Abstract
NF-κB is a heterodimeric transcription activator consisting of the DNA binding subunit p50 and the transactivation subunit p65/RelA. NF-κB prevents cell death caused by tumor necrosis factor (TNF) and other genotoxic insults by directly inducing antiapoptotic target genes. We report here that the tumor suppressor PTEN, which functions as a negative regulator of phosphatidylinositol (PI)-3 kinase/Akt-mediated cell survival pathway, is down regulated by p65 but not by p50. Moreover, a subset of human lung or thyroid cancer cells expressing high levels of endogenous p65 showed decreased expression of PTEN that could be rescued by specific inhibition of the NF-κB pathway with IκB overexpression as well as with small interfering RNA directed against p65. Importantly, TNF, a potent inducer of NF-κB activity, suppressed PTEN gene expression in IKKβ+/+ cells but not in IKKβ−/− cells, which are deficient in the NF-κB activation pathway. These findings indicated that NF-κB activation was necessary and sufficient for inhibition of PTEN expression. The promoter, RNA, and protein levels of PTEN are down-regulated by NF-κB. The mechanism underlying suppression of PTEN expression by NF-κB was independent of p65 DNA binding or transcription function and involved sequestration of limiting pools of transcriptional coactivators CBP/p300 by p65. Restoration of PTEN expression inhibited NF-κB transcriptional activity and augmented TNF-induced apoptosis, indicating a negative regulatory loop involving PTEN and NF-κB. PTEN is, thus, a novel target whose suppression is critical for antiapoptosis by NF-κB.
Homeostasis in eukaryotic tissues is tightly regulated by an intricate balance of the prosurvival and antisurvival signals received from the microenvironment as well as the intracellular genetic and phenotypic context. Deviation from homeostasis involves sequential and cumulative genetic alterations that contribute to the loss of the normal cell phenotype and acquisition of the transformed or malignant state, resulting in cancer development (18). These alterations are accompanied by the reorganization of the intracellular signaling network, often functionally dominated by the prosurvival determinants that either directly or indirectly cross-regulate the growth-inhibitory responses (18). Oncogenic factors are the prime prosurvival determinants that can act to suppress a broad range of antitumor pathways. The findings of the present study identify one such novel regulatory loop involving the prosurvival transcription factor nuclear factor κB (NF-κB) and the tumor suppressor PTEN (phosphatase and tensin homologue deleted from chromosome 10).
The tumor suppressor PTEN is a dual-specificity phosphatase that plays a functional role in cell cycle arrest and apoptosis (11, 41). The PTEN gene was identified by loss-of-heterozygosity studies directed toward a search for a putative tumor suppressor gene on chromosome 10 and was localized to chromosome 10q23, a region that is often deleted from genes of many human cancers. Germ line mutations in PTEN have also been detected in three human autosomal dominant disorders: Cowden disease, Lhermitte-Duclos disease, and Bannayan-Zonana syndrome, which share similar pathological features such as the formation of multiple benign tumors and an increased susceptibility to malignant cancers (12, 32). Somatic deletions or mutations of the PTEN gene have been identified in a large fraction of tumors, including glioblastomas and endometrial and advanced prostate cancers, thus placing PTEN among the most commonly mutated genes in human cancer (11). The genetic evidence supporting PTEN as an important tumor suppressor protein is corroborated by the finding that heterozygous disruption of the PTEN gene in knockout mice results in the spontaneous development of tumors in several tissues (12). PTEN is involved in the regulation of cell survival signaling through the phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway (43). PTEN dephosphorylates the D3 position of the key lipid second messenger phosphatidylinositol 3,4,5-triphosphate, which is produced by PI3K following activation by upstream receptor tyrosine kinases, activated Ras, or G proteins and leads to the stimulation of several downstream targets, including Akt. Consistently, constitutive activation of Akt is a common event in cancer cells that have lost PTEN function because of either chromosomal deletion or mutation (53). Activated Akt protects cells from apoptotic death by phosphorylating and inactivating proapoptotic substrates such as BAD, procaspase-9, and forkhead family transcription factors (34). Akt also prolongs cell survival by delaying p53-dependent apoptosis through Mdm2 phosphorylation (59). Expression of exogenous PTEN in mutant cells restores the endogenous pattern of Akt phosphorylation and corresponding sensitivity to apoptosis induced by various proapoptotic stimuli. Thus, PTEN exercises its role as a tumor suppressor by negative regulation of the PI3K/Akt signaling pathway. In addition to genetic alteration in the PTEN locus in certain tumors, many other cancers, such as those of the lung and thyroid, possess wild-type PTEN alleles but expression of the PTEN gene is often diminished (14, 56). Recent studies have shown that the PTEN pseudogene but not the wild-type PTEN gene is methylated in a wide variety of tumor cells, indicating that DNA methylation is not a potential mechanism for silencing the PTEN promoter, leading to loss of PTEN gene expression in human cancers (60). Despite the significance of PTEN in cancer and apoptosis, very little is known about the regulation of PTEN gene expression as a mechanism for alleviation of PTEN growth-inhibitory and apoptotic functions (42, 47).
NF-κB plays a central role in prevention of apoptosis, promotion of tumor growth, and activation of inflammatory responses (26). Classical NF-κB is a heterodimer consisting of the DNA binding subunit p50 and the transactivation subunit RelA/p65. In nonstimulated cells, the NF-κB dimer exists as a cytoplasmic latent complex through binding of specific IκB inhibitor proteins, which mask their nuclear localization signal (NLS). Stimulation with various proinflammatory stimuli, including tumor necrosis factor (TNF) or interleukin 1 (IL-1), or genotoxic stress unmasks the NLS and activates NF-κB by phosphorylation and degradation of IκB. This allows NF-κB to translocate into the nucleus and transcriptionally activate NF-κB target genes that include antiapoptotic genes and growth-promoting genes (42). The prosurvival function of NF-κB was established by the finding that mice lacking the p65 subunit of NF-κB die at embryonic day 12 as a result of massive liver apoptosis triggered by endogenous TNF (1, 3). Importantly, many tumors show constitutively elevated levels of NF-κB activity and the antiapoptotic function of NF-κB represents a major obstacle to effective radiation therapy and chemotherapy of cancer (25, 50). The mechanism by which NF-κB activation promotes cell survival is, however, still unclear. Although NF-κB up-regulates several prosurvival genes, such as Bcl-xL, TRAF1, TRAF2, SOD, and A2, or inhibitor of apoptosis proteins (IAPs) (25, 50, 52), none of the knockout mice null for a single NF-κB-inducible antiapoptotic gene show the extensive liver apoptosis associated with loss of p65 or its upstream activator IKKβ, suggesting either functional redundancy among the NF-κB target genes or that the critical target gene has not been identified yet (26). Generally, NF-κB induces gene expression by transcription activation following direct binding to consensus DNA binding motifs in the promoter of the target genes. However, NF-κB can also negatively regulate gene expression in certain settings (40, 49) by a transcription-independent mechanism through modulation of coactivator usage. We present here evidence that NF-κB negatively regulates proapoptotic tumor suppressor PTEN expression by a mechanism involving modulation of transcriptional coactivator usage, which promotes cell survival.
MATERIALS AND METHODS
Cell culture.
p65−/− immortalized mouse embryo fibroblasts (MEFs) (RelA 3T3 cells), IKKβ+/+, and IKKβ−/− MEFs were from Michael Karin (University of California at San Diego, La Jolla). Human lung cancer cells A549, H157, H838, and H460 were from John Yannelli (Internal Medicine Department, University of Kentucky). PTEN+/+ and PTEN−/− immortalized MEFs were obtained from Hong Wu, University of California at Los Angeles (29). Human thyroid cancer cells WRO, NPA, ARO, DRO, and MRO were from G. F. Juillard (University of California at Los Angeles). NIH 3T3 cells and COS-7 cells were purchased from the American Type Culture Collection, Manassas, Va. The thyroid cancer cells were grown in phenol red-free Dulbecco modified Eagle medium supplemented with 10% charcoal-stripped fetal bovine serum, 5% sodium pyruvate, and 5% nonessential amino acids. All other cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, l-glutamine (2 mM), penicillin (1,000 U/ml), and streptomycin (1,000 μg/ml).
