Abstract
Ligands by binding to G protein coupled receptors (GPCRs) stimulate dissociation of heterotrimeric G proteins into Gα and Gβγ subunits. Released Gα and Gβγ subunits induce discrete signaling cues that differentially regulate focal adhesion kinase (FAK) activity and endothelial barrier function. Activation of G proteins downstream of receptors such as protease activated receptor 1 (PAR1) and histamine receptors rapidly increases endothelial permeability which reverses naturally within the following one to two hours. However, activation of G proteins coupled to the sphingosine-1-phosphate receptor 1 (S1P1) signal cues that enhance basal barrier endothelial function and restore endothelial barrier function following the increase in endothelial permeability by edemagenic agents. Intriguingly, both PAR1 and S1P1 activation stimulates FAK activity, which associates with alteration in endothelial barrier function by these agonists. In this review, we focus on the role of the G protein subunits downstream of PAR1 and S1P1 in regulating FAK activity and endothelial barrier function.
Keywords: Heterotrimeric G Proteins, PAR1, thrombin, S1P1, sphingosine-1-phosphate FAK, endothelial barrier function
Introduction
Heterotrimeric G proteins consisting of Gα and Gβγ subunits regulate a variety of endothelial functions including migration, proliferation, differentiation, survival, and tissue-fluid homeostasis (Hamm, 1998; Wieland and Mittmann, 2003; Shajahan et al., 2004; Andreeva et al., 2005; Andreeva et al., 2006; Offermanns, 2007; Knezevic et al., 2009; Liu et al., 2009). These proteins act as an intracellular partner downstream of seven-transmembrane domain G protein coupled receptors (GPCRs), the largest family of membrane proteins within the human genome, to transmit diverse signals from extracellular GPCR ligands (Gilman, 1987; Hamm, 1998; Offermanns, 2007). Thus far 16 genes encode for 23 known Gα proteins which are divided into four major classes based on sequence homology: Gα (s/olf), Gα (i1/i2/i3/o/ t-rod/ t-cone/gust/z), Gα (q/11/14/16), and Gα (12/13) (Simon et al., 1991). There are five known Gβ and twelve human Gγ subunit genes, resulting in a large number of potential Gβγ heterodimer combinations (Hurowitz et al., 2000). Under naïve conditions GPCRs are generally linked with the heterotrimer made of the Gα and Gβγ complex. Ligand binding to GPCRs leads to dissociation and/or uncoupling of Gα from Gβγ subunits (McCudden et al., 2005), which by inducing discrete signaling cues differentially regulates the activity of focal adhesion kinase (FAK), a non-receptor tyrosine kinase, and endothelial barrier function (Schlaepfer et al., 1999; Mehta and Malik, 2006; Rozengurt, 2007). For example, activation of G proteins downstream of receptors such as protease activated receptor 1 (PAR1) and histamine receptors rapidly increases endothelial permeability which reverses naturally within the next one to two hours (Kouklis et al., 2004; Holinstat et al., 2006; Kaneider et al., 2007). In contrast, G proteins coupled to sphingosine-1-phosphate receptor 1 (S1P1) signal cues that enhance endothelial barrier function (English et al., 1999; McVerry and Garcia, 2004; Finigan et al., 2005; Mehta et al., 2005; Tauseef et al., 2008). Interestingly, as we will discuss, activated PAR1 and S1P1 receptor signaling appears to converge at FAK which in turn is associated with alteration in endothelial barrier function by thrombin and S1P. Herein, we review the role of the G protein subunits downstream of PAR1 and S1P1 in regulating FAK activity and how the activation of FAK by these two discrete receptors alters endothelial barrier function.
1. PARs and endothelial barrier function
Protease activated receptors (PARs) are seven-transmembrane spanning receptors, typical of all GPCRs, that are activated by thrombin, a procoagulant generated during vascular injury (Coughlin, 2005; Landis, 2007). These PARs thereby connect the coagulation proteases to cellular responses that induce inflammation and local edema by recruiting activated leukocytes. To date, four members of the PAR family (PAR1, PAR2, PAR3 and PAR4) have been identified in endothelial cells. Whereas PAR-1 -3 and -4 are the essential receptors for thrombin, PAR2 is activated by trypsin-like serine proteases (Coughlin, 2005; Landis, 2007). PAR3 is not thought to directly elicit intracellular signal transduction, but function as a cofactor for PAR1 (McLaughlin et al., 2005) and PAR4 (Nakanishi-Matsui et al., 2000). Therefore, PAR1 and PAR4 are considered the major receptors which mediate the effects of thrombin.
