Abstract
CMI responses, combined with quantification of CMV DNA (DNAemia), may identify transplantation recipients at risk for invasive disease. PBMC were collected in pediatric transplantation candidates at one, three, and six months post-transplant in 10 subjects (six renal, three cardiac, one stem cell) and at single time points in eight HC and 14 children greater than one yr post-transplant (LTTx). Cells were stimulated with anti-CD3mAb or CMV pp65 peptide pools and responses assessed by IFNG enzyme-linked immunosorbent spot assay and cytokine secretion. IFNG responses to anti-CD3mAb were significantly lower pretransplant relative to HC and were further decreased at one and three months post-transplant, but recovered to levels comparable to HC by six months. Responses to pp65 among CMV-seropositive recipients followed a similar pattern but recovered by three months. CMV-seropositive LTTx and HC showed a Th1 cytokine response to pp65 stimulation. Three LTTx subjects developed CMV DNAemia; two demonstrated decreased responses to anti-CD3mAB (and pp65 in the CMV seropositive subject) at the onset of DNAemia, which recovered as DNAemia resolved. Monitoring CMI in children is feasible and may provide an adjunct biomarker to predict CMV progression and recovery.
Keywords: cytomegalovirus, transplant, pediatrics, T lymphocytes
Although antiviral prophylaxis and preemptive monitoring for viral DNA in the blood have reduced the morbidity associated with CMV, it remains a major opportunistic pathogen in transplantation recipients. Primary infection and/or reactivation can lead to tissue-invasive disease, graft loss, and rejection (1-4). CMV infection results in an increased net state of IS and is associated with increased risk of secondary bacterial infections, invasive fungal infections, endothelial dysfunction, and post-transplant lymphoproliferative disorder (5-9). Prior to the introduction of prophylaxis, the incidence of CMV DNAemia (defined as the presence of detectable CMV DNA or antigen in the blood) and tissue-invasive disease ranged from 14–85% to 7–39%, respectively (10-13). The risk of infection and disease varies with the type of organ transplanted, intensity and duration of IS, and CMV serostatus prior to transplant, with seropositive donor (D+)/seronegative recipient (R−) patients representing the highest risk cohort (1, 10-14). Thus, pediatric transplantation recipients, who are often CMV naïve prior to transplantation, are at increased risk of infection and its associated adverse sequelae.
Universal prophylaxis with VGCV for the first three months post-transplant has significantly reduced the risk of early-onset CMV (less than or equal to three months post-transplant) but is associated with an increased incidence of late-onset disease, viral resistance, and drug-related toxicities including neutropenia. In animal studies, GCV/VGCV was carcinogenic and teratogenic and caused aspermatogenesis, although the clinical significance of these findings is not known (15-22). The risks of prolonged prophylaxis may be more significant for pediatric recipients, as VGCV pharmacokinetic data are less robust for infants and small children (23, 24).
Alternatively, preemptive management, in which antiviral therapy is initiated and/or IS reduced when CMV is detected in the blood, has also been shown to be effective in reducing risk of CMV while minimizing drug-related adverse effects (10, 25, 26). However, preemptive management relies heavily on screening tests, such as quantitative nucleic acid testing with real-time PCR or the measurement of CMV pp65 antigenemia, to determine when to institute antiviral therapy or reduce IS. A study of 364 CMV D+/R− adult SOT recipients found that the positive and negative predictive values of PCR for invasive CMV disease were only 17% and 82%, respectively (27). This suggests screening for CMV replication alone may fail to identify patients who are at risk for disease or, conversely, may result in unnecessary exposure to antiviral agents and/or increased risk of rejection following premature reduction in immunosuppressive therapy. Thus, adjunct biomarkers that better identify those patients at risk for CMV disease are urgently needed.
An adequate host T-cell response is critical for control of viral replication. Several studies of CMI responses in adult transplantation recipients indicate that impaired CMV-specific T-cell responses, measured by the detection of IFNG following ex vivo stimulation with CMV-specific antigens, are associated with an increased risk of CMV disease (28-31). Conversely, restoration of CMI responses by adoptive immunotherapy for prophylaxis or treatment of CMV in HSC transplantation recipients has been shown to reduce risk of CMV DNAemia or progression to tissue-invasive disease (32, 33). A few studies of CMI have been performed in pediatric HSC transplantation recipients, but no studies have been conducted in pediatric SOT recipients (34-39). Therefore, the objective of this pilot study was to evaluate CMI and assess the feasibility of monitoring T-cell responses in infants and children in the first six months post-transplant as an adjunct to routine monitoring of CMV viral load by PCR. For comparison, we also examined the CMV-specific and global T-cell responses in a cohort of healthy children and in a cohort of pediatric transplantation recipients who are greater than one yr post-transplantation.
Subjects and methods
Subjects
Pediatric cardiac, renal, and HSC transplantation candidates ≤ 21 yr of age awaiting transplantation at the Children’s Hospital at Montefiore in Bronx, New York, were recruited from their respective clinics between November 2009 and March 2010 (longitudinal cohort). Exclusion criteria included medical conditions that would have precluded study blood sample collection. Pediatric HC subjects and children more than one yr post-renal transplantation (LTTx cohort) who were enrolled in a concurrent influenza vaccine immunogenicity study and had sufficient PBMC for testing were also evaluated. The study protocols were approved by the Albert Einstein College of Medicine’s Institutional Review Board (2008-499 and 2009-270). Written informed consent or assent was obtained from parents/guardians or subjects; subjects enrolled in the influenza vaccine immunogenicity study had also provided general consent for participation in transplant-related research.
Blood (3 mL/kg per visit, maximum 20 mL) was collected for isolation of PBMC within the three months prior to transplantation and at one, three, and six months post-transplant in the longitudinal cohort. Additional blood from subjects who developed CMV was collected biweekly from onset of viral detection until resolution.