Plasmids, antibodies, and chemical reagents.
The human PTEN gene promoter-luciferase reporter construct was a gift from Eileen Adamson (Burnham Institute, La Jolla, Calif.). The PTEN-Luc reporter construct was made by subcloning the 1,978-bp genomic DNA region upstream of the human PTEN gene into the empty pGL3 basic-Luc vector (47). Wild-type p50 and p65 expression constructs and p65 deletion construct p65ΔC (containing p65 amino acids 1 to 337) were from Nancy Rice (National Cancer Institute, Frederick, Md.). Point mutants of p65, including 276A, 529A, 529E, and IκBα-SR, were from Albert Baldwin (University of North Carolina at Chapel Hill). Wild-type PTEN and PTEN C124S point mutant expression constructs were obtained from Ramon Parsons, Columbia University, New York, N.Y. The green fluorescent protein (GFP)-PTEN expression construct was obtained from K. M. Yamada (45). The expression vector for pRSV-p300 was obtained from D. Livingston (13). Hemagglutinin-tagged CBP was obtained from R. H. Goodman (9). p300-C, an amino-terminal deletion mutant of p300 lacking the C/H1 and KIX domains containing amino acids 1135 to 2414, was from Christophe Nicot, University of Kansas Medical Center (37). Dominant-negative (DN) Akt1 (K179A, T308A, and S473A) was from Naoya Fujita, University of Tokyo, Tokyo, Japan (15). The expression construct for constitutively active Akt (myr-Akt) has been previously described (23). The pSVβgal construct (Promega Corp., Madison, Wis.) and the p65 luciferase reporter system composed of a Gal4-luciferase (Gal4-Luc) plasmid, which contains four Gal4 DNA consensus binding sites derived from the Saccharomyces cerevisiae GAL4 gene upstream of luciferase, and a Gal4-p65 construct, which has the yeast Gal4 DNA binding domain fused to the transactivation domain (TAD) of p65, have been previously described (31). The Tal-Luc vector was from Clontech, Palo Alto, Calif. The PTEN-Luc Δ600 deletion mutant was constructed by restriction enzyme digestion of the wild-type PTEN Luc construct with KpnI and XhoI to remove the upstream 600-bp region containing the two NF-κB binding sites followed by end filling with Klenow and religation. The PTEN 600 Tal-Luc construct was constructed by inserting the 600-bp PTEN promoter region fragment into Tal-Luc after digesting Tal-Luc with KpnI and XhoI followed by religation. Adenovirus expression vectors for IκB-SR and GFP have been previously described (5).
Antibodies, cytokines, and other reagents.
Mouse monoclonal antibodies for PTEN (A2B1) and IKKβ (H-4) and rabbit polyclonal antibodies for p65 (C-20), p50 (H-119), IκB-α (C-21), Akt1/2 (H-136), X chromosome-linked IAP (XIAP) (H-202), and cIAP1 (H-83) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). The monoclonal antibody for β-actin was purchased from Sigma Chemical Corp. Rabbit polyclonal antibody for phospho-Akt (Ser-473) was purchased from Cell Signaling Corp. The annexin V apoptosis kit was from Clontech. TNF, IL-1β, forskolin, IBMX, and 8-bromo cyclic AMP (cAMP) were purchased from Sigma Chemical Corp. Small interfering (siRNA) oligonucleotides against p65 (catalog number M-003533-00-05) and control nonspecific siRNA oligonucleotides (catalog number D-001206-13-05) were purchased from Dharmacon, Inc., Lafayette, Co.
Northern and Western blot analysis.
Cells were treated with murine or human TNF (20 ng/ml, as indicated) or vehicle and harvested after various time points for preparation of total RNA by the guanidinium isothiocyanate extraction method. In our optimization experiments (data not shown), we used various concentrations of TNF (20 to 100 ng/ml) and found that 20 ng/ml was sufficient to cause repression of PTEN expression. Hence, this concentration was used in all of the experiments in this study. The RNA was resolved on formaldehyde-agarose gels, transferred onto nylon membranes, and subjected to Northern blot analysis by using human PTEN cDNA or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA as a probe, as previously described (39). Whole-cell protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and subjected to Western blot analysis for PTEN, p65, IKKβ, cIAP1, XIAP, or actin expression with the indicated antibodies, as described previously (39).
Transfection and reporter assays.
Cells were transfected transiently with the Luc reporter and various driver plasmids at a reporter-to-driver ratio of 1:4, along with the β-galactosidase expression construct as an internal control. Transfections were performed for 48 h as described previously (5, 35), and whole-cell extracts from the transfectants were examined for luciferase activity by using the LucLite kit (from Packard Bioscience) or for β-galactosidase activity. The Luc activity in each reaction was normalized with respect to the corresponding β-galactosidase activity and expressed as relative Luc activity. For adenovirus infection, cells were transduced with the adeno-GFP control virus or adeno-IκB-SR virus as described previously (5).
Transfection of cells with siRNA.
Cells, in six-well plates, were transiently transfected with siRNAs by using Oligofectamine (Invitrogen Corp.) according to the manufacturer's instructions. Briefly, 2.6 μg of p65 siRNA or control siRNA was mixed with 175 μl of Opti-MEM (fresh RPMI medium without antibiotics) and then complexed with a mixture of 3 μl of Oligofectamine and 15 μl of Opti-MEM for 20 min at room temperature. The RNA-Oligofectamine complex was diluted with 800 μl of Opti-MEM to obtain a final volume of 1 ml and added to the cells. After 8 h, the cells were replenished with 500 μl of Opti-MEM containing 30% fetal calf serum and incubated for another 24 h. siRNA transfections were repeated after 24 h, mixtures were incubated for another 48 h, and the cells were processed for Western blot analysis of p65, PTEN, and actin.
Apoptosis assay.
NIH 3T3 cells were transfected with GFP-vector, GFP-PTEN myr-Akt1, or DN-Akt1, and 24 h after transfection, the cultures were treated with either vehicle or murine TNF (20 ng/ml) and incubated for another 24 h. Cells were fixed with 3% paraformaldehyde in phosphate-buffered saline for 20 min and subjected to indirect immunofluorescence with rabbit Akt1/2 antibody or preimmune antibody and a secondary anti-rabbit antibody conjugated with the fluorescent dye Alexa Fluor 594 (red) from Molecular Probes, Inc., as previously described (5). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) for 15 min and subsequently washed with phosphate-buffered saline. The apoptotic morphology of the cells (i.e., nuclear condensation and fragmentation and membrane blebbing) was examined under a fluorescence microscope equipped with a 63× oil immersion objective and the appropriate filter set. Apoptotic cells were also scored by annexin V staining (with ApoAlert annexin V-fluorescein isothiocyanate from Clontech Laboratories) as previously described (5). A total of three independent experiments were performed, and in each experiment, about 200 cells expressing the transfected construct were scored.
Statistical considerations.
All experiments were performed at least three times to ascertain reproducibility, and representative Western or Northern analysis data or means of four readings from each of the multiple luciferase or apoptosis experiments ± standard deviation error bars are shown in the figures.
RESULTS
PTEN expression is down-regulated by NF-κB.
