Abstract
Spinal cord transection silences neuronal activity in the deafferented cortex to cutaneous stimulation of the body and untreated animals show no improvement in functional outcome (weight-supported stepping) with time after lesion. However, adult rats spinalized since neonates that receive exercise therapy exhibit greater functional recovery and exhibit more cortical reorganization. This suggests that the change in the somatotopic organization of the cortex may be functionally relevant. To address this issue, we chronically implanted arrays of microwire electrodes into the infragranular layers of the hindlimb somatosensory cortex of adult rats neonatally transected at T8/T9 that received exercise training (spinalized rats) and of normal adult rats. Multiple, single neuron activity was recorded during passive sensory stimulation, when the animals were anesthetized, and during active sensorimotor stimulation during treadmill-induced locomotion when the animal was awake and free to move. Our results demonstrate that cortical neurons recorded from the spinalized rats that received exercise 1) had higher spontaneous firing rates, 2) were more likely to respond to both sensory and sensorimotor stimulations of the forelimbs, and also 3) responded with more spikes per stimulus than those recorded from normal rats, suggesting expansion of the forelimb map into the hindlimb map. During treadmill locomotion the activity of neurons recorded from neonatally spinalized rats was greater during weight-supported steps on the treadmill compared with the neuronal activity during nonweight supported steps. We hypothesize that this increased activity is related to the ability of the animal to take weight supported steps and that, therefore, these changes in cortical organization after spinal cord injury are relevant for functional recovery.
Keywords: treadmill, hindlimb, electrophysiology, multiple, single neuron
the role of cortical reorganization in the sensorimotor cortex of the brain after traumatic injury has been studied for over fifty years. Reorganization is generally demonstrated by the representation of skin surfaces along the edge of the deprived cortex that appear to expand topographically into part of the deprived cortex somatotopically (Merzenich et al. 1983). Although it is generally accepted that cortical reorganization can occur after amputation of digits (Kelahan and Doetsch 1984; Rasmusson et al. 1992; McCandlish et al. 1996), limbs (Rasmusson and Nance 1986), or peripheral nerve damage (Merzenich et al. 1983; Kalaska and Pomeranz 1979; Wall and Cusick 1984), the reorganization of the sensorimotor cortex in response to spinal cord injury is severely restricted due, in part, to a reduced opportunity for axonal sprouting in the central nervous system compared with the peripheral nervous system. In fact, in one of the first studies of the effects of spinal cord injury on neuronal activity in the cortex, Levitt and Levitt (1968) showed that regions of the cortex that normally respond to cutaneous stimulation below the level of the lesion (deafferented cortex) are silent (e.g., do not increase their responsiveness to cutaneous stimulation above the level of the lesion) after the spinal cord injury. McKinley and Smith (1990) confirmed these results in studies of adult spinalized cats, demonstrating that the deafferentated cortex remains unresponsive and is not reactivated by forelimb afferents. More recent studies by Kaas and colleagues shed light on these issues (Jain et al. 1995) by showing that unilaterally lesioning the dorsal funiculus at the thoracic level (T6–T8) silenced the hindlimb somatosensory cortex contralateral to the lesion, indicating that the spared spinothalamic tract is not sufficient to generate cortical responses to cutaneous stimulation. It is noteworthy that hindlimb motor deficits did not recover in these animals. Extending this work to neonatal injuries, they demonstrated that a spinal overhemisection at the cervical level (C3) at postnatal day 3 resulted in a complete lack of responses in the deafferented parts of the forepaw somatosensory cortex to stimulation below C3, even in rats that received an enriched environment until adulthood (Jain et al. 2003). More recent studies using electrical stimulation (1 mA) showed silencing of the hindlimb sensorimotor cortex in adult spinalized rats (Endo et al. 2007; Ghosh et al. 2010; Aguilar et al. 2010). These results are in agreement with our recent data showing that the deafferented hindpaw somatosensory cortex in adult rats spinalized as neonates that do not receive any therapy is silenced (Kao et al. 2009).
In contrast, when animals received some form of exercise, there are measurable changes in the organization of the sensorimotor cortex. For example, neurons in the affected somatosensory cortex of spinalized kittens that received passive exercise responded to sensory stimulation of peripheral areas innervated rostral to the injury (Chau and McKinley 1991). Moreover, when neonatally spinalized rats received treadmill exercise, motor cortex for upper trunk regions (Giszter et al. 1998a) and sensory cortex for forepaw regions (Kao et al. 2009) expanded into the hindlimb sensorimotor cortex. The expansion of sensory and motor cortex was correlated to the ability of these animals to take weight-supported steps on the treadmill. Therefore, exercise after spinal cord injury modifies the organization of the somatosensory cortex, and this reorganization maybe functionally relevant.
Improvement in functional outcome after rehabilitative therapy is generally thought to be the result of plasticity in response to increased activation of the spared neuronal networks (Edgerton et al. 2008; Lynskey et al. 2008, Barrière et al. 2008). In the case of spinal cord injury, these strategies have generally focused on plasticity below the level of the lesion. Because reorganization of the somatoptic maps in the cortex may play a critical role in functional recovery (Kaas et al. 2008; Kao et al. 2009; Ghosh et al. 2009; Nishimura et al. 2007), it has been suggested that rehabilitative strategies should not be limited to targeting spinal cord plasticity but should address plasticity at all levels of the sensorimotor system (Beekhuizen and Field-Fote 2005; Thomas and Gorassini 2005; Winchester et al. 2005; Girgis et al. 2007; Hoffman and Field-Fote 2007; Martinez et al. 1995). Nevertheless, the impact of rehabilitative strategies on cortical organization of the somatotopic maps in the cortex remains poorly understood.
To address this issue, we chronically implanted arrays of microwire electrodes into the hindlimb sensorimotor cortex (HL-SMC) of the cortex of adult rats that received a complete, midthoracic spinal transection as neonates and daily treadmill exercise therapy. Populations of single neurons were recorded in response to tactile stimuli when the animals were lightly anesthetized and when the animals were awake and locomoting on a treadmill and compared with that of normal rats. Our data demonstrate that the novel cortical organization in the brains of neonatally spinalized rats that received exercise is evident in the awake, freely moving animal, and this activity is greater during weight-supported stepping than during nonweight-supported stepping, suggesting that this novel organization is used to enhance motor function.
METHODS
Overview.
The present study used multiple, single neuron electrophysiology in awake, freely moving rats and behavioral testing to identify the effect of exercise therapy (treadmill exercise) on cortical remodeling of the HL-SMC after a midthoracic transection (TX) in neonates. The complete TX eliminates hindlimb input to the HL-SMC cortex while leaving forelimb input intact. We used neonatal injury because treadmill exercise enables a percentage of adult animals spinalized as neonates to support their hindquarters during treadmill exercise (Kao et al. 2009; Giszter et al. 1998a; Giszter et al. 1998b; Miya et al. 1997). We measured changes in cortical organization in two ways. First, we recorded neuronal activity during passive stimulation of the cutaneous paw surface above the level of the lesion when the animal was lightly anesthetized. Second, we recorded neuronal activity during paw placements while the animals performed treadmill-induced locomotion. By studying changes in cortical organization of spinalized rats during both the awake and anesthetized conditions, we were able to correlate these changes with functional behavioral measures (percentage of weight-supported steps) and determine whether the changes were relevant to functional recovery. We did not make a direct comparison between spinalized rats that received exercise and spinalized rats that did not receive exercise because we had shown previously that the HL-SMC of rats that did not receive any exercise do not show any novel cortical reorganization (i.e., probability of responding to forelimb inputs was the same as a normal rat; Kao et al. 2009). Therefore, the effect of exercise therapy after complete spinal transaction on cortical organization was compared with the cortical organization of normal rats.
All procedures used in this study were performed under the guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine.
Neonatal spinalization and postoperative care.
The transection procedure for the pups was performed as in our methods published previously (Kao et al. 2006; Shumsky et al. 2005). Briefly, 2- to 3-day-old Sprague Dawley pups (Charles River) were anesthetized by hypothermia, and the spinal cord was exposed by laminectomy at the T8/T9 level and transected with iridectomy scissors followed by aspiration to ensure completeness. A collagen matrix, Vitrogen, was injected into the site of the transection to fill the cavity. The muscle and skin were sutured in layers with 5-0 silk. Pups were then warmed and, when they became active, were returned to the mothers and littermates. The pups were weaned at 4 weeks and housed in controlled conditions of temperature and humidity under a 12-h:12-h light/dark cycle (lights on at 07:00) with free access to food and water. Sixteen neonatally spinalized rats that survived weaning were used in this study.
