Abstract
After gravistimulation of Ceratodon purpureus (Hedw.) Brid. protonemata in the dark, amyloplast sedimentation was followed by upward curvature in the wild-type (WT) and downward curvature in the wwr mutant (wrong way response). We used ponderomotive forces induced by high-gradient magnetic fields (HGMF) to simulate the effect of gravity and displace the presumptive statoliths. The field was applied by placing protonemata either between two permanent magnets at the edge of the gap, close to the edge of a magnetized ferromagnetic wedge, or close to a small (<1 mm) permanent magnet. Continuous application of an HGMF in all three configurations resulted in plastid displacement and induced curvature in tip cells of WT and wwr protonemata. WT cells curved toward the HGMF, and wwr cells curved away from the HGMF, comparable to gravitropism. Plastids isolated from protonemal cultures had densities ranging from 1.24 to 1.38 g cm−3. Plastid density was similar for both genotypes, but the mutant contained larger plastids than the WT. The size difference might explain the stronger response of the wwr protonemata to the HGMF. Our data support the plastid-based theory of gravitropic sensing and suggest that HGMF-induced ponderomotive forces can substitute for gravity.
The force exerted by gravity is proportional to an object's volume and density. Therefore, objects denser than the surrounding medium fall or sediment. Much evidence suggests that gravity sensing in higher plants depends on the sedimentation of dense, starch-filled amyloplasts inside specialized cells, so-called statocytes (Sack, 1991, 1997; Kuznetsov and Hasenstein, 1996, 1997b; Balus̆ka and Hasenstein, 1997).
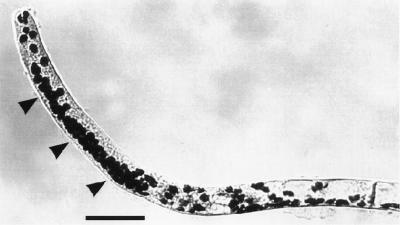
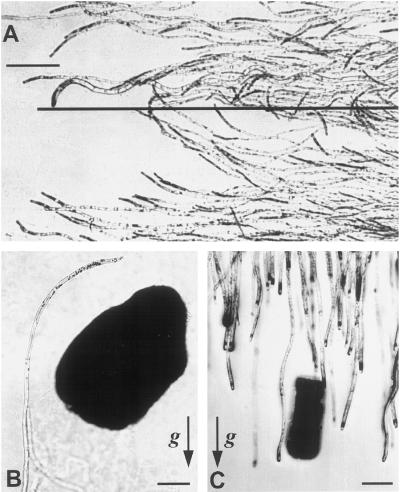
Dark-grown protonemata of the moss Ceratodon purpureus are tip-growing cells that are negatively gravitropic, i.e. they grow upward (Fig. 1). The wwr mutant (wrong way response) of C. purpureus is positively gravitropic, with reaction kinetics similar to the WT (Wagner et al., 1997). In horizontal WT (Fig. 1) and wwr protonemata, amyloplasts sediment in a specific zone located behind the apical dome. Plastid sedimentation is probably responsible for gravity sensing in both genotypes because it precedes curvature and because the recovery of gravitropism after basipetal centrifugation correlates with the return and sedimentation of amyloplasts (Walker and Sack, 1990, 1991; Wagner et al., 1997; Sack et al., 1998).
Figure 1.
Gravitropic curvature and amyloplast sedimentation (arrowheads) in WT protonemata of C. purpureus that were rotated from the vertical to the horizontal 4 to 5 h before fixation.
To study further the possible role of amyloplast sedimentation in gravity sensing, it is helpful to displace amyloplasts without reorienting the cell in the gravitational field. This can be achieved by exposing cells to an HGMF, thereby inducing the intracellular magnetophoretic displacement of starch-containing plastids (Kuznetsov and Hasenstein, 1996, 1997a, 1997b).