Apoptosis induced by TNF or other stimuli is inhibited by p65 (2, 51). In studies aimed at characterization of the mechanistic basis of p65-induced antiapoptosis, we identified the PTEN gene as a downstream target whose expression is suppressed by NF-κB. When p65−/− immortalized MEFs were transiently transfected with constructs expressing p65, p50, or p65 and p50 together, and PTEN expression was examined by Western blot analysis, we noted that p65 alone or p65 plus p50, but not p50 alone, caused a 50 to 70% reduction in PTEN protein levels (Fig. 1A). These findings indicated that p65 acting alone or as a component of NF-κB can suppress PTEN expression. Since PTEN is a negative regulator of Akt activation, we determined whether down-regulation of PTEN by p65 results in concomitant activation of Akt. When p65−/− cells were transiently transfected with vector, p65, or p50 constructs and whole-cell extracts were analyzed by Western blot analysis, a significant increase in the levels of phospho-Akt was noted in p65 transfected cells (Fig. 1B). By contrast, cells transfected with either the control vector or p50 construct did not show an increase in phospho-Akt levels. There was no change in the total Akt protein levels in the p65-transfected cells compared to vector-transfected cells. These results indicated that down-regulation of PTEN expression by NF-κB is accompanied by activation of Akt.
FIG. 1.
PTEN is down-regulated by NF-κB. p65−/− immortalized MEFs were transiently transfected for 48 h with constructs expressing p50, p65, or vector (as a control), and the expression of p65, PTEN, or actin (as loading control) was examined by Western blot analysis (A). The signal intensity was quantified by densitometry and normalized to the corresponding signal for actin, and the relative PTEN protein levels (in arbitrary units) are shown at the bottom of panel A. p65−/− cells were transiently transfected for 48 h with constructs expressing p50, p65, or vector (as a control), and expression of phospho-Akt, total Akt, p65, p50, or actin was examined by Western blot analysis (B). NIH 3T3 cells were treated with murine TNF or IL-1, and whole-cell protein extracts prepared after 24 h were subjected to Western blot analysis for PTEN, cIAP1, or actin (C). Human MCF-7 breast cancer cells were treated with human TNF, and total RNA, prepared after the indicated time points, was subjected to Northern blot analysis for PTEN or GAPDH (as a control) (D). The RNA signal intensity was quantified by densitometry and normalized to the corresponding signal for GAPDH, and the relative PTEN RNA levels (in arbitrary units) are shown at the bottom of panel D. MCF-7 cells were treated with human TNF for the indicated time points, and whole-cell protein extracts were subjected to Western blot analysis for PTEN and actin (D). NIH 3T3 cells were treated with murine TNF for the indicated time periods, and total RNA or whole-cell lysates were subjected to either Northern blot analysis for PTEN (E) or Western blot analysis for PTEN and actin expression (E). The RNA signal intensity was quantified by densitometry and normalized to the corresponding signal for 28S RNA on the corresponding ethidium bromide gel, and the relative PTEN RNA levels (in arbitrary units) are shown at the bottom of panel E. IKKβ−/− or wild-type (IKKβ+/+) MEFs were treated with murine TNF or vehicle for 24 h, and whole-cell extracts were analyzed for PTEN, IKKβ, or actin expression by Western blot analysis (F). NIH 3T3 cells were transfected with GFP-PTEN expression plasmid along with vector or p65 expression construct used at a 1:1 ratio, and whole-cell protein extracts prepared after 36 h were subjected to Western blot analysis for expression of PTEN or actin (G).
Similar to ectopic p65, activation of endogenous NF-κB with either TNF or IL-1, two potent physiological inducers of NF-κB, in NIH 3T3 cells resulted in down-regulation of PTEN protein levels compared to vehicle-treated cells (Fig. 1C). On the other hand, TNF or IL-1 treatment resulted in up-regulation of NF-κB target gene cIAP1, which served as the positive control. To determine the kinetics of PTEN down-regulation by NF-κB, MCF-7 or NIH 3T3 cells were treated with human or murine TNF, respectively, and PTEN expression was analyzed on Western blots at various time points. TNF treatment resulted in a time-dependent decrease in PTEN protein levels (Fig. 1D, right panel, and E, lower panel). Consistently, MCF-7 and NIH 3T3 cells treated with TNF showed a rapid decrease in PTEN mRNA levels (Fig. 1D, left panel, and E, upper panel). The PTEN mRNA down-regulation response to TNF treatment was biphasic. The mRNA levels were strongly reduced at 3 or 6 h posttreatment, but at 12 h, the down-regulation was modest. Then at 24 h, the levels were again strongly reduced (Fig. 1D, left panel, and E, upper panel). This pattern of down-regulation of mRNA levels may be explained by the NF-κB activation kinetics model proposed by Baltimore and coworkers (20) that describes the temporal control of NF-κB activation by the coordinated degradation and synthesis of IκB proteins. The model suggests that IκBα is responsible for strong negative feedback that allows for a fast turn-off of the NF-κB response, whereas IκBβ and IκBɛ function to reduce the system's oscillatory potential and stabilize NF-κB responses during longer stimulations (20). Interestingly, the PTEN protein down-regulation response was relatively more uniform either because the fluctuations in the mRNA were highly transient and inconsequential or because Western blot analysis was not sensitive enough to detect the fluctuations. Overall, the findings suggested that TNF down-regulates PTEN mRNA and protein levels.
To confirm the requirement of NF-κB activation for PTEN repression by TNF, we used IKKβ−/− MEFs, which are defective in TNF-inducible NF-κB activation (27, 28). IKKβ−/− or IKKβ+/+ MEFs were treated with TNF or vehicle for 24 h, and whole-cell extracts were analyzed for PTEN expression by Western blot analysis. In these experiments, TNF caused down-regulation of PTEN protein in IKKβ+/+ cells but not in IKKβ−/− cells (Fig. 1F). TNF also caused a drop in the IKKβ protein levels in IKKβ+/+ cells, as has been reported previously (46), and this served as a positive control. These findings suggested that NF-κB activation by TNF was necessary for down-regulation of PTEN. To assess whether down-regulation of PTEN expression by NF-κB is due to decreased mRNA or protein stability (i.e., posttranscriptional or posttranslational regulation), we transfected NIH 3T3 cells with plasmid vector encoding GFP-PTEN along with p65 expression construct or empty vector as a control and determined the expression of ectopic GFP-PTEN expression after 36 h of transfection by Western blot analysis. p65 transfection did not result in down-regulation of ectopic GFP-PTEN driven by a heterologous promoter (Fig. 1G), suggesting that NF-κB caused down-regulation of PTEN expression not by decreasing mRNA or protein stability but possibly by transcriptional repression of PTEN promoter.
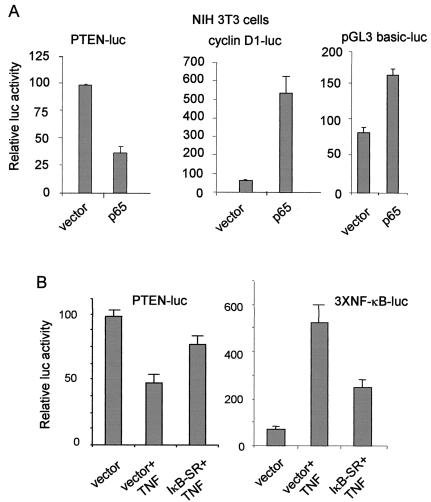
To ascertain that NF-κB regulates PTEN expression at the transcription level, we cotransfected NIH 3T3 cells with a luciferase reporter construct containing ∼2 kb of the promoter upstream region of the human PTEN gene along with a p65 expression construct or an empty vector for control and determined the effect of p65 on PTEN promoter activity. Consistent with the changes noted in the PTEN protein levels, p65 repressed the PTEN promoter (Fig. 2A). p65 induced the control cyclin D1 promoter (Fig. 2A) as well as luciferase reporter constructs containing the IL-8 promoter or NF-κB-binding sites (data not shown). Moreover, as seen in Fig. 2A, p65 marginally stimulated the expression of pGL3-basic-Luc (which is the basic reporter in which the PTEN promoter region is subcloned to generate PTEN-Luc). Together, these results indicated that repression by p65 was specific for the PTEN promoter.
FIG. 2.