The expression profile of PARs varies in endothelial cells isolated from various vascular beds (Fujiwara et al., 2004). Fujiwara et al. (2004) compared the incorporation of BrdU, a read out of endothelial proliferation, in human endothelial cells isolated from aorta, pulmonary artery and umbilical veins to determine whether differences in endothelial functions in response to thrombin in various vascular beds could be explained by distinct PARs expression. They showed the highest BrdU incorporation rate in response to a selective PAR1-activating peptide, SFLLRN, in aortic endothelial cells. The PAR4-activating peptide, GYPGQV, induced DNA synthesis in pulmonary arterial and aortic cells but not in venular cells (Fujiwara et al., 2004). Since endothelial cells isolated from pulmonary microvessels form the most restrictive barrier to macromolecules (Del Vecchio et al., 1992; Malik, 1993; Schnitzer et al., 1994; Kelly et al., 1998), a possibility exists that differences in PAR expression in endothelial cells from different vascular beds could contribute to the observed segmental variations in their barrier properties.
PAR1 is most likely the first receptor to respond during coagulation because it is constitutively expressed on the endothelium cell surface (Ellis et al., 1999; Landis, 2007). In fact, the permeability increasing effects of thrombin in lung endothelium are predominantly mediated through PAR1 since selective PAR1 peptide agonists and thrombin fail to induce endothelial contraction and lung microvascular permeability increase in mice lacking PAR1 (Vogel et al., 2000). The roles of the other PARs in mediating increases in endothelial barrier permeability have not been fully elucidated, but they may work in concert with PAR1 to alter endothelial barrier function. For example, thrombin ligation of PAR1 was shown to induce the transactivation of PAR2 in COS7 and human umbilical vein endothelial cells (HUVECs) which in turn regulated thrombin responses (Mirza et al., 1996; Nystedt et al., 1996; O’Brien et al., 2000). In another study, transactivation of PAR2 by PAR1 switched PAR1 from being an endothelial-disruptive receptor to an endothelial-protective receptor during the progression of sepsis in mice (Kaneider et al., 2007). PAR3 also modulated PAR1 responses by altering its affinity with Gαq to Gα13 (McLaughlin et al., 2005). Furthermore, ligation of PAR1 by activated protein C (APC) or thrombin at concentrations below its EC50 of 50 pM for PAR1 activation, induces barrier enhancement (Feistritzer and Riewald, 2005; Ludeman et al., 2005). APC was shown to dampen thrombin-induced endothelial hyperpermeability (Feistritzer and Riewald, 2005; Ludeman et al., 2005). Thrombin and APC are closely related serine proteases with distinct substrate specificities. It is important therefore that the PAR1 interactions with the PAR2, PAR3 and APC should be considered as an essential component by which thrombin alters endothelial barrier function.
1.1 G proteins involved in PAR1 regulation of endothelial barrier function
Thrombin via PAR1 induces the formation of minute intercellular gaps between endothelial cells and thus has been well established as a potent inducer of permeability in cells and intact tissues such as the lung (Mehta and Malik, 2006). PAR1 couples to the heterotrimeric G proteins of the Gq, G12/13, Gi, and Gs families (Macfarlane et al., 2001). Because thrombin-induced increase in endothelial permeability is restored towards basal levels even in the presence of agonist, it is likely that different G proteins are ligated with PAR1 that modulate thrombin alteration of endothelial barrier function. Gαq and Gα12/13 subunits have been generally accepted to mediate the increase in endothelial permeability in response to thrombin by activating myosin light chain kinase (MLCK) and RhoA (Holinstat et al., 2003; Andreeva et al., 2005; McLaughlin et al., 2005; Mehta and Malik, 2006; Singh et al., 2007; Gavard and Gutkind, 2008; Korhonen et al., 2009). Gαs has been shown to enhance endothelial barrier function through cAMP generation (Qiao et al., 2003; Bundey and Insel, 2006). Additionally, dissociated Gβγ subunits have recently emerged to be barrier protective (Knezevic et al., 2009). However, the affinity and the time frame of activation of these G proteins by PAR1 remain enigmatic. Evidence indicates that in COS7 cells PAR1 preassembles with Gαi subunits, but not Gα12 subunits (Ayoub et al., 2010) raising the possibility that preassembled G protein/PAR1 complexes may dictate selective recruitment of other Gα or Gβγ subunits, thereby accounting for the differential effect of PAR1 on endothelial permeability.
Inconclusive evidence to define which G protein(s) is involved in altering endothelial monolayer permeability by PAR1 stems from the lack of adequate tools. Several studies have used pertussis toxin (Ptx) to assess the impact of Gi proteins in regulating endothelial barrier function, but these results have not been conclusive because Ptx prevents GDP release from the Gαi subunit, as well as alters Gβγ function (Smrcka, 2008). Global and endothelial specific deletion of Gα13 in mice resulted in embryonic lethality (Offermanns, 2007), hampering the analysis of its role in regulating endothelial barrier function. Recently, Offermanns’ group generated mice in which Gαq/Gα11- and Gα12/Gα13-mediated signaling pathways can be conditionally deleted in endothelial cells by inducing Tie2-Cre recombinase activity (Korhonen et al., 2009). These authors for the first time identified the specific role of Gαq/Gα11 and Gα12/Gα13 in regulating endothelial barrier function in the adult mice vasculature. Surprisingly, their findings showed that Gαq/Gα11 mediated signaling is required for disruption of endothelial barrier function (Korhonen et al., 2009) (described in detail in the following section).