Spinalized rats were handled and examined 5 days/week for skin lesions and other health concerns. After weaning, rats were placed on a motorized treadmill for 3 min/day at a speed of 6.5 m/min, 5 days per week. Previous work has shown that neonatally spinalized rats that received treadmill training can step under speeds of 6 m/min but not 12 m/min (Miya et al. 1997). This treadmill regimen was selected because this level of exercise is known to enhance the representation of the forelimb to tactile stimulation by cells in the hindlimb sensorimotor cortex of neonatally spinalized rats (Kao et al. 2009). Exercise continued until the animals were 6- to 8 months old. Four animals did not survive past the end of training; two were euthanized due to autophagia, and two died, presumably due to complications from bladder infections. At this point, animals were chronically implanted with arrays of microeletrodes (see below). A total of 12 neonatally spinalized rats and 10 age-matched normal animals were implanted with arrays of microwire electrodes. Seven neonatally spinalized rats and eight normal rats survived the implant surgery and had sufficient single neurons (>10 per array; see spike discrimination below) to continue with the study.
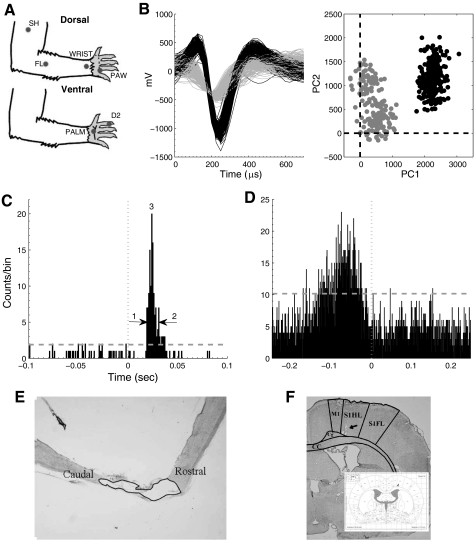
To assess the completeness of the transection, spinal cords were stained with Nissl-myelin staining or Nissl-myelin combined with 5-HT at the end of the experiments. For both these procedures, rats were perfused transcardially with buffered saline, followed by buffered 4% paraformaldehyde. Spinal cords were removed and placed in phosphate buffer containing 30% sucrose for 72 hours. Specimens were frozen in tissue freezing medium (Tissue-Tek) and sectioned on a freezing microtome at 20 μm. The lesion segments were either 1) sectioned parasagitally, and alternate sections were Nissl-myelin stained to confirm completeness of transaction (Kao et al. 2009) or 2) sectioned transversely, rostral and caudal to the spinal injury (T8/9) and stained with Nissl-myelin and a polyclonal antibody to 5-HT on alternate slices. For this second approach, frozen sections mounted on slides were incubated at 4 °C with the primary antibody (diluted 1:40,000; Immunostar, Stillwater, MN) for 16 hours, with biotinylated goat anti-rabbit IgG for 2 hours, and with avidin-biotinylated horseradish peroxidase complex for 2 hours, as specified by the manufacturer (ABC Standard Kit; Vector Laboratories, Burlingame, CA). Peroxidase reactivity was visualized with 0.05% diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05 mM Tris buffer. This second approach was used for the animals that had a high percentage of weight-supported stepping to ensure no possibility of spared fibers. The resulting sections were examined under a microscope to confirm completeness of the transection (Fig. 1).
Fig. 1.
Single neurons were recorded from the infragranular hindlimb somatosensory cortex under 2 experimental paradigms: passive sensory stimulation and active sensorimotor stimulation. A: six discrete locations on each forelimb were repeatedly touched with a punctate stimulus (100 times). Three locations were on the paw [digit 2 (D2), palm pad 5 (PLM), and a single location on the dorsal forepaw (PAW)], and 3 locations were on the forelimb [wrist (WR), distal forelimb (FL), and the shoulder (SH)]. Locations D2, PLM, and WR were stimulated on the ventral glabrous skin surface; locations PAW, FL, and SH were stimulated on the dorsal hairy skin surface. B: single units were discriminated using the first and second principal components (PC1 and PC2). The black and gray represent 2 distinct neurons. C: a representative peristimulus time histogram (PSTH) from a single cell during passive stimulation of a single location on the forepaw under anesthesia. The response magnitude was defined as the integral of the PSTH between the first significant bin (1) and the last significant bin (2). The peak response (3) was defined as the single bin with the most spikes. The stimulus was presented at time zero. The dashed line represents the threshold for a significant response. D: a representative unsmoothed perievent time histogram (PETH) from a single cell during forepaw placement on the treadmill (for a complete analysis of types of responses see Chapin and Woodward 1986). The response magnitude (RM), first significant bin, the last significant bin, and the peak response (PR) are defined as in B. The forepaw placement was made at time zero. E: verification of the completeness of the transection using Nissl staining. F: histological verification of electrode placement into the hindlimb sensory-sensorimotor overlap region of the infragranular layer of the cortex. Arrow indicates location of electrode tips. Inset is reprinted from Paxinos and Watson 1986.
Implantation of electrode arrays.
Six to eight months after spinalization, rats were chronically implanted bilaterally with arrays of microwires (stainless steel, 50 micron diameter insulated with Teflon; Neurolinc, Baskinridge, NJ) into the hindlimb representation of the sensorimotor cortex (HL-SMC) using the procedure from our hindlimb mapping study (Moxon et al. 2008). The wires were first bundled and then separated into two rows of eight wires spaced 250 microns apart so that the relative position of any wire in the array and the pins on the connector were unknown. Therefore, it was not possible to perform a topographical analysis of the response across recording sites. A similar procedure was performed on age-matched normal adult control rats. Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (45 mg/kg), placed in a stereotaxic frame, and craniotomies were performed over both the right and left cortices to expose the hindlimb representation. A craniotomy was made between coordinates relative to bregma: (0 AP, 2.5 ML), (−0.5 AP, 3 ML), (−2 AP, 2 ML), (−2.5 AP, 2.5 ML), where ML is the medial-lateral coordinate and negative AP coordinates are posterior to bregma (units are in millimeters). These coordinates center the microwires over the sensory and sensorimotor overlap region of the hindpaw granular cortex (Chapin and Lin 1984). As the electrode array was slowly lowered, the signals were monitored, one channel at a time, on the oscilloscope and audio speakers. When the characteristic large amplitudes of layer V (infragranular layer) neurons were recorded on the majority of electrodes, the array was cemented in place with dental cement. It should be noted that the distinction between granular and agranular cortex relies on characteristics of layer IV cells and clearly refers to the locations where thalamocortical neurons project (Akers and Killackey 1978). In our study, we recorded from layer V/VI cells directly below this granular cortex. Because the precise position of each electrode could not be verified as being below the granular or agranular cortex, it is possible that some of these cells were recorded from cortex outside the granular zone (Fig. 1). However, the possibility is equally likely for neonatally spinalized rats as the normal adult rats. Animals were allowed 7–10 days to recover from the implantation surgery before physiological evaluations of the neurons were performed. Recordings were completed within 4 weeks of implant.
Single neuron discrimination.
Single neurons were discriminated from the analog signal recorded from each microwire immediately before physiological assessment of the cells using our standard methods (Moxon et al. 2008; Tutunculer et al. 2006; Foffani et al. 2004), similar to those used to study the receptive fields of cells in the trigeminal somatosensory system of the rat (Ghazanfar and Nicolelis 1999; Leiser and Moxon 2006). To assess the responsiveness of cells to passive sensory stimulation, each animal received an induction dose of Nembutal (35 mg/kg), which immobilized the rat but ensured minimal interference of the anesthesia on the neural recordings (stage III-2; Friedberg et al. 1999). Stable levels of light anesthesia were maintained by giving small supplements when the rat responded to tail-pinch. No anesthesia was given to animals before recording sessions during treadmill locomotion. Even though any movement of the arrays was likely to be small (e.g., micromotion), cells were rediscriminated each day. Although we do not know whether the same cell was recorded during active and passive recordings sessions, they clearly belonged to the same population of cells. Therefore, for statistical purposes, the activity recorded from each cell was considered an independent sample.
