found that the cumulative probability of mEPSCs from DE and NRscaled are significantly different (Kolmogorov-Smirnov test, P < 0.005), suggesting that the change is not multiplicative (Fig. 1F). This was also the case for DE and LE groups (DEscaled: scaling factor 0.71; Kolmogorov-Smirnov test, P < 0.02) (Fig. 1G). These results suggest that visual experience-dependent homeostatic plasticity does not affect all synapses in L6 neurons equally.
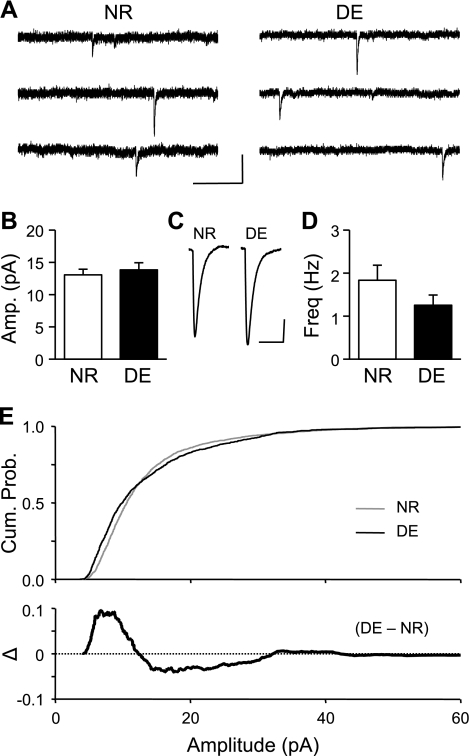
Lack of homeostatic synaptic plasticity in L6 of P21 mice.
Next, we determined whether DE-induced changes in L6 neurons are restricted to an early critical period, as in L4 (Desai et al. 2002). To test this, mice were dark-exposed for 2 days from P21 to P23. In contrast to younger mice, at this later age 2 days of DE did not significantly change either the average amplitude of the mEPSCs [P23: NR, 13.1 ± 0.9 pA, n = 10 cells from 5 mice; DE, 13.8 ± 1.1 pA, n = 10 cells from 7 mice; t-test, P > 0.59] (Fig. 2, A–C) or their average frequency [P23: NR, 1.8 ± 0.3 Hz, n = 10; DE, 1.3 ± 0.2 Hz, n = 10; t-test, P > 0.19] (Fig. 2D). Interestingly, however, DE significantly altered the amplitude distribution of the mEPSC amplitudes (Kolmogorov-Smirnov test, P < 0.0001) (Fig. 2E, top). In particular, there was an increase in the fraction of smaller and larger mEPSCs at the expense of medium-sized mEPSCs in the DE group compared with NR (Fig. 2E, bottom). This suggests that excitatory synapses on L6 neurons are malleable with visual deprivation at this later age, but the direction and magnitude of changes balance each other such that there is no net alteration in average synaptic weight. In any case, our results support the idea that L6 and L4 share similarities of having an early critical period for homeostatic synaptic plasticity with brief duration of DE.
Fig. 2.
Two days of DE initiated at P21 fails to change the average mEPSC amplitude in L6 neurons. A: representative mEPSC traces from NR (3 traces at left) and DE cells (3 traces at right). Scale bars: 20 pA, 250 ms. B: no significant change in average mEPSC amplitudes of NR and 2-day DE. C: average mEPSC traces. Scale bars: 3 pA, 15 ms. D: no significant change in average mEPSC frequency between NR and 2-day DE. E, top: comparison of mEPSC cumulative probability of NR (gray line) and DE (black line). The cumulative probability curves of NR and D are significantly different (Kolmogorov-Smirnov test, P < 0.0001). Bottom: subtraction of cumulative probability graphs of DE and NR (Δ = DE − NR).