CMV serostatus was assessed pretransplant as part of routine clinical care for the longitudinal cohort using the Immulite 2000 CMV IgG chemiluminescence assay (Siemens, Washington, DC, USA). CMV serostatus was determined by CMV IgG Capture ELISA kit (Trinity Biotech, Wicklow, Ireland) for HC and LTTx participants from serum obtained at the time of enrollment. Quantification of CMV viral DNA by automated real-time PCR (lower limit of detection 50 copies/mL) was performed for all transplantation subjects as part of routine clinical care (Abbott Diagnostics, Santa Clara, CA, USA); frequency of testing was conducted according to program-specific protocols. CMV DNAemia and tissue-invasive disease were defined as previously described (40).
PBMC isolation and storage
PBMC were isolated by density gradient centrifugation using Ficoll-Histopaque (Sigma-Aldrich, St Louis, MO, USA). PBMC were counted, divided into aliquots of 107 PBMC, and stored in liquid nitrogen following graded cryopreservation. Freezing media for PBMC consisted of RPMI-1640 (Invitrogen, Grand Island, NY, USA) with 10% FBS and 10% dimethylsulfoxide (DMSO) (Sigma-Aldrich) with 2 mmol glutamine, 100 units (U)/mL penicillin, and 100 μg/mL streptomycin. Longitudinal samples collected from each subject were thawed simultaneously at the time of testing. After thawing, PBMC were rested overnight in RPMI-1640, 10% heat-inactivated FBS, 2 mmol glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin prior to stimulation. PBMC viability was determined using 0.4% Trypan blue staining (Sigma-Aldrich) and ranged from 80% to 95%. Prior testing of fresh vs. frozen PBMC isolated from healthy adult volunteers yielded comparable CMI responses.
ELISPOT assay
CMI response was measured by IFNG ELISPOT assay, according to the manufacturer’s protocol (ELISpotPRO for Human Interferon-Gamma; Mabtech, Nacka, Sweden). Briefly, 2 × 105 PBMC per well were stimulated with either overlapping peptide pools spanning the CMV late gene tegument protein, pp65 (1 μg/mL) (JPT Peptide Technologies, Berlin Germany), anti-CD3mAb (0.1 μg/mL) (Mabtech) as a global T-cell activator, or media negative control (RPMI-1640, 10% heat-inactivated FBS, 2 mmol glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cells were incubated for 24 h at 37°C in 5% CO2 humidified air. Plates were washed with phosphate-buffered saline (PBS), incubated with alkaline phosphatase-conjugated biotinylated mAb 7-B6-1-ALP (Mabtech) for two h at room temperature, and developed using 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt/nitro-blue tetrazolium chloride. All tests were performed in triplicate, read on an AID ELISPOT reader (Autoimmune Diagnostika GmbH, Strasberg, Germany), and analyzed using elispot 3.2.3. software. Negative control wells were subtracted from responder wells, and the mean values were calculated. ELISPOT results were expressed as the number of SFU per 105 PBMC.
Multiplex assay for cytokines
PBMC (2 × 105 per well) were incubated in 96-well plates with either pp65 overlapping peptide pools (1 μg/mL), anti-CD3mAB (0.1 μg/mL), or RPMI-1640 media alone at 37 °C for 24 h. Culture supernatants were collected and analyzed for T helper (Th) 1 (IL2, IFNG, TNFa) and Th2 (IL4, IL5, IL10, IL13) cytokines using beads from Milliplex Human Cytokine kits (Millipore, Billerica, MA, USA), measured by Luminex100 (Austin, TX, USA), and analyzed using Star-Station (Applied Cytometry Systems, Sacramento, CA, USA). Culture supernatants were tested in duplicate and mean values calculated. Values less than the MDC as determined by the manufacturer were assigned the MDC value.
Flow cytometry
Frequency of CD4+ and CD8+ T cells was measured by flow cytometry as previously described (41, 42). Briefly, 5 × 105−1 × 106 PBMC were suspended in 100 μL of SB (BD Biosciences, San Jose, CA, USA) and then stained for 20 min at 4 °C with the following antibody cocktail: anti-CD3-PerCp, anti-CD4-Pacific Blue, and anti-CD8-PeCy7 (BD Biosciences). PBMC were then washed in SB, fixed with Cytofix (BD Biosciences) for 20 min at 4 °C, washed, and resuspended in SB for cell acquisition. At least 100 000 CD3+ events were acquired by flow cytometer (Becton Dickinson LSR II, San Jose, CA, USA). Analysis was conducted using FlowJo v9.2 software (Tree Star, Inc., Ashland, OR, USA). Results were expressed as percent frequency of CD3+/CD8+ or CD3+/CD4+ cells among total lymphocytes.
Statistical analysis
Descriptive statistics were generated for all continuous outcomes of interest. Median values for each group are presented with the corresponding IQR. Comparisons between independent groups of subjects were performed via Wilcoxon Mann–Whitney U-test; two-sided p-values were generated for each comparison. The effect of time with respect to log CD3 and log pp65 outcomes in transplant subjects was explored via linear models with a random-intercept term included to account for the longitudinal nature of the data. The overall effect of time (time treated as a categorical variable) was examined. Additionally, time and time2 were entered into the model to explore a non-linear relationship. Because of the exploratory nature of this study, no adjustment of p-values for multiple testing was performed. Statistical analyses were performed with GraphPad Prism v5 (San Diego, CA, USA) for continuous outcomes and sas v9.2 (Raleigh, NC, USA) for evaluation of trends over time.
Results
Study subjects
Nineteen pediatric transplantation candidates were assessed for eligibility in the longitudinal cohort study; 10 (three cardiac, six renal, and one HSC) were enrolled and transplanted during the study period (Table 1). The median age was nine yr (range three months to 18 yr). CMV donor/recipient serostatus was D−/R− (n = 3), D−/R+ (n = 2), D+/R− (n = 1), and D+/R+ (n = 4). Nine subjects received induction therapy with Th. Maintenance IS was administered per protocol by each respective transplant service and included TAC (n = 9), MMF (n = 9), CSA (n = 1), and/or Prd (n = 5). Administration of VGCV prophylaxis (n = 6) or preemptive monitoring for CMV (n = 4) was determined by each transplant service according to program-specific protocols. One subject developed chronic skin and gut GVHD and received daclizumab post-transplant. Six subjects developed 12 infectious complications during the study period including bacteremia with MSSA (n = 1), BK DNAemia/viruria (n = 2), EBV DNAemia (n = 1), parvovirus DNAemia with anemia (n = 1), and upper respiratory tract infections with adenovirus (n = 2), influenza type A virus (n = 1), human metapneumovirus (n = 1), parainfluenza virus type 3 (n = 1), RSV (n = 1), and rhinovirus (n = 1).
