Abstract
The medial septum-vertical limb of the diagonal band of Broca (MSvDB) is important for normal hippocampal functions and theta oscillations. Although many previous studies have focused on understanding how MSVDB neurons fire rhythmic bursts to pace hippocampal theta oscillations, a significant portion of MSVDB neurons are slow-firing and thus do not pace theta oscillations. The function of these MSVDB neurons, especially their role in modulating hippocampal activity, remains unknown. We recorded MSVDB neuronal ensembles in behaving rats, and identified a distinct physiologically homogeneous subpopulation of slow-firing neurons (overall firing <4 Hz) that shared three features: 1) much higher firing rate during rapid eye movement sleep than during slow-wave (SW) sleep; 2) temporary activation associated with transient arousals during SW sleep; 3) brief responses (latency 15∼30 ms) to auditory stimuli. Analysis of the fine temporal relationship of their spiking and theta oscillations showed that unlike the theta-pacing neurons, the firing of these “pro-arousal” neurons follows theta oscillations. However, their activity precedes short-term increases in hippocampal oscillation power in the theta and gamma range lasting for a few seconds. Together, these results suggest that these pro-arousal slow-firing MSvDB neurons may function collectively to promote hippocampal activation.
Keywords: medial septum; small-amplitude irregular activity, auditory response; theta oscillations; cholinergic
the medial septum-vertical limb of the diagonal band of Broca (MSvDB) heavily innervates the hippocampus through cholinergic, GABAergic, and glutamatergic projections (Gritti et al. 2006; Kiss et al. 1990; Kohler et al. 1984; Lewis et al. 1967). These projections are essential for normal hippocampal functions. Lesion or inactivation of MSvDB recapitulates the memory deficit resulting from hippocampal lesions and eliminates hippocampal theta oscillations (Gray and McNaughton 1983; Green and Arduini 1954; Mahut 1972; Mizumori et al. 1990; Winson 1978).
To achieve a mechanistic understanding of the critical role of MSvDB in hippocampal functioning, it is essential to elucidate how the neuronal activity in MSvDB modulates hippocampal activity, in particular its dynamic interactions on fine temporal scales. Indeed, this issue has been studied extensively at the electrophysiological level over the last few decades. Those studies have largely focused on the fast-firing, rhythmic-bursting MSvDB neurons and have demonstrated that these cells are the pacemaker of hippocampal theta oscillations (Ford et al. 1989; Hangya et al. 2009; Morales et al. 1971; Petsche et al. 1962).
However, in parallel with their heterogeneity in terms of neurotransmitters, the physiological properties of MSvDB neurons are similarly diverse. In addition to the extensively studied fast-firing, rhythmic-bursting neurons, many MSvDB neurons are nonetheless slow-firing and do not fire theta-range rhythmic bursts (Ford et al. 1989; Gaztelu and Buno 1982; Stewart and Fox 1989). As such, these latter cells are unlikely candidates to participate in pacing theta oscillations. The function of these slow-firing neurons remains unknown, in part because these slow-firing MSvDB neurons have received little attention in the literature. However, a recent study demonstrated for the first time that neurochemically identified MSvDB cholinergic neurons are slow-firing and do not pace theta oscillations (Simon et al. 2006). Given the well-established role of MSvDB cholinergic neurons in normal hippocampal theta oscillations and hippocampal-dependent learning and memory (representative reviews: Hasselmo 2006; Jerusalinsky et al. 1997; Vanderwolf 1988), this new finding calls for further investigation of the functions of the slow-firing MSvDB neurons.
Therefore, the first goal of the present current study was to comprehensively characterize the physiological properties of slow-firing MSvDB neurons in different behavioral contexts in freely moving rats. More importantly, to provide new insights on how the slow-firing MSvDB neurons modulate hippocampal activity, we aimed at determining how their neuronal activity is correlated with changes in hippocampal oscillatory activities, including theta oscillations. By using chronically implanted multielectrode arrays (MEA) in freely moving rats to record simultaneously neuronal ensembles in MSvDB and local field potentials (LFPs) in the hippocampus, we first investigated the firing properties of MSvDB slow-firing neurons during the sleep-waking cycle and during exposure to auditory stimuli. On the basis of neurophysiological properties measured during three distinct behavioral contexts, we identified a functionally homogenous MSvDB subpopulation. We then investigated how the firing of these neurons is correlated with the oscillatory activities in the hippocampus and demonstrated that the activity of these slow-firing MSvDB neurons precedes hippocampal activations for a few seconds. Our results therefore provide new insights on how a functionally identified subpopulation of slow-firing MSvDB neurons may dynamically enhance hippocampal activity on the time scale of a few seconds.
MATERIALS AND METHODS
Animals
Animal use and procedures were approved by the Duke Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines. Eight adult male Long-Evans rats (300–500 g) were used in the experiments.
Electrodes and Surgery
The multielectrode assembly or array (MEA) was constructed similarly to earlier studies (Lin and Nicolelis 2008; Nicolelis et al. 2003). On each assembly, two 29-gauge stainless-steel cannulas were secured, with tips separated by 0.8 mm horizontally [anterior-posterior (AP)] and 0.5 mm vertically, targeting anterior (deeper) and posterior (shallower) parts of the MSvDB. As a bundle, 8 or 16 35-μm tungsten wire electrodes were threaded together through each cannula. The bundles could be advanced by microdrives with precision over the course of the experiments. The total range of advancement was 2 mm in length. Wires spread slightly from the cannula tip after being pushed out, covering a semi-cone-shaped area for ∼1–1.5 mm in diameter at the end (Fig. 1A). Since the implant was slightly angled (below), the semi-cone-shaped spreading would start from midline and then advance with a slight preference toward one hemisphere (Fig. 1A). Ipsilateral to this preferred hemisphere, the hippocampal MEA was arranged in a 4 × 4 form (250-μm spacing) for hippocampal LFP recordings. Two of the four rows, aimed at dentate gyrus (DG), were 0.7 mm longer than the rest, which aimed at CA1.
Fig. 1.
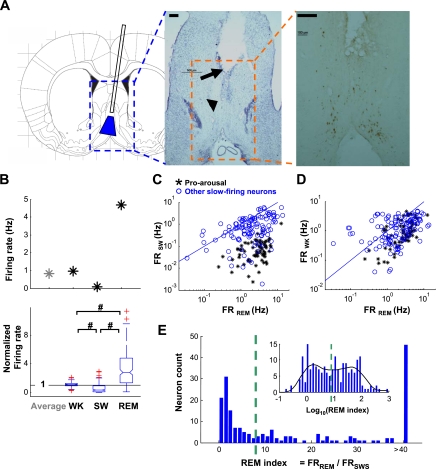
Slow-firing neurons have higher firing rate in rapid eye movement (REM) sleep. A: schematic and examples of the recording site demonstrated with histology and immunohistochemistry. Left: the cannula implant design and estimated target area (blue area) in the medial septum-vertical limb of the diagonal band of Broca (MSvDB). Blue dashed rectangle indicates the area in middle. Middle: histology (Nissl staining) image. The empty space (arrow) resulted from cannula implantation. The arrowhead indicates initial spreading of the bundled electrodes from the tip of the cannula. Electrodes advanced progressively for another 1 mm, during which time the recordings were obtained. Scale bar at top left, 200 μm. Orange dashed rectangle indicates area at right. Right: choline acetyltransferase staining demonstrating cholinergic neurons in MSvDB, dispersed in the area being recorded. Scale bar at top left, 200 μm. B: firing rates of a representative (top) and all slow-firing MSvDB neurons (bottom) change across waking (WK), slow-wave (SW), and REM sleep. Box plot (bottom) shows firing rates in different behavioral states normalized to average firing rate of each individual neuron. Horizontal line indicates 1. For each box plot, the central mark (middle of notch) is the median; box edges mark the 25th and 75th percentiles; red crosses are outliers; the whiskers extend to the most extreme data points not including outliers; and nonoverlapping notches between groups indicate a significant difference between medians (significance level = 0.05). Firing rate was highest in REM, lowest in SW, and intermediate in WK (#P < 0.001, Wilcoxon signed rank test). C and D: 2-dimensional (2-D) plots of firing rate comparison for individual neurons between REM and SW (C) and between REM and WK (D). Black symbols represent pro-arousal slow-firing neurons as defined in Fig. 4; blue symbols represent other slow-firing neurons; and blue lines indicate unity. E: histogram of REM index for individual neurons. Inset: histogram of log-transformed index values with local fitting of distribution curve (black). Green dashed line indicates local minimum used as the cutoff threshold (REM index = 8.0). Neurons were therefore grouped into REM− (92/190) and REM+ (98/190) groups. FRSW, FRREM, and FRWK, firing rate of individual neurons in SW, REM, and WK states, respectively.