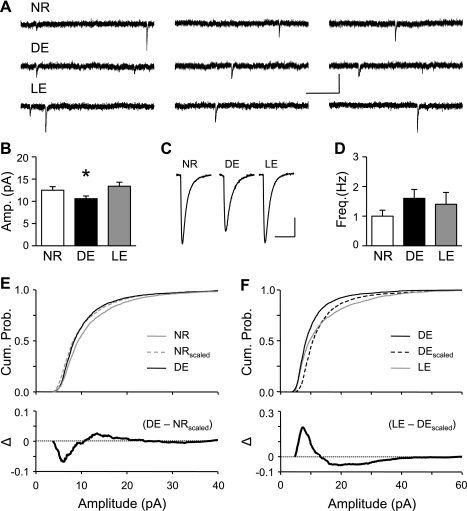
A longer duration of DE decreases L6 mEPSCs in P21 mice.
To determine whether the absence of homeostatic synaptic plasticity in L6 at later ages is due to a complete termination of the plasticity mechanisms or to a requirement of a longer duration of visual deprivation, we repeated the study using 7 days of DE (7d-DE) initiated at P21. Surprisingly, we found that the longer duration of DE now decreased the average mEPSC amplitude in L6 neurons, which reversed back to normal levels with 1 day of LE [P28: NR, 12.5 ± 0.8 pA, n = 12 cells from 7 mice; DE, 10.6 ± 0.6 pA, n = 14 cells from 8 mice; LE, 13.4 ± 0.9 pA, n = 13 cells from 5 mice; ANOVA F(2,36) = 3.382, P < 0.05] (Fig. 3, A–C). There was no significant change in mEPSC frequency [P28: NR, 1.0 ± 0.2 Hz, n = 12; DE, 1.6 ± 0.3 Hz, n = 14; 1d-LE, 1.4 ± 0.4, n = 13; ANOVA F(2,36) = 1.105, P > 0.34] (Fig. 3D), suggesting a postsynaptic change. The 7d-DE group showed an increase in the mEPSC decay time constant (τ), which reversed back to NR levels with 1-day of LE (Table 1). This further corroborates postsynaptic regulation of AMPAR function. The decrease in mEPSC amplitude following 7d-DE was not multiplicative in nature, because the cumulative probability curve of mEPSCs of NR scaled down with a scaling factor of 0.85 (NRscaled) was significantly different from that of DE (Kolmogorov-Smirnov test, P < 0.0001) (Fig. 3E). Reexposure to light for 1 day after 7d-DE was sufficient to increase the mEPSC amplitude to NR levels with a scaling factor of 1.26, but again in a nonmultiplicative manner (Kolmogorov-Smirnov test, P < 0.0001) (Fig. 3F). These data suggest that later in development, L6 neurons respond to a longer duration of visual deprivation by decreasing the strength of their excitatory synapses, but this novel form of synaptic plasticity is in an opposite direction to what is predicted from the homeostatic synaptic scaling hypothesis.
Fig. 3.
L6 neurons undergo nonhomeostatic regulation of mEPSCs with 7 days of DE initiated at P21. A: representative mEPSC traces from NR (3 traces at top), DE (3 traces at middle), and LE cells (3 traces at bottom). Scale bars: 20 pA, 250 ms. B: average mEPSC amplitude significantly decreased with 7 days DE, which reversed with 1 day of LE. *P < 0.05 (1-way ANOVA, followed by Fisher's PLSD post hoc test, P < 0.002). C: average mEPSC traces. Scale bars: 3 pA, 15 ms. D: no significant change in mEPSC frequency across NR, 7-day DE, and 1-day LE. E, top: cumulative probability of mEPSC amplitudes from NR (solid gray line) and 7-day DE (solid black line) groups. The curve for NRscaled (dashed gray line) represents mEPSCs of NR that were scaled down by a scaling factor of 0.84 to match the average mEPSC amplitude to that of DE. There was a significant difference between NRscaled and DE (Kolmogorov-Smirnov test, P < 0.0001), suggesting a nonmultiplicative change in synaptic strength. Bottom: subtraction of cumulative probability graphs of DE and NRscaled (Δ = DE − NRscaled). F, top: cumulative probability of mEPSC amplitudes of 7-day DE (solid black line), 1-day LE (solid gray line), and DEscaled (dashed black line). mEPSC amplitudes of DE were multiplied by a scaling factor of 1.37 to obtain DEscaled, which matched in average mEPSC amplitude to that of LE. There was a statistically significant difference between LE and DEscaled (Kolmogorov-Smirnov test, P < 0.0001). Bottom: subtraction of cumulative probability graphs of LE and DEscaled (Δ = LE − DEscaled).