NF-κB down-regulates the PTEN promoter. NIH 3T3 cells were cotransfected with pGL3 basic-Luc, PTEN promoter-Luc, or cyclin D1-Luc reporter construct, as indicated, and p65 expression construct or vector as a control along with β-galactosidase expression plasmid. Relative Luc activity, normalized to the corresponding β-galactosidase activity is shown (A). Relative Luc activity of 100 corresponds to 16,000 to 25,000 U of the raw Luc activity values normalized to β-galactosidase activity for all of the luciferase reporter experiments. NIH 3T3 cells were cotransfected with the PTEN promoter-Luc reporter construct or NF-κB-Luc reporter construct, as indicated, and IκB-SR expression construct or vector as a control along with β-galactosidase expression plasmid. Cells were treated with TNF (20 ng/ml) for 6 h before harvesting them for luciferase activity. Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown (B).
To further determine whether activation of endogenous NF-κB also represses the PTEN promoter, we cotransfected the NIH 3T3 cells with PTEN-Luc reporter construct and β-galactosidase expression plasmid along with IκB-SR expression plasmid or vector as control, and treated the cells with murine TNF for 6 h. As a positive control, the NF-κB-Luc reporter construct was used in place of PTEN-Luc in these experiments. Consistent with the repressor effect of ectopic p65 on PTEN promoter activity, activation of endogenous NF-κB by TNF also resulted in repression of the PTEN promoter and the repression could by significantly rescued by specific inhibition of NF-κB activation by cotransfection of IκB-SR (Fig. 2B). On the other hand, TNF induced the NF-κB Luc reporter expression about eightfold compared to the untreated cells, and TNF-inducible NF-κB activity was significantly inhibited by cotransfection with IκB-SR (Fig. 2B). Together, these findings suggested that ectopic as well as endogenous NF-κB down-regulated PTEN expression at the promoter level.
High levels of p65 repress PTEN expression in a subset of human lung and thyroid cancer cells.
We then examined the relationship between p65 and PTEN protein levels in human cancer cells. Human non-small-cell lung cancer cells and thyroid cancer cells were chosen for this study because these particular tumor types and the corresponding cell lines have wild-type PTEN alleles but often show a decreased expression of PTEN protein (55, 56). PTEN and p65 protein levels were determined by Western blot analysis in four lung cancer cell lines and five thyroid cancer cell lines, all of which were randomly chosen for analysis. A striking inverse correlation between p65 and PTEN protein levels was detected in seven of nine cancer cell lines examined (Fig. 3A). Three lung cancer cell lines, A549, H157, and H838, expressed high levels of endogenous p65 but very low or undetectable levels of PTEN protein (Fig. 3A). On the other hand, H460 cells had low levels of p65 protein but high levels of PTEN protein (Fig. 3A). Of the thyroid cancer cell lines examined, ARO, DRO, and MRO cells had high levels of p65 protein but low levels of PTEN protein (Fig. 3A). However, in two other thyroid cancer cell lines tested, WRO (which had relatively lower levels of p65) and NPA (which had relatively higher levels of p65), there was no reciprocal relationship between p65 and PTEN expression level (Fig. 3A), suggesting that in these two cell lines PTEN expression may be regulated by p65-independent mechanisms. Importantly, in all of the cell lines with high levels of p65 expression, the activity of the PTEN promoter was rescued by inhibition of NF-κB with ectopic IκB-SR (Fig. 3B and data not shown). Moreover, inhibition of NF-κB in lung cancer cells A549 and H838 or thyroid cancer cells MRO and ARO by adenovirus-mediated gene transfer of IκB-SR resulted in up-regulation of PTEN protein in 48 h (Fig. 3C).
FIG. 3.
Elevated levels of p65 protein in certain human lung and thyroid cancer cells repress PTEN expression. Whole-cell extracts were prepared from non-small-cell lung carcinoma or thyroid cancer cell lines and subjected to Western blot analysis for p65, PTEN, or actin (A). MRO and A549 cells were cotransfected with the PTEN promoter-Luc reporter construct and IκB-SR expression constructs or vector as a control along with β-galactosidase expression plasmid. Relative Luc activity measured after 48 h of transfection, normalized to the corresponding β-galactosidase activity, is shown (B). A549 and H838 lung cancer cells and MRO and ARO thyroid cancer cells were infected with adenovirus expression vector for IκB-SR or GFP as a control, and whole-cell protein extracts collected after 24 or 48 h were subjected to Western blot analysis for PTEN, IκB-α, or actin. Cell extracts from PTEN+/+ and PTEN−/− MEFs were loaded as controls for PTEN expression (C).
To further confirm the inverse relationship between p65 and PTEN expression, we used siRNA to directly inhibit the p65 transcript. When H838 lung cancer cells, which express high levels of endogenous p65 protein, were transfected with siRNA for p65, a significant reduction in p65 protein was observed along with a concomitant increase in PTEN protein relative to cells transfected with control siRNA (Fig. 3D). Thus, in a subset of human lung or thyroid cancer cells, high levels of endogenous p65 protein actively repress PTEN expression, suggesting the existence of a hitherto unidentified mechanism for limiting PTEN expression in human cancers.
PTEN is down-regulated by NF-κB by a mechanism that is independent of potential NF-κB binding sites in the PTEN promoter.
Because the PTEN promoter contains two potential NF-κB binding sites (GGGAATCTCT at nucleotide position −1565 and GGGTATTCCC at nucleotide position −1441) in the far upstream region, we made a 600-bp deletion in the PTEN promoter to eliminate both putative NF-κB binding sites (Fig. 4A). Surprisingly, deletion of these sites in construct Δ600 did not eliminate the ability of p65 to repress the promoter (Fig. 4A), suggesting that these binding sites are not required for p65 to repress PTEN expression. Consistent with this finding, when the 600-bp PTEN promoter region containing two putative NF-κB binding sites was subcloned in Tal-Luc upstream of a heterologous herpes simplex virus-thymidine kinase minimal promoter, it did not confer p65-mediated repression function to the thymidine kinase promoter (Fig. 4B). These findings indicated that NF-κB binding sites in the PTEN promoter or the sequences adjacent to them in cis do not mediate repression by p65 and that p65 may target other regulatory elements to repress the PTEN promoter.
FIG. 4.
Putative NF-κB binding sites in the PTEN promoter do not mediate repression by p65. NIH 3T3 cells were cotransfected with either full-length PTEN promoter-Luc reporter construct or Δ600 PTEN promoter-Luc reporter construct, in which the two putative NF-κB binding sites were deleted, along with either vector or p65 constructs, and luciferase activity was determined at 48 h posttransfection (A). NIH 3T3 cells were cotransfected with either empty Tal-Luc reporter construct or PTEN 600 Tal-Luc reporter construct, which contained the 600-bp region of the PTEN promoter with the two putative NF-κB binding sites subcloned in Tal-Luc, along with either vector or p65 constructs, and luciferase activity was determined at 48 h posttransfection (B). Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown (A and B).
The TAD of NF-κB is not essential, but the serine 276 residue of NF-κB is critical for PTEN inhibition.
p65 is characterized by an N-terminal conserved Rel homology domain, which contains regions required for DNA binding and dimerization, and a protein kinase A (PKA) phosphorylation site at serine 276, an NLS, and a C-terminal TAD (Fig. 5A). To identify the mechanism by which p65 causes repression of PTEN gene expression, we examined the effects of various p65 deletion and point mutants on the PTEN promoter (Fig. 5A). The point mutants included 276A, a phosphorylation-defective serine-to-alanine mutation of the conserved PKA phosphorylation site at serine 276; 529A, a phosphorylation-defective serine-to-alanine mutation of the casein kinase II phosphorylation site at serine 529; and 529E, a phosphorylation mimetic serine-to-glutamic acid mutation at serine 529. Interestingly, the deletion mutant ΔC, which lacks TAD, as well as the point mutants 529A and 529E retained the ability to repress the PTEN promoter, but the 276A mutant had lost the ability to repress the PTEN promoter in both NIH 3T3 and Cos-7 cells (Fig. 5B and C). These findings indicated that DNA binding or TAD functions of p65 were not required for inhibition of PTEN promoter activity. Most importantly, because the 276A point mutant completely lost its ability to repress the PTEN promoter, serine 276 is a critical residue essential for repression of the PTEN promoter by p65.