Table 1.
Characteristics of longitudinal cohort subjects
| Group, age in yr (sex, race) |
Transplant type |
Reason for transplant |
Induction IS* |
Maintenance IS† |
CMV (D/R) |
CMV prophylaxis‡ |
CMV DNAemia/disease (months post-tx) |
Intercurrent infections or rejection (months post-tx) |
|---|---|---|---|---|---|---|---|---|
| 0.4 (M, H) | Heart | Cardiomyopathy | Th | TAC, MMF | −/− | Preempt | N/N | Parainfluenza (1 month) Adenovirus (9.5 months) Metapneumovirus (9.5 months) |
| 0.7 (M, H) | Heart | Cardiomyopathy | Th | TAC, MMF | −/− | Preempt | Y/hepatitis (5.5–7 months) |
Rhinovirus (5.5 months) |
| 0.8 (F, H) | Heart | Cardiomyopathy | Th | TAC, MMF | −/+ | GCV/VGCV | N/N | None |
| 3 (M, A) | Renal | Renal dysplasia | Th | Prd, TAC, MMF | −/− | Preempt | N/N | BKV (DNAemia: 3.5 months, viruria: 10 months) |
| 3 (M, B) | Renal | ARPKD | Dac | TAC, MMF | +/+ | GCV/VGCV | N/N | None |
| 15 (M, B) | HSCT | Aplastic anemia | Th | Prd, CSA | +/+ | GCV/VGCV | Y/N (4.5–6.25 months) |
EBV DNAemia (2.5, 5 months) MSSA bacteremia (3 months) RSV (5 months) Chronic GVHD (2 months) |
| 17 (F, A) | Renal | Obstructive uropathy | Th | Prd, TAC, MMF | +/+ | GCV/VGCV | N/N | Parvovirus DNAemia (3 months) |
| 17 (M, H) | Renal | IC-mediated glomerulo-nephropathy |
Th | Prd, TAC, MMF | +/− | GCV/VGCV | N/N | None |
| 18 (F, H) | Renal | Renal dysplasia | Th | Prd, TAC, MMF | +/+ | GCV/VGCV | N/N | BKV (DNAemia: 3.5, 5 months; viruria: 7 months) Influenza A (0.5 month) |
| 18 (F, H) | Renal | CKD (unknown etiology) |
Th | TAC, MMF | −/+ | Preempt | Y/N (2.25 months) |
None |
ARPKD, autosomal-recessive polycystic kidney disease; A, Asian; B, Black; BKV, BK virus; CKD, chronic kidney disease; D, donor; F, female; H, Hispanic; HSCT, hematopoietic stem cell transplant; IC, immune complex; M, male; post-tx, post-transplant; Preempt, preemptive; R, recipient; W, white.
Th induction per protocol. cardiac: 1.5 mg/kg/day × 5 days, cumulative dose 7.5 mg/kg; renal: Th 1.5 mg/kg × 4 doses, cumulative dose 6 mg/kg.
Target TAC levels per protocol. cardiac: 1–3 months post-transplant: 8–12 ng/mL, 3–12 months: 6–10 ng/mL; renal (deceased donor): 0–12 months: 5–10 ng/mL; renal (living donor): 0–6 wk: 10–15 ng/mL, 7–12 wk: 5–10 ng/mL, >12 wk: 3–7 ng/mL.
Preemptive monitoring. Viral load testing every two wk by CMV PCR for three months, then monthly; antiviral therapy instituted if DNAemia or signs/symptoms of invasive disease develop. GCV/VGCV prophylaxis includes intravenous GCV followed by oral VGCV.
An additional 14 renal LTTx subjects (median 3.9 yr post-transplant; median 15 yr of age, 10 CMV seropositive) and eight HC (median 11.8 yr of age; five CMV seropositive) were enrolled in the cross-sectional study (Table 2).
Table 2.
Characteristics of long-term renal transplant subjects greater than one yr post-transplant (cross-sectional cohort)
| Group, age in yr (sex, race) |
Time from transplant (yr) |
Current IS | CMV status* | CMV DNAemia/disease† |
Other infections or rejection‡ |
|---|---|---|---|---|---|
| 3 (M, W) | 1.5 | Prd, TAC, MMF | + | + | Gingivostomatitis (HSV) |
| 5 (M, H) | 8.3 | Prd, TAC, MMF | − | − | N/A |
| 5 (M, H) | 1.2 | Prd, TAC, MMF | − | − | EBV DNAemia |
| 7 (M, B) | 4.0 | Prd, TAC | + | − | EBV DNAemia |
| 8 (F, B) | 2.3 | Prd, TAC | − | − | N/A |
| 10 (F, A) | 2.4 | Prd, TAC, MMF | + | + | N/A |
| 14 (M, B) | 2.8 | Prd, TAC, MMF | + | − | N/A |
| 16 (M, H) | 5.6 | Prd, TAC, MMF | + | − | Chronic allograft rejection |
| 16 (M, A) | 2.3 | Prd, TAC, MMF | + | − | N/A |
| 18 (M, B) | 11.1 | Prd, TAC, MMF | + | − | N/A |
| 18 (F, B) | 3.8 | Prd, TAC, MMF | + | + | N/A |
| 19 (M, H) | 7.5 | Prd, AZA, MMF | + | + | N/A |
| 19 (M, H) | 4.6 | Prd, TAC, MMF | + | − | N/A |
| 21 (F, W) | 10.4 | TAC, MMF | − | − | N/A |
A, Asian; AZA, azathioprine; B, Black; F, female; H, Hispanic; M, male; V, DNAemia; W, White.
CMV serostatus at time of sample blood collection.
CMV DNAemia or disease within one yr prior to sample blood collection.
Other infection or rejection within three months prior to sample blood collection.