DISCUSSION
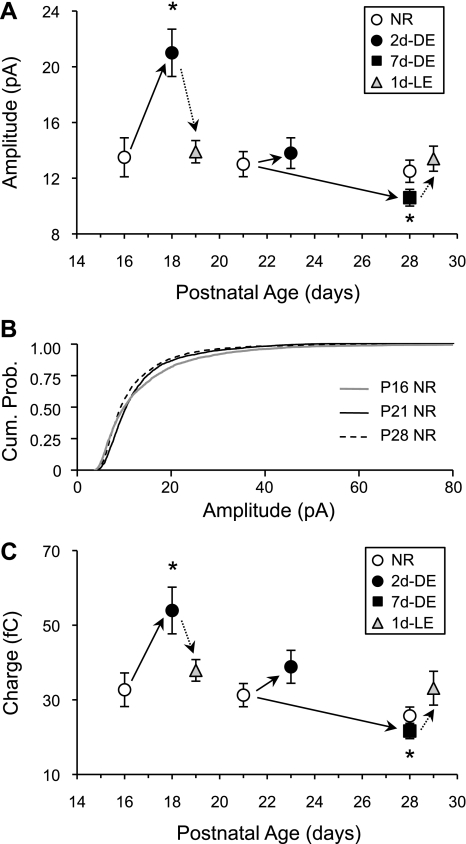
We have demonstrated that L6 neurons share similarities with L4 neurons in that they display an early critical period for homeostatic synaptic plasticity with a brief duration of DE. The homeostatic increase in mEPSC amplitude of L6 neurons triggered by 2 days of DE was rapidly reversed by 1 day of LE. Whereas 2 days of DE initiated later in life (at P21) was ineffective at causing a net change in the average mEPSC amplitude, a longer duration of DE decreased the average mEPSC amplitude (Fig. 4A). The visual experience-induced changes in mEPSC amplitude at both younger and older ages did not accompany alterations in mEPSC frequency but was associated with changes in mEPSC decay kinetics, which suggests that they occur via postsynaptic regulation of AMPARs.
Fig. 4.
A summary of mEPSC amplitude changes in L6 neurons induced by manipulation of visual experience across different ages. A: there was no significant change in the average mEPSC amplitude across ages between P16 and P28 in NR controls (open circles). Two days of DE (solid circles) initiated at P14 increased the average mEPSC amplitude, but when initiated at P21, DE failed to alter the average mEPSC amplitude. However, 7 days of DE (solid squares) initiated at P21 significantly decreased the average mEPSC amplitude. One day of LE (1d-LE; shaded triangles) reversed the changes in mEPSC amplitude caused by DE. *P < 0.05 (1-way ANOVA followed by Fisher's PLSD post hoc test, P < 0.05). B: difference in cumulative probability curves of mEPSC amplitude in P16, P21, and P28 NR groups (Kolmogrov-Smirnov test: P < 0.0001 across all groups). Data shown in previous figures are replotted here for direct comparison. C: comparison of average charge transfer of mEPSCs. Symbols are the same as in A. *P < 0.05 (1-way ANOVA followed by Fisher's PLSD post hoc test).