FIG. 5.
p65 represses the PTEN promoter independent of its transcriptional activity. Schematic diagram illustrating various deletion and phosphorylation-defective point mutant constructs of p65 used in this study (A). NIH 3T3 cells were cotransfected with vector, p65, or various p65 deletion-point mutant expression constructs, and the PTEN promoter-Luc reporter construct. Luciferase activity was determined at 48 h posttransfection (B). The bottom panel shows Western blot analysis performed on whole-cell lysates from the transfectants (B). Note the expression of p65 (arrow) or Flag-tagged p65 mutants (asterisk, thick arrow for ΔC, which contains amino acids 1 to 337 of p65) and actin (B). Cos-7 cells were cotransfected with vector, IκB-SR, p65, or various p65 deletion-point mutant expression constructs, and the PTEN promoter-Luc reporter construct. Luciferase activity was determined at 48 h posttransfection (C). Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown.
PKA signaling induces PTEN expression, but p65 blocks this function.
Because serine 276 is a well-defined target site for PKA-dependent phosphorylation of p65 (57) and mutation of this site abolished the repressor function of p65 (Fig. 5B), we examined whether the PKA signaling pathway regulated the expression of PTEN. To activate endogenous PKA, we used two different approaches. First, we treated the cells with a combination of forskolin and IBMX. Forskolin is an adenylate cyclase activator that increases the production of intracellular cAMP levels. IBMX prevents degradation of cAMP by inhibiting the enzyme phosphodiesterase, thereby increasing the intracellular levels of cAMP. The second approach involved treatment of cells with nonhydrolyzable analog of cAMP, 8-bromo cAMP, to increase the intracellular concentration of cAMP to activate endogenous PKA. Treatment of NIH 3T3 cells with a combination of forskolin and IBMX or cAMP analog, 8-bromo cAMP (500 μm), which activates endogenous PKA, resulted in induction of the PTEN promoter as well as of control CRE-Luc activity (Fig. 6A). Treatment of cells with 8-bromo cAMP also resulted in up-regulation of PTEN protein levels as early as 3 h posttreatment, and the levels continued to increase for up to 48 h posttreatment (Fig. 6B). These results indicated that PTEN gene expression was induced by cAMP-PKA-activated signals. We then analyzed the effect of wild-type p65 or the 276A mutant, which is incapable of being phosphorylated by PKA, on cAMP-PKA-induced PTEN promoter activity. Overexpression of wild-type p65, but not 276A mutant, inhibited the cAMP-PKA-induced PTEN promoter activity (Fig. 6C). Together, these findings suggested that the serine 276 residue of p65 was essential for the repression of both basal and PKA-inducible expression of the PTEN promoter.
FIG. 6.
cAMP-PKA pathway induces PTEN expression, but p65 blocks this induction. NIH 3T3 cells were transfected with either PTEN promoter-Luc reporter construct or CRE (cAMP response element)-Luc reporter construct and left untreated or treated with forskolin (FK) plus IBMX or 8-bromo-cAMP for 24 h before harvesting the cells. Luciferase activity was determined at 48 h posttransfection (A). NIH 3T3 cells were treated with 8-bromo-cAMP for the indicated time periods, and whole-cell lysates were subjected to Western blot analysis for PTEN or actin (B). NIH 3T3 cells were cotransfected with the PTEN promoter-Luc reporter construct along with p65 or 276A mutant of p65, and 8-bromo-cAMP was added 6 h before harvesting the cells. Luciferase activity was determined at 48 h posttransfection (C). Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown.
p65 suppresses the PTEN promoter by sequestering transcriptional coactivators CBP/p300.
CBP/p300 transcriptional coactivator proteins play a central role in coordinating and integrating multiple signal-dependent events with the transcription apparatus, allowing the appropriate level of gene activity in response to diverse physiological cues that influence, for example, proliferation, differentiation, and apoptosis (6). Viral oncoproteins, such as adenoviral E1A and simian virus 40 large T antigen, specifically target these proteins by direct interaction and inactivate CBP/p300 tumor suppressor-like activity (17). Transcription can be regulated by the competitive binding of specific transcription factors to CBP/p300. Compelling evidence indicates that CBP/p300 proteins are rate-limiting in the nucleus, and competition for a limiting pool of CBP/p300 upon NF-κB activation has been previously proposed as a plausible mechanism for transcriptional regulation or induction of apoptosis (21, 22, 24, 36, 54). p65 and the p53 tumor suppressor gene compete for binding to limiting amounts of CBP/p300 (48). Recent studies have reported phosphorylation of p65 on serine 276 by PKA and that this phosphorylation is essential for efficient binding of p65 to CBP/p300 (37, 58). Because the S276A mutant did not inhibit PTEN promoter activity in the present study and because the S276A mutant is defective in binding to CBP/p300 (37, 58), it is possible that direct interaction between p65 and CBP/p300 may be the primary mechanism underlying inhibition of PTEN promoter activity. Studies were therefore performed to test the possibility that p65 represses PTEN gene transcription by competing with the binding of other factors to CBP/p300, with the rationale that if this was indeed the mechanism, ectopic expression of CBP/p300 would relieve repression mediated by p65. Because p300 and CBP are general transcriptional coactivators and can enhance the basal PTEN promoter activity, we used p300- or CBP-encoding plasmid in amounts that did not significantly enhance the basal PTEN-Luc activity (Fig. 7A, left panel). Expression of p65 alone repressed PTEN promoter activity, and cotransfection with either p300 or CBP expression vector partially reversed this effect (Fig. 7A, left panel). Because p65 interacts with the N-terminal region of p300 containing the C/H1 and KIX domains (37), sequestration of p300 by p65 may account for the partial effect. We therefore used p300-C, a mutant of p300 lacking the C/H1 and KIX domains that is unable to interact with p65 but fully capable of functioning as a coactivator. When cotransfected with p65, p300-C completely rescued the NF-κB-mediated repression of PTEN promoter activity (Fig. 7A, right panel). Consistent with the sequestration model, the rescue function of p300-C most likely results from lack of sequestration by p65, making it available for PTEN transcription. These results suggested that p65 represses PTEN gene transcription through competitive interaction with, and sequestration of, limiting pools of coactivators p300/CBP from other transcription factors that mediate both basal and PKA-inducible induction of the PTEN gene (Fig. 7B).
FIG. 7.
Cotransfection of CBP or p300 relieves p65-mediated repression of PTEN. NIH 3T3 cells were cotransfected with the PTEN promoter-Luc reporter construct and p65 expression construct alone or vector for control or with a combination of p65 and wild-type p300 or CBP expression constructs (A, left panel) or a combination of p65 and p300-C, an amino-terminal deletion mutant of p300 lacking the C/H1 and KIX domains (A, upper panel), used at a 1:1 ratio, along with β-galactosidase expression plasmid. Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown (A). A model for the mechanism of PTEN suppression by NF-κB is shown (B). In unstimulated cells, the NF-κB complex consisting of p65 and p50 is sequestered by IκB. Stimulation with proinflammatory cytokines such as TNF or IL-1 causes phosphorylation and degradation of IκB, with the release of p65/p50 complex resulting in exposure of the serine-276 residue on p65. Phosphorylation of p65 at the serine-276 site by PKA results in efficient binding with, and sequestration of, transcriptional coactivators CBP/p300, thereby blocking the transcriptional activation by certain transcription factors responsible for basal and PKA-inducible expression of the PTEN gene and causing repression of PTEN. It is currently unknown which one of the PKA-activated transcription factors are targeted by p65 via CBP or p300 sequestration. Transcription factors C/EBP-α and -β and RXR are potential targets with 3 sites for C/EBP (at nucleotide positions −1311, −1208, and −819; shown in pink), and 4 sites for RXR (at nucleotide positions −1377, −1227, −1028, and −977; shown in purple) present in the PTEN promoter region. The transcription start site is at position −1031.