To perform the discrimination, a VLSI headstage with 10× gain (HST/16o50-G1; Plexon, Dallas, TX) was attached to each of the two connectors on the rat's head, and the headstage was connected to a multineuron acquisition system (MNAP; Plexon). Signals from the electrodes were further amplified reaching a total gain of 10,000–20,000 and filtered (bandpass 154 Hz–13 kHz). The resulting analog signals were displayed on an oscilloscope and amplified through audio speakers to aid in online neuronal spike sorting. Real-time spike sorting software (SortClient; Plexon) captured action-potential waveform segments around a voltage threshold crossing and sorted these in real time according to their shape. Neural signals were monitored via a computer screen using the SortClient software, an oscilloscope, and audio speakers. To discriminate single units, template matching was used (Wheeler 1999) and the first three principal components of the template were monitored to define the waveform shape and ensure clear separation between units before the recording session began (Chapin and Nicolelis 1999). Waveforms were saved for offline analysis to ensure single unit separation by testing for no significant changes (F-statistic, WaveTracker) in their waveform shape or principal components collected during a single recording session using methods identical to our previous studies (Leiser and Moxon 2007) ensuring reliability in our single-unit separation. A total of 367 cells were recorded from the infragranular layer of the seven neonatally spinalized animals and 343 neurons were recorded from the eight intact rats during the passive sensory stimulation procedure described next.
Passive sensory stimulation procedure.
Cells were recorded from the lightly anesthetized animals while the cutaneous surface of the forelimbs was stimulated with a triggered stimulus using methods similar to our previous mapping study of the HL-SMC (Fig. 1A) (Moxon et al. 2008). Stimulation locations were chosen based on the rat's use of these areas for tasks involving complex discrimination (Whishaw and Pellis 1990; Whishaw and Gorny 1994). Six sparse locations were chosen for stimulation: three on each forepaw (digit 2, ventral palm, dorsal paw) and three on each forelimb (ventral wrist, a location on the proximal limb, and a location on the distal limb; Fig. 1). These locations were chosen to maximize the number of responding neurons, while maintaining a reasonable compromise between spatial sampling precision on the body and experimental feasibility. Each location was consecutively tapped 100 times at 0.5 Hz with a fine-tipped metal probe, which was controlled by a precision stepper motor (Gemini GV6) that was in turn controlled by a servo drive (Parker Hannifin Corporation, Compumotor Division, Rohnert Park, CA), and which delivered squared-pulse tactile stimuli (duration: 100 ms, frequency: 0.5 Hz), similar to previous studies (Chapin 1986; Foffani et al. 2004; Tutunculer et al. 2006). To ensure that only tactile receptors at the sight of contact were activated, the tip of the metal probe moved 0.5 mm in response to the square-pulse stimuli. To control the magnitude of the stimuli at each location, the metal probe was first positioned on the skin, ensuring contact but no visual indentation under 10× magnification. The metal probe was then moved 0.5 mm away from the skin, and the stimulation was begun. The effect of the stimulus was viewed under 10× magnification to ensure no movement of the digits or limb. All locations were tapped within the same recording session to ensure that the same neurons were recorded in response to stimulation of all locations. All 100 stimuli were given to a location, and the stimulator was then moved to the next location. The frequency of stimulation of 0.5 Hz corresponds to twice the interstimulus interval shown previously not to influence subsequent responses (Chapin 1986). The motor stimulator simultaneously sent pulses to the data acquisition system for precise timing of the stimulus onsets. The waveforms and action potential times of all the discriminated neurons were recorded, and data were stored in NeuroExplorer (Nex Technologies, Littleton, MA, version 2.66) for offline analysis. For each stimulus location, peristimulus time histograms (PSTHs) of all neurons (1 ms bin size) were calculated using NeuroExplorer (Fig. 1B), and exported to Matlab (version 6.5, The Mathworks) for further analysis as in our work that was published previously (Tutunculer et al. 2006; Moxon et al. 2008).
Data analyses.
Although the activity of all cells was continuously recorded during stimulation at all 10 locations, each cell was responsive to only a small number of stimulated locations. For each cell, only significant responses were processed for further analysis. Significant responses were identified from the PSTHs using three tests for each discriminated neuron and peripheral location stimulated: 1) A threshold was set as the average background firing rate of the neuron (evaluated from 100 ms to 5 ms before the stimulus) plus 3 standard deviations and the first and the last significant bin (1 ms bin size) that exceeded the threshold in a window between 5 ms and 90 ms after the stimulus was identified, 2) at least three bins had to be over the threshold, and 3) the response between the first and last significant bin had to be significantly greater than the average background activity (unpaired t-test, P < 0.001).
For each neuron with a significant response, we first identified the location that gave the greatest response. We then determined the percentage of cells with their largest response ipsilateral to the stimulus and the percentage of cells with their largest response contralateral to the stimulus. In addition, for each neuron, eight measures of neuronal responsiveness were extracted from the PSTH for the largest response (1 response per neuron): 1) spontaneous activity measured in spikes per second; 2) response magnitude, using all the spike, the average number of spikes per stimulus between the first and the last significant bin minus the background firing rate (i.e., probability of spike per stimulus); 3) the average peak response minus the background firing rate (i.e., the number of spikes per stimulus per bin); 4) first spike latency or time to the first spike after the stimulus; 5) the first bin latency, or the time interval between the stimulus onset and the first significant bin of the response; 6) the peak latency, or the time interval between the first significant bin and the peak of the response; 7) the last bin latency, or the time interval between the stimulus onset and the last significant bin of the response; and 8) the receptive field size or number of locations to which the cell was responsive time. The duration of the response was calculated by subtracting the first bin latency from the last bin latency. The jitter of the first spike was measured as the variance in the latency to the first spike.
This procedure is similar to our previous study (Kao et al. 2009) except here we extend that work in the following ways. First, we examine for the first time, the duration of the response, the jitter of the first spike, and changes in the size of the receptive field. Second, as explained below, we compare the responses that were produced from stimuli ipsilateral to the stimulus as well as contralateral. The eight measures defined above and the percentage of responding cells were then compared between animal groups (neonatally transected vs. normal) and between stimulus position (ipsilateral to the neuron recorded or contralateral) using a two-way ANOVA. The first factor, animal group, had two levels: spinalized and normal. The second factor, stimulus position, had two levels: ipsilateral or contralateral to the neuron being recorded. A Tukey post-hoc was used to identify differences between groups following a significant interaction.
Active sensorimotor (treadmill) stimulation procedure.
To extend our previous work (Kao et al. 2009) we also measured the responsiveness of HL-SMC neurons to active sensorimotor stimulation by recording neuronal activity during locomotion on a motorized treadmill (Fig. 1C). The rat was placed on the nonmoving treadmill in an enclosed chamber while the single neuron discrimination procedure, as defined above, was performed at each recorded channel. When the single neuron discriminations were complete, a video camera (Mintron, Taipei, Taiwan) was placed in a position that allowed a lateral view of the rat during treadmill locomotion. A mirror was placed behind the animal and the lateral view of the back of the rat was also recorded. The camera was connected to a VCR (SONY SL-HF750), which captured 60 frames per second. The VCR was connected to a synchronization signal generator and time/date text inserter (FOR-A VTG-55) that recorded the duration of the rat's awake, freely moving session with millisecond resolution on each frame. At the start of the neuronal recording, the clock was reset to zero by the Plexon MNAP system's start-recording TTL pulse synchronizing the video with the neural data. Neural signals and synchronized high-speed video were recorded simultaneously during the entire recording session. When the experimenter was ready, the treadmill was turned on to run at a speed of 6.5 m/min. After the animal began treadmill-induced locomotion, neural recordings were started and the Plexon system synchronized the video recording with neural data. Each recording session lasted 10 minutes. A total of 212 cells were recorded from the infragranular layer of six of the neonatally spinalized animals (the dataset from the 7th animal was corrupted with noise artifact and unusable), and 419 neurons were recorded from the eight intact rats.
Offline video analysis of behavior.