ELISPOT responses are reduced in transplantation candidates and recipients
IFNG responses to anti-CD3mAb were lower among subjects pretransplant (median 76 SFU/105 PBMC; IQR: 31–308) compared with HC (median 521 SFU/105 PBMC; IQR: 199–985), possibly reflecting the effects of chronic disease in children awaiting transplantation (p = 0.02) (Fig. 1a). Relative to HC, IFNG responses further decreased one month post-transplant (median 15 SFU/105 PBMC; IQR: 4–310; p = 0.01) and remained significantly lower at three months post-transplant (median 55 SFU/105 PBMC; IQR: 14–320; p = 0.01). By six months post-transplant (median 172 SFU/105 PBMC; IQR: 71–725), responses for transplant recipients were similar to those observed among HC (p = 0.19). CD3 responses demonstrated a significant non-linear trend across time (pretransplant to six months post-transplant), reflecting gradual recovery during the post-transplant period (quadratic term: p = 0.04, overall effect: p = 0.023). There were no significant differences in responses between HC and LTTx (median 851; IQR: 588–1650; p = 0.16).
Fig. 1.
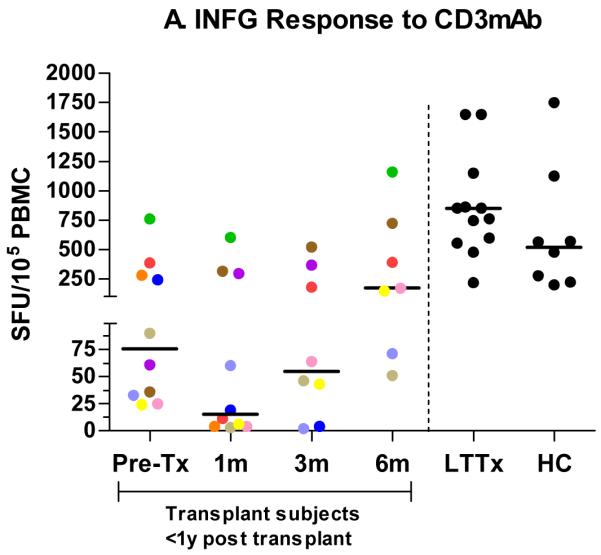
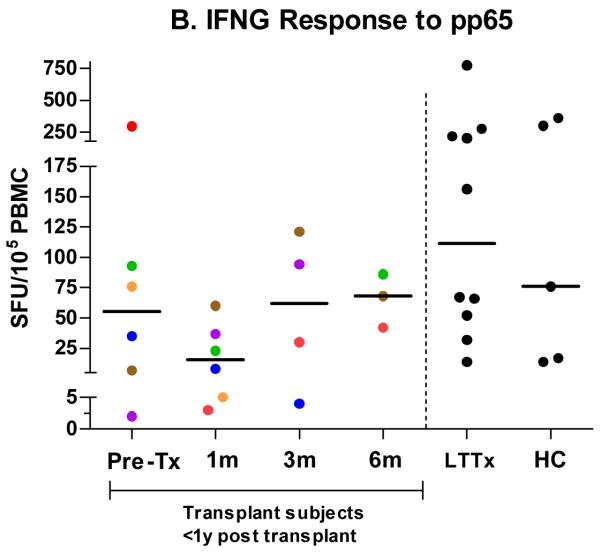
IFNG responses in SFU/105 PBMC by ELISPOT to CD3mAb (a) in the longitudinal cohort (pretransplant (n = 10) and then one month (n = 10), three months (n = 8), and six months (n = 7) post-transplant) and at single time points in a cross-sectional cohort of transplant subjects greater than one yr post-transplant (LTTx) (n = 14) and HC (n = 8). IFNG responses by ELISPOT to pp65 among CMV-seropositive subjects (b) in the longitudinal cohort (pretransplant (n = 6) and then one month (n = 6), three months (n = 4), and six months (n = 3) post-transplant) and at single time points in LTTx (n = 10) subjects and HC (n = 5). Each dot (●) represents the median SFU at a particular time point for an individual subject. In the longitudinal cohort, dots are color coded to show each subject’s median IFNG response across time.
Among the six subjects who were CMV seropositive prior to transplant, responses to pp65 peptide pools at the pretransplant visit (median 55 SFU/105 PBMC; IQR: 6–143) were slightly lower than, but not significantly different from, those of HC (median 76 SFU/105 PBMC; IQR: 16–330; p = 0.46) (Fig. 1b). Compared with HC, responses subsequently decreased at one month post-transplant (median 16 SFU/105 PBMC; IQR: 5–43; p = 0.13), but recovered by three (median 62 SFU/105 PBMC; IQR: 11–114; p = 0.73) and six (median 68 SFU/105 PBMC; IQR: 42–86; p = 1.0) months post-transplant. HC and LTTx (median 112 SFU/105 PBMC; IQR: 47–230) subjects had comparable pp65 responses (p = 1.0). Responses to pp65 among CMV-seronegative subjects were negligible (<5 SFU/105) at all time points.
Evaluation of Th1/Th2 cytokine responses to anti-CD3mAb and pp65 peptide pools
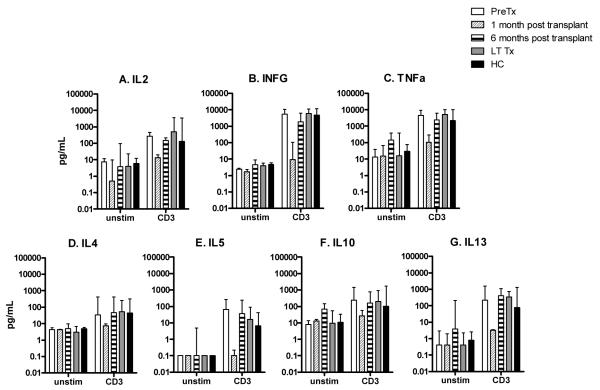
The observation that transplant recipients manifest reduced global IFNG responses in the first six months following transplantation was further explored by evaluating a panel of cytokine responses to anti-CD3mAb in a subset of subjects for whom sufficient PBMC were available: pretransplant (n = 3); one month post-transplant (n = 3); six months post-transplant (n = 3); LTTx (n = 10); and HC (n = 5) (Fig. 2). Th1 (IL2, IFNG and TNFa) and Th2 (IL4, IL5, IL10 and IL13) responses at one month post-transplant were similar compared to that of unstimulated PBMC, but depressed compared to HC. Recovery was seen by six months post-transplant. Cytokine responses to anti-CD3mAb between LTTx and HC were similar.