Surgeries for electrode implantation have been described earlier (Nicolelis et al. 1997). Animals were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) and positioned in a stereotaxic frame. Atropine (0.02 mg) was used to reduce airway secretion. Stainless steel screws were secured above frontal cortex and cerebellum, serving as grounds. Craniotomies were opened above the MSvDB region [anterior-posterior (AP) +1.5–0 mm, mediolateral (ML) 0.5–1.5 mm relative to Bregma] and hippocampus unilaterally (AP −4.0 mm, ML 2.5 mm, ∼1 × 1 mm) (Paxinos and Watson 2005). The MEA with two movable electrode bundles was lowered into MSvDB with an angle of 9.5° on the coronal plane, targeting two locations in MSvDB: AP +1.1/+0.3 mm, ML 0 mm, −6.2/−5.7 mm below dura. A 4 × 4 array was lowered into one hemisphere to record from CA1 (−2.2 mm below dura) and DG (−2.9 mm below dura). Dental acrylic was used to cover and secure the implant with the help of anchoring screws. Rats were allowed to recover for at least 14 days after surgery before recordings.
Undisturbed Sleep-Waking Behavior
Animal behavior was monitored online with a camera and recorded on videotape. Rats were allowed to freely move around or sleep for 1∼1.5 h in the recording session in the light/sound-attenuated chamber to which they had been habituated to and the same one in which they would perform the behavioral task. To minimize disturbance from the experimenter, sleep-waking states were monitored remotely using an online detection algorithm based on LFP spectral features (Gervasoni et al. 2004).
Behavioral Task and Auditory Stimuli
In each recording session, well-trained rats performed a variant of the Go/NoGo behavioral task for 1∼1.5 h. The apparatus (Med Associates, St. Albans, VT), behavioral training, and task details have been described elsewhere (Lin and Nicolelis 2008). Three cues, an 80-dB 6-kHz tone, an 80-dB white noise sound, or a light (on), each lasting 2 s, were presented in a random order during the task, with intertrial interval (ITI) randomly varied between 6 and 18 s (6, 8, 10, 14, or 18 s). Each animal was assigned to respond to one of the cues within 5 s of the cue onset to get reward (∼0.04 ml of 10% sucrose). After they retrieved their reward or if they did not respond, a new trial (new ITI) would start. If they licked during the ITI, the trial would be reset. There was no punishment for responding to the other two cues or for not responding to the rewarding cue. For each type of auditory stimulus, it could be either a rewarding cue, a distracter when the other auditory cue was associated with reward, or nonrelevant when the light cue was associated with reward.
Electrophysiological Recording
Electrical signals were amplified with TBSI 2× or 1× head stages (Triangle BioSystems, Durham, NC). Neuronal and LFP signals were filtered at 154–8.8 kHz and 0.4–240 Hz, digitized at 40 and 2 kHz, respectively, and recorded with a Multichannel Acquisition Processor (Plexon, Dallas, TX). MSvDB electrode bundles were advanced at 60- or 125-μm steps between recording sessions to minimize sampling from overlapping neuronal populations. Single units were identified online based on spike waveform to aid off-line processing of single-unit data (see Data Analysis). LFP signals were also transmitted online to a remote computer to monitor the sleep-waking states of the animal.
Data Analysis
Single-unit isolation (spike sorting).
Spike waveforms were processed with an Offline Sorter (Plexon) to obtain single units. Effort was used to ensure the isolation of single units, with signal-to-noise ratio larger than three and a minimal amount (<0.1%) of “spike collision” (spikes occurred within the action potential refractory period, set as 1.2 ms) based on the ITI histogram (Nicolelis et al. 2003). The time stamps of the single units and LFP signals were further analyzed in Matlab (The MathWorks, Natick, MA).
Sleep-waking analysis and small-amplitude irregular activity definition.
Sleep states were characterized as described earlier (Gervasoni et al. 2004). Spectral features were obtained to determine the behavioral state of the animal as three gross categories: waking (WK), slow-wave (SW) sleep, and rapid eye movement (REM) sleep. Firing rates of individual neurons were calculated for the whole recording session (overall or average firing rate) and individual sleep-waking states (Fig. 1A). Instantaneous firing rates were calculated by binning spikes into each second and smoothed for ±4 s around each bin.
Small-amplitude irregular activity (SIA) epochs were further determined in the SW sleep episodes, based on methods described elsewhere (Jarosiewicz and Skaggs 2004). Briefly, root-mean-square amplitude of hippocampal LFP was calculated and smoothed for 0.5-s bins. An amplitude threshold was set at a local minimum in the amplitude distribution around the 20th percentile of the distribution (Fig. 2A). Individual SIA epochs were defined as a continuous period with 80% of the amplitude below amplitude threshold and no gaps (large-amplitude LFP) longer than 1.5 s. The SIA onset was initially identified as amplitude trace crossing the threshold (large to small) and was then slightly adjusted for 1 bin/0.5 s at most (most often no adjustment) to find the sharpest drop of amplitude. Minimal duration of SIA was set to 1.5 s. With the choice of amplitude threshold, about 20% of the SW sleep was identified as SIA, in agreement with earlier studies (Jarosiewicz et al. 2002). The remaining part of the SW sleep was defined as large-amplitude irregular activity (LIA) epochs.
Fig. 2.
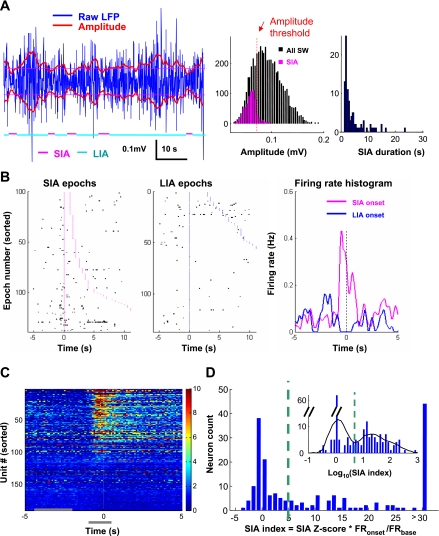
Many slow-firing neurons increase firing around small-amplitude irregular activity (SIA) onset. A, left: example of SIA and large-amplitude irregular activity (LIA) epochs during SW sleep. Blue traces indicate local field potentials (LFP) in the dentate gyrus (DG); red traces indicate smoothed root-mean-square amplitude of LFP (0.5-s bins); thin magenta lines, amplitude threshold; magenta and cyan lines at bottom indicate identified SIA and LIA epochs. Middle: histogram of amplitude distributions for the whole recording session. Red dotted line indicates the amplitude threshold used to define SIA epochs (see materials and methods). Right: histogram of SIA duration in the same recording session. B: example of transient firing around SIA onset. Left and middle: raster plots of spikes (black) around SIA or LIA onset (aligned, onset time is 0). Magenta or blue dots indicate the beginning and end of individual SIA or LIA epochs. Epochs are sorted according to length (display cutoff, 10 s). Right: averaged firing rate around the onset of SIA or LIA. C: pseudocolored peristimulus time histograms (PSTHs; time 0 indicates SIA onset) of all slow-firing MSvDB neurons, sorted according to the SIA index. Firing rates are normalized to average firing rates of individual neurons during SW sleep. Horizontal gray bars at bottom indicate the peak (−1 to +0.5 s) and baseline (−4 to −1.5 s) periods used to calculate SIA index. D: histogram of SIA index for all slow-firing neurons. Inset: histogram of log-transformed index values with local fitting of distribution curve (black). Green dashed line indicates the local minimum used as the cutoff threshold (SIA index = 5.0). Neurons were grouped as SIA− (91/190) or SIA+ (99/190).
Firing property indexes for individual neurons.
The REM index for each neuron was defined as the ratio between firing rates of REM and SW sleep. Response to auditory stimuli was characterized using peristimulus time histograms (PSTH) with 5-ms bins. The auditory response index (Aud index) was defined as the ratio between averaged PSTH values during 15–35 ms after and 300–5 ms before the stimulus onset (see Fig. 3B). Unless specified, the Aud index was calculated for the white noise stimulus. Aud index >1 represents excitatory response, whereas Aud index <1 represents inhibitory response.