The videotape of each recorded session was viewed offline, one frame at a time (17 ms per frame), to identify the time at which each paw made contact with the treadmill. When the appropriate frame was identified, the timestamp on that frame was entered into the NeuroExplorer data file containing the times of action potentials for each individual cell recorded. For each recording session, the times of the first 100 forepaw footfalls for each paw were determined. Finally, to compare the neural responses during hindlimb weight-supported steps with those during nonweight-supported steps, the times of both forepaw and hindpaw placements during weight-supported steps were assessed as well as the times of forepaw and hindpaw placements during nonweight-supported steps when the animal made a sweep. A weight supported step was defined as a sequential flexion and extension of the hindlimbs so that the hindquarters were elevated above the surface of the treadmill, and a nonweight-supported step was defined as when the animal swept its hindlimb for hindpaw contact on the treadmill, but the hindquarters were not elevated above the surface of the treadmill.
Data analysis.
Not every recorded cell responded to forepaw footfalls on the treadmill. Only those cells that showed a significant response to the footfalls were used for further analysis. To determine whether a cell had a significant response, perievent time histograms (PETHs) were generated around footfalls for every neuron recorded using the timestamps obtained during offline video analysis of the rat's behavior (5 ms bin size). The PETHs were then exported to Matlab (version 6.5; The Mathworks) for further analysis. Because the animals were moving during the recordings, spontaneous activity was defined as the activity of the cells during the entire recording procedure. To define a significant response, first a threshold was defined for each cell as the 99% confidence interval around a random Poisson process, with the same mean as that cell's firing rate for the entire recording period being investigated. The PETHs were then smoothed in a window, 500 ms long, centered on the time of the footfalls with a sliding window, 25 ms long using a zero phase digital filter. For each smoothed waveform, the first and last bin greater in amplitude than the chosen threshold defined a region around the peak that had a finite width and position with respect to the overall PETH. The first and last bins identified were then applied to the original, unsmoothed PETH for each neuron. To be classified as significant, the response of the cell required the presence of at least three bins between the first and last bin that exceeded threshold in the unsmoothed PETH (Fig. 1D).
With the use of the largest response from each neuron, six of the measures defined above for the passive sensory stimulation paradigm were extracted from the PETH: 1) spontaneous activity, 2) the response magnitude, 3) the peak response, 4) the first bin latency, 5) the peak latency, and 6) the last bin latency. The duration of the response was calculated as the last bin latency minus the first bin latency. These measures and the number of responding cells were then compared between groups (neonatally transected vs. normal) and between paw placement (paw placement is ipsilateral to the neuron recorded or contralateral) using a two-way ANOVA similar to that described above for passive sensory stimulation paradigm. The first factor, animal group, had two levels: spinalized and normal. The second factor, paw placement, had two levels: ipsilateral or contralateral to the neuron being recorded. A Tukey post-hoc was used to identify differences between groups following a significant interaction.
Assessment of functional recovery.
Some adult rats that are spinalized as neonates and receive treadmill exercise are able to support their hindquarters through a step cycle during treadmill-induced locomotion (Kao et al. 2009; Giszter et al. 1998a; Giszter et al. 1998b; Miya et al. 1997). We define a step cycle as a sequential flexion and extension of the hindlimb on the treadmill. Not all step cycles on a treadmill are weight supported. Weight-supported step cycles, in which the hindlimbs support the hindquarters so that they are elevated above the surface of the treadmill, were distinguished from nonweight-supported step cycles, in which the hindlimbs flex and extend, but the knee remains in contact with the treadmill, and the hindquarters are not elevated above the surface of the treadmill. To assess the functional behavior of the spinalized rats, treadmill sessions were videotaped in the lateral view, and the percentage of weight-supported steps (%WSS) was assessed over 3 minutes of treadmill locomotion before the animals were connected to the tethers used for electrophysiological recordings to ensure that there was no interference of the tethers on the assessment.
Data analysis.
To evaluate the effect of the transect on the animal's stride during treadmill locomotion, the time between left and right forepaw footfalls was calculated and compared between the two groups. The average time between forepaw footfalls for each animal was calculated and the value for spinalized rats was compared with that of normal animals using Student's t-test. The locomotor behavior of the spinalized rats was also assessed by calculating the percentage of hindlimb weight-supported steps (%WSS) during the first 100 steps of the video. The time between forepaw footfalls was correlated to the %WSS using Spearman's rho.
Finally, to evaluate the functional significance of the changes in cortical reorganization, the neuronal responsiveness, as measured by the PETH, during weight-supported steps was compared with the neuronal responsiveness around nonweight-supported steps for which there was some identifying movement (e.g., sweeps) for the six parameters from the perievent histogram (response magnitude, peak, first bin latency, peak latency, last bin latency, and duration). A multivariate ANOVA was used to assess statistical differences in the neuronal responsiveness between the two types of steps. Step cycles in which the hindlimbs did not move were not included. Six spinalized rats that took weight-supported steps were included in the analysis.
RESULTS
Passive sensory stimulation.
A total of 61 of 343 neurons (18%) recorded from eight normal animals and 256 of 367 (68%) neurons recorded from seven spinalized animals responded to stimulation of at least one forelimb location. As expected, based on our previous work with single microelectrodes (Kao et al. 2009), a significantly greater proportion of HL-SMC cells recorded from the neonatally spinalized animals responded to forelimb stimulation compared with that of normal animals [animal group factor: F(1, 31) = 15.6; P < 0.001]. Here we extend these results to show that this was true whether the cells primarily responded to a contralateral stimulus (spinalized contralateral cells: 40.6 ± 24.5%; normal contralateral cells: 13.1 ± 12.4%) or an ipsilateral stimulus [spinalized ipsilateral cells: 32.4 ± 25.6%; normal ipsilateral cells: 8.1 ± 7.3; stimulus position factor: F(1,31) = 1.0; P = 0.32; interaction factor: F(1,31) = 0.80]. Therefore, HL-SMC neurons from spinalized animals that received exercise therapy are approximately four times more likely to respond to forelimb tactile stimulation than HL-SMC cells recorded from normal animals.
Cells recorded from the spinalized rats were also more likely to respond to more locations than those recorded from the normal rats [animal group factor: F(1,313) = 52.4; P < 0.0001] with cells more likely to respond to contralateral locations than ipsilateral locations [stimulus position factor: F(1,313) = 10.69; P < 0.05; interaction factor: F(1,313) = 0.076; P = 0.78]. Contralateral cells from spinalized animals responded to 3.2 ± 1.6 locations, whereas contralateral cells from normal animals responded to 1.6 ± 1.1 locations. This represents a twofold increase. A similar difference was found when comparing ipsilateral cells from spinalized animals (2.5 ± 1.3 locations) with ipsilateral cells from normal animals (1.03 ± 0.2). The largest proportion of both contralateral and ipsilateral cells from spinalized animals (64.7% and 70.8%, respectively) had their greatest response to locations on the paw while contralateral or ipsilateral cells from normal animals had their greatest response (68.0% or 86.7%, respectively) to locations on the forearm rather than the paw. Therefore, the receptive field of HL-SMC cells recorded from spinalized rats covered a large portion of the forelimb/forepaw but the locations were predominately on the paw.
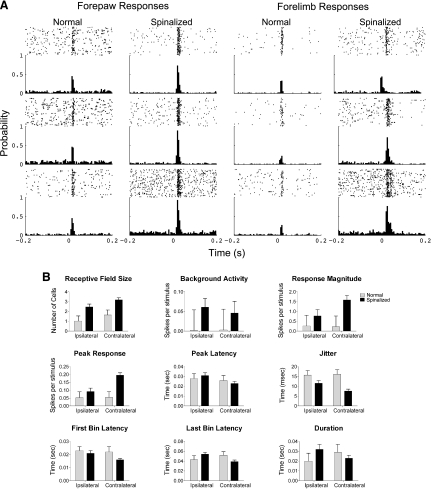
As reported previously, there were significant differences between the neuronal activity of HL-SMC cells recorded from neonatally spinalized rats that received exercise compared with those from normal rats during passive sensory stimulation (Fig. 2). We now extend that result to compare changes in neuronal activity when the stimulus was contralateral with the neurons and when the stimulus was ipsilateral. For both contralateral and ipsilateral stimuli, the average spontaneous firing rate for HL-SMC cells recorded from the spinalized rats was significantly greater than that recorded from normal adult rats [animal group factor: F(1,313) = 5.9, P < 0.02; stimulus location factor: F(1,313) = 0.1, P = 0.7; interaction factor: F(1,313) = 0.2, P = 0.7]. Despite this increase in spontaneous activity, these cells responded with more spikes per stimulus [animal group factor: F(1,313) = 5.9, P < 0.001; stimulus position factor: F(1,313) = 3.2, P = 0.08; interaction factor: F(1,313) = 3.7, P = 0.06]. This increased responsiveness was also observed when the peaks of the PSTHs were compared [animal group factor: F(1,313) = 37.8, P < 0.001; stimulus position factor: F(1,313) = 12.9, P < 0.001; interaction factor: F(1,313), P < 0.005]. However, the difference was only significant when the stimulus was contralateral to the cell (Tukey; P < 0.0001). Moreover, the variance in the latency to the first spike (jitter) was significantly reduced for spinalized rats compared with normal rats [animal group factor: F(1,313) = 47.7, P < 0.001; stimulus position factor: F(1,313) = 3.6, P = 0.06; interaction factor: F(1,313) = 5.8, P < 0.05; Tukey, contralateral P < 0.0001 and ipsilateral P < 0.05].