Dense plastids such as amyloplasts and the cytoplasm differ in their chemical composition and physical properties, including their magnetic characteristics. When subjected to a nonuniform magnetic field, magnetically heterogeneous systems experience ponderomotive forces that depend on their relative magnetic susceptibilities (Kuznetsov and Hasenstein, 1996). Therefore, a magnetic field of sufficient intensity and gradient should be able to displace plastids inside the cell and provide an excellent test for plastid-based gravity sensing.
If gravity sensing is plastid dependent, negatively gravitropic WT protonemata should curve toward stronger field intensities. In contrast, wwr cells should curve toward lower field intensities or in a positive gravitropic sense, similar to previous experiments with positively gravitropic roots (Audus, 1960; Kuznetsov and Kuznetsov, 1989; Kuznetsov and Hasenstein, 1996) and negatively gravitropic shoots (Schwarzacher and Audus, 1973; Kuznetsov and Hasenstein, 1997b). These experiments suggest that intracellular magnetophoresis is equivalent to plastid-based gravity sensing. However, these experiments were performed with higher plant organs, where the sites for perception and response are different, rather than with single cells that are capable of both sensing and responding to gravity. Moreover, the small size of moss protonemata and the availability of genotypes with opposite gravitropic responses warrants the use of HGMFs to study the possible involvement of plastid-based sensing in C. purpureus. If gravitropic sensing depends on the amyloplast sedimentation, then exposure to a magnetic field should induce both amyloplast displacement and the curvature of the tip cells in directions that are genotype dependent.
This hypothesis was tested using several configurations to produce magnetic fields of different intensities and geometries. We report here that exposure to HGMF caused magnetophoretic displacement of amyloplasts and induced curvature in both WT and wwr protonemata in the predicted directions.
MATERIALS AND METHODS
Plant Material
WT (strain “WT3”) and wwr protonemata of Ceratodon purpureus (Hedw.) Brid. were vegetatively propagated from stock cultures (Walker and Sack, 1990). Protonemata were transferred onto nutrient medium overlaid with cellophane in 35-mm-diameter plastic Petri dishes (Schwuchow and Sack, 1993). The dishes were sealed with Parafilm and the protonemata were kept in darkness for 5 d with the surface of the agar in a vertical position. After several days the protonemata were oriented parallel to the agar surface due to upward growth of the WT and downward growth of the wwr mutant. Cultures were transferred to the experimental systems under dim green light (<2 μmol s−1 m−2).
For gap experiments, we transferred 5-d-old cultures to plastic sheets (56 × 30 × 0.8 mm) with an opening of 35 × 16 mm, both sides of which were taped with cover glasses. Agar medium (0.35 mL) was spread onto the inner surface of the first cover glass to a thin film. A cellophane strip with protonemata was placed on top and a second cover glass was taped to the frame to provide an enclosed chamber.
For experiments with a ferromagnetic wedge, we filled plastic chambers (24 × 8 × 4 mm) with nutrient medium and cultures on cellophane. The chamber was covered with a slitted lid to allow the wedge to be in close proximity to the protonemata without making contact.
We applied magnetic particles to WT and wwr cultures growing in 35-mm Petri dishes containing growth medium; we placed the particles on top of the agar about 4 mm from the tips of the protonemata and then arranged the dishes vertically so that the cells would grow toward the magnetic particles.
Magnetic Systems
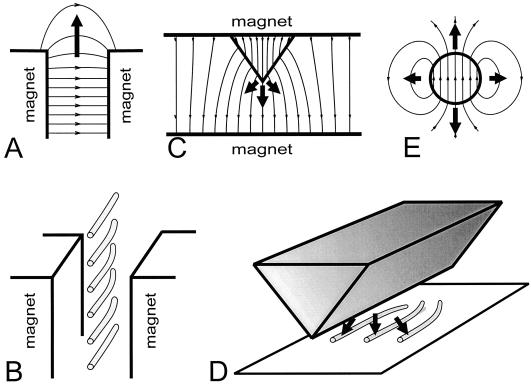
We used three types of magnetic systems to generate an HGMF with the required parameters (Fig. 2). The first system employed an HGMF at the edge of a 1.2-mm gap between two 50- × 50- × 12.5-mm neodymium iron boron magnets that generated a magnetic field of 8 kOe (Fig. 2A), producing a ponderomotive force for plastids equivalent to about 0.1g (Kuznetsov and Hasenstein, 1996) directed away from the gap. The cuvette with the cultures was inserted into the gap between the two magnets and positioned such that the protonemata were oriented parallel to the edge of the magnets (Fig. 2B) so that most protonemata were exposed to the HGMF. Control protonemata were placed either in the gap between two nonmagnetic plates or in a uniform magnetic field. Both cultures exposed to HGMF and controls were rotated on a 1-rpm clinostat with the protonemata growing perpendicular to the horizontal axis of rotation.