Our observation that L6 neurons of young mice homeostatically increase their excitatory synapses with brief DE only early in development is similar to observations made in L4 (Desai et al. 2002). These results corroborate the idea that thalamic recipient layers are highly plastic during an early critical period. Although the direction of change in mEPSCs with 2 days of DE is consistent with what is expected of a homeostatic adaptation, it did not occur via a multiplicative synaptic scaling mechanism. This is qualitatively different from multiplicative synaptic scaling observed in L2/3 at a later developmental time point (i.e., P21–P28 range) (Gao et al. 2010; Goel et al. 2006; Goel and Lee 2007). Whether the L4 neurons scale multiplicatively with DE was not determined in a previous study (Desai et al. 2002). The nonmultiplicative changes in L6 mEPSCs could be due to many factors. One possibility is that the changes triggered by DE are restricted to a subset of synapses. L6 neurons not only receive direct geniculocortical inputs (LeVay and Gilbert 1976; Ribak and Peters 1975), like L4, but they also receive diverse sets of inputs as shown from synaptic responses elicited in response to uncaging glutamate in L2/3, L4, L5, and L6 (Zarrinpar and Callaway 2006). It is interesting to note that L6 receives fewer LGN inputs than L4 (Binzegger et al. 2004; da Costa and Martin 2009; Ribak and Peters 1975). In a recent anatomical study, it was estimated that corticothalamic L6 neurons in cat V1 receive about 20 geniculocortical synapses, mainly onto their basal dendrites (da Costa and Martin 2009). Collectively, these results would suggest that intracortical inputs are likely highly represented in the recorded mEPSCs. Whether visual experience differentially affects geniculocortical and intracortical inputs or intracortical inputs originating from specific layers requires further study. As a note of caution, we cannot rule out the possibility that visual experience may have altered the dendritic cable properties, which could potentially influence the sampling of a subset of synaptic populations. However, it is unlikely that this could happen at a gross level, because we did not see a correlation between dendritic filtering and experimental manipulations (data not shown), and the reversal potential for mEPSCs was as expected (Erev = 6 ± 2.5 mV, n = 4 cells). Furthermore, comparing mEPSC charge transfer, which is less affected by dendritic filtering and space clamp than the peak current amplitude (Spruston et al. 1993), showed comparable changes with visual experience (Fig. 4C).
An unexpected finding from our work is that at P21, a short duration (2 days) of DE did not cause a net change, but a longer duration (7 days) of DE decreased the average amplitude of mEPSCs. Even though 2 days of DE did not result in a net change in the average mEPSC amplitude, there was a significant shift in the distribution of mEPSCs. This suggests that a short duration of DE increases and decreases the strength of individual synapses on L6 neurons, but these cancel each other such that there is no net change in the average (Fig. 2E, bottom). However, with a longer duration DE, L6 synapses weakened overall. The decrease in mEPSCs with 7 days of DE is contrary to what is expected of a homeostatic adaptive change, which predicts that loss of visually driven activity in V1 would scale up excitatory synapses. Furthermore, it distinguishes L6 plasticity from L2/3 plasticity at this developmental age. We reported previously that the same durations of DE scales up mEPSC amplitude in L2/3 (Gao et al. 2010; Goel et al. 2006; Goel and Lee 2007). The unexpected decrease in the average mEPSC amplitude of L6 neurons in response to 1 wk of DE may reflect adaptation to an increase in input activity from other cortical layers, which project back to L6. However, this is unlikely, considering a recent study showing that visual deprivation decreases the intrinsic excitability of L5 (Nataraj et al. 2010), which provides a major input to L6 (Zarrinpar and Callaway 2006). Alternatively, the DE-induced decrease in mEPSCs may be a manifestation of a nonhomeostatic synaptic plasticity, such as LTD, which is expected from a reduction of input activity to specific sets of synapses. It is known that L6 neurons undergo pairing-induced LTD of intracortical inputs originating from superficial layers, which depends on the activation of metabotropic glutamate receptors (mGluRs) (Rao and Daw 2004). The average decrease in mEPSC amplitude with 7 days of DE (∼18%) is within the range of the magnitude of LTD (10–20%) observed in mouse visual cortex (Choi et al. 2002; Kirkwood et al. 1997). Such a decrease in synaptic weight will bring inputs that were previously just above threshold for producing action potentials to subthreshold, which would alter the information propagation in the L6 circuit. In any case, the decrease in mEPSC amplitude was not multiplicative and was readily reversed by 1 day of LE. Furthermore, the decrease in mEPSC amplitude with 7 days of DE was associated with a concomitant increase in the mEPSC decay kinetics, which suggests that the changes are mediated by regulation of postsynaptic AMPAR function. Specifically, our data suggest that the mEPSC amplitude changes may be due to regulation of AMPAR subunit composition, because AMPARs containing the edited form of GluA2 (or GluR2) subunit display slower decay kinetics and lower conductance than GluA2-lacking receptors (Mosbacher et al. 1994). The regulation of GluA2-lacking AMPARs was also observed in L2/3 accompanying multiplicative homeostatic synaptic plasticity (Goel et al. 2006). These results suggest that the regulation of AMPAR subunit composition may be a general mechanism for adjusting synaptic gain in V1 regardless of the mode of synaptic plasticity. Although the exact nature of synaptic changes in L6 at older developmental ages requires further investigation, our findings suggest that L6 synapses, at least a subpopulation of them, are capable of undergoing plastic changes with visual deprivation even after the short early critical period for homeostatic synaptic plasticity.