Fig. 3.
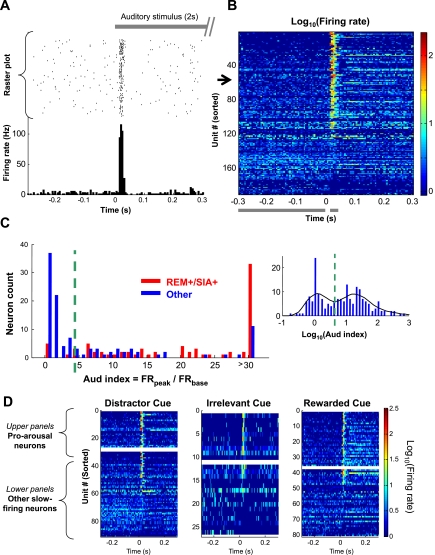
Most REM+/SIA+ neurons have transient auditory responses. A: example of a neuron with transient auditory response. Gray bar at top indicates a 2-s auditory stimulus (an 80-Hz white noise sound). Raster plot in middle shows the trial-by-trial firing of this neuron (black dot, single spike) aligned to the onset of the auditory stimuli (time 0). PSTH at bottom summarizes the response of this neuron, showing a very strong but transient response, tightly locked at 15–30 ms after the stimulus onset. B: pseudocolored PSTHs of all slow-firing MSvDB neurons, sorted according to the auditory response index (Aud index; see materials and methods). The example in A is unit 53 on this plot (black arrow). Horizontal gray bars at bottom indicate the peak (15 to 35 ms) and baseline (−300 to −5 ms) periods used to calculate Aud index. C: histogram of Aud index for 2 groups of neurons, REM+/SIA+ (red) and the remaining ones (blue). Most REM+/SIA+ neurons had a higher Aud index, whereas the majority of the remaining neurons had a low Aud index. Inset: histogram of log-transformed index values of all slow-firing neurons with local fitting of distribution curve (black). Green dashed line indicates the local minimum used as the cutoff threshold (Aud index = 4.5). Neurons were grouped as Aud− (81/190) or Aud+ (109/190). D: responses to auditory cue (white noise) not depending on the role of the cue. Pseudocolored PSTHs of all slow-firing MSvDB neurons are categorized based on the role of the white noise cue for individual animals. Horizontal white bars (middle) separate pro-arousal slow-firing neurons (top) and other slow-firing neurons (bottom). See Fig. 4 for definition of pro-arousal and other slow-firing neurons.
Histograms similar to PSTH were calculated for individual neurons, with spikes aligned to SIA onset at 100-ms bins (Fig. 2B). Z scores of firing associated with SIA onset were calculated, defined as the difference between averaged PSTH values during −1 to +0.5 s around SIA onset and baseline time −4 to −1.5 s, divided by the standard deviation of PSTH values during the baseline time. The ratio between averaged PSTH values around SIA onset and the baseline was also calculated. The SIA index was subsequently defined as the product of the Z score and the ratio. These two components incorporate the substantial firing changes associated with SIA onset in two types of neurons we observed: one with extremely low firing during baseline (LIA) and a sudden firing at SIA onset (high ratio but low Z score due to high variance caused by the sporadic firing during baseline), and another with relatively stable firing during baseline and a relatively small (low ratio) but very significant increase of firing at SIA onset (high Z score). SIA index >1 represents an excitatory association, whereas SIA index less than −1 suggests inhibition. SIA indexes between −1 and 1 suggest no clear firing rate changes and were treated as 0 for log-transformed value. Except for these values, log transform of SIA index was performed on the absolute value, with original signs assigned to the log value (SIA index >1 became Log index >0; SIA index less than −1 became Log index <0).
Histograms of log-transformed indexes were plotted and fitted with the local fit function (Chronux analysis software; Mitra 2008; Mitra and Bokil 2008). The local minima between the two modes were used as threshold values. Principle component analysis on the three indexes was performed for all the slow-firing neurons, and the first principle component was extracted to aggregate the indexes.
K-means clustering, a standard algorithm in the Matlab program, was used to cluster neurons based on the log-transformed indexes. This algorithm partitions data points into a defined number of clusters and, with iteration, minimizes the summed Euclidean distance to cluster centroids.
Theta-related analysis.
One of the DG LFPs was filtered between 4.5 and 9 Hz for theta oscillations, and theta phases were calculated using Hilbert transform on the filtered signal. The rhythmicity index was calculated based on autocorrelogram in REM sleep (Jobert et al. 1989; Kitchigina et al. 2003). Phase of individual spikes was calculated as the Hilbert phase at the time of the spike. Phase preference of each neuron was displayed with phase distributions (histograms). Phase-locking strength (Z values) and P values were calculated with circular statistics (Rayleigh test, Matlab toolbox; Berens and Velasco 2009). The Z-shift method to analyze the fine temporal relationship between phase-locked neuronal activity and LFP theta oscillations was adopted from earlier studies (Hangya et al. 2009; Siapas et al. 2005). For the Z-shift analysis, one LFP from MSvDB was also used to quantify local theta oscillations in MSvDB. Neuronal spikes were shifted temporally relative to LFPs, to calculate phase-locking Z values (see Fig. 6A). The temporal shift that results in maximal Z values indicates the temporal relationship between the neuronal activity and the microstructures of the LFP theta oscillations. A positive spike shift suggests spikes leading LFP theta, and vice versa.
Fig. 6.
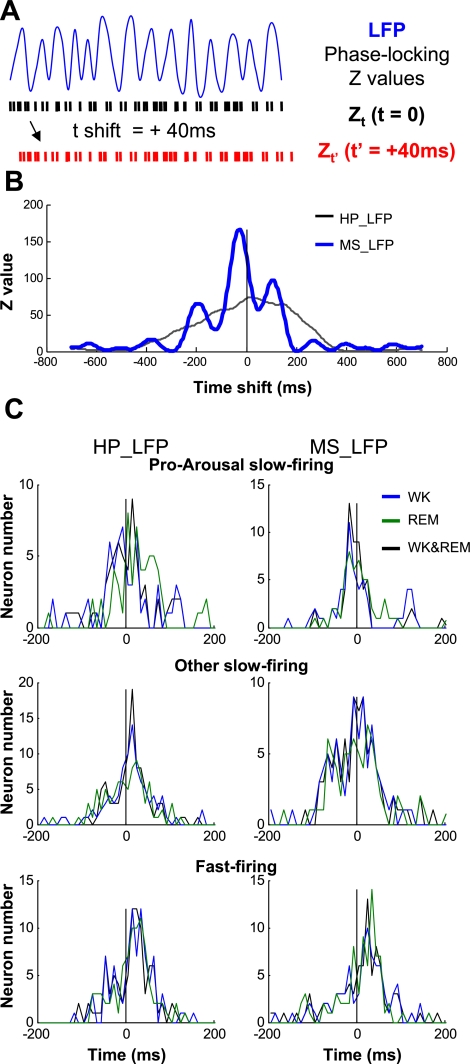
Pro-arousal slow firing follows local theta oscillations in MSvDB. A: schematics of the Z-shift method. Blue trace indicates LFP; black and red rasters represent original and time-shifted neuronal spikes, respectively. Phase-locking Z values were calculated while shifting neuronal spikes relative to LFP signals. Positive shift in time, if resulting in a larger Z value, suggests that the neuronal spikes are better phase-locked to LFP signals in the future. B: an example of Z values from 1 pro-arousal slow-firing neuron, calculated for theta oscillations for both WK and REM episodes concatenated together. HP_LFP, LFP theta oscillations in the hippocampus; MS_LFP, LFP theta oscillations in MSvDB. The maximal Z value for phase-locking to theta oscillations in MSvDB occurred before 0, suggesting that the neurons spikes followed the theta oscillations in MSvDB LFP in the past. C: histogram of time-shift distribution corresponding to maximal Z values of significantly phase-locked (P < 0.01) MSvDB neurons during WK, REM sleep, or both states. Positive spike shift suggests spike leading LFP theta oscillation, and vice versa. Pro-arousal slow-firing neurons mostly follow LFP theta oscillations in MSvDB, whereas fast-firing neurons mostly lead theta oscillations in both the hippocampus and MSvDB.