1.1.1 Role of Gαq
Activated Gαq predominantly regulates the increase in intracellular Ca2+ which in turn signals cues to induce endothelial cell contraction (Nilius and Droogmans, 2001; Tiruppathi et al., 2002b; Holinstat et al., 2003; Ahmmed and Malik, 2005; Mehta and Malik, 2006) and posttranslational modification of endothelial junctional proteins such as vascular endothelial (VE)-cadherin, p120-catenin and β-catenin, leading to increased endothelial permeability (Sandoval et al., 2001; Tiruppathi et al., 2002a; Konstantoulaki et al., 2003; Reynolds and Carnahan, 2004; Adam et al., 2010). Gαq increases intracellular Ca2+ by stimulating phospholipase C (PLC) that generates second messengers such as inositol 1, 4, 5 triphosphate (IP3) and diacylglycerol (DAG). DAG directly activates receptor-operated Ca2+ (ROC) channels at the plasma membrane to induce Ca2+ entry in the endothelial cells (Tiruppathi et al., 2002b; Gudermann et al., 2004; Mehta and Malik, 2006; Singh et al., 2007; Birnbaumer, 2009). IP3 on the other hand binds IP3 receptors at the endoplasmic reticulum (ER) leading to mobilization of stored Ca2+. Depletion of ER stores in turn activates store-operated Ca2+ (SOC) channels on the plasma membrane that refill the ER stores and sustains the increase in intracellular Ca2+ concentration (Nilius and Droogmans, 2001; Cioffi and Stevens, 2006; Mehta and Malik, 2006; Singh et al., 2007; Birnbaumer, 2009). We and others have shown that transient receptor potential canonical (TRPC) channels 1, 4 and 6 act as constituents of ROC and SOC channels in endothelial cells (Tiruppathi et al., 2002b; Gudermann et al., 2004; Cioffi and Stevens, 2006; Mehta and Malik, 2006; Singh et al., 2007; Kini et al., 2010) and thereby act as a critical determinant of thrombin-induced endothelial cell contraction (Cioffi and Stevens, 2006; Singh et al., 2007; Cioffi et al., 2010; Kini et al., 2010).
Endothelial cell contraction is regulated by the phosphorylation state of myosin light chain (MLC), which interacts with actin to generate contractile forces (Dudek and Garcia, 2001). MLC kinase (MLCK) and small GTPase RhoA have been established as critical regulators of actin-myosin-induced endothelial contraction (Dudek and Garcia, 2001; Mehta and Malik, 2006). A rise in intracellular Ca2+ is required for MLCK activity (Garcia et al., 1996). Consistent with this notion, Korhonen et al. (2009) showed that thrombin-induced MLC phosphorylation requires Gαq/Gα11 signaling by using Gαq/Gα11 and Gα12/Gα13 null endothelial cells.
The activation of RhoA requires its dissociation from the guanosine dissociation inhibitor (GDI-1) followed by GTP exchange mediated by guanine nucleotide exchange factors (GEFs) (Schmidt and Hall, 2002). We and others have shown that Gαq can induce RhoA activity (Chikumi et al., 2002b; Rojas et al., 2007; Singh et al., 2007). For example, Gαq directly interacted with p63RhoGEF, which specifically activates RhoA (Rojas et al., 2007). Singh et al. (2007) showed that Gαq activated RhoA via TRPC6-mediated increase in intracellular Ca2+. They also found that TRPC6 activated protein kinase Cα (PKCα) which by phosphorylating GDI-1 stimulated RhoA activity thereby increasing endothelial permeability (Singh et al., 2007). Using mutational analysis, Knezevic et al. (2007) identified S96 in GDI-1 as the phosphor-residue that selectively lead to RhoA activation which increased endothelial cell contraction, intercellular gap formation, and endothelial permeability. Additionally, PKCα can alter endothelial permeability by modifying the phosphorylation status of endothelial adherens junction proteins such as p120-catenin (Sandoval et al., 2001; Konstantoulaki et al., 2003; Brown et al., 2009; Vandenbroucke et al., 2009). Surprisingly, thrombin induced RhoA activity was not altered in Gαq/Gα11 null cells (Korhonen et al., 2009), indicating Gα12/Gα13 may compensate for RhoA activity. Moreover, endothelial Gαq/Gα11 deletion significantly reduced endothelial permeability increases by PAR1 (Figure 1A), histamine, PAF, and LPA stimulation, whereas Gα12/Gα13 deletion did not (Korhonen et al., 2009). Therefore, Gαq/ Gα11 appear to be required for increasing endothelial permeability induced by activation of diverse GPCRs by agonists such as LPA, PAF, histamine and PAR1.