Fig. 2.
TH1 (a–c) and TH2 (d–g) cytokines (pg/mL) in PBMC culture supernatants following stimulation with CD3mAB in samples obtained from subjects pretransplant (n = 3), one month post-transplant (n = 3), six months post-transplant (n = 3), subjects greater than one yr post-transplant (LTTx) (n = 10), or HC (n = 5).
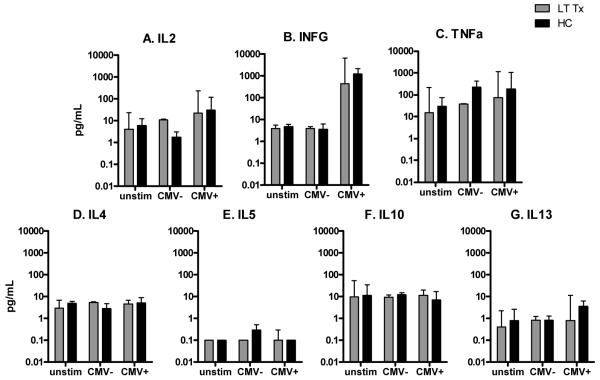
We also examined the cytokine responses to pp65 in a subset of LTTx (n = 8 CMV+, 2 CMV−) and HC (n = 3 CMV+, 2 CMV−) subjects (Fig. 3); insufficient PBMC were available from subjects in the longitudinal cohort. Relative to unstimulated cells, stimulation with pp65 in CMV-seropositive LTTx and HC subjects induced strong IFNG responses (p = 0.01 and p = 0.06, respectively) and moderate IL2 responses (p = 0.01 and p = 0.06, respectively). There was no Th2 response to CMV, which is consistent with the Th1 bias previously observed in response to CMV antigens (43). Responses to pp65 peptides among CMV-seronegative subjects were similar to that of unstimulated PBMC.
Fig. 3.
TH1 (a–c) and TH2 (d–g) cytokines (pg/mL) in PBMC culture supernatants following stimulation with CMV pp65 peptide pools in samples obtained from renal transplant subjects more than one yr post-transplant (LTTx) (n = 10) or from HC (n = 5).
Frequency of CD4 T-cells is reduced in the post-transplant period
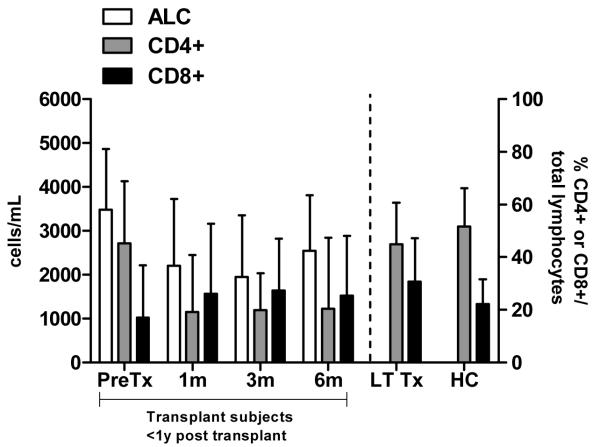
Immunosuppressive therapy may also modulate the relative numbers of CD4+ and CD8+ T-cells. Therefore, we examined the frequency of CD4+ and CD8+ cells among total lymphocytes pretransplant (n = 7) and one (n = 6), three (n = 5), and six (n = 5) months post-transplant and at single time points for LTTx (n = 12) and HC (n = 5) by flow cytometry (Fig. 4). The median ratios of CD4/CD8 cells among subjects pretransplant and HC were 2.7:1 and 2.8:1, respectively. However, the percentage of CD4+ cells was significantly decreased at one month post-transplant (median: 19.1%, IQR: 5–29%) compared with HC (median: 51.5%; IQR: 49–63%; p = 0.004), resulting in an inversion of the CD4/CD8 ratio (median 0.5:1). This depression persisted until at least six months post-transplant (median percentage of CD4+ cells: 20.4%, IQR: 13–35%; median ratio of CD4/CD8 cells: 0.8:1) and was significantly reduced compared with HC (p = 0.016). Although the percentage of CD4+ cells of LTTx subjects (median: 44.9%, IQR: 42–52%) was similar to that of HC (p = 0.10), the CD4/CD8 ratio of LTTx (median 1.5:1) remained significantly different from that of HC (p = 0.018).
Fig. 4.
Frequency of CD4+ and CD8+ cells among total lymphocytes by flow cytometry (right Y-axis) and absolute lymphocyte count (ALC) (left Y-axis) in LTTx subjects and HC.
T-cell responses in subjects with CMV DNAemia
Three subjects developed CMV DNAemia during the course of the study; one of whom also developed tissue-invasive disease (hepatitis). The kinetics of their IFNG responses by ELI-SPOT, viral loads, and management of CMV are depicted in Fig. 5.
Fig. 5.
Longitudinal IFNG responses to CD3mAb and pp65 and CMV viral load in a HSC (a), cardiac (b), and renal (c) transplant subject.
The CMV D+/R− HSC transplant recipient (Fig. 5a) had developed moderate chronic GVHD three months post-transplant and subsequently received 10 doses of daclizumab until five months post-transplant. While receiving daily prophylaxis with VACV for herpes simplex virus, CMV DNAemia occurred at four months post-transplant with a peak CMV viral load of 1900 copies/mL. Treatment with intravenous GCV was initiated and continued for three wk, followed by a brief course of VGCV. Notably, there was a decline in ELISPOT responses to both pp65 and CD3mAb in PBMC isolated at the time DNAemia was first detected. Recovery of CMI responses was observed concurrently with resolution of the CMV DNAemia.