Restoration of PTEN inhibits NF-κB-induced antiapoptosis.
We analyzed the functional relevance of PTEN down-regulation by NF-κB by determining the effect of PTEN on NF-κB transcription activity. Because activated PI3K or activated Akt can increase the transcription activity of NF-κB (30, 31), we sought to determine whether PTEN could prevent NF-κB activation by targeting TAD1 (amino acids 519 to 551) of the p65 subunit. To address this question, we used a construct encoding the Gal4-p65 fusion protein, where sequences encoding the DNA binding domain of Gal4 were fused to sequences encoding TAD1 of p65 (31). When cotransfected with a Gal4-Luc reporter, this chimera allowed us to determine whether overexpression of PTEN inhibited NF-κB-dependent gene expression by specifically targeting TAD1. NIH 3T3 cells were cotransfected with Gal4-p65 chimera, Gal4-Luc reporter, expression constructs for wild-type PTEN, or a tumor-derived phosphatase-defective C124S mutant of PTEN incapable of preventing Akt activation. Cotransfection with the Gal4 expression construct was used as a control. As shown in Fig. 8A, wild-type PTEN, but not the C124S mutant of PTEN, inhibited p65-dependent reporter activity (Fig. 8A). These results indicated that PTEN blocks NF-κB transcriptional activity by targeting the TAD of the p65 subunit.
FIG. 8.
PTEN inhibits transactivation and antiapoptotic functions of p65. NIH 3T3 cells were cotransfected with Gal4-p65 fusion construct as a driver and 5× Gal4-Luc reporter in the presence or absence of vector, wild-type PTEN, or the phosphatase-defective C124S mutant of PTEN. Luciferase activity was determined at 48 h posttransfection (A). Relative Luc activity, normalized to the corresponding β-galactosidase activity, is shown. Cell extracts from PTEN+/+ and PTEN−/− MEFs were subjected to Western blot analysis for expression of XIAP, PTEN, and actin (B). NIH 3T3 cells were transfected with GFP-vector, GFP-PTEN, myr-Akt1, or DN-Akt1, and 24 h after transfection, the cultures were treated with either vehicle or murine TNF (20 ng/ml) and incubated for another 24 h. Apoptotic cells were scored as described in Materials and Methods (C). Whole-cell extracts prepared from transfected cells where subjected to Western blot analysis for expression of Akt, PTEN, and actin (C). A model for the negative regulatory loop between NF-κB and PTEN involved in the modulation of TNF-inducible apoptosis is depicted (D). TNF-inducible apoptosis is known to be prevented by NF-κB activation. We suggest that activation of NF-κB by TNF results in down-regulation of PTEN expression and prevention of apoptosis. Overexpression of PTEN results in inhibition of NF-κB transcriptional activity and potentiation of TNF-inducible apoptosis (D).
To further ascertain that endogenous PTEN blocks the expression of NF-κB-dependent target genes, we examined the expression of the antiapoptotic NF-κB target gene XIAP in wild-type and PTEN knockout MEFs. NF-κB-inducible XIAP gene expression has previously been shown to protect cells from TNF-induced apoptosis (44). As seen in Fig. 8B, XIAP levels were elevated in PTEN-deficient cells compared to wild type cells, indicating that endogenous PTEN effectively blocks the expression of NF-κB-inducible antiapoptotic target genes. Together with the above data, these findings suggested a negative regulatory loop between PTEN and NF-κB, in which the two proteins reciprocally inhibit each other at the expression or functional level.
Finally, because TNF down-regulated endogenous PTEN, we examined the effect of PTEN restoration on TNF-inducible apoptosis. NIH 3T3 cells were transfected with constructs expressing GFP control vector, GFP-PTEN, myr-Akt, or DN-Akt (K179A, T308A and S473A), treated with TNF for 24 h, and analyzed for apoptosis of transfectants as described in Materials and Methods. As seen in Fig. 8C, treatment of the cells with TNF alone did not significantly increase apoptosis, and ectopic expression of PTEN in the absence of TNF resulted in a marginal increase in the number of apoptotic cells. However, ectopic expression of PTEN greatly sensitized the TNF-treated cells to apoptosis (Fig. 8C). Importantly, PTEN-inducible sensitization to apoptosis by TNF was effectively prevented by coexpression of myr-Akt (Fig. 8C). Moreover, similar to PTEN, overexpression of DN-Akt sensitized TNF-treated cells to apoptosis (Fig. 8C). Together, these data suggest that PTEN greatly sensitizes cells to apoptosis by TNF by inhibiting Akt-mediated activation of NF-κB and cell survival. Thus, down-regulation of PTEN expression by TNF via NF-κB is critical for prevention of apoptosis.
DISCUSSION
The present study identified the proapoptotic tumor suppressor gene PTEN as a novel target of NF-κB. In contrast to the antiapoptotic target genes that are induced by NF-κB, PTEN is down-regulated by NF-κB. Because NF-κB is a transcriptional activator and its role in antiapoptosis is mediated by induction of genes such as Bcl-xL and IAPs (52), suppression of the proapoptotic tumor suppressor PTEN gene by NF-κB to prevent apoptosis, is a unique finding that suggests a broader regulatory role for NF-κB than previously anticipated. Moreover, our studies showed that high levels of NF-κB activity in several tumor cells cause suppression of wild-type PTEN expression. These results uncovered a novel mechanism by which PTEN expression can be negatively regulated in human tumors. The promoter, RNA, and protein levels of PTEN are down-regulated by NF-κB. The significance of this finding stems from the fact that the apoptotic and tumor suppressor functions of PTEN are often ablated in tumor cells by mutation or chromosomal deletion, and suppression of PTEN expression by NF-κB offers a novel mechanism to counteract apoptosis and growth suppression in tumors where the PTEN gene is intact and functional. Furthermore, we identified a closed negative regulatory loop involving NF-κB and PTEN that may serve to balance their mutually antagonistic functions.
Endogenous NF-κB released from IκB after treatment of cells with TNF or IL-1 caused repression of PTEN gene expression. Moreover, in cells that are deficient in NF-κB activation, TNF did not repress PTEN. These results suggest that activation of endogenous NF-κB inhibits PTEN expression, thereby underscoring the physiological relevance of the findings. These findings have broader implications in apoptosis, inflammation, and cancer development. Down-regulation of PTEN is accompanied by corresponding up-regulation of phospho-Akt levels. Because haplo-insufficiency of the PTEN gene is widely accepted as the cause of a functionally relevant increase in Akt activity and corresponding downstream prosurvival alterations (10-12), we suggest that the 50 to 70% reduction in PTEN expression noted in this study is functionally relevant, and in fact, it protects the cells from TNF-inducible apoptosis (Fig. 8). Given the established role of Akt in the inhibition of apoptosis (43), the activation of Akt by NF-κB-mediated suppression of PTEN expression may serve as a powerful stimulus in the development or progression of cancer.
Several investigators have speculated on PTEN promoter methylation as a possible mechanism of PTEN inactivation. On the contrary, however, a recent study showed that the PTEN pseudogene, and not PTEN, is predominantly methylated in cancer cell lines and tumors (60). Genetic analysis of PTEN in non-small-cell lung cancers and thyroid cancers revealed that PTEN mutations or deletions are rare in these two types of cancer (14, 55, 56). However, reduced expression of PTEN protein was often observed in these cancers, and the underlying mechanism was unknown (4, 14, 55). In our study, we noted that in a subset of lung cancer and thyroid cancer cells, p65 protein levels are elevated and correlated with decreased PTEN protein levels, suggesting that elevated p65 protein may repress PTEN expression in these cancers. This interpretation was supported by our observation that inhibition of NF-κB by overexpression of IκB-SR induced PTEN promoter activity as well as PTEN protein levels in cancer cells containing high levels of p65 protein.