Figure 1. Role of G proteins in regulating endothelial barrier function.
A. Endothelial specific depletion of Gαq/Gα11 in mice prevents PAR1-induced increase in endothelial permeability. Evans blue extravasation was determined in mice skin implants lacking indicated Gα subunits in endothelium. In control and endothelial depleted Gα12/13 mice, PAR1-activating peptide increased Evans blue accumulation. Deletion of endothelial Gαq/11 inhibited local Evans blue extravasation in response to PAR1 activation, as well as other GPCR ligands including histamine, LPA, and PAF (data not shown). (© Korhonen et al., 2009. Originally published in The Journal of Experimental Medicine. doi:10.1084/jem.20082150) B. Gβγ restores basal endothelial barrier function following thrombin-induced increase in endothelial permeability. Human pulmonary artery endothelial cells (HPAEC) transducing control siRNA (SiSc) or Gβ1 siRNA (SiGβ1) were stimulated with 50 nM thrombin and transendothelial electrical resistance (TER) across the endothelial monolayer was recorded. Thrombin induced a rapid decrease in HPAEC transducing control siRNA, indicating an increase in intercellular junction permeability. The permeability naturally reverses to the basal level within next 120 min. However, thrombin induced a persistent increase in endothelial monolayer permeability in Gβ1 depleted cells, indicating that Gβ1 is required for restoring basal endothelial permeability. (© Knezevic et al., 2009. Originally published in Journal of Experimental Medicine. doi: 10.1084/jem.20090652).
1.1.2 Role of Gα12/13
Gα12 and Gα13 have several overlapping functions, but differ in their abilities to couple to various ligands and to recruit different signaling pathways for effectors. For example, gene knockout studies showed that Gα13 deficiency resulted in impaired angiogenesis and embryonic lethality (Offermanns et al., 1997), whereas Gα12 knockout did not (Gu et al., 2002). However, the regulatory factors controlled by the distinct signaling pathways triggered by Gα12 and Gα13 have not yet been identified. The Gα12 and Gα13 family of heterotrimeric G proteins were shown to bind RhoGEFs such as p115RhoGEF and LARG (leukemia-associated RhoGEF), thereby controlling RhoA activation, a key mechanism of actin cytoskeleton reorganization and focal adhesion assembly (Kozasa et al., 1998; Bhattacharyya et al., 2009; Suzuki et al., 2009). Additionally, in Gα12/13 null endothelial cells thrombin failed to activate RhoA, whereas RhoA activity was comparable to control cells in Gαq/11 depleted cells (Korhonen et al., 2009), thus identifying Gα12/13 as a critical determinant of RhoA-mediated endothelial cell contraction. Moreover, Fromm et al. (1997) showed that heterotrimeric G proteins of the Gα12/13 family promote transcriptional activation of the serum response factor and cellular transformation through RhoA. Gα13 was also shown to interact with radixin, an actin polymerizing protein of the ERM (ezrin, radixin, moesin) family (Vaiskunaite et al., 2000). Thus, Gα12 and Gα13 were proposed to regulate endothelial cell permeability by the RhoA pathway. Consistently, Holinstat et al. (2003) showed that transduction of the regulator of G protein signaling (RGS) domain of p115RhoGEF, a GAP for Gα12 and Gα13 (Kozasa et al., 1998), in HUVECs inhibited RhoA activation as well as endothelial permeability in response to PAR1 activation. Interestingly, in a human dermal microvessel endothelial cell line (HMECs) thrombin-induced barrier permeability remained unaltered following chelation of intracellular Ca2+ (McLaughlin et al., 2005; Minshall et al.) or inhibition of Gαi/o by Pertussis toxin (Ptx) (Vanhauwe et al., 2002) indicating Gα12 and Gα13 can independently regulate endothelial permeability. However, endothelial permeability increases in response to PAR1, LPA, PAF and histamine were preserved in Gα12/13 null mouse lung microvessel endothelial cells (Korhonen et al., 2009). RhoA via Rho kinase inhibits myosin phosphatase (PP1) and sustains MLC phosphorylation induced by MLCK (Mehta and Malik, 2006). As discussed above, Gαq-induced Ca2+ signaling is required for MLCK activity and thereby MLC phosphorylation (Nilius and Droogmans, 2001; Tiruppathi et al., 2002b; Ahmmed and Malik, 2005; Mehta and Malik, 2006). Thus, in the absence of Gα12/13, the Gαq/11→RhoA→Rho kinase axis may be able to sustain MLC phosphorylation and hence endothelial barrier disruption will ensue. Since a direct interaction between Gα12, vascular endothelial (VE)-cadherin and α-SNAP was shown to be critical for limiting the increase in endothelial barrier function by PAR1 (Andreeva et al., 2005), an additional possibility exists that Gα12 (or Gα13) may contribute as a barrier protecting mechanism which needs to be considered.