Fig. 2.
A: peristimulus time rasters and histograms generated from cells simultaneously recorded from the hindlimb sensorimotor cortex during stimulation of the cutaneous forelimbs under light anesthesia. The responses of 6 representative cells are shown from each group of rats. Each cell responded to passive stimulations of the cutaneous forepaw digit 2. The PSTH of cells recorded from the spinalized rats had greater spontaneous activity, greater response magnitudes relative to the spontaneous activity, and their peak latency was shifted in time but the duration of their response was not significantly different from the normal rats. The rasters above each PSTH show the spikes (dots) per trial (row of dots). B: response measures of cells in the hindlimb somatosensory cortex recorded during passive sensory stimulations of the cutaneous forelimbs separated by whether the stimulus was ipsilateral or contralateral to the neuron for spinalized rats and normal rats: receptive field size, background activity, response magnitude, peak response, latency of the peak of the response, jitter to the first response, first bin latency, last bin latency, and duration of the response.
There was also a difference in the first bin latency of the response regardless of whether the stimulus was ipsilateral or contralateral [animal group factor: F(1,313) = 8.2, P < 0.005; stimulus location factor: F(1,313) = 5.0, P < 0.05; interaction factor: F(1,313) = 3.1, P = 0.08], but there were no significant differences in the peak latency, the last bin latency or the duration of the response, or between cells recorded from spinalized and normal rats.
These data extend our previous results to show that the significant increase in response magnitude of neurons under anesthesia after spinal cord injury (SCI) compared with neuronal activity recorded from normal animals is in addition to a significant increase in spontaneous activity. Furthermore, this increase in response magnitude is well time-locked to the stimulus and not due to an increase in the duration of the response.
Behavioral assessment on treadmill.
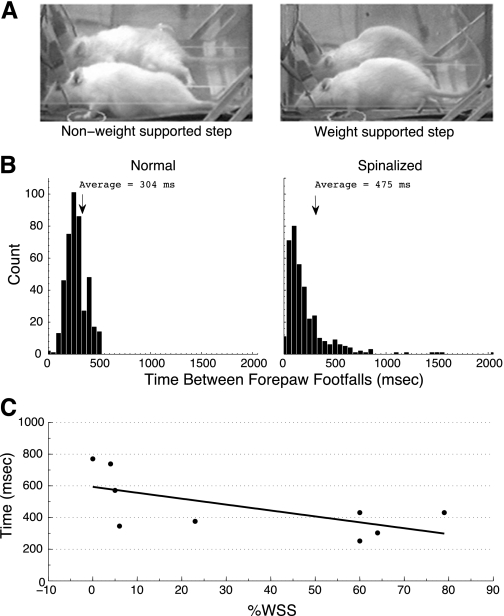
The spinalized animals that received treadmill exercise displayed the usual broad range of percentage of weight-supported steps during treadmill induced locomotion (0%–79%) (Miya et al. 1997). The average time difference between placements of sequential right and left forepaws during treadmill locomotion for the spinalized rats (average 475 + 17 ms) was significantly greater than that of normal rats (average 304 + 5 ms; P < 0.001; Fig. 3A). The average time between sequential right-left forepaw placements was negatively correlated with the percentage of weight-supported steps each rat was able to take (Spearman's rho, r-squared = 0.42, P < 0.05; Fig. 3B).
Fig. 3.
A: comparison of a weight-supported step where the animal clears the surface of the treadmill and a nonweight-supported step. B: average time differences in sequential forepaw footfalls between spinalized and normal rats. During treadmill induced locomotion, the average time between forepaw footfalls was less than that for spinalized rats. C: average time differences in sequential forepaw placements of spinalized rats negatively correlated with percentage of weight-supported steps (%WSS) during treadmill-induced locomotion. R = -0.66; P < 0.05. For the spinalized rats with lower %WSS, more time elapsed between sequential right and left forepaw placements.
Active sensorimotor stimulation.
There were also significant differences between the neuronal activity of HL-SMC cells recorded from neonatally spinalized rats and that of normal rats when the animals were walking on the treadmill (Fig. 4). A total of 142 of 419 neurons (33.9%) recorded from eight normal animals had a significant response to forepaw placement on the treadmill, whereas 121 of 212 (57.1%) neurons recorded from six spinalized animals had a significant response. Similar to the somatosensory responses, there were differences in the proportion of cells that responded to a stimulus across groups [F(1,28) = 4.9, P < 0.05]. Cells recorded from spinalized rats were, therefore, more likely to respond to a stimulus (29.91 ± 23.9) than cells recorded from normal rats (15.11 ± 13.9).
Fig. 4.
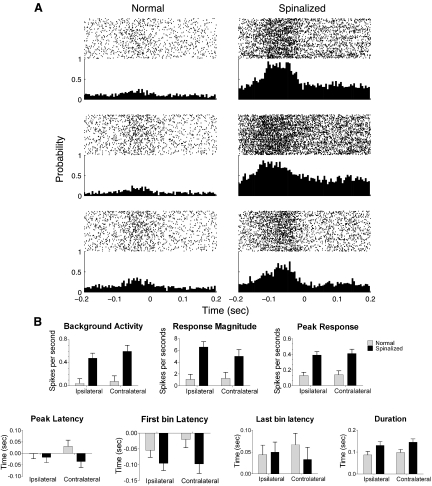
A: perievent time rasters and histograms generated from cells recorded in the hindlimb somatosensory cortex while rats underwent treadmill-induced locomotion. Three cells simultaneously recorded from 1 normal (left) and 3 simultaneously recorded from 1 spinalized (right) rat are shown. Each cell had a significant response to the forepaw placements. Time zero on the x-axis indicates time of forepaw placement on the treadmill. The HL-SMC, unlike the forelimb, has significant overlap of sensory and motor function and, since the paw placements are sensorimotor events, it is not unusual for the responses to occur before paw placements (see Chapin and Woodward 1986 for a more complete description). B: response measures of cells in the hindlimb somatosensory cortex recorded around the time of forepaw placements during treadmill-induced locomotion. The background activity, response magnitude, peak response, and duration of response were significantly greater for cells recorded from spinalized rats compared with those from normal animal. No significant difference was observed in the peak latency for spinalized rats compared with normal.
For these responsive cells, there were significant differences across groups for each of the PSTH variables regardless of whether the paw placement was ipsilateral or contralateral to the cell recorded (F statistic and P value for factor stimulus location and interaction not presented). Neurons recorded from spinalized rats had significantly higher baseline firing rates [animal group factor: F(1,259) = 105.23, P < 0.001] and response magnitudes [animal group factor: F(1,259) = 84.23, P < 0.001] to forepaw placements on the treadmill than neurons recorded from normal animals. Therefore, cortical cells within the hindlimb somatosensory cortex devoid of its normal inputs respond with increased robustness to forepaw placements during active sensorimotor stimulation induced by treadmill locomotion.
In addition to increased spontaneous firing and response magnitude of HL-SMC neurons recorded from spinalized awake animals, the peak of the PETH for cells recorded from spinalized rats was greater than that of normal rats, suggesting that this activity was well time-locked to the event [F(1,259) = 118.81, P < 0.001]. The first bin latency and the peak latency of cells recorded from spinalized rats were also shifted significantly earlier than that of normal rats [F(1,259) = 20.79, P < 0.001 for first bin latency and F(1,259) = 7.26, P < 0.01 for peak latency]. Moreover, the duration of the response to forepaw footfalls was greater for cells recorded from spinalized rats compared with those recorded from normal rats [F(1,259) = 3.97, P < 0.001]. Therefore, in contrast with the similar duration of the response of these neurons to passive sensory stimulation, the duration of the neurons' response during active, sensorimotor events was greater in the spinalized rats than that of the normal rats.