Figure 2.
Magnetic systems generating HGMF. Bold arrows indicate the direction of force acting on diamagnetic substances. A, HGMF at the edge of a gap between two magnets or magnetic poles repels diamagnetics from the gap. The dispersion of the magnetic field at the edge of the gap between two magnets results in a decrease of field intensity. The field in the depth of the gap reached 8 kOe, the dynamic factor ▿(H2/2) at the edge of the gap was estimated to be 5.3 × 108 Oe2 cm−1 (Kuznetsov and Hasenstein, 1996). B, Position and the predicted curvature of wwr protonemata near the edge of the gap between two magnets. C, The field in the vicinity of a ferromagnetic wedge between two magnets concentrates the field near its protruding edge such that LH is directed toward the edge. Therefore, diamagnetic plastids experience a force that is directed away from the wedge. L(H2/2) in close proximity of the edge can exceed 1010 Oe2 cm−1. D, Relative position of the magnetized wedge and protonemata with the projected curvature of the WT. E, Gradient of the field around a spherical magnet is directed toward the particle, hence diamagnetic particles will be repelled from it. Near a small (<1 mm) but strong magnet, the dynamic factor of the field L(H2/2) can exceed 109 Oe2 cm−1.
The second system (Fig. 2C) used a ferromagnetic wedge that was magnetized by a uniform magnetic field (4.5 kOe) in an 8-mm-wide gap between two permanent magnets (see Kuznetsov and Hasenstein, 1996, 1997b). The dynamic factor ▿(H2/2) of HGMF around the wedge tip was approximately 109 to 5 × 1010 kOe cm−1 within the cylinder of a diameter of 0.2 to 0.3 mm around and along the edge of the wedge and generated a force on plastids of about 1g. Outside this volume, the dynamic factor of the field decreased by 2 orders of magnitude over the next 1 mm. A nonferromagnetic, plastic-coated brass wedge of the same shape was used as a control. Chambers containing the protonemata were mounted in the gap between the two magnets so that the filaments were oriented parallel to and in close proximity to the wedge edge (Fig. 2D). The magnets with the cultures were rotated on a 1-rpm clinostat with protonemata oriented perpendicular to the horizontal axis of rotation.
The third configuration (Fig. 2E) used small permanent magnets (SmCo5, diameter < 0.6 mm) coated with plastic or nonmagnetic brass particles as controls. The protonemata were allowed to grow toward the particles without clinorotation. The experiments were terminated by fixing the cultures after the protonemata reached the vicinity of the particles.
Documentation
Protonemata were fixed in darkness for 45 to 90 min by dropwise application of fixative (1% paraformaldehyde, 2% glutaraldehyde, and 5 mm CaCl2 in 50 mm sodium cacodylate buffer, pH 7.1). Starch was stained with IKI (1% iodine in 2% potassium iodide) and cells were photographed on a light microscope. The negatives were digitized and the tip angles were measured using the Optimas image analysis program (version 5.2, Media Cybernetics, Silver Spring, MD). All possible cells within 0 to 0.6 mm from the wedge were measured and average values (±se) were calculated for 0 to 0.2, 0.2 to 0.4, and 0.4 to 0.6 mm.
Determination of Amyloplast Size and Density
We isolated the intact plastids from 5- to 6-d-old dark- and light-grown protonemata, and determined their density by centrifugation in metrizamide solutions (Kuznetsov and Hasenstein, 1997a). We used microscopy to find the diameter of the IKI-stained amyloplasts.