1.1.3 Role of Gαs
The ubiquitously expressed Gαs protein couples many receptors including PAR1 and mediates receptor-dependent adenylyl cyclase activation which results in an increase in intracellular cAMP concentrations. An increase in intracellular cAMP is known to strengthen basal endothelial barrier function and also prevent PAR1-induced increases in endothelial permeability (Mehta and Malik, 2006). cAMP stimulates protein kinase A (PKA) activity which in turn prevents endothelial cell contraction by inhibiting the activities of RhoA (Qiao et al., 2003) and MLCK (Garcia et al., 1997; Verin et al., 1998). cAMP can also strengthen endothelial barrier function by activating Epac (exchange factor directly activated by cAMP) that stabilizes endothelial barrier by inducing VE-cadherin-based intercellular adhesions (Cullere et al., 2005). Since PAR1 activation rapidly increases endothelial monolayer permeability, it is likely that Gαs signaling operates at later time points to facilitate endothelial barrier recovery.
1.1.4 Role of Gαi
Gαi activates the Ras/MAPK cascade, which in turn induces gene transcription. For example, PAR1 activation was shown to enhance its own gene transcription through the Ras pathway (Ellis et al., 1999). Evidence indicates that the Gαi/o family of heterotrimeric G proteins negatively regulates adenylyl cyclase activity (Kanthou et al., 1996). Thrombin inhibited isoproterenol or 3-isobutyl-1-methylxanthine (IBMX) - stimulated cAMP production in rat lung endothelial cells, whereas inhibition of Gαi by Ptx prevented this response (Manolopoulos et al., 1997). Inhibition of Gαi by Ptx also prevented the thrombin-induced increase in endothelial permeability (Vanhauwe et al., 2002), suggesting that Gαi may contribute in PAR1 disruption of the endothelial barrier. It has been shown that Ptx can inhibit both Gαi and Gβγ subunits (Smrcka, 2008). However, as we will discuss in the following section, inhibition of Gβγ using siRNA or c-terminal domain of β-adrenergic receptor (CT-βARK)-1 which sequesters Gβγ (Drazner et al., 1997) did not perturb endothelial cell contraction (Knezevic et al., 2007) (Figure 1B) suggesting that Gαi plays a role in PAR1- induced endothelial disruption. Since Gαq deletion in mice prevented increases in endothelial permeability by various GPCR ligands (Korhonen et al., 2009) these findings imply that Gαi may work downstream of Gαq to regulate endothelial barrier function in response to thrombin.
1.1.5. Role of Gβγ
Gβγ has a large number of effectors including phospholipases (Rhee and Bae, 1997), adenylyl cyclases (Sunahara et al., 1996), ion channels (Schneider et al., 1997), and G protein receptor kinases (Pitcher et al., 1992). Activation of certain GPCRs results in Gβγ-mediated stimulation of ERK1/2, JNK, and p38 mitogen activated protein kinases (MAPKs) (Sun Y., 1999), cAMP generation (Tang and Gilman, 1992; Taurin et al., 2007), Ca2+ signaling (Herlitze et al., 1996; Blackmer et al., 2001), oxidant generation (Niu et al., 2003), neurotransmission (Blackmer et al., 2005), chemotaxis (Neptune and Bourne, 1997; Jin et al., 2000), and caveolae-mediated transcytosis (Shajahan, 2004).
In human pulmonary arterial endothelial cells, Gβ1 is the predominant Gβ isoform expressed (Knezevic et al., 2009). This study showed that targeted suppression of Gβ1 did not alter thrombin-induced increase in endothelial permeability and intracellular gap formation but prevented adherens junction reannealing (Figure 1B), thereby leading to persistent increase in endothelial permeability by thrombin. Gβ1 does not have enzymatic activity, and thus modulates G protein signaling through protein interactions (Smrcka, 2008). Knezevic et al. (2009) showed that Gβ1 interacts with Fyn, a Src family kinase (SFK), which led to Fyn activation. Fyn in turn induced FAK phosphorylation which promoted FAK interaction with p120-catenin at the adherens junctions leading to recovery of endothelial barrier to the normal levels post thrombin challenge (Knezevic et al., 2009). These findings were the first to demonstrate that Gβγ signaling works in opposition to Gαq/Gαi to restore basal endothelial barrier function. However, how FAK activation by Gβ1 selectively targets FAK to translocate at the adherens junctions to induce adherens junction re-annealing remains to be teased out. Since Gβ1 can couple with various γ subunits, further studies are needed to clearly identify which Gβ1γ dimer restores endothelial barrier function following the increase in endothelial permeability by PAR1 activation.