We have shown that visual deprivation leads to two distinct outcomes at L6 excitatory synapses depending on the age of the animal and the duration of visual deprivation. Although we did not observe a significant change in the average mEPSC amplitude across the developmental ages examined (Fig. 4A), we nonetheless found that that distribution of mEPSC amplitudes significantly changed (Fig. 4B). This suggests that there is considerable adjustment of excitatory synaptic gain during this developmental period, which may alter the rules of experience-dependent synaptic plasticity. Considering that L6 neurons provide cortical input to the LGN, their synaptic regulation with visual deprivation is likely to alter LGN processing of visual information.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 EY014882 and R21 NS070645 (to H.-K. Lee), NIH Diversity Supplement 3R01 EY014882-05A1S1 (to T. T. Anguh), and Howard Hughes Medical Institute Undergraduate Research Fellowship (University of Maryland) (to A. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. A. Kirkwood for helpful discussions on this manuscript.
REFERENCES
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci 24: 8441–8453, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Deschenes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66: 253–263, 1995 [DOI] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron 62: 135–146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A. Intrinsic connections of rat primary visual cortex: laminar organization of axonal projections. J Comp Neurol 279: 171–186, 1989 [DOI] [PubMed] [Google Scholar]
- Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic acid decarboxylase-65 knock-out mice. J Neurosci 22: 5271–5276, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa NM, Martin KA. Selective targeting of the dendrites of corticothalamic cells by thalamic afferents in area 17 of the cat. J Neurosci 29: 13919–13928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Labra C, Rivadulla C, Grieve K, Marino J, Espinosa N, Cudeiro J. Changes in visual responses in the feline dLGN: selective thalamic suppression induced by transcranial magnetic stimulation of V1. Cereb Cortex 17: 1376–1385, 2007 [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789, 2002 [DOI] [PubMed] [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci 30: 7168–7178, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci 9: 1001–1003, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci 27: 6692–6700, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci USA 94: 3380–3383, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Gilbert CD. Laminar patterns of geniculocortical projection in the cat. Brain Res 113: 1–19, 1976 [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7: 1353–1359, 2004 [DOI] [PubMed] [Google Scholar]
- Marrocco RT, McClurkin JW, Alkire MT. The influence of the visual cortex on the spatiotemporal response properties of lateral geniculate nucleus cells. Brain Res 737: 110–118, 1996 [DOI] [PubMed] [Google Scholar]
- McClurkin JW, Marrocco RT. Visual cortical input alters spatial tuning in monkey lateral geniculate nucleus cells. J Physiol 348: 135–152, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266: 1059–1062, 1994 [DOI] [PubMed] [Google Scholar]
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron 68: 750–762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distributions of potential in cylindrical coordinates and time constants for a membrane cylinder. Biophys J 9: 1509–1541, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Daw NW. Layer variations of long-term depression in rat visual cortex. J Neurophysiol 92: 2652–2658, 2004 [DOI] [PubMed] [Google Scholar]
- Ribak CE, Peters A. An autoradiographic study of the projections from the lateral geniculate body of the rat. Brain Res 92: 341–368, 1975 [DOI] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Williams SH, Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol 70: 781–802, 1993 [DOI] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat 4: 13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Callaway EM. Local connections to specific types of layer 6 neurons in the rat visual cortex. J Neurophysiol 95: 1751–1761, 2006 [DOI] [PubMed] [Google Scholar]