Functional relevance of increased responsiveness of HL-SMC neurons.
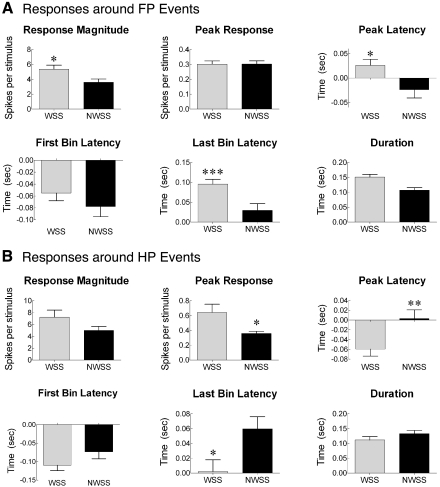
To assess the functional relevance of increased responsiveness of HL-SMC cells to forepaw placement during treadmill induced locomotion, the neuronal responses during weight-supported steps were compared with those during nonweight-supported steps that had some type of identifying locomotor movement (e.g., sweep of the hindpaw; Fig. 5). The magnitude of the response of cells to forepaw placement was greater when the animal took a weight-supported step compared with when the animal took a step that did not support the weight of its hindquarters [F(1,130) = 5.37, P < 0.05] but there was no difference in the magnitude of the peak of the responses. The latency of the peak was shifted later [F(1,130) = 5.98, P < 0.05] as was the latency of the last bin [F(1,130) = 10.89, P < 0.005], therefore increasing the duration of the response [F(1,130) = 10.85, P < 0.005]. To further understand differences in the responses, the responses around hindlimb paw placements were compared for weight-supported steps and nonweight-supported steps (sweeps). There was no significant difference in the magnitude of the response [F(1,108) = 2.76, P = 0.10], but the peak of the response was significantly greater around weight-supported steps compared with nonweight-supported steps [F(1,108) = 6.23, P < 0.05]. Moreover, the peak of the latency occurred earlier [F(1,108) = 7.30, P < 0.01] and the last bin latency was later [F(1,108) = 6.40, P < 0.05], but there was no difference in the duration of the response [F(1,108) = 1.78, P = 0.19]. Therefore, the magnitude of the responses around footfalls was greater when the animal took a weight-supported step compared with steps when the animal was unable to support its weight.
Fig. 5.
The responsiveness of cells in the hindlimb SMC of neonatally spinalized rats was greater during weight-supported steps (WSS) than during nonweight-supported steps (NWSS). A: responsiveness measures derived from perievent histograms centered on forelimb paw (FP) placement (see methods). The number of spikes was greater when the animal took a weight-supported step compared with when the animal took a step that did not support the weight of its hindquarters. There was no difference in the peak of the response for WSS compared with NWSS. However, the latency to the peak of the response was significantly later, and the duration of the response was significantly longer when the animal took a WSS compared with when the animal took a NWSS. *P < 0.05, **P < 0.01, ***P < 0.005. B: responsiveness measures derived from perievent histograms centered around hind paw (HP) events (see methods). The measures are the same as in A.
DISCUSSION
This is the first demonstration of the effect of spinal cord injury on single neuron activity in awake, freely moving animals that sustained a spinal injury. Taken together with previous studies on spinal cord injury demonstrating a general silencing of the affected area of the brain in the absence of exercise therapy, the results of this study suggest that exercise therapy can promote novel organization of the somatotopic maps of the cortex that is functionally relevant during awake exercise. As shown previously (Kao et al. 2009), treadmill therapy increased the probability that neurons in the HL-SMC would respond to functionally relevant stimuli of peripheral areas innervated by spinal segments rostral to the injury compared with those of normal animals or spinalized rats that did not receive therapy. Here we extend those results to show that during passive tactile stimulation responses recorded from spinalized rats were greater and better time-locked to the stimulus than those recorded from normal animals. Moreover, when recording from animals in the awake state, the neuronal activity around footfalls was greater when the animal took a weight-supported step compared with nonweight-supported steps. Therefore, the novel cortical organization associated with rehabilitative therapy in animals that received daily treadmill exercise after neonatal spinal cord injury is likely to be directly involved in the functional recovery.
In normal animals, the hindpaw–forepaw overlap in the primary somatosensory cortex can be useful for sensory and sensorimotor integration between paws (Alenda and Nunez 2004; Moxon et al. 2008) during functionally relevant behaviors and is involved with postural adjustments made during locomotion (Beloozerova et al. 2003). Moreover, neonatally spinalized rats that receive treadmill exercise and develop some weight-supported locomotion have increased representation of axial musculature in the motor cortex (Giszter et al. 1998a). Furthermore, the ability of these animals to make appropriate postural adjustments to maintain balance contributes to their ability to take weight-supported steps (Giszter et al. 2008). Therefore, the responses of HL-SMC neurons of spinalized rats during treadmill-induced locomotion could represent sensorimotor integration in the form of afferent feedback for motor control signals to the forelimb, upper trunk, and long axial muscles of the trunk. This may allow the spinalized rats to not only lift but also to stabilize their hindquarters during treadmill induced locomotion, and thus be able to produce more weight-supported steps.
Changes in cortical spontaneous activity.
Cortical responses to sensory stimuli strongly depend on the ongoing spontaneous activity of cortical networks (Arieli et al. 1996; Wörgötter et al. 1998; Tsodyks et al. 1999; Kisley and Gerstein 1999; Azouz and Gray 1999; Kenet et al. 2003; Lakatos et al. 2008). However, it is not possible to assess the impact of the reorganized cortical network on spontaneous activity when indirect measurements of neuronal activity are used (Girgis et al. 2007; Endo et al. 2007; Ghosh et al. 2010). We recently showed that immediately after spinal cord injury, functional reorganization is evident and this functional reorganization is due, at least in part, to a state change in which cortical spontaneous activity becomes strikingly silent (Aguilar et al. 2010). In the chronically spinalized animals examined here, the organization is stable and the state of the systems is represented by higher background firing rates both during light anesthesia and when awake. It is noteworthy that the increases in the responsiveness of these cells were in addition to this overall increase in spontaneous activity, suggesting a substantial overall increase in neuronal activity, especially during treadmill locomotion.
Changes in neuronal responsiveness.
We showed previously that the proportion of hindlimb cells responding to forelimb stimulation in spinalized rats that did not receive exercise was less than 8% (Kao et al. 2009), confirming an effective silencing of the hindpaw cortex in response to tactile stimulation of any part of the cutaneous body surface in the absence of exercise found in other spinal injury models (Levitt and Levitt 1968; McKinley and Smith 1990; Jain et al. 1995; Jain et al. 2003; Endo et al. 2007; Ghosh et al. 2010; Aguilar et al. 2010). In contrast, the proportion of hindlimb cells recorded from animals that received daily treadmill exercise was greater than 50% (Kao et al. 2009), and this result is confirmed in the present work using a fixed array. The majority of these cells responded to passive stimulation of the paw but not to stimulation of the limb or shoulder whose cortical representations is adjacent to the hindpaw somatosensory cortex. Therefore, the increase in proportion of responding cells in the neonatally spinalized rats that receive exercise is biased toward stimulation related to functional use of the paws rather than the closest anatomical brain structure.
The fact that this represents an increase in probability of responding and not a de novo type of response suggests that existing cortical pathway between forepaw and hindpaw areas is not lost after complete spinal lesion and is, in fact, enhanced, both in number of connections and strength of connection, by exercise. Recent studies in adult spinalized rats using electrical stimulation confirmed that low intensity stimulation (1 mA) of the forepaws does not activate cells in the deafferented hindlimb cortex after complete midthoracic spinal transaction; however, stimuli at 5 mA does produce responses (Endo et al. 2007; Ghosh et al. 2010; Aguilar et al. 2010). Therefore, even in adult spinalized rats, the substrate for reorganization appears to be present. It will be interesting to know if therapy that improves functional outcome in rats spinalized as adults also induces cortical reorganization that would be evident at tactile stimulation levels.