We noted that p65 represses PTEN expression independent of NF-κB sites in the promoter. It has been suggested that repression by p65 is mediated by direct competition for the coactivator CBP/p300. Recently, it was shown that NF-κB-dependent transcription is regulated through phosphorylation of p65 by PKA. The catalytic subunit of PKA (PKAc) associates with p65 in the cytoplasm promoting p65 phosphorylation at S276 (38, 57). Several groups have shown that p300/CBP functionally interacts with the phosphorylated S276 residue in p65 (14, 58). The observation that p65 completely lost the ability to inhibit PTEN transcription when mutated at the conserved S276 PKA phosphorylation site, directly implicates PKA in NF-κB mediated suppression of PTEN. Consistent with this rationale, treatment of cells with activators of endogenous PKA, such as cAMP or forskolin and IBMX, caused induction of the PTEN promoter, thereby identifying PTEN as a novel target of cAMP signaling. However, the cAMP-induced PKA can have a dual effect on the PTEN promoter depending upon the levels of IκB-unbound or free p65 (i.e., active p65) in the cell (Fig. 6C). This dual effect can be explained by the following observation reported by Zhong and coworkers (57, 58). PKAc interacts with IκB-α and IκB-β through sequences from the N terminus of the protein, and this interaction inhibits the catalytic activity of PKAc. Stimulation of cells with inducers of NF-κB activity, such as lipopolysaccharide or TNF, that do not alter the levels of intracellular cAMP leads to degradation of IκB proteins and activation of PKAc. Thus, this pathway represents a novel mechanism by which PKA is regulated in a cAMP-independent manner. Activated PKAc phosphorylates the p65 subunit of NF-κB at the PKA consensus site Ser-276 in the Rel homology domain and leads to a strong increase in the transcriptional activity of NF-κB (57, 58). However, activation of endogenous PKA with cAMP alone, in the absence of activation of the NF-κB pathway, does not result in phosphorylation of endogenous p65 because, in the nonstimulated state, the Ser-276 residue of p65 is masked by IκB and rendered inaccessible to PKA. PKA can only phosphorylate IκB-unbound or free p65 occurring, for example, after activation of the NF-κB pathway by TNF or ectopic overexpression of p65 (Fig. 6C). cAMP-induced PKA, in the absence of activated p65 or ectopic p65, therefore, does not phosphorylate p65 or lead to p300 sequestration by p65 in NIH 3T3 cells (Fig. 6) but is expected to activate transcription factors (such as RXR or C/EBP) that may induce the PTEN promoter. A similar mechanism was reported for regulation of the PEPCK gene by p65 or cAMP (49). PTEN may be a potential mediator of the cellular functions of the cAMP-PKA pathway because both PTEN and PKA cause similar phenotypic changes including growth inhibition, apoptosis, and differentiation (7).
By using p65 mutants that cannot bind DNA but can interact with CBP/p300, we ruled out the possibility that p65 transcriptional activity was required to suppress PTEN transcription. The repression of PTEN promoter activity by p65 occurred through competition for binding to the coactivators p300 and CBP. This model was supported by the finding that p300 or CBP partially restored PTEN promoter activity in the presence of p65. Moreover, the S276A mutant of p65, which was defective in binding to p300/CBP, was unable to suppress PTEN promoter activity, and the p300-C mutant of p300, which was unable to bind to p65, completely reversed the p65-mediated repression of the PTEN promoter. NF-κB may play a role in the regulation of both the basal and hormone-stimulated activity of the PTEN gene. The mechanism of inhibition of PTEN gene expression by NF-κB can be explained as illustrated in Fig. 7C. Phosphorylation of p65 at the serine 276 site by PKA results in efficient binding and sequestration of transcriptional coactivators p300 and CBP, thereby blocking the transcriptional activation by certain transcription factors responsible for basal and PKA-inducible expression of the PTEN gene and causing repression of PTEN. It is currently unknown which of the PKA-activated factors are targeted by p65 via p300 sequestration. Multiple transcription factors, such as CREB, C/EBP family members, retinoid receptors RAR and RXR, and glucocorticoid receptor, are dependent upon PKA-induced phosphorylation for their activation (8). Consensus DNA binding motifs for many of these transcription factors are present in the PTEN promoter (Fig. 7B). Studies designed to identify the downstream transcription factors inhibited by p65 in a PKA-dependent manner are currently under way. Our preliminary experiments suggest that C/EBPα, C/EBPβ, and RXRα, but not glucocorticoid receptor or CREB, are potential targets of NF-κB action (K. M. Vasudevan and V. M. Rangnekar, unpublished data). Similar to these findings, p65 repressed the PKA-C/EBP-inducible promoter of the metabolic gene PEPCK and c-Myb-dependent transcription in a serine 276 phosphorylation-dependent manner via sequestration of p300/CBP (37, 49).
Ectopic expression of PTEN inhibits the transactivation potential of p65 in a phosphatase-dependent manner (33) (Fig. 8A), implying that PTEN negatively regulates NF-κB at a juncture when its activity is amenable to positive regulation by phosphorylation (16). Constitutively activated Akt stimulates NF-κB transcriptional activity predominantly through signaling pathways that targeted the TAD of the p65 subunit (30, 31). Consistent with the importance of Akt in cell survival, we found that the inhibition of endogenous Akt activity by PTEN overexpression resulted in a loss of NF-κB transcriptional activity and sensitization of cells to TNF-induced apoptosis. This finding suggests that PTEN down-regulation confers long-term cell survival signaling by activating pathways that target NF-κB-dependent transcription. This interpretation is further supported by the observation that XIAP, a key antiapoptotic gene target of NF-κB, is aberrantly elevated in cells that lack PTEN (Fig. 8B) because XIAP can directly bind to caspases and prevent their activation upon apoptotic stimuli, and XIAP itself has been shown to activate NF-κB transcriptional activity (19).
Thus, repression of PTEN, which can otherwise hamper the full activation of NF-κB, facilitates the amplification of p65 transcriptional activity (Fig. 8D). Because ectopic PTEN blocks the NF-κB transcriptional activity and promotes cell death induced by TNF (Fig. 8C) (33, 51), it is conceivable that down-regulation of PTEN is an essential step in maximal activation of NF-κB and prevention of cell death. Finally, PTEN is a unique target of NF-κB in establishing a cross-regulatory loop (Fig. 8D), involving NF-κB and PTEN, which determines cell survival or apoptosis.
Acknowledgments
This study was supported by NIH and NCI grants CA60872 and CA84511 (to V.M.R.).
We thank Eileen Adamson, Burnham Institute, for the kind gift of the PTEN promoter construct.