Cross-correlation analysis.
Cross-correlation functions were calculated for neuronal pairs, with spikes from the whole recording session or from selected states during the recording session, such as waking, SW, or REM sleep. Cross-correlation functions were normalized by firing rates (overall or during specific states) of the two neurons and the length of recording or state. The long tails of the function ([−20 −10] and [10 20] s) were used as an empirical baseline for individual pairs (Lin et al. 2006) during which the neuronal firing is supposedly not correlated. The average baseline values were subtracted out, and the fluctuations during the baseline were used to estimate the significant cross-correlation between −10 and 10 s. One-way ANOVA with Bonferroni-corrected multiple comparisons was used for analysis of cross-correlation values. To estimate the width of the cross-correlation functions, the average cross-correlation value within the window of [−1 1] s was calculated. If this value was larger than baseline fluctuations (mean + 3SD), the two temporal points at which the smoothed cross-correlation function dropped to one-half of the average value were determined, and the time window between these two points was deemed as the half-width of the cross-correlation function.
Spectral analysis.
Spectral analyses were performed with the Chronux application based on multitaper spectral methods (Mitra 2008). For different frequency ranges, slightly different moving windows were used: 0–20 Hz, 2-s window with 0.5-s moving steps; 30–300 Hz, 0.5 s/0.25 s. To calculate spike-triggered or event-triggered spectrograms, spectrograms around individual spikes/events were aligned and averaged. For plots during the waking state, spikes occurring [−1 +1] s around behavioral events (cues, licks, etc.) were excluded to prevent potential confounding factors. Spike-triggered spectrograms were normalized by the baseline period [−8 −5] s before the spike. Z scores for each spectrogram were calculated based on the fluctuations during the baseline. Individual time-frequency pixels in the spectrogram with Z scores larger than a predetermined Z value (5.7, 8.1 for the 2 frequency ranges, square root of the number of time bins) were counted as significant pixels. The number of these significant pixels was counted in Fig. 8B.
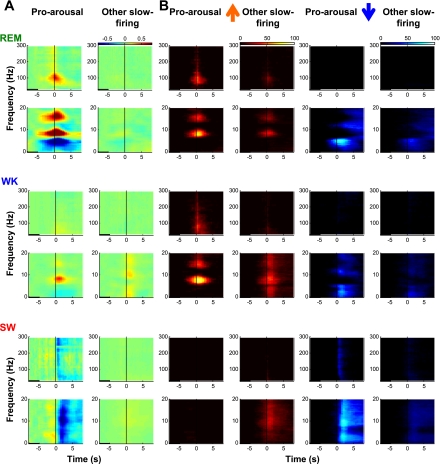
Fig. 8.
Pro-arousal slow-firing cholinergic neurons promote hippocampal activation. A: example of averaged spectrograms around spikes of a pro-arousal slow-firing and an other slow-firing neuron recorded in the same session. Low- and high-frequency ranges (0–20 and 30–300 Hz) are displayed separately. Spectrograms are aligned to the spikes of individual neurons (time 0, black vertical line in center). Black horizontal bar at bottom left indicates the baseline period. For normalization, average log power during the baseline period at each frequency was subtracted, and pseudocolors indicate the power increase or decrease. During REM and WK states, the firing of pro-arousal slow-firing neurons promoted increase of theta power (7–10 Hz and 2× harmonics), and in some neurons (this example) the power is in the high gamma range. Their firing was also associated with a decrease in power in frequency bands below 6 Hz. In SW sleep, the firing of these neurons was associated with a marked decrease of power in the low-frequency range (0–20 Hz). Firing of other slow-firing neuron did not lead to comparable spectral changes. B: significance plots for spectrograms of all pro-arousal slow-firing and other slow-firing neurons. Significance is based on Z scores calculated with baseline (see materials and methods). Heat plots indicate the percentage of neurons beyond Z-score threshold for positive (left 2 columns, warm color) and negative (right 2 columns, cool color) Z scores, corresponding to power increase and decrease, respectively. The pattern is similar to the individual example.
RESULTS
We recorded from 300 MSvDB single units in freely moving and behaving animals (n = 8 rats). In the following report, we focus mostly on the slow-firing neurons with an average firing rate below 4 Hz (n = 190) (4-Hz demarcation defined by Simon et al. 2006).
Physiological Properties of Slow-Firing MSvDB Neurons in Three Behavioral Contexts
To comprehensively characterize the physiological properties of these slow-firing neurons, we investigated their activity in three distinct behavioral contexts that are known to modulate MSvDB neuronal activity: 1) different sleep states, 2) neuronal activity associated with transient arousal epochs during sleep, and 3) neuronal responses to auditory stimulus.
First, we investigated the sleep-waking state-dependent firing property of the slow-firing MSvDB neurons. Similar to the rhythmic-bursting neurons (Morales et al. 1971; Simon et al. 2006; Sweeney et al. 1992), the firing rates of the entire population of slow-firing MSvDB neurons are lowest during SW sleep (0.76 ± 1.14 Hz, mean ± SD), intermediate during waking (WK; 1.14 ± 1.17 Hz), and highest during REM sleep (2.98 ± 2.35 Hz). Figure 1B shows the state-dependent firing rates for a representative neuron and normalized firing rates for the population (Friedman test, P < 0.001 for all 3 states; Wilcoxon signed rank test, P < 0.001 between each of 2 states).
To understand how uniform such state-dependent firing rate fluctuations manifest on the single-neuron level, we compared the firing rates of individual MSvDB neurons in REM with SW sleep (Fig. 1C) and WK with REM sleep (Fig. 1D). The scatter plots indicate that not all neurons have the firing rate modulation similar to that shown in the population. To quantify the state-dependent firing of individual neurons, especially the propensity to fire more frequently during REM than during SW sleep, we devised a REM index for each neuron. This REM index was obtained as the ratio between the neuron's firing rates during REM and SW sleep. The distribution of REM index was roughly bimodal, with a subset of MSvDB neurons showing very high REM indexes (Fig. 1E). Slightly more than one-half (52%, 98/190) of the slow-firing neurons were categorized as REM+ (see materials and methods for the selection of threshold).
Second, we investigated the detailed firing properties of MSvDB neurons during SW sleep, when the average firing rate is the lowest among the major arousal states. Particularly, we identified transient arousal epochs, the SIA epochs (Fig. 2A) (Jarosiewicz et al. 2002; Jarosiewicz and Skaggs 2004), and studied how these transient states were associated with the neuronal activities in the MSvDB. A subset of MSvDB neurons increased their activity starting around 1 to 0.5 s before SIA onsets (example in Fig. 2B, population results in Fig. 2C), suggesting that the activity of these neurons may promote the transition into SIAs. Compared with their firing rates during SIA epochs, these neurons fired much less frequently during the slow-wave LIA epochs, which accounts for most of the SW sleep (data not shown). To quantify the transient firing around SIA onsets, we calculated an SIA index for each MSvDB neuron (Fig. 2D, see materials and methods for detail). Overall, 52% (99/190) of MSvDB neurons showed a significant firing rate increase around the SIA onset and were categorized as SIA+ (see materials and methods for the selection of threshold).
Third, we investigated the firing properties of slow-firing MSvDB neurons in response to auditory stimuli (2 s of white noise) in a reward-related behavioral context. Consistent with previous reports (Miller and Freedman 1993), we found a subset of MSvDB neurons with prominent auditory responses in the form of very transient firing (single spikes or short bursts) at 15∼35 ms after stimulus onset (see example in Fig. 3A). Such an auditory-evoked neuronal response was sensory related but not reward related, because the response magnitudes covaried with sound intensity levels (data not shown) but did not depend on whether or not the sound was associated with the prediction of reward (Fig. 3D). To characterize this auditory response, we devised an Aud index (see materials and methods for detail). Similar to the distributions of REM index and the SIA index, the Aud index distribution was also bimodal (Fig. 3C). More than one-half (57%, 109/190) of the MSvDB neurons were categorized as Aud+ (Fig. 3C, see materials and methods for the selection of threshold).