Although the anatomical substrate for these differences in proportion of responding cells, response magnitude, and latency are unknown, one possibility is the strengthening of cortico-cortical connections. Because it has been shown previously that the proportion of responding cells in the granular layer (layer IV) of either exercised spinalized rats (Kao et al. 2009) or spinalized rats that did not receive exercise (Jain et al. 2003) is similar compared with normal rats, changes in cortico-cortical activity are most likely to be responsible. The functional organization of the cortex supports the idea that sensory information is processed between layers within a column before it is transmitted to other columns via the extragranular layers (Silva and Koretsky 2002). The infragranular layers are particularly significant for sensory integration, because they represent the main output of the primary somatosensory cortex and can exert direct influence on the early stages of subcortical somatosensory processing (Martinez et al. 1995; Mariño et al. 1999; Canedo and Aguilar 2000; Aguilar et al. 2003; Temereanca and Simons 2004). However, a primate study using a unilateral cervical spinal cord injury demonstrated reorganization in the thalamus that paralleled in the cortex after 2 years (Jain et al. 2008). Therefore, a thalamocortical influence on the effects seen in the cortex cannot be ruled out without further study.
Our results support corticocortical changes but we cannot determine whether these changes are attributable to changes in synaptic efficacy, sprouting, or the formation of new connections across cortical regions. In normal animals after exercise, in vitro studies of local circuits to identify mechanisms for changes in cortical organization suggested that the ability of cells to alter synaptic efficacy depends on levels of incoming activity by increasing synaptic efficacy attributable to a general excitation of the cortical cells (Abraham and Bear 1996; Hickmott and Merzenich 2002). Peripheral injury studies to identify the mechanism for cortical reorganization of affected regions of the cortex also support corticocortical plasticity (Donoghue and Sanes 1988; Wang et al. 1995; Lane et al. 1999). Finally, synaptic plasticity has been shown in the motor cortex of adult rats with C4 over hemisections. In these cells, dendritic spine length increased and spine density decreased within 7 days but returned to normal by day 28 after hemisection, indicating a more immature and modifiable pattern of synaptic connectivity (Kim et al. 2006). However, because no exercise was reported for the spinal cord-injured rats of that study, it is possible that the reversal of changes in spine morphology could be made permanent with appropriate therapy. It remains unclear if more long-term changes in the cortex would have been observed if the rats had received any form of exercise.
Role of exercise therapy.
Exercise therapy has been shown to improve functional outcome and induce reorganization of the somatotopic maps in the cortex of patients with SCI. For example, a longitudinal study using fMRI in human patients with spinal cord injury at the cervical level showed evidence that improvement in function after exercise therapy was associated with the extent of motor cortex activation (Jurkiewicz et al. 2007). Mapping studies with transcranial magnetic stimulation and electroencephalographic recordings in SCI patients revealed changes of cortical sensorimotor areas (Topka et al. 1991). Preserved muscle groups above the level of lesion in SCI patients showed an enlargement of the related cortical sensorimotor areas compared with healthy subjects (Levy et al. 1990). In addition, during finger movements, SCI patients showed an increased volume of M1 activation. Increased activation was also found in nonprimary motor and parietal areas, as well as in the cerebellum during movements of the fingers, wrist, and elbow, whereas no changes were present during tongue movements (Curt et al. 2002b). Our data are consistent with these studies and support an increase in cortical activation for sensory as well as sensorimotor events. Furthermore, they document that these expansions are the results of both more cells responding to the stimulus and, of the cells that respond, an increase in the number of spikes per stimulus.
Our data also support studies in human subjects, suggesting that the increased representation is relevant for functional recovery. For example, PET studies demonstrate that in tetraplegic patients the strength of wrist extension is related to an increased area of activation in the contralateral SMC (Bruehlmeier et al. 1998; Curt et al. 2002a). In addition, a case report on intensive, bimanual training of a C6 motor complete spinal injury resulted in functional improvement and an increased representation of the involved muscles in the cortex (Hoffman and Field-Fote 2007). Finally, cortical reorganization demonstrated with PET in primates trained to use their digits in dexterous gripping task can be causal to functional recovery (Nishimura et al. 2007). The direct method used here to assess the firing rate of single neurons shows that the increased neuronal activity around paw movements was associated with increased neuronal activity during weight-supported steps compared with activity during nonweight-supported steps. Therefore, during treadmill-induced locomotion, the activity in the HL-SMC is directly related to the ability of the animal to take weight-supported steps.
These results may have important consequences for the development of therapies for spinal cord injury. The fact that after a neonatal, midthoracic transection, cells in the hindlimb cortex are more likely to be engaged in the processing of sensorimotor information above the level of the lesions and that their activity is related to functional recovery supports the development of physical therapy paradigms devoted to functional changes at all levels of the sensorimotor system.
GRANTS
This work was funded by National Institutes of Health Grants PO1-NS-24707 and R01-NS-05741.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 [DOI] [PubMed] [Google Scholar]
- Aguilar J, Rivadulla C, Soto C, Canedo A. New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol 89: 3328–3339, 2003 [DOI] [PubMed] [Google Scholar]
- Aguilar J, Humanes-Valera D, Alonso-Calviño E, Yague JG, Moxon KA, Oliviero A, Foffani G. Spinal cord injury immediately changes the state of the brain. J Neuroscience 30: 7528–7537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers RM, Killackey HP. Organization of cortical connections in the parietal cortex of the rat. J Comp Neuro 181: 513–538, 1978 [DOI] [PubMed] [Google Scholar]
- Alenda A, Nunez A. Sensory-interference in rat primary somatosensory cortical neurons. Eur J Neurosci 19: 766–770, 2004 [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the largevariability in evoked cortical responses. Science 273: 1868–1871, 1996 [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci 19: 2209–2223, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci 28: 3976–3987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen EC, Field-Fote KS. Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair 19: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA, Orlovsky GN, Popova LB, Deliagina TG. Activity of different classes of neurons on the motor cortex during postural corrections. J Neurosci 23: 7844–7853, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci 10: 3918–3922, 1998 [DOI] [PubMed] [Google Scholar]
- Canedo A, Aguilar J. Spatial and cortical influences exerted on cuneothalamic and thalamocortical neurons of the cat. Eur J Neurosci 12: 2515–2533, 2000 [DOI] [PubMed] [Google Scholar]
- Chapin JK. Laminar differences in sizes, shapes, and response profiles of cutaneous receptive fields in the rat SI cortex. Exp Brain Res 62: 549–559, 1986 [DOI] [PubMed] [Google Scholar]
- Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol 229: 199–213, 1984 [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Distribution of somatic sensory and active-movement neuronal discharge properties in the MI-SI cortical border area in the rat. Exp Neurol 91: 502–523, 1986 [DOI] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MA. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods 94: 121–140, 1999 [DOI] [PubMed] [Google Scholar]
- Chau CW, McKinley PA. Chronological observations of primary somatosensory cortical maps in kittens following low thoracic (T12) spinal cord transection at 2 weeks of age. Somatosens Mot Res 8: 355–376, 1991 [DOI] [PubMed] [Google Scholar]
- Cohen LG, Ziemann U, Chen R. Mechanisms, functional relevance and modulation of plasticity in the human central nervous system. Electroencephalogr Clin Neurophysiol Suppl 51: 174–182, 1999 [PubMed] [Google Scholar]
- Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain 128: 2941–2950, 2005 [DOI] [PubMed] [Google Scholar]
- Curt A, Alkadhi H, Crelier GR, Boendermaker SH, Hepp-Reymond MC, Kollias SS. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain 125: 2567–2578, 2002a [DOI] [PubMed] [Google Scholar]
- Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma 19: 43–51, 2002b [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci 8: 3221–3232, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick RR, Roy JW. Training locomotor networks. Brain Res Rev 57: 241–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 130: 2951–2961, 2007 [DOI] [PubMed] [Google Scholar]