A CMV D−/R− cardiac transplant recipient developed CMV DNAemia with hepatitis five months post-transplant (Fig. 5b). Although the subject initially showed recovery of CD3 IFNG responses following transplantation, IFNG responses precipitously dropped at the onset of CMV DNAemia (peak viral load 1.2 × 105 copies/mL). Intravenous GCV was initiated upon detection of CMV DNAemia and was continued for two wk, followed by six wk of VGCV. Treatment was discontinued when the CMV viral load fell to 50 copies/mL and transaminases normalized. Notably, although CD3 IFNG responses recovered with resolution of DNA-emia, CMV-specific responses remained undetectable. One month after completing treatment with VGCV, the CMV viral load slightly increased to 150 copies/mL with a concomitant rise in transaminases. VGCV was reinitiated, and within one month, the CMV PCR became undetectable, transaminases normalized, and VGCV was discontinued. PBMC for evaluation of CMI were unavailable during this second episode of DNAemia.
A CMV D−/R+ renal transplant subject developed transient, low-level CMV DNAemia (100 copies/mL) approximately two months post-transplant. In contrast to the other two subjects, this subject demonstrated no decline in CD3 or pp65 IFNG responses at the time of DNAemia. No recurrences of CMV DNAemia were observed by one yr post-transplant.
Discussion
This pilot study represents the first longitudinal evaluation of the kinetics of global and CMV-specific T-cell responses in a cohort of pediatric SOT recipients. Our results indicate that ELI-SPOT assays are feasible to perform in pediatric populations and may be a useful tool to predict risk for CMV disease. CMV DNAemia developed in three of the 10 subjects (30%) studied longitudinally and was associated with loss of global CD3 response in the two who exhibited sustained DNAemia. This observation suggests that IFNG responses to anti-CD3mAB may provide a biomarker to identify those patients at risk for persistent CMV replication and its sequelae. Monitoring CD3 responses may have broader applicability than evaluating pp65 responses as they are independent of CMV serostatus, HLA type, and selection and testing of appropriate peptides for ex vivo stimulation.
This study also provides insights into the kinetics of functional and quantitative T-cell recovery in pediatric transplantation patients. There was a significant depression in ELISPOT responses and Th1 and Th2 cytokine secretion following stimulation of PBMC with anti-CD3mAb one month post-transplant, which recovered by six months. IFNG responses at six months were greater than pretransplant levels, possibly reflecting improvement in overall health and reduction in IS. However, depression in the frequency of CD4+ cells persisted for at least six months post-transplant. Furthermore, the CD4/CD8 ratio remained significantly lower in children who were more than one yr post-transplant when compared with HC. The decline in CD4+ cells in the early post-transplant period likely reflects the susceptibility of rapidly dividing CD4+ cells to Th (44, 45), whereas the lag in recovery of CD4/CD8 ratio may reflect the impact of maintenance immunosuppressive therapy (46). These more modest and persistent abnormalities in CMI may contribute to overall impaired memory responses and increased susceptibility to infections.
Recovery of CMV-specific responses is more difficult to evaluate because of greater variability in the IFNG ELISPOT responses to pp65 in CMV-seropositive subjects compared with anti-CD3mAb responses. This variability was also evident in HC and LTTx cohorts (Fig. 1b) and may reflect differences in the frequency of sub-clinical reactivation leading to variable boosting of CMV-specific T-cell responses among subjects (47).
We are unable to discern whether the reduction in CMI responses in the two subjects who developed persistent CMV DNAemia was triggered by viral replication or whether the decrease in CMI responses predisposed these subjects to CMV. However, our findings support the notion that preemptive monitoring of T-cell responses may still be a useful indicator of risk for CMV disease. While no prior studies have been reported in pediatric SOT recipients, a study of 108 adult SOT recipients found IFNG responses to be a significant predictor of CMV disease. During the first three months following transplantation, only 5% of subjects with IFNG responses to ex vivo stimulation with CMV peptides developed CMV disease, whereas 23% of subjects without IFNG responses developed CMV disease (p = 0.038) (28).
Evaluating CMV-specific CD4+ and CD8+ T-cell responses may also identify transplant subjects at risk for other CMV-related morbidities, such as secondary infections or graft dysfunction. In a study of 19 CMV-seropositive adult heart transplant recipients, delayed reconstitution of CMV-specific CD4+ cells was associated not only with higher peak CMV viral loads but also with greater risk of acute rejection and transplant arteriopathy (48).
Monitoring both global and CMV-specific T-cell responses could inform and guide clinical care practices during the post-transplant period. The inclusion of global CMI responses may be particularly beneficial for pediatric transplant recipients, who are often seronegative prior to transplantation, and therefore have minimal immunological memory. Universal prophylaxis has a number of associated complications; immune monitoring could identify those subjects who reconstitute their CMI responses early and could therefore discontinue prophylaxis and be followed preemptively. Conversely, subjects with delayed immune reconstitution may benefit from more prolonged prophylaxis and more aggressive monitoring. Furthermore, once a patient manifests CMV DNAemia or tissue-invasive disease, monitoring global and CMV-specific CMI could potentially predict severity of disease and timing of recovery. For example, persistently poor CMV-specific T-cell responses in the cardiac transplant recipient who developed DNAemia and hepatitis (Fig. 5b) could have predicted the subject’s clinical relapse and the possible need for additional antiviral therapy or more aggressive reduction of IS.
Assessing global T-cell responses could also have implications for management of other OI and timing of vaccinations post-transplant. For example, the duration of prophylaxis for pneumocystis jirovecii (PCP) is controversial and varies by transplant center. Similarly, data are limited regarding the optimal time to safely and effectively vaccinate pediatric SOT recipients. Restoration of T-cell numbers and global function could provide a biomarker to determine duration of prophylaxis of other OI or when vaccinations, particularly live attenuated vaccines, can be safely and effectively administered.