Multiple Firing Features Uniquely Define a Distinctive Population of Slow-Firing MSvDB Neurons
Interestingly, most of the neurons that were REM+ and SIA+ were also Aud+ (71 of 81, 87.7%), whereas the majority (72 of 109, 66%) of the remaining neurons were Aud− (Fig. 3C), suggesting that these properties which we have investigated may be tightly related. To systematically investigate the relationship between the three physiological properties of MSvDB slow-firing neurons, we first calculated the correlation between any two of the three indexes. Strong correlations were found for all three pairs of indexes when all the slow-firing neurons were pooled together (Fig. 4C, red lines, all P < 0.001, significance tested with nonparametric Spearman rank correlation; correlation coefficients: r = 0.55, 0.59, 0.51 pairs of indexes, left to right).
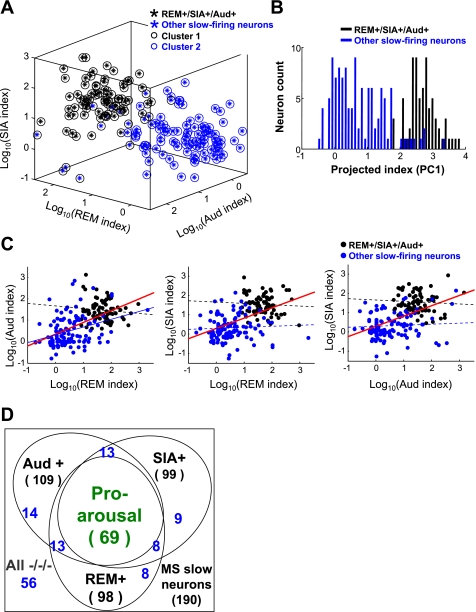
Fig. 4.
Multiple firing features uniquely define a distinctive population of slow-firing MSvDB neurons. A: REM+/SIA+/Aud+ neurons formed a distinctive population. Three-dimensional plots of the 3 log-transformed indexes are shown. Circles indicate clustering results from unsupervised K-means algorithm. The 2 clusters were very consistent with their division based on firing indexes, suggesting that REM+/SIA+/Aud+ neurons are distinct from the remaining neurons. B: aggregation of the 3 indexes into the first principle component (PC1) shows similar separation of the 2 groups of neurons. C: correlation between indexes on 2-D views of plots in A. Black and blue lines indicate correlation calculated in the respective group indicated; red lines indicate correlation for all neurons. Solid lines indicate significant correlation (all red lines and the blue line in left panel, P < 0.001; significance tested with nonparametric Spearman rank correlation); dotted lines indicate no significant correlation. D: diagram showing the distribution of neurons among the REM+/−, Aud+/−, and SIA+/− groups. Neuron numbers of other individual categories are shown in blue.
Using the three indexes to construct a three-dimensional (3-D) plot (Fig. 4A), we observed that the MSvDB neurons bearing all three properties (REM+, SIA+, Aud+) formed a cluster separated from the remaining neurons, indicating the existence of two groups of slow-firing neurons (REM+/SIA+/Aud+ and the remaining neurons). To confirm this observation, we used an unsupervised K-means clustering algorithm on all the MSvDB slow-firing neurons to separate them into two clusters based on these three indexes. The result matched very well with our two groups (REM+/SIA+/Aud+, 82% overlapping with cluster 1) (Fig. 4A). In fact, similar results could be achieved when clustering with any two of these indexes (data not shown). Very similar separation could also be seen if the first principal component (PC) of the 3-D plot was used to aggregate the three indexes into one dimension (PC1, the first principal component), which showed a bimodal distribution matching the two groups (Fig. 4B).
Furthermore, the correlations between indexes within either group were not significant (Fig. 4C, except between REM index and Aud index for the remaining neurons), corroborating the notion that two separate and homogenous subpopulations of neurons were present. All these results suggest that the REM+/SIA+/Aud+ neurons form a distinctive MSvDB subpopulation that is physiologically homogeneous (Fig. 4D). Since these firing properties are associated with behavioral or electrophysiological arousal, we refer to this subpopulation as “pro-arousal” slow-firing neurons and to the remaining slow-firing neurons as “other” slow-firing neurons.
Fine Temporal Relationship of MSvDB Pro-Arousal Slow-Firing Neurons With Theta Oscillations
To provide a context in which to compare our pro-arousal slow-firing neurons with earlier studies examining theta-related properties of MSvDB neurons, we characterized their theta-related firing modulations during REM sleep, which has the highest level of theta oscillations and engages rhythmic firing of MSvDB neurons the most (Sweeney et al. 1992). Particularly, we characterized the rhythmic firing in the theta frequency range, phase-locking to theta oscillations, and interspike interval (ISI) distributions of MSvDB neurons.
Figure 5, A–D, shows neuronal activities during one REM episode for one pro-arousal slow-firing, one other slow-firing, and one fast-firing rhythmic-bursting neuron. The theta-related analysis shown in Fig. 5 was based entirely on hippocampal (DG) LFP. Despite the high levels of theta oscillations in the REM episode (Fig. 5A), pro-arousal slow-firing neurons showed little rhythmic firing in the theta frequency range compared with the other two types of neurons, visualized as the theta-related side bands in the autocorrelation function (Fig. 5, D and H). In general, it is known that many nonrhythmic neurons can phase-lock to theta oscillations (e.g., type II neurons defined in King et al. 1998). Even though not rhythmic, most of the pro-arousal slow-firing neurons were phase-locked to theta oscillations (Fig. 5, B and E), with a lower level of phase preference compared with the two groups (Fig. 5F). Interestingly, for the neurons that were consistently phase-locked to theta oscillations, the pro-arousal neurons, but not the other two groups of neurons, preferentially fired at the falling phase of the local theta oscillations in MS (data not shown). Consistent with the phase-locking, their ISI distributions showed a prominent peak in the theta interval (100–180 ms) (Fig. 5, C and G). These results suggest that pro-arousal slow-firing neurons have weaker but consistent theta-related firing compared with other slow-firing and the fast-firing populations.
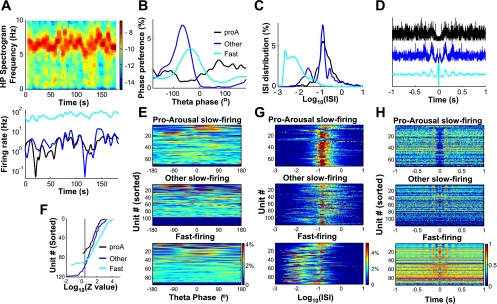
Fig. 5.
Theta-related firing properties of pro-arousal slow-firing neurons in REM sleep. A–D: example of theta-related properties of 3 types of neurons recorded in the same REM episode. A, top: spectrogram of hippocampal LFP during the REM episode; bottom: smoothed instantaneous firing rates. B: phase preferences of the 3 individual neurons. The pro-arousal slow-firing neuron (proA) had weaker phase modulation. C: interspike interval (ISI; in s) distribution of the 3 neurons. D: autocorrelation during REM episode. The pro-arousal slow-firing neuron was not rhythmic-firing, whereas the other slow-firing neuron and the fast-firing neuron had characteristic rhythmic side peaks in their autocorrelation functions. Autocorrelation functions are normalized to their individual maximal values. E: population plots of phase-locking during REM sleep for the 3 groups of neurons. Units are sorted according to phase-locking strength (Rayleigh test critical value Z). Nonsignificant (P > 0.05) neurons are displayed as null (bottom). Pseudocolor indicates phase distribution (%). F: sorted phase-locking strength (Z values) for the 3 groups in E. Vertical line indicates significant phase-locking (to right of the line, P < 0.05). Among phase-locked neurons, Z values were generally small for the pro-arousal slow-firing neurons compared with the other 2 groups. G: ISI distribution for the 3 groups. Pro-arousal slow-firing neurons had a clear peak around 0.1–0.18 s. H: population plots of autocorrelation (pseudocolor, normalized to maximum of individual correlation). Units are sorted according to rhythmicity index. Many fast-firing neurons were rhythmic firing, unlike most pro-arousal slow-firing neurons.
To further understand the relationship between the firing of MSvDB neurons and the pacing of theta oscillations, we investigated the fine temporal structure of MSvDB spike timing relative to the microstructure of theta oscillations using Z-shift analysis (Fig. 6, A and B), which has been used to infer the causal relationship between neuronal spiking activity and theta oscillations (Siapas et al. 2005). Among the neurons that were significantly phase-locked to theta oscillations (phase-locking P < 0.01; 84, 81, and 94% of pro-arousal slow-firing, other slow firing, and fast-firing neurons, respectively), the spike timing of most pro-arousal slow-firing neurons was best correlated with local theta oscillations in the MSvDB that occurred 10–20 ms before their spiking. Such results suggest that pro-arousal slow-firing neurons are likely followers of theta paces in the MSvDB. In contrast, the spike timing of most other slow-firing and fast-firing MSvDB neurons consistently leads the microstructure of theta oscillations by 10–40 ms (Fig. 6C). Overall, our analysis of the millisecond-level spike timing indicates that the spiking of pro-arousal slow-firing neurons is likely driven, as least partially, by local theta oscillations.