- Foffani G, Tutunculer B, Moxon KA. Role of spike timing in the forelimb somatosensory cortex of the rat. J Neurosci 24: 7266–7271, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999 [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MA. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex 9: 348–361, 1999 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, Mueggler T, Baltes C, Rudin M, Weber B, Schwab ME. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 13: 97–104, 2010 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci 29: 12210–12219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron 54: 677–696, 2007 [DOI] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge K, Fouad V. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 130: 2993–3003, 2007 [DOI] [PubMed] [Google Scholar]
- Giszter SF, Davies MR, Graziani V. Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates. Exp Brain Res 190: 53–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ, Davies M, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol 80: 3021–3030, 1998a [DOI] [PubMed] [Google Scholar]
- Giszter S, Graziani V, Kargo W, Hockensmith G, Davies MR, Smeraski CS, Murray M. Pattern generators and cortical maps in locomotion of spinal injured rats. Ann NY Acad Sci 860: 554–555, 1998b [DOI] [PubMed] [Google Scholar]
- Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury: an electroencephalographic study. Neurology 50: 1115–1121, 1998 [DOI] [PubMed] [Google Scholar]
- Hall RD, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res 66: 23–38, 1974 [Google Scholar]
- Hickmott PW, Merzenich MM. Local circuit properties underlying cortical reorganization. J Neurophysiol 88: 1288–1301, 2002 [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger M. Is the hindlimb representation of the rat's cortex a “sensorimotor amalgam”? Brain Res 346: 75–81, 1985 [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther 87: 208–223, 2007 [DOI] [PubMed] [Google Scholar]
- Jain N, Florence SL, Kaas JH. Limits on plasticity in somatosensory cortex of adult rats: hindlimb cortex is not reactivated after dorsal column section. J Neurophysiol 73: 1537–1546, 1995 [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature 386: 495–498, 1997 [DOI] [PubMed] [Google Scholar]
- Jain N, Diener PS, Coq JO, Kaas JH. Patterned activity via spinal dorsal quadrant inputs is necessary for the formation of organized somatosensory maps. J Neurosci 23: 10321–10330, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci 28: 11042–11060, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair 21: 527–538, 2007 [DOI] [PubMed] [Google Scholar]
- Kalaska J, Pomeranz B. Chronic paw denervation causes an age-dependent appearance of novel responses from forearm in “paw cortex” of kittens and adult cats. J Neurophysiol 142: 618–633, 1979 [DOI] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Murray M, Moxon KA. Exercise induces cortical plasticity after neonatal spinal cord injury in the rat. J Neurosci 29: 7549–7557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Jacob-Vadakot S, Himes BT, Murray M, Moxon KA. Role of the 5-HT2C receptor in improving weight-supported stepping in adult rats spinalized as neonates. Brain Res 11: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol 209: 407–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelahan AM, Doetsch GS. Time-dependent changes in the functional organization of somatosensory cerebral cortex following digit amputation in adult raccoons. Somatosens Res 2: 49–81, 1984 [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003 [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198: 401–415, 2006 [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Trial-to-trial variability, and state-dependent modulation of auditory evoked responses in cortex. J Neurosci 19: 10451–10460, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Stojic AS, Killackey HP, Rhoades RW. Source of inappropriate receptive fields in cortical somatotopic maps from rats that sustained neonatal forelimb removal. J Neurophysiol 81: 625–633, 1999 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320: 110–113, 2008 [DOI] [PubMed] [Google Scholar]
- Leiser S, Moxon KA. Somatotopic organization of the rat trigeminal ganglion. Journal of Neurophysiology 95: 3129–3145, 2006 [DOI] [PubMed] [Google Scholar]
- Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron 53: 117–133, 2007 [DOI] [PubMed] [Google Scholar]
- Levitt M, Levitt J. Sensory hind-limb representation in the SmI cortex of the cat after spinal tractotomies. Exp Neurol 22: 276–302, 1968 [DOI] [PubMed] [Google Scholar]
- Levy WJ, Jr, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res 510: 130–134, 1990 [DOI] [PubMed] [Google Scholar]
- Lotze M, Laubis-Hermann U, Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci 24: 97–107, 2006 [PubMed] [Google Scholar]
- Lynskey JV, Belanger R, Jung A. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev 45: 229–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarrez E, Rojas-Piloni G, Vazquez D, Flores A. Cortical neuronal ensembles driven by dorsal horn spinal neurones with spontaneous activity in the cat. Neurosci Lett 318: 145–148, 2002 [DOI] [PubMed] [Google Scholar]
- Martinez L, Lamas JA, Canedo A. Pyramidal tract, and corticospinal neurons with branching axons to the dorsal column nuclei of the cat. Neuroscience 68: 195–206, 1995 [DOI] [PubMed] [Google Scholar]
- Mariño J, Aguilar J, Canedo A. Cortico-subcortical synchronization in the chloralose-anesthetized cat. Neuroscience 93: 409–411, 1999 [DOI] [PubMed] [Google Scholar]
- McCandlish CA, Li CX, Waters RS, Howard EM. Digit removal leads to discrepancies between the structural and functional organization of the forepaw barrel subfield in layer IV of rat primary somatosensory cortex. Exp Brain Res 108: 417–426, 1996 [DOI] [PubMed] [Google Scholar]
- McKinley PA, Smith JL. Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal cord transection in cats. J Neurosci 10: 1429–1443, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley PA, Swyter E. Progressive changes in somatosensory cortical maps in 6-week-old kittens cord-transected at T12. Brain Res 484: 378–382, 1989 [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b, and 1 in adult owl and squirrel monkeys. Neuroscience 10: 639–665, 1983 [DOI] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. J Neurosci 17: 4856–4872, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Hale LL, Aguilar J, Foffani G. Responses of infragranular neurons in the rat primary somatosensory cortex to forepaw, and hindpaw tactile stimuli. Neuroscience 156: 1083–1092, 2008 [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada T, Isa H. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science 318: 1150–1155, 2007 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 2nd Edition Orlando: Academic Press, 1986 [Google Scholar]
- Rasmusson DD, Nance DM. Non-overlapping thalamocortical projections for separate forepaw digits before and after cortical reorganization in the raccoon. Brain Res Bull 16: 399–406, 1986 [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Webster HH, Dykes RW. Neuronal response properties within subregions of raccoon somatosensory cortex 1 week after digit amputation. Somatosens Mot Res 9: 279–289, 1992 [DOI] [PubMed] [Google Scholar]
- Shumsky J, Kao T, Amato N, Simansky K, Murray M, Moxon KA. Partial 5-HT1A receptor agonist activity by the 5-HT2C receptor antagonist SB 206,553 is revealed in rats spinalized as neonates. Exp Neurol 191: 361–365, 2005 [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci USA 99: 15182–15187, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon S, Kambi N, Lazar L, Mohammed H, Jain N. Large-scale expansion of the facerepresentation in somatosensory areas of the lateral sulcus after spinal cord injuries in monkeys. J Neurosci 29: 12009–12019, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004 [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 94: 2844–2855, 2005 [DOI] [PubMed] [Google Scholar]
- Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology 41: 1276–1283, 1991 [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons, and the underlying functional architecture. Science 286: 1943–1946, 1999 [DOI] [PubMed] [Google Scholar]
- Tutunculer B, Foffani G, Himes BT, Moxon KA. Structure of the excitatory receptive fields of infragranular forelimb neurons in the rat primary somatosensory cortex responding to touch. Cereb Cortex 16: 791–810, 2006 [DOI] [PubMed] [Google Scholar]
- Wall JT, Cusick CG. Cutaneous responsiveness in primary somatosensory (S.-I.) hindpaw cortex before, and after partial hindpaw deafferentation in adult rats. J Neurosci 4: 1499–1515, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Egger MD. Formation of new connexions in adult rat brains after partial deafferentation. Nature 232: 542–545, 1971 [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75, 1995 [DOI] [PubMed] [Google Scholar]
- Wheeler BC. Automatic discrimination of single units. In: Methods for Neural Ensemble Recordings (MAL Nicolelis, ed.). New York: CRC Press, 1999, pp. 61–77 [Google Scholar]
- Whishaw IQ, Gorny B. Arpeggio and fractionated digit movements used in prehension by rats. Behav Brain Res 60: 15–24, 1994 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res 41: 49–59, 1990 [DOI] [PubMed] [Google Scholar]
- Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey J, Williamson K. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair 19: 313–324, 2005 [DOI] [PubMed] [Google Scholar]
- Worgotter F, Suder K, Zhao Y, Kerscher N, Eysel UT, Funke K. State-dependent receptive-field restructuring in the visual cortex. Nature 396: 165–168, 1998 [DOI] [PubMed] [Google Scholar]