Advances in functional immunoassays for evaluation of CMI have greatly facilitated immunologic studies of immunocompromised pediatric patients. The ELISPOT and multiplex cytokine assays offer the advantage of relatively simple methodology and high reproducibility (49, 50). Despite the advantages of more detailed analyses of T-cell populations with flow cytometry, intracellular cytokine staining, and tetramer staining, these additional studies often require greater volumes of blood than are typically available from younger pediatric transplant patients. Although functional T-cell assays are technically demanding and expensive, it is anticipated future advances in automation will render CMI testing more feasible. The ELISPOT and multiplex cytokine assays used in this study required only 2–3 mL of blood and provide a relatively rapid approach to evaluate CMI function. Many centers now offer comparable assays for other pathogens as part of their laboratory portfolio, such as the QuantiFERON test for latent TB.
Our study has several additional limitations. First, the number of children in the longitudinal cohort was small. Secondly, although pp65 is considered an immunodominant epitope and use of pp65 overlapping peptide pools has been shown to increase the sensitivity and specificity of detecting CMV-specific immune responses, not all subjects may have recognized the represented epitopes (51). Evaluating responses to other CMV antigens, including the immediate early antigens, may further characterize these responses. Finally, follow-up periods beyond six months would clarify the dynamics of reconstitution of T-cell responses during the late transplant period. Larger prospective studies are necessary to fully evaluate the quantitative and qualitative T-cell responses required for control of viral replication and to determine the level of global or virus-specific T-cell responses necessary to prevent CMV and its complications.
Acknowledgments
This publication was supported in part by UL1RR025750, KL2RR025749 and TL1RR025748 funding from the NCRR, a component of the NIH, and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. We thank the Clinical Virology laboratory at Montefiore Medical Center for performing CMV serology tests on HC and LTTx cohorts. We also thank the Immunology/Pathology Core lab of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519) for the use of the ELISPOT reader.
Abbreviations
- CMI
cell-mediated immunity
- CMV
cytomegalovirus
- CSA
cyclosporine
- EBV
Epstein Barr virus
- FBS
fetal bovine sera
- GCV
ganciclovir
- GVHD
graft-versus-host disease
- HC
healthy controls
- HSC
hematopoietic stem cell
- HSV
herpes simplex virus
- IFNG
interferon gamma
- IQR
interquartile range
- IS
immunosuppression
- LTTx
long-term transplant
- MDC
minimum detectable concentration
- MMF
mycophenolate mofetil
- MSSA
methicillin-sensitive Staphylococcus aureus
- NCRR
National Center for Research Resources
- NIH
National Institutes of Health
- OI
opportunistic infections
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- Prd
prednisone
- RSV
respiratory syncytial virus
- SB
staining buffer
- SFU
spot-forming units
- SOT
solid organ transplantation
- TAC
tacrolimus
- Th
thymoglobulin
- VACV
valacyclovir
- VGCV
valganciclovir
Footnotes
Disclosure
This manuscript was not prepared or funded in any part by a commercial organization, including educational grants. The authors of this manuscript have no conflicts of interest to disclose.
Author contributions
M. Patel: concept/design, data collection, data analysis/ interpretation, drafting article, statistics, approval of article; M. Stefanidou, L. Tesfa: data analysis/interpretation, critical revision of article, approval of article; C.B. Long, M. Del Rio, J. Lamour, R. Ricafort: data collection, critical revision of article, approval of article; M.J. Fazzari: statistics, data analysis/interpretation, critical revision of article, approval of article; R.P. Madan: concept/design, data collection, critical revision of article, approval of article; B.C. Herold: concept/design, data analysis/interpretation, critical revision of article, funding secured by, approval of article.
References
- 1.Danziger-Isakov LA, Worley S, Michaels MG, et al. The risk, prevention, and outcome of cytomegalovirus after pediatric lung transplantation. Transplantation. 2009;87:1541–1548. doi: 10.1097/TP.0b013e3181a492e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Paya C, Ruthazer R, et al. Significance of cytomegalovirus for long-term survival after orthotopic liver transplantation: A prospective derivation and validation cohort analysis. Transplantation. 1998;66:1020–1028. doi: 10.1097/00007890-199810270-00010. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann A, Sagedal S, Hjelmesaeth J. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation. 2006;82:S15–S17. doi: 10.1097/01.tp.0000230460.42558.b0. [DOI] [PubMed] [Google Scholar]
- 4.Von Muller L, Schliep C, Storck M, et al. Severe graft rejection, increased immunosuppression, and active CMV infection in renal transplantation. J Med Virol. 2006;78:394–399. doi: 10.1002/jmv.20552. [DOI] [PubMed] [Google Scholar]
- 5.Arend SM, Westendorp RG, Kroon FP, et al. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22:920–925. doi: 10.1093/clinids/22.6.920. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: Biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manez R, Breinig MK, Linden P, et al. Factors associated with the development of post-transplant lymphoproliferative disease (PTLD) in Epstein–Barr virus (EBV)-seronegative adult liver transplant recipients. Transpl Int. 1994;7(Suppl 1):S235–S237. doi: 10.1111/j.1432-2277.1994.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds J, Fenton M, Dewar C, et al. Endothelial dysfunction and cytomegalovirus replication in pediatric heart transplantation. Circulation. 2008;117:2657–2661. doi: 10.1161/CIRCULATIONAHA.107.718874. [DOI] [PubMed] [Google Scholar]
- 9.Toyoda M, Puliyanda DP, Amet N, et al. Co-infection of polyomavirus-BK and cytomegalovirus in renal transplant recipients. Transplantation. 2005;80:198–205. doi: 10.1097/01.tp.0000165110.78397.93. [DOI] [PubMed] [Google Scholar]
- 10.Madan RP, Campbell AL, Shust GF, et al. A hybrid strategy for the prevention of cytomegalovirus-related complications in pediatric liver transplantation recipients. Transplantation. 2009;87:1318–1324. doi: 10.1097/TP.0b013e3181a19cda. [DOI] [PubMed] [Google Scholar]