1.2 G proteins involved in PAR1 activation of FAK
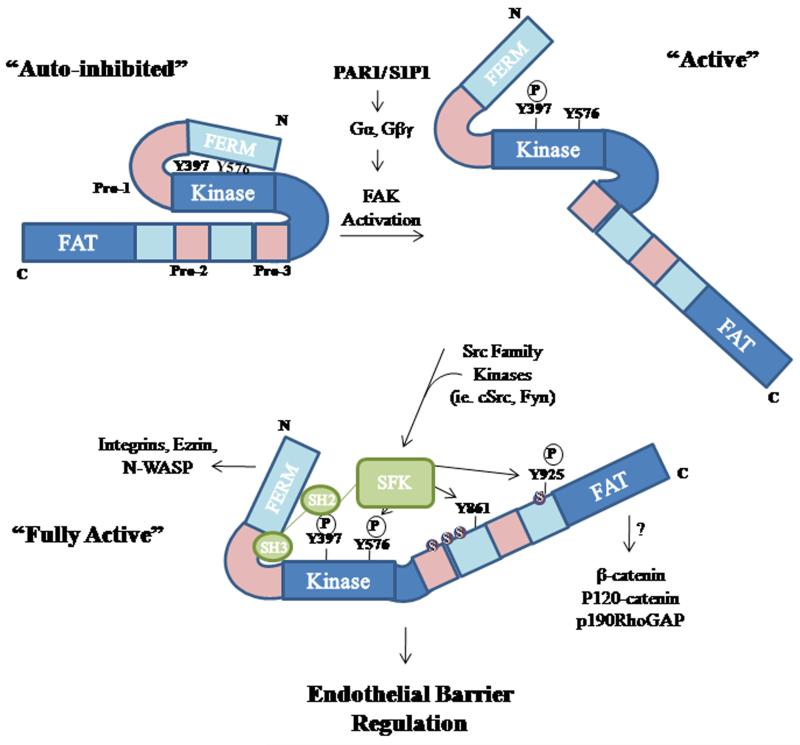
FAK plays a key role in focal adhesion formation and turnover (Ilic et al., 1995; Li et al., 2002; Romer et al., 2006). FAK contains several serine, threonine and tyrosine residues (Parsons, 2003). Under basal conditions, intramolecular interactions between the FAK 4.1 ezrin, radixin, moesin (FERM) domain and kinase domain keep FAK in an inactive conformation by sterically blocking tyrosine residues from potential kinases (Figure 2). Dissociation of the FERM domain from the kinase domain exposes Y397 residue leading to its autophosphorylation. Phospho-Y397 is a high affinity binding site for Src homology (SH) 2 domain-containing molecules, such as SFKs (Cooper et al., 2003; Cohen and Guan, 2005; Jacamo and Rozengurt, 2005). When SFKs interact with FAK through their SH2 domain, as well as an adjacent SH3 domain, the FAK-SFK complex formation further facilitates FAK phosphorylation at Y576/577 residues maximizing FAK enzymatic activity (Lietha et al., 2007). FAK phosphorylation at Y397 and Y576/577 has been well studied, but SFKs can induce phosphorylation at other sites including Y861 and Y925, both of which are located at the C-terminus (Laird et al., 2003; Sanders and Basson, 2004; Mitra et al., 2006). For example, it has been shown that FAK phosphorylation at Y925 leads to ERK activation as Y925 creates an SH3 binding site for the Grb2, a small adaptor protein, which activates the Ras-MAPK pathway (Schlaepfer and Hunter, 1996). ERK activation increases MLCK activity and MLC phosphorylation, leading to actin stress fiber formation and the generation of force required for cell movement (Klemke et al., 1997).
Figure 2. Model of G protein activation of FAK.
Intramolecular interactions between the FAK FERM domain and its Kinase domain keep FAK in an inactive conformation by sterically blocking Y397 residues from potential substrates. Dissociation of FAK FERM domain from the kinase domain leads to rapid autophosphorylation of FAK at Y397 residue. Phospho-Y397 residue forms a high affinity binding site for Src family kinases including p60cSrc and Fyn, which by phosphorylating FAK at Y576/577 fully activate FAK. Src family kinases can also induce FAK phosphorylation at Y861 and Y925 at the C-terminus. FAK may also be phosphorylated at serine residues and their proximity to proline-rich regions may influence FAK-mediated protein-protein interactions and thereby endothelial barrier function.