MSvDB Pro-Arousal Slow-Firing Neurons Fire Together and Promote Hippocampal Activation in a State-Dependent Manner
To further understand how MSvDB neurons operate in relation to each other, we investigated their propensity to fire together, using cross-correlation analyses of pro-arousal slow-firing, other slow-firing, and fast-firing MSvDB neurons. As shown in the example in Fig. 7A and the population results in Fig. 7, B and C, almost all pairs of pro-arousal slow-firing neurons showed significant positive correlation, indicating that these neurons are more likely to fire action potentials when another pro-arousal slow-firing neuron fires within a few seconds (Fig. 7B). However, this propensity was much reduced between other types of MSvDB neurons and between pro-arousal slow-firing neurons and other neuronal types (Fig. 7B). Moreover, almost none of the pro-arousal slow-firing neuron pairs showed anticorrelated firing (negative peak in cross-correlations), whereas 10–20% of other pair combinations were significantly anticorrelated (Fig. 7B). This pattern was consistently observed in all behavioral states, although the width of correlation functions (positive correlations) differed among states (cross-correlation half-width: WK, 3.2 ± 1.3 s; SW, 0.2 ± 1.3 s; REM, 3.8 ± 2.8 s; REM > WK > SW, Wilcoxon signed rank test between each pair). The strength of correlated firing, as determined by the average amplitude of cross-correlation functions, was significantly higher between pro-arousal slow-firing neurons than between other neuronal pairs (Fig. 7C). These results indicate that pro-arousal slow-firing neurons fire together within a few seconds of each other in all behavioral states and suggest that these cells may operate as a coherent neuronal ensemble.
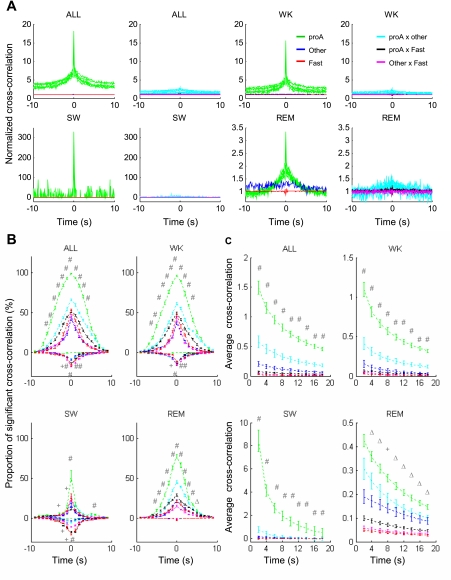
Fig. 7.
Pro-arousal slow-firing neurons fire together. A: example of cross-correlation between neuronal pairs. Panels show cross-correlation in each recording session as a whole (ALL) or cross-correlation broken down by states. Three types of neurons are shown: pro-arousal slow-firing (proA), other slow-firing (Other) and fast-firing (Fast) neurons. For every pair of panels, left panel shows cross-correlation of a pair of neurons from the same type and right panel shows cross-correlation of a pair of neurons from 2 different types. Cross-correlation between 2 pro-arousal slow-firing neurons is more prominent than that from other pairs in all behavioral states. B: proportion (average ± SE) of significant cross-correlation of all neuronal pairs at different time lags. Positive values depict original cross-correlations above the upper significant threshold (mean + 3SD); negative values depict those below the lower significant threshold (mean − 3SD). The proportion of positively significant cross-correlation is higher for pro-arousal slow-firing pairs than for other types of pairs. No pro-arousal slow-firing pairs showed negative cross-correlations. Color scheme is the same as in A. C: average cross-correlation values (average ± SE) of all neuronal pairs for different time windows. Cross-correlation between 2 pro-arousal slow-firing neurons is stronger than for other types of pairs. Color scheme is the same as in A. All comparisons tested were using 1-way ANOVA with Bonferroni-corrected multiple comparisons. Significance levels are labeled only when the value of the pair from 2 pro-arousal slow-firing neurons is significantly larger than that of any other types of pairs. ΔP < 0.05; +P < 0.01; #P < 0.001.
Finally and most importantly, to better understand how MSvDB neurons modulate large-scale oscillatory activity patterns in the hippocampus, we investigated how the activity of individual MSvDB neurons are correlated with simultaneously recorded hippocampal LFP activity by analyzing spike-triggered spectrograms. Single-neuron (Fig. 8A) and population data (Fig. 8B) show that during REM and WK, the firing of pro-arousal slow-firing neurons was associated with increased theta oscillations (7–10 Hz) and its harmonics. The peak of theta power increase occurred, on average, after the timing of the spike. This suggests that the firing of pro-arousal slow-firing neurons promotes the increase of theta oscillation power. Increases in the power of high gamma oscillations (70∼130 Hz) was also observed. These increases lasted about 2 s. In addition, the firing of pro-arousal slow-firing neurons also preceded a decrease in the power for oscillations below 6 Hz. In contrast, other slow-firing neurons were not associated with such changes. During SW sleep, which does not favor theta oscillations, the firing of pro-arousal slow-firing neurons was associated with a decrease of power in the 0- to 20-Hz oscillatory range for several seconds. Such a decrease in low-frequency power corresponds to the diminished LFP amplitude during the SIA epochs.
Overall, these results suggest that, in contrast to the precise spike timing of pro-arousal slow-firing neurons being influenced by individual theta cycles on a millisecond level (Figs. 5 and 6), the firing of pro-arousal slow-firing neurons promotes an increase in theta and gamma oscillation power during theta-dominant WK and REM states and a decrease in low-frequency power and the onset of SIA during SW sleep. This pattern of modulation was not associated with the spiking of other slow-firing neurons. It is important to note that the modulations of power (but not phase) of hippocampal oscillatory patterns operate on the time scale of a few seconds, consistent with the time scale of the collective behavior of the pro-arousal slow-firing neurons (cross-correlation results in Fig. 7).
DISCUSSION
Unlike previous MSvDB studies, which most often focused on fast-firing, rhythmic bursting neurons and theta pacing, the current study was designed to investigate the neurophysiological properties of the slow-firing MSvDB neurons. We successfully identified a homogeneous subpopulation of slow-firing neurons, comprehensively characterized their physiological properties, and investigated how their activity may modulate large-scale hippocampal activity patterns. Specifically, we demonstrated that a subpopulation of slow-firing MSvDB neurons can be uniquely defined and separated from other MSvDB neurons by three highly correlated features (Fig. 4): higher firing rate during REM sleep (Fig. 1), firing rate increase before transient arousals during SW sleep (Fig. 2), and short-latency transient responses to auditory stimuli at 15–35 ms (Fig. 3). These pro-arousal slow-firing neurons were also distinct in their intrinsic firing properties compared with other MSvDB neurons: nonrhythmic firing, less-prominent theta phase-locking, and likely followers of theta oscillations rather than theta pace makers (Figs. 5 and 6). Furthermore, they fire together as a group (Fig. 7), and their firing precedes and likely promotes an increase in power of theta and gamma oscillations during waking and REM sleep, as well as suppressing slow-waves while promoting SIA epochs during SW sleep (Fig. 8). Our data suggest that these pro-arousal slow-firing neurons may act as a coherent neuronal ensemble in vivo to collectively promote the activation of hippocampal networks in all behavioral states. These results represent one of the few studies that delineate how a functionally distinctive group of neurons in a subcortical neuromodulatory system influences the activity of its cortical target.
A Distinctive, Putatively Cholinergic Subpopulation of Neurons in MSvDB
Previous studies on MSvDB neuronal activities have mostly focused on fast-firing neurons as well as disparate physiological properties in isolated domains, e.g., firing rate modulations in all major arousal states (Morales et al. 1971; Sweeney et al. 1992), responses to behavioral events (Mercer and Remley 1979; Zin et al. 1977), and intrinsic firing properties in relation to theta oscillations (King et al. 1998; Macadar et al. 1970; Petsche et al. 1962). The lack of comprehensive characterization across those feature domains has made it difficult to compare and integrate results collected in different studies. Our study provides for the first time a comprehensive characterization of slow-firing MSvDB neurons in normal behaving animals and shows that many disparate features are in fact highly related (Fig. 4). Most importantly, the convergence of these features uniquely defines a group of homogeneous MSvDB neurons that are distinct from other MSvDB neurons.