- 11.Manez R, Kusne S, Green M, et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation. 1995;59:1010–1014. doi: 10.1097/00007890-199504150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.San Juan R, Aguado JM, Lumbreras C, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: Data from the RESITRA study group. Am J Transplant. 2007;7:964–971. doi: 10.1111/j.1600-6143.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 14.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 15.Arthurs SK, Eid AJ, Pedersen RA, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 16.Eckle T, Prix L, Jahn G, et al. Drug-resistant human cyto-megalovirus infection in children after allogeneic stem cell transplantation may have different clinical outcomes. Blood. 2000;96:3286–3289. [PubMed] [Google Scholar]
- 17.Hodson EM, Craig JC, Strippoli GF, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008:CD003774. doi: 10.1002/14651858.CD003774.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 19.Limaye AP, Bakthavatsalam R, Kim HW, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78:1390–1396. doi: 10.1097/01.tp.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 20.Reischig T, Opatrny K, Jr, Bouda M, Treska V, Jindra P, Svecova M. A randomized prospective controlled trial of oral ganciclovir versus oral valacyclovir for prophylaxis of cytomegalovirus disease after renal transplantation. Transpl Int. 2002;15:615–622. doi: 10.1007/s00147-002-0475-0. [DOI] [PubMed] [Google Scholar]
- 21.Sia IG, Patel R. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin Microbiol Rev. 2000;13:83–121. doi: 10.1128/cmr.13.1.83-121.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wutzler P, Thust R. Genetic risks of antiviral nucleoside analogues – a survey. Antiviral Res. 2001;49:55–74. doi: 10.1016/s0166-3542(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 23.Pescovitz MD, Ettenger RB, Strife CF, et al. Pharmaco-kinetics of oral valganciclovir solution and intravenous ganciclovir in pediatric renal and liver transplant recipients. Transpl Infect Dis. 2010;12:195–203. doi: 10.1111/j.1399-3062.2009.00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Vaudry W, Ettenger R, Jara P, et al. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am J Transplant. 2009;9:636–643. doi: 10.1111/j.1600-6143.2008.02528.x. [DOI] [PubMed] [Google Scholar]
- 25.Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134–2143. doi: 10.1111/j.1600-6143.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh N. Preemptive therapy versus universal prophylaxis with ganciclovir for cytomegalovirus in solid organ transplant recipients. Clin Infect Dis. 2001;32:742–751. doi: 10.1086/319225. [DOI] [PubMed] [Google Scholar]
- 27.Humar A, Paya C, Pescovitz MD, et al. Clinical utility of cytomegalovirus viral load testing for predicting CMV disease in D+/R− solid organ transplant recipients. Am J Transplant. 2004;4:644–649. doi: 10.1111/j.1600-6143.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 29.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633–644. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 30.Radha R, Jordan S, Puliyanda D, et al. Cellular immune responses to cytomegalovirus in renal transplant recipients. Am J Transplant. 2005;5:110–117. doi: 10.1111/j.1600-6143.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 31.Sester U, Gartner BC, Wilkens H, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant. 2005;5:1483–1489. doi: 10.1111/j.1600-6143.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 32.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;3:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 34.Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2007;9:286–294. doi: 10.1111/j.1399-3062.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheng FW, Chan PK, Lee V, et al. Lymphoproliferative response to herpes simplex virus type 1, cytomegalovirus, Epstein–Barr virus, varicella zoster virus, human herpes virus 6, 7, and 8 antigen stimulation in pediatric allogeneic stem cell transplant recipients. Pediatr Transplant. 2010;14:761–769. doi: 10.1111/j.1399-3046.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 36.Guerin V, Dalle JH, Pedron B, et al. Cellular immune parameters associated with spontaneous control of CMV in children who underwent transplantation. Bone Marrow Transplant. 2010;45:442–449. doi: 10.1038/bmt.2009.179. [DOI] [PubMed] [Google Scholar]
- 37.Koehl U, Dirkwinkel E, Koenig M, et al. Reconstitution of cytomegalovirus specific T cells after pediatric allogeneic stem cell transplantation: Results from a pilot study using a multi-allele CMV tetramer group. Klin Padiatr. 2008;220:348–352. doi: 10.1055/s-0028-1086029. [DOI] [PubMed] [Google Scholar]
- 38.Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood. 2006;108:1406–1412. doi: 10.1182/blood-2005-11-012864. [DOI] [PubMed] [Google Scholar]
- 39.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 40.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 41.Maecker HT. Multiparameter flow cytometry monitoring of T cell responses. Methods Mol Biol. 2009;485:375–391. doi: 10.1007/978-1-59745-170-3_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maecker HT, Maino VC. Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry. Hum Immunol. 2004;65:493–499. doi: 10.1016/j.humimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Van De Berg PJ, Heutinck KM, Raabe R, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis. 2010;202:690–699. doi: 10.1086/655472. [DOI] [PubMed] [Google Scholar]
- 44.Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10:2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller TF, Grebe SO, Neumann MC, et al. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997;64:1432–1437. doi: 10.1097/00007890-199711270-00010. [DOI] [PubMed] [Google Scholar]
- 46.Kogina K, Shoda H, Yamaguchi Y, et al. Tacrolimus differentially regulates the proliferation of conventional and regulatory CD4(+) T cells. Mol Cells. 2009;28:125–130. doi: 10.1007/s10059-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 47.Dunn HS, Haney DJ, Ghanekar SA, Stepick-Biek P, Lewis DB, Maecker HT. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J Infect Dis. 2002;186:15–22. doi: 10.1086/341079. [DOI] [PubMed] [Google Scholar]
- 48.Tu W, Potena L, Stepick-Biek P, et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation. 2006;114:1608–1615. doi: 10.1161/CIRCULATIONAHA.105.607549. [DOI] [PubMed] [Google Scholar]
- 49.Ganepola S, Gentilini C, Hilbers U, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:293–299. doi: 10.1038/sj.bmt.1705585. [DOI] [PubMed] [Google Scholar]
- 50.Ohnishi M, Sakurai T, Heike Y, et al. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol. 2005;131:472–479. doi: 10.1111/j.1365-2141.2005.05800.x. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmeister B, Kiecker F, Tesfa L, Volk HD, Picker LJ, Kern F. Mapping T cell epitopes by flow cytometry. Methods. 2003;29:270–281. doi: 10.1016/s1046-2023(02)00349-3. [DOI] [PubMed] [Google Scholar]