REFERENCES
- 1.Alcamo, E., J. P. Mizgerd, B. H. Horwitz, R. Bronson, A. A. Beg, M. Scott, C. M. Doerschuk, R. O. Hynes, and D. Baltimore. 2001. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-kappa B in leukocyte recruitment. J. Immunol. 167:1592-1600. [DOI] [PubMed] [Google Scholar]
- 2.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 3.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 4.Brognard, J., A. S. Clark, Y. Ni, and P. A. Dennis. 2001. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 61:3986-3997. [PubMed] [Google Scholar]
- 5.Chakraborty, M., S. G. Qiu, K. M. Vasudevan, and V. M. Rangnekar. 2001. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 61:7255-7263. [PubMed] [Google Scholar]
- 6.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 7.Chen, T. C., D. R. Hinton, R. Zidovetzki, and F. M. Hofman. 1998. Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas. Lab. Investig. 78:165-174. [PubMed] [Google Scholar]
- 8.Chin, K. V., W. L. Yang, R. Ravatn, T. Kita, E. Reitman, D. Vettori, M. E. Cvijic, M. Shin, and L. Iacono. 2002. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann. N. Y. Acad. Sci. 968:49-64. [DOI] [PubMed] [Google Scholar]
- 9.Chrivia, J. C., R. P. Kwok, N. Lamb, M. Hagiwara, M. R. Montminy, and R. H. Goodman. 1993. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365:855-859. [DOI] [PubMed] [Google Scholar]
- 10.Di Cristofano, A., P. Kotsi, Y. F. Peng, C. Cordon-Cardo, K. B. Elkon, and P. P. Pandolfi. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science 285:2122-2125. [DOI] [PubMed] [Google Scholar]
- 11.Di Cristofano, A., and P. P. Pandolfi. 2000. The multiple roles of PTEN in tumor suppression. Cell 100:387-390. [DOI] [PubMed] [Google Scholar]
- 12.Di Cristofano, A., B. Pesce, C. Cordon-Cardo, and P. P. Pandolfi. 1998. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19:348-355. [DOI] [PubMed] [Google Scholar]
- 13.Eckner, R., M. E. Ewen, D. Newsome, M. Gerdes, J. A. DeCaprio, J. B. Lawrence, and D. M. Livingston. 1994. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8:869-884. [DOI] [PubMed] [Google Scholar]
- 14.Frisk, T., T. Foukakis, T. Dwight, J. Lundberg, A. Hoog, G. Wallin, C. Eng, J. Zedenius, and C. Larsson. 2002. Silencing of the PTEN tumor-suppressor gene in anaplastic thyroid cancer. Genes Chromosomes Cancer 35:74-80. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, N., S. Sato, K. Katayama, and T. Tsuruo. 2002. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277:28706-28713. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 17.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 18.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 19.Hofer-Warbinek, R., J. A. Schmid, C. Stehlik, B. R. Binder, J. Lipp, and R. de Martin. 2000. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J. Biol. Chem. 275:22064-22068. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, A., A. Levchenko, M. L. Scott, and D. Baltimore. 2002. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298:1241-1245. [DOI] [PubMed] [Google Scholar]
- 21.Horvai, A. E., L. Xu, E. Korzus, G. Brard, D. Kalafus, T. M. Mullen, D. W. Rose, M. G. Rosenfeld, and C. K. Glass. 1997. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc. Natl. Acad. Sci. USA 94:1074-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hottiger, M. O., and G. J. Nabel. 1998. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J. Virol. 72:8252-8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu, A. L., T. T. Ching, D. S. Wang, X. Song, V. M. Rangnekar, and C. S. Chen. 2000. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 275:11397-11403. [DOI] [PubMed] [Google Scholar]
- 24.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 25.Karin, M., Y. Cao, F. R. Greten, and Z. W. Li. 2002. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2:301-310. [DOI] [PubMed] [Google Scholar]
- 26.Karin, M., and A. Lin. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 27.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 28.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liliental, J., S. Y. Moon, R. Lesche, R. Mamillapalli, D. Li, Y. Zheng, H. Sun, and H. Wu. 2000. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10:401-404. [DOI] [PubMed] [Google Scholar]
- 30.Madrid, L. V., M. W. Mayo, J. Y. Reuther, and A. S. Baldwin, Jr. 2001. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276:18934-18940. [DOI] [PubMed] [Google Scholar]
- 31.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh, D. J., P. L. Dahia, Z. Zheng, D. Liaw, R. Parsons, R. J. Gorlin, and C. Eng. 1997. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 16:333-334. [DOI] [PubMed] [Google Scholar]
- 33.Mayo, M. W., L. V. Madrid, S. D. Westerheide, D. R. Jones, X. J. Yuan, A. S. Baldwin, Jr., and Y. E. Whang. 2002. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J. Biol. Chem. 277:11116-11125. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, N., S. Ramaswamy, F. Vazquez, S. Signoretti, M. Loda, and W. R. Sellers. 2000. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20:8969-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalca, A., S. G. Qiu, N. El-Guendy, S. Krishnan, and V. M. Rangnekar. 1999. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J. Biol. Chem. 274:29976-29983. [DOI] [PubMed] [Google Scholar]
- 36.Nicot, C., and R. Harrod. 2000. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol. Cell. Biol. 20:8580-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicot, C., R. Mahieux, C. Pise-Masison, J. Brady, A. Gessain, S. Yamaoka, and G. Franchini. 2001. Human T-cell lymphotropic virus type 1 Tax represses c-Myb-dependent transcription through activation of the NF-κB pathway and modulation of coactivator usage. Mol. Cell. Biol. 21:7391-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki, T., S. Sakon, T. Sasazuki, H. Sakurai, T. Doi, H. Yagita, K. Okumura, and H. Nakano. 2003. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem. Biophys. Res. Commun. 300:807-812. [DOI] [PubMed] [Google Scholar]
- 39.Qiu, S. G., S. Krishnan, N. el-Guendy, and V. M. Rangnekar. 1999. Negative regulation of Par-4 by oncogenic Ras is essential for cellular transformation. Oncogene 18:7115-7123. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard, K. A., K. M. Phelps, A. J. Williams, D. Thanos, C. K. Glass, M. G. Rosenfeld, M. E. Gerritsen, and T. Collins. 1998. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J. Biol. Chem. 273:29291-29294. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, L., and R. Parsons. 2001. PTEN: life as a tumor suppressor. Exp. Cell Res. 264:29-41. [DOI] [PubMed] [Google Scholar]
- 42.Stambolic, V., D. MacPherson, D. Sas, Y. Lin, B. Snow, Y. Jang, S. Benchimol, and T. W. Mak. 2001. Regulation of PTEN transcription by p53. Mol. Cell 8:317-325. [DOI] [PubMed] [Google Scholar]
- 43.Stambolic, V., A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29-39. [DOI] [PubMed] [Google Scholar]
- 44.Stehlik, C., R. de Martin, I. Kumabashiri, J. A. Schmid, B. R. Binder, and J. Lipp. 1998. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 188:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura, M., J. Gu, K. Matsumoto, S. Aota, R. Parsons, and K. M. Yamada. 1998. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280:1614-1617. [DOI] [PubMed] [Google Scholar]
- 46.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKbeta suppresses TNF-alpha-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 47.Virolle, T., E. D. Adamson, V. Baron, D. Birle, D. Mercola, T. Mustelin, and I. de Belle. 2001. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat. Cell Biol. 3:1124-1128. [DOI] [PubMed] [Google Scholar]
- 48.Wadgaonkar, R., K. M. Phelps, Z. Haque, A. J. Williams, E. S. Silverman, and T. Collins. 1999. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J. Biol. Chem. 274:1879-1882. [DOI] [PubMed] [Google Scholar]
- 49.Waltner-Law, M., M. C. Daniels, C. Sutherland, and D. K. Granner. 2000. NF-kappa B inhibits glucocorticoid and cAMP-mediated expression of the phosphoenolpyruvate carboxykinase gene. J. Biol. Chem. 275:31847-31856. [DOI] [PubMed] [Google Scholar]
- 50.Wang, C. Y., J. C. Cusack, Jr., R. Liu, and A. S. Baldwin, Jr. 1999. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat. Med. 5:412-417. [DOI] [PubMed] [Google Scholar]
- 51.Wang, C. Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 52.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 53.Wang, S. I., R. Parsons, and M. Ittmann. 1998. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin. Cancer Res. 4:811-815. [PubMed] [Google Scholar]
- 54.Webster, G. A., and N. D. Perkins. 1999. Transcriptional cross talk between NF-κB and p53. Mol. Cell. Biol. 19:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng, L. P., O. Gimm, J. B. Kum, W. M. Smith, X. P. Zhou, D. Wynford-Thomas, G. Leone, and C. Eng. 2001. Transient ectopic expression of PTEN in thyroid cancer cell lines induces cell cycle arrest and cell type-dependent cell death. Hum. Mol. Genet. 10:251-258. [DOI] [PubMed] [Google Scholar]
- 56.Yokomizo, A., D. J. Tindall, H. Drabkin, R. Gemmill, W. Franklin, P. Yang, K. Sugio, D. I. Smith, and W. Liu. 1998. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 17:475-479. [DOI] [PubMed] [Google Scholar]
- 57.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]
- 58.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]
- 60.Zysman, M. A., W. B. Chapman, and B. Bapat. 2002. Considerations when analyzing the methylation status of PTEN tumor suppressor gene. Am. J. Pathol. 160:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]