The constellation of these features suggests that this homogeneous group of neurons likely shares the same neurochemical identity, in particular cholinergic. Multiple lines of supporting evidence indicate that the properties of the pro-arousal slow-firing neurons remarkably resemble the known physiological attributes of the MSvDB cholinergic neurons. First, the overall low firing rates were consistent with the findings from neurochemically identified MSvDB cholinergic neurons (Simon et al. 2006). Second, their firing rate modulations are consistent with the fluctuations in hippocampal ACh concentration during sleep-waking cycles (Bianchi et al. 2003; Marrosu et al. 1995). Third, their firing precedes and may promote transient arousal epochs during SW sleep, where a nicotinic cholinergic mechanism has been shown as a contributing mechanism (Lena et al. 2004). Fourth, a previous study suggested that neurons with the transient response to auditory stimulus might be cholinergic (Miller and Freedman 1993). Therefore, we propose that the pro-arousal slow-firing MSvDB neurons that we identified in the present study are putative MSvDB cholinergic neurons.
Although converging evidence supports the view that these are cholinergic neurons, difficulties in determining the exact neurochemical identity of chronically recorded neurons in behaving animals have prevented us from definitively confirming their cholinergic identity. Future experiments employing juxtacellular labeling or optogenetic tools (Zhang et al. 2007) will likely resolve this issue. In fact, our comprehensive characterization points out a promising direction for identifying MSvDB cholinergic neurons and will likely reduce the otherwise tremendous effort required in these studies.
Relationship Between MSvDB Pro-Arousal Slow-Firing Neurons and Theta Oscillations
Previous studies have presented conflicting views on the roles of different types of MSvDB neurons in pacing and generating hippocampal theta activity (Apartis et al. 1998; Brazhnik and Fox 1997; Hangya et al. 2009; Markram and Segal 1990; Simon et al. 2006; Sotty et al. 2003). Our large-scale survey of MSvDB neurons indicates that other slow-firing and fast-firing neurons are more tightly phase-locked with theta oscillations compared with pro-arousal slow-firing neurons. The former two groups also lead theta oscillations by tens of milliseconds, supporting their roles in pacing theta oscillations (Fig. 6). On the contrary, pro-arousal slow-firing neurons are only mildly phase-locked (Fig. 5) and follow, rather than lead, theta oscillations (Fig. 6). In fact, the pro-arousal neurons may be theta followers regardless of the behavioral context of the theta oscillations: their spiking lags behind both REM and WK theta (Fig. 6), and their firing rates during theta in REM or type I theta in WK (particularly, active exploration of objects) were not different statistically (data not shown).
Despite following local theta oscillations at the millisecond level, our results indicate that the firing of pro-arousal slow-firing neurons likely modulates hippocampal oscillatory power, lasting for several seconds (Fig. 8). Particularly, during WK and REM states, in which theta oscillations are favored, these neurons likely promote the increase in power of the higher frequency theta/gamma oscillations in the hippocampus. Therefore, our results suggest that the pro-arousal slow-firing neurons are not involved in the cycle-by-cycle pacing of hippocampal theta oscillations. Rather, they are entrained to and recruited by theta oscillations, and their firing in turn may sustain and enhance the power of ongoing hippocampal theta oscillations. Taking into account that the rhythmic bursting neurons pace the frequency of theta oscillations (Hangya et al. 2009), we suggest that the frequency and amplitude (power) of hippocampal theta oscillations may be dynamically modulated by separate populations of MSvDB neurons.
Despite the wealth of literature on the pharmacology and lesion of cholinergic actions (Dougherty et al. 1998; Lee et al. 1994; Parent and Baxter 2004; Steckler et al. 1995), surprisingly little is known about how the firing of MSvDB cholinergic neurons is related to, and modulates, in turn, the activity of hippocampal networks in normal behaving animals. This is largely due to the lack of knowledge about how cholinergic neurons behave in vivo. If this unique subpopulation we identified is cholinergic, our results suggest that MSvDB cholinergic neurons do not pace theta oscillations. Rather, their precise spike timing is influenced by the preceding theta cycle in the local circuit, and their firing in turn promotes theta oscillation power, lasting for a few seconds. Our investigation therefore provides new insights on theta-related properties of MSvDB cholinergic neurons and a novel mechanistic hypothesis on how they modulate hippocampal activity, particularly theta oscillation amplitude. These conclusions are also consistent with our recent finding in urethane-anesthetized rats that hippocampal ACh level and the activity of the putative MSvDB cholinergic neurons lag behind, and therefore do not participate in, the fast onset and pacing of theta oscillations (Zhang et al. 2010).
New Insights on the Function of MSvDB Pro-Arousal Slow-Firing Neurons
As discussed above, one function of the pro-arousal slow-firing neurons may be to promote a power increase of theta/gamma oscillations and to suppress low-frequency oscillations (0–6 Hz) in the hippocampus during WK and REM states. On the other hand, in behavioral states in which theta oscillations are not favored, such as SW sleep, the firing of the pro-arousal slow-firing neurons likely suppresses low-frequency oscillations (0–20 Hz). In line with our observation that they increased their activity just before SIA onsets (Fig. 2), another function of these neurons may be to promote the onset of SIA epochs during SW sleep. Although the neural mechanisms responsible for generating the transient arousal state SIA are largely unknown (Jackson et al. 2008; Jarosiewicz et al. 2002), it has been shown that the cholinergic system plays an important role: knockout mice without the β2-subunit of nicotinic receptors showed decreased frequency of SIA occurrence (Lena et al. 2004). This finding not only supports our proposal that the specific neuronal population we identified is likely cholinergic neurons but also suggests that MSvDB cholinergic neurons may be a crucial component of the SIA generation mechanism. Furthermore, these pro-arousal neurons may also be generally associated with transitions to behavioral arousal or electrophysiologically activated states, because they gradually increased their firing rates starting from 2–3 s before the transitions from SW sleep to WK or from intermediate sleep (IS; also called transition to paradoxical sleep, t-PS) to REM sleep, and transiently increased firing in the 1 s before SW-to-IS transition (data not shown).
Together, their strong association with SIA onsets, tight coupling with an increase in theta/gamma oscillation power, and decrease in low-frequency oscillation power suggest that MSvDB pro-arousal slow-firing neurons may act as a generalized mechanism for hippocampal activation and electrophysiological arousal. Direct evidence beyond the temporal relationship between spike timing and power changes warrants future investigations to confirm this hypothesis. Beyond the above-discussed association and potential promotion of electrophysiological activation of the hippocampus, by naming these neurons “pro-arousal,” we are not suggesting that their activity could directly produce behavioral arousal or awakening. Behavioral arousal is controlled by more caudal nuclei (for example, reviewed in Jones 2008), and the MSvDB pro-arousal neurons may be an important node to relay such arousal to their target areas, i.e., hippocampus and associated areas.
The mechanism by which the pro-arousal neurons potentially produce hippocampal activation is not known. Direct projection from these pro-arousal neurons to the hippocampus may be a parsimonious mechanism. However, septohippocampal projection is said to have a rough topographic organization, with different groups of neurons preferentially innervating different part of the hippocampus along the longitudinal axis (Amaral and Kurz 1985; Segal and Landis 1974), whereas LFPs along the longitudinal axis may not be entirely synchronized. Our current techniques limit our capability to identify whether the recorded MSvDB neurons project directly to the dorsal hippocampus, where we recorded the LFPs. This issue and the activation mechanism need to be investigated in future studies.
Last but not least, if this subpopulation is indeed cholinergic, this putative cholinergic network not only participates in the wake-sleep states modulation, but also is activated in a behavioral context such as responding to external stimuli. In behaving animals, the short-lasting increase of theta power caused by the firing of these neurons may serve as short-term tags (Vertes 2005) and a favorable network state for synaptic plasticity (Larson et al. 1986; Pavlides et al. 1988). The consequent release of ACh in the hippocampus may, in parallel, act on muscarinic receptors to produce relatively long-lasting effects on promoting signaling pathways that lead to activation of kinases (Giovannini et al. 2005) and immediate-early genes (Wirtshafter 2005). Through these concerted actions, the activity of MSvDB cholinergic neurons can modulate both the short-term activity and long-term plastic changes of the hippocampal network, thus fulfilling the role of the cholinergic system in hippocampal synaptic plasticity and learning and memory.