RESULTS
Magnetophoretic Curvature of WT and wwr Mutants
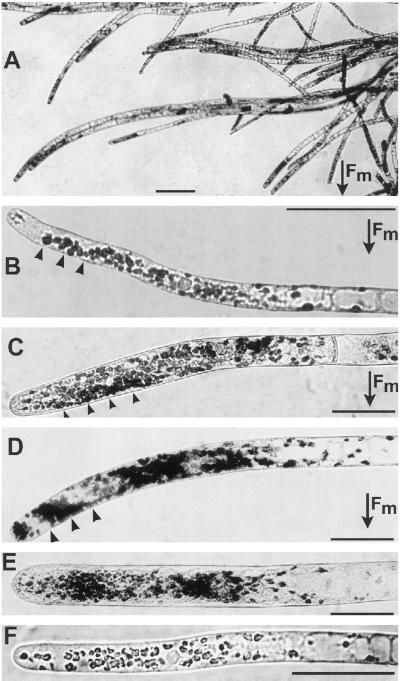
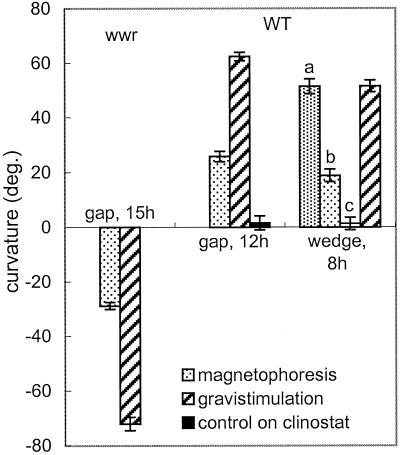
Cultures exposed to an HGMF and rotated on a clinostat showed both amyloplast displacement and curvature (Fig. 3). WT cells curved toward the HGMF and wwr cells curved away from it, in the direction of amyloplast displacement (Fig. 3, compare with Fig. 2B). The angles of curvature of cultures exposed to the HGMF in the gap for 12 (WT) and 15 h (wwr) were 41% and 40%, respectively, of the curvature of the controls after horizontal orientation for the same time period (Fig. 4).
Figure 3.
Photographs of clinorotated protonemata in the gap. Bars = 50 μm. In A to D the direction of the magnetic force is toward the bottom of the micrographs. The arrowheads in B to D indicate HGMF-induced amyloplast displacement. A, wwr cells curving toward the outside of the gap after 24 h in the HGMF, in a pattern similar to Figure 2B. B, WT protonema curving toward the inside of the gap after 12 h in the HGMF. C, wwr tip cell in the gap for 6 h. D, wwr tip cell in the gap for 5 h. E, wwr control cell inside the gap between the magnets for 5 h. Cells were exposed to a uniform magnetic field (about 8 kilo-oersteds), but not to an HGMF. Plastids were distributed symmetrically around the longitudinal axis of the cell and no curvature was observed. F, WT control cell after 12 h outside the gap, away from an HGMF. No lateral amyloplast displacement or curvature occurred. Fm, magnetic force.
Figure 4.
Curvature of WT and wwr protonemata after magnetophoresis or gravistimulation. After 8 h the curvature in the wedge system equaled that of protonemata that were gravistimulated for the same time period. The HGMF-induced curvature (stippled bars, 8 h WT) decreased with the distance from the wedge edge (bar a, 0–0.2 mm; bar b, 0.2–0.4 mm; bar c, 0.4–0.6 mm). The WT control was rotated on a clinostat (1 rpm) without a magnetic field.
In both the WT and wwr protonemata, tip-cell amyloplasts were displaced toward the outside of the gap (arrowheads in Fig. 3, B–D, direction of arrow in Fig. 2A). HGMF-induced plastid displacement occurred in the same subapical sedimentation zone where amyloplast sedimentation occurred after a 90° reorientation in the gravity field (Fig. 1). Although cells exposed to an HGMF exhibit opposite curvature in WT and in wwr, the direction of plastid displacement is the same, emulating the gravitropic phenotypes of the WT (Fig. 1) and the wwr cells (Wagner et al., 1997). However, the extent of amyloplast displacement by HGMF was less obvious compared with the horizontally placed controls, presumably because of a weaker force acting upon the plastids. This force was estimated to be only 0.1g.