Thrombin induces sustained phosphorylation of FAK on Y397, Y576, and Y925 residues (Shikata et al., 2003a; Holinstat et al., 2006; Knezevic et al., 2009) (Figure 3A). Thrombin also induces the redistribution of FAK within the cell. For example, in quiescent endothelial cells, FAK is mainly localized in the cytoplasm and only few FAK containing focal adhesions are apparent (Mehta et al., 2002; Knezevic et al., 2009; Sun et al., 2009). Thrombin markedly enhances the reorganization of FAK into focal adhesions (Schaphorst et al., 1997; Mehta et al., 2002; Holinstat et al., 2006). These focal adhesions were localized at the ends of actin stress fibers (Schaphorst et al., 1997; Mehta et al., 2002; Holinstat et al., 2006). At focal adhesions, FAK-mediates phosphorylation of various focal adhesion proteins, including paxillin, vinculin, tensin, and p130CAS, which may regulate their recruitment into complexes to mediate cell shape change (Schaller, 2001; Wu, 2005; Mehta and Malik, 2006). Since FAK organization into focal adhesions occurred in conjunction with increased endothelial permeability, FAK was initially considered as an inducer of endothelial barrier dysfunction (Schaphorst et al., 1997; Wu et al., 2003). However, impairment of FAK function in endothelial cells using anti-sense oligonucleotides or a dominant negative (dn)-FAK mutant revealed that FAK in fact was required for maintaining endothelial barrier function and for limiting the persistent increase in endothelial barrier function following thrombin challenge (Mehta et al., 2002; Holinstat et al., 2006) (Figure 3B). Other studies showed that FAK function was required for strengthening the endothelial barrier in response to hyperosmolar stress (Quadri and Bhattacharya, 2007) further supporting the role of FAK in maintaining endothelial barrier integrity. Recently, we have shown that tamoxifen-induced endothelial specific deletion of FAK in adult mice grossly disrupts alveolar-capillary barrier resulting in lung vascular leak and edema formation (Thennes et al., 2010). Additionally, thrombin causes FAK redistribution to the cell periphery where it interacts with p120-catenin dependent on Y397/Y576 FAK phosphorylation leading to adherens junction re-annealing and restoration of endothelial barrier function (Knezevic et al., 2009). Assessment of the role of FAK phosphorylation at Y397 and Y576 versus Y925 in regulating spatial localization of FAK at focal adhesions or adherens junctions may be useful in defining the mechanism by which FAK phosphorylation regulates the repair of the endothelial barrier.
Figure 3. FAK regulation of endothelial barrier function.
A. Thrombin induces sustained activation of FAK. HPAE cells were stimulated with 50 nM thrombin for indicated times and lysates were immunoblotted with Y397 or Y576 residue specific anti-phospho-FAK antibodies or pan anti-FAK as control to determine the phosphorylation of FAK. FAK phosphorylation was increased within 10 min, and remained elevated at this level for up to 120 min (© Knezevic et al., 2009. Originally published in Journal of Experimental Medicine. doi: 10.1084/jem.20090652). B. Impairment of FAK function causes persistent increase in endothelial permeability. HPAEC infected with control virus (Adv-GFP) or dominant-negative FAK viruses (Ad-FRNK) were stimulated with 50 nM thrombin and TER across the endothelial monolayer was recorded. Thrombin also decreased TER to the same extent in dominant negative FAK (GFP-FRNK) expressing cells, but in contrast to control cells, the monolayer did not recover within 2 hr (This research was originally published in the Journal of Biological Chemistry. Holinstat et al. JBC. 2005; Vol: 281, NO.4, pp2296-2305. © The American Society for Biochemistry and Molecular Biology.) C. Gβγ depletion prevents FAK activation. HPAE cells were transfected with control (Sc) or Gβ1 siRNA and after 48 hr post transfection cells were stimulated with 50 nM thrombin for indicated times. Lysates were immunoblotted with Y397 or Y576 residue specific anti-phospho-FAK antibodies or pan anti-FAK as control to determine the phosphorylation of FAK. Thrombin failed to induce FAK activity in Gβ1 depleted transfected cells (© Knezevic et al., 2009. Originally published in Journal of Experimental Medicine. doi: 10.1084/jem.20090652).
A few studies have addressed the role of G proteins in regulating FAK activity. Chikumi et al. (2002) transfected HEK cells with GTPase-deficient (i.e. constitutively active forms) Gα subunits and assessed their ability to activate FAK. They showed that constitutively active Gαq and Gα12/13 signal FAK activity indicating these proteins may participate in FAK activation by PAR1 (Chikumi et al., 2002a). Moreover, based on the notion that actin polymerization and increased RhoA activity all lead to FAK activation (Mehta et al., 2002), it can be further presumed that Gαq and Gα12/13 primarily regulate FAK activation. In fact, thrombin failed to induce FAK activity in cells pretreated with C3 transferase, which ribosylates RhoA and thus inhibits its activity (Carbajal and Schaeffer, 1999). Inhibition of actin filament polymerization by latrunculin also inhibited FAK activity (Abedi and Zachary, 1997; Vepa et al., 1999; Mehta et al., 2002). However, the role of Gαq-induced rise in intracellular Ca2+ in mediating FAK activity remains controversial. Schaplafrost et al. (1997) showed that Ca2+ ionophore ionomycin decreased FAK phosphorylation in endothelial cells. In contrast, Ca2+ influx was shown to be required for FAK-mediated spreading of HUVECs on type IV collagen (Alessandro et al., 1998).