GRANTS
This research was supported by National Institutes of Health Grants 5R01 DE011451-11 and R33 NS049534 to M. A. L. Nicolelis and a National Alliance for Research on Schizophrenia and Depression 2008 Young Investigator Award and the Intramural Research Program of the National Institute on Aging to S.-C. Lin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.Z., S.-C.L., and M.A.N. conception and design of research; H.Z. performed experiments; H.Z. analyzed data; H.Z. and S.-C.L. interpreted results of experiments; H.Z. prepared figures; H.Z. and S.-C.L. drafted manuscript; H.Z. and M.A.N. edited and revised manuscript; H.Z., S.-C.L., and M.A.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Romulo Fuentes and Kafui Dzirasa for critical discussions and comments on the manuscript, Gary Lehew and Jim Meloy for technical assistance, and Susan Halkiotis for proofreading the manuscript. M.A. Nicolelis acknowledges a visiting professorship, Chaire Blaise Pascal, from the Région Ile de France at the Ecole Supérieure de Physique et de Chimie Industrielles, Paris.
REFERENCES
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol 240: 37–59, 1985 [DOI] [PubMed] [Google Scholar]
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. J Neurophysiol 79: 1633–1642, 1998 [DOI] [PubMed] [Google Scholar]
- Berens P, Velasco MJ. Toolbox for circular statistics with Matlab (Online) The MathWorks, Natick, MA: http://www.mathworks.com/matlabcentral/fileexchange/10676 [ACCESS DATE] [Google Scholar]
- Bianchi L, Ballini C, Colivicchi MA, Della Corte L, Giovannini MG, Pepeu G. Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochem Res 28: 565–573, 2003 [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Intracellular recordings from medial septal neurons during hippocampal theta rhythm. Exp Brain Res 114: 442–453, 1997 [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Turchin PI, Walsh TJ. Septocingulate and septohippocampal cholinergic pathways: involvement in working/episodic memory. Brain Res 810: 59–71, 1998 [DOI] [PubMed] [Google Scholar]
- Ford RD, Colom LV, Bland BH. The classification of medial septum-diagonal band cells as theta-on or theta-off in relation to hippocampal EEG states. Brain Res 493: 269–282, 1989 [DOI] [PubMed] [Google Scholar]
- Gaztelu JM, Buno W., Jr Septo-hippocampal relationships during EEG theta rhythm. Electroencephalogr Clin Neurophysiol 54: 375–387, 1982 [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci 24: 11137–11147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Pazzagli M, Malmberg-Aiello P, Della Corte L, Rakovska AD, Cerbai F, Casamenti F, Pepeu G. Inhibition of acetylcholine-induced activation of extracellular regulated protein kinase prevents the encoding of an inhibitory avoidance response in the rat. Neuroscience 136: 15–32, 2005 [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev 7: 119–188, 1983 [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol 17: 533–557, 1954 [DOI] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143: 1051–1064, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci 29: 8094–8102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: 710–715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Dickson CT, Bland BH. Median Raphe stimulation disrupts hippocampal theta via rapid inhibition and state-dependent phase reset of theta-related neural circuitry. J Neurophysiol 99: 3009–3026, 2008 [DOI] [PubMed] [Google Scholar]
- Jarosiewicz B, McNaughton BL, Skaggs WE. Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci 22: 1373–1384, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, Skaggs WE. Level of arousal during the small irregular activity state in the rat hippocampal EEG. J Neurophysiol 91: 2649–2657, 2004 [DOI] [PubMed] [Google Scholar]
- Jerusalinsky D, Kornisiuk E, Izquierdo I. Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochem Res 22: 507–515, 1997 [DOI] [PubMed] [Google Scholar]
- Jobert A, Bassant MH, Lamour Y. Hemicholinium-3 selectively alters the rhythmically bursting activity of septo-hippocampal neurons in the rat. Brain Res 476: 220–229, 1989 [DOI] [PubMed] [Google Scholar]
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann NY Acad Sci 1129: 26–34, 2008 [DOI] [PubMed] [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur J Neurosci 10: 464–477, 1998 [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. J Comp Neurol 298: 362–372, 1990. 2212109 [Google Scholar]
- Kitchigina VF, Kutyreva EV, Brazhnik ES. Modulation of theta rhythmicity in the medial septal neurons and the hippocampal electroencephalogram in the awake rabbit via actions at noradrenergic alpha2-receptors. Neuroscience 120: 509–521, 2003 [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat Embryol (Berl) 169: 41–44, 1984 [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res 368: 347–350, 1986 [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62: 1033–1047, 1994 [DOI] [PubMed] [Google Scholar]
- Lena C, Popa D, Grailhe R, Escourrou P, Changeux JP, Adrien J. Beta2-containing nicotinic receptors contribute to the organization of sleep and regulate putative micro-arousals in mice. J Neurosci 24: 5711–5718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PR, Shute CC, Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol 191: 215–224, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol 96: 3209–3219, 2006 [DOI] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron 59: 138–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadar O, Roig JA, Monti JM, Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav 5: 1443–1449, 1970 [DOI] [PubMed] [Google Scholar]
- Mahut H. A selective spatial deficit in monkeys after transection of the fornix. Neuropsychologia 10: 65–74, 1972 [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the rat medial septum-diagonal band complex. Brain Res 513: 171–174, 1990 [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res 671: 329–332, 1995 [DOI] [PubMed] [Google Scholar]
- Mercer LF, Jr, Remley NR. Mapping of sensory-responsive cells in the septal area of the rat. Brain Res Bull 4: 483–490, 1979 [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. Medial septal neuron activity in relation to an auditory sensory gating paradigm. Neuroscience 55: 373–380, 1993 [DOI] [PubMed] [Google Scholar]
- Mitra P. Chronux. National Institute of Mental Health; (Online) http://www.chronux.org [ACCESS DATE] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. New York: Oxford University Press, 2008 [Google Scholar]
- Mizumori SJ, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res 528: 12–20, 1990 [DOI] [PubMed] [Google Scholar]
- Morales FR, Roig JA, Monti JM, Macadar O, Budelli R. Septal unit activity and hippocampal EEG during the sleep-wakefulness cycle of the rat. Physiol Behav 6: 563–567, 1971 [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron 18: 529–537, 1997 [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem 11: 9–20, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res 439: 383–387, 1988 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. San Diego, CA: Elsevier Academic, 2005 [Google Scholar]
- Petsche H, Stumpf C, Gogolak G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol 14: 202–211, 1962 [DOI] [PubMed] [Google Scholar]
- Segal M, Landis S. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Res 78: 1–15, 1974 [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46: 141–151, 2005 [DOI] [PubMed] [Google Scholar]
- Simon AP, Poindessous-Jazat F, Dutar P, Epelbaum J, Bassant MH. Firing properties of anatomically identified neurons in the medial septum of anesthetized and unanesthetized restrained rats. J Neurosci 26: 9038–9046, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 551: 927–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Keith AB, Wiley RG, Sahgal A. Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: role of the septohippocampal projection. Neuroscience 66: 101–114, 1995 [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Firing relations of medial septal neurons to the hippocampal theta rhythm in urethane anesthetized rats. Exp Brain Res 77: 507–516, 1989 [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Lamour Y, Bassant MH. Arousal-dependent properties of medial septal neurons in the unanesthetized rat. Neuroscience 48: 353–362, 1992 [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol 30: 225–340, 1988 [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus 15: 923–935, 2005 [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201: 160–163, 1978 [DOI] [PubMed] [Google Scholar]
- Wirtshafter D. Cholinergic involvement in the cortical and hippocampal Fos expression induced in the rat by placement in a novel environment. Brain Res 1051: 57–65, 2005 [DOI] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci 8: 577–581, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin SC, Nicolelis MA. Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo. J Neurosci 30: 13431–13440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zin R, Conforti N, Feldman S. Sensory responsiveness of single cells in the medial septal nucleus in intact and hypothalamic-deafferented rats. Exp Neurol 54: 7–23, 1977 [DOI] [PubMed] [Google Scholar]