Controls inside the gap in the area of homogenous field density showed no plastid displacement or curvature (Fig. 3E). Controls rotated on a clinostat in the absence of a magnetic field also showed no preferential curvature or lateral displacement of plastids (Fig. 3F). The angles of tip cells from controls rotated on a clinostat exhibited much greater variability than vertical, stationary controls, regardless of the presence or absence of a magnetic field.
As in gap experiments, exposure of protonemata to a magnetized wedge also induced curvature. wwr protonemata close to the edge curved away from the wedge (data not shown), and WT cells curved toward the wedge (Figs. 2D and 5A). The extent of curvature increased with the proximity of protonemata to the wedge (Fig. 5A), so that some WT protonemata not only curved on the plane of the agar, but upward, away from the agar surface toward the wedge. The angle of curvature of WT cultures exposed to the HGMF in the area less than 0.2 mm from the wedge (where the ponderomotive force is comparable to gravity) was similar to the curvature of the controls that were kept horizontally for the same time period (Fig. 4). The curvature of protonemata decreased with increasing distance from the wedge edge to negligible values at distances greater than 0.4 mm (Fig. 4). Controls placed near a nonmagneticwedge showed neither lateral amyloplast displacement nor curvature.
Figure 5.
Protonemata growing in the presence of magnetized wedges and magnetic and nonmagnetic particles. A, WT cells exposed to an HGMF for 8 h on a clinostat curve toward the wedge from both sides, as in Figure 2D. The projection of the wedge edge is shown as a long, horizontal line. Note that curvature decreases with increasing distance from the wedge edge. B, WT protonema curving around a magnetic particle. The protonemal culture was not clinorotated and curvature is thus the result of the magnetic force and gravity. C, Control wwr protonemata grow downward and are not influenced by the presence of a nonferromagnetic, brass particle. Arrows in B and C indicate the g vector. Bars = 200 μm.
HGMF derived from magnetic particles also induced curvature. Dark-grown tip cells of WT protonemata curved toward the magnetic particle (Fig. 5B) and eventually grew around it. Nonmagnetic particles did not induce curvature (Fig. 5C).
Density and Size Distribution of Plastids
The densities of IKI-stained plastids isolated from WT and wwr protonemal mats ranged from 1.24 to 1.38 g cm−3 and 1.26 to 1.38 g cm−3, respectively. In contrast, amyloplasts from flax, corn, barley, and tomato showed a narrower range of densities (1.36–1.39 g cm−3 [Kuznetsov and Hasenstein, 1997a]). The larger density range indicates higher variability in plastid composition of moss plastids. The densities of plastids from light-grown WT and wwr cells did not differ measurably from those of dark-grown cultures.
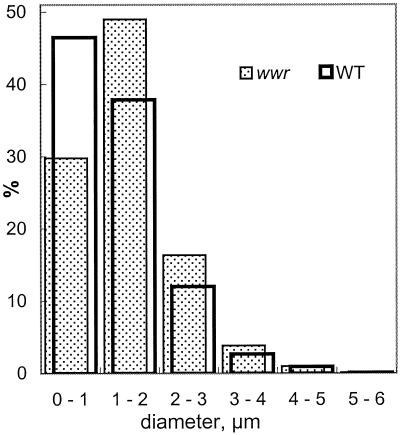
The diameter of plastids from WT and wwr protonemata ranged from 1 to 5 μm (Fig. 6). The average diameter (±se) of dark-grown wwr plastids (1.97 ± 0.03 μm) was slightly larger than that of WT plastids (1.74 ± 0.03 μm).
Figure 6.
Distribution of sizes of amyloplasts isolated from WT (open) and wwr (stippled) mutant protonemata.
DISCUSSION
This study shows that an HGMF induces protonemal curvature and amyloplast displacement that mimics gravitropism in the moss C. purpureus. Such effects can be attributed to the ponderomotive force rather than to the presence of a magnetic field, because protonemata failed to curve in a uniform magnetic field. These data provide significant support for plastid-based gravitropic sensing in C. purpureus. The pattern of magnetophoretic displacement was comparable to the location of amyloplast sedimentation in horizontal tip cells of C. purpureus (Walker and Sack, 1990).
Amyloplasts were displaced away from the HGMF in both the WT and wwr mutant protonemata, yet the WT curved toward the field and wwr curved away, similar to the direction of gravitropic curvature in both genotypes (Wagner et al., 1997). Thus, exposure to magnetic gradients does not impede the directionality of the growth response. Because amyloplasts were likely to be the major intracellular component displaced by the HGMF and because this displacement correlates with the correct sense of curvature for each genotype, our data strongly suggest that amyloplast displacement is important for gravity sensing.
Despite the correct direction of curvature, the extent of curvature due to the HGMF in a gap system was less than gravity-induced curvature for a comparable period of exposure (Fig. 4). This is probably due to the reduced force of approximately 0.1g that is experienced by the plastids. Because the HGMF-induced force in a wedge system can reach 1g within less than 0.3 mm of the wedge, the rate of curvature should be stronger than in the gap system. This reasoning is confirmed by the stronger curvature obtained from 8 h in the wedge system compared with the curvature after 12 h in the gap design (Fig. 4). The stronger curvature in the more effective HGMF suggests that gravitropic sensing relies on a force exerted by amyloplasts on some structure. The gravitropic receptor in moss protonemata is unknown, but candidates include the ER and microtubules (Schwuchow et al., 1990; Walker and Sack, 1995; Sack et al., 1998).
The importance of a force exerted by plastids is also supported by the more reliable and stronger response of the wwr protonemata compared with the WT (Fig. 4). Although plastid density was comparable in the two genotypes, wwr protonemata contained more and 13% larger amyloplasts than the WT. The size difference suggests that the forces produced by the displacement of amyloplasts by HGMF would be stronger in wwr than in WT protonemata. This observation conflicts with data showing that WT and wwr protonemata respond gravitropically at the same rate (Wagner et al., 1997), and suggests a rate-limited response mechanism in either system for a 1g stimulus. The differential response to HGMF may be due to a reduced force that did not saturate the sensing mechanism. The difference in the directions of curvature of WT and wwr protonemata probably resulted from transduction events downstream of sensing rather than from differences in the mechanism of sensing itself (Wagner et al., 1997; Sack et al., 1998).
An alternate hypothesis of gravitropic sensing is based on pressure exerted by the mass of the entire protoplast on tension- or compression-sensitive components of the plasma membrane and cell wall (Wayne et al., 1992; Staves et al., 1997). If this hypothesis were true for moss protonemata, then an HGMF should produce curvature in the direction opposite to that observed. The polysaccharide-rich cell wall has a magnetic susceptibility comparable to starch. The cell itself (the protoplast) has a much lower magnetic susceptibility because most of its volume is filled with cytosol, which has a magnetic susceptibility close to that of water (Audus, 1960). Thus, an HGMF would repel the cell wall more than the cell, and the cell membrane would be compressed to the cell wall closer to the HGMF and would be under tension on the side away from the HGMF. If the pressure of the cell mass functioned in gravitropic sensing, then the WT should curve toward the weaker part of the field (away from the gap), and the wwr mutant should curve toward the inside of the gap. However, Kuznetsov and Hasenstein, (1996, 1997b) found the reverse in this and previous studies using roots and shoots of higher plants.
In experiments with magnetic particles, the moss protonemata were exposed to an HGMF and gravity field without clinostat rotation. The protonemata did not curve toward the particle but curved around it, presumably because of an equilibrium between the stimuli of gravity and ponderomotive forces. As the protonema leaves the vicinity of the magnetic particle, the HGMF weakens and the tip begins to curve upward (Fig. 5B). Because magnetophoresis initiated strong curvature and because the HGMF repelled the amyloplasts, the results from the particle studies also support plastid-based rather than whole-cell-based gravity sensing.
Collectively, these HGMF data extend previous evidence consistent with the importance of the plastid mass in gravitropic sensing in moss protonemata (Sack et al., 1998) and in higher plants (Sack, 1991, 1997). Our work also shows that an HGMF can substitute for gravity in inducing differential growth in a single cell.
Abbreviations:
- HGMF
high-gradient magnetic field
- Oe
oersteds
- WT
wild-type
Footnotes
This research was supported by the National Aeronautics and Space Administration (grant nos. NAG10-0179 to F.D.S. and NAG10-0190 to K.H.H.).
LITERATURE CITED
- Audus LJ. Magnetotropism: a new plant growth response. Nature. 1960;185:132–134. [Google Scholar]
- Baluška F, Hasenstein KH. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH. Magnetophoretic induction of root curvature. Planta. 1996;198:87–94. doi: 10.1007/BF00197590. [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH (1997a) Magnetophoretic characterization of plant gravity receptors. In U Häfeli, W Schütt, J Teller, M Zborowski, eds, Scientific and Clinical Applications of Magnetic Carriers. Plenum Press, New York, pp 429–444
- Kuznetsov OA, Hasenstein KH. Magnetophoretic induction of curvature in coleoptiles. J Exp Bot. 1997b;48:1951–1957. doi: 10.1093/jexbot/48.316.1951. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AA, Kuznetsov OA. Simulation of gravity force for plants by high gradient magnetic field. Biofizika. 1989;35:835–840. [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Sack FD, Kern VD, Wagner TA (1998) Gravitropism in moss protonemata. In JW Bates, NW Ashton, JG Duckett, eds, Bryology for the Twenty-First Century. Maney Publishing and The British Bryological Society, Leeds, UK, pp 247–260
- Schwarzacher JC, Audus LJ. Further studies in magnetotropism. J Exp Bot. 1973;24:459–474. [Google Scholar]
- Schwuchow J, Sack FD. Effects of inversion on plastid position and gravitropism in Ceratodon protonemata. Can J Bot. 1993;71:1243–1248. doi: 10.1139/b93-147. [DOI] [PubMed] [Google Scholar]
- Schwuchow J, Sack FD, Hartmann E. Microtubule distribution in gravitropic protonemata of the moss Ceratodon. Protoplasma. 1990;159:60–69. doi: 10.1007/BF01326635. [DOI] [PubMed] [Google Scholar]
- Staves MP, Wayne R, Leopold CA. The effect of the external medium on the gravitropic curvature of rice (Oryza sativa, Poaceae) roots. Am J Bot. 1997;84:1522–1529. [PubMed] [Google Scholar]
- Wagner TA, Cove DJ, Sack FD. A positively gravitropic mutant mirrors the wild-type protonemal response in the moss Ceratodon purpureus. Planta. 1997;202:149–154. doi: 10.1007/s004250050113. [DOI] [PubMed] [Google Scholar]
- Walker LM, Sack FD. Amyloplasts as possible statoliths in gravitropic protonemata of the moss Ceratodon purpureus. Planta. 1990;181:71–77. [PubMed] [Google Scholar]
- Walker LM, Sack FD. Recovery of gravitropism after basipetal centrifugation in protonemata of the moss Ceratodon purpureus. Can J Bot. 1991;69:1737–1744. doi: 10.1139/b91-221. [DOI] [PubMed] [Google Scholar]
- Walker LM, Sack FD. An ultrastructural analysis of cell component distribution in apical cells of Ceratodon protonemata. Protoplasma. 1995;189:238–248. doi: 10.1007/BF01280178. [DOI] [PubMed] [Google Scholar]
- Wayne R, Staves MP, Leopold AC. The contribution of the extracellular matrix to gravisensing in characean cells. J Cell Sci. 1992;101:611–623. doi: 10.1242/jcs.101.3.611. [DOI] [PubMed] [Google Scholar]