Interestingly, we have recently shown that sequestration of Gβγ in endothelial cells with CT-βARK-1 or suppressing Gβ1 expression using siRNA failed to promote thrombin-induced FAK tyrosine phosphorylation at Y397 or Y576 (beginning at 10, 60 and 120 min post thrombin) (Knezevic et al., 2009), indicating a critical role for the Gβγ heterodimer in regulating FAK activation (Figure 3C). Thus, Gβ1-mediated FAK activation was shown to integrate FAK with adherens junction assembly which prevented persistent increase in endothelial monolayer permeability. Additionally, FAK upregulation of p190RhoGAP activity, which negatively regulates RhoA activity (Holinstat et al., 2006), is most likely Gβγ dependent since inhibition of Gβγ increased basal RhoA activity and potentiated RhoA activity in response to thrombin stimulation (Knezevic et al., 2009). Since p120-catenin also cross-talks with p190RhoGAP (Wildenberg et al., 2006), the Gβγ→ FAK-mediated cell flattening and adherens junction assembly may work concomitantly with p120-catenin and p190RhoGAP proteins to restore endothelial cell-cell contacts following endothelial barrier disruption by inflammatory mediators. Further studies in Gα12/Gα13/Gαq null endothelial cells will be useful to delineate the role of Gα12/Gα13/Gαq versus Gβγ in mediating FAK activation during endothelial cell contraction and recovery.
1.3 Role of RACK1 in regulating FAK activity and endothelial barrier function downstream of Gα and Gβγ proteins
As discussed above, downstream of PAR1 Gα-mediated signaling precedes Gβγ dependent signaling leading to sustained FAK activation and reversible increase in endothelial barrier function. These findings suggest that a common linker, which has the ability to bind both Gα and Gβγ but enforce delayed activation of Gβγ, may be a determinant of endothelial barrier function regulation by PAR1. Receptor for activated kinase 1 (RACK1) is a member of the WD40 repeat protein family that is predicted to adopt a β-propeller structure similar to that of Gβ, and was originally identified as a homolog of Gβ (Chen et al., 2004b). RACK1 was shown to specifically alter functions induced by Gβγ subunits by sterically hindering the access of effector proteins to Gβγ (Chen et al., 2004a; Chen et al., 2005). Upon GPCR activation, RACK1 dissociates from Gβγ, allowing Gβγ interaction with downstream effectors (Chen et al., 2004a).
RACK1 interacts with p60cSrc (Chang et al., 1998; Chang et al., 2001) and Fyn (Yaka et al., 2002; Thornton et al., 2004) which binds FAK and regulates its kinase activity (Knezevic et al., 2009). We showed that in naïve cells RACK1 forms a complex with Gβ1 and Fyn (Knezevic et al., 2009). Suppression of RACK1 expression by siRNA potentiated the interaction between Gβ1 with Fyn and FAK leading to increased FAK activity, reannealing of adherens junctions thereby speeding the recovery of the endothelial barrier after thrombin stimulation (Knezevic et al., 2009) (Figure 4). Therefore, these findings suggest that RACK1 negatively regulates Gβ1 restoration of barrier function by preventing Gβ1-Fyn interaction and restricting the upregulation of FAK activity by this complex (Figure 5). However, the mechanism by which thrombin facilitates the dissociation of RACK1 from Gβγ and Fyn remains unclear.
Figure 4. RACK1 negatively regulates Gβ1 function.
A. Depletion of RACK1 promotes Gβ1 interaction with FAK and Fyn. HPAEC were transfected with control siRNA (Sc) or RACK1 siRNA (SiRACK1) and 48 hr post transfection cells were stimulated with 50 nM thrombin for indicated times. Cell lysates were immunoprecipitated with anti-Fyn antibody and probed with anti-Gβ1 or anti-FAK antibodies to assess interaction. RACK1 knockdown basally potentiated the interaction between Gβ1, Fyn and FAK, which did not further increase following stimulation with thrombin. B. Knockdown of RACK1 increases FAK tyrosine phosphorylation. siSc and siRACK1 expressing cells were assessed for FAK tyrosine phosphorylation following thrombin challenge using site-specific FAK phosphor-antibodies. Results indicate that suppression of endogenous RACK1 basally activated FAK and the phosphorylation did not increase further after thrombin challenge. C. Knockdown of RACK1 accelerates adherens junction assembly. HPAE cells transfected with siSc or SiRACK1 were immuno-stained with anti-VE-cadherin antibody to quantify interendothelial gap formation following thrombin stimulation. Results showed that suppression of RACK1 potentiated adherens junction re-annealing after thrombin challenge indicating RACK1 prevents endothelial barrier recovery by blocking the interaction between Gβ1, Fyn and FAK. (© Knezevic et al., 2009. Originally published in Journal of Experimental Medicine. doi: 10.1084/jem.20090652).
Figure 5. PAR1 signaling inducing FAK activity and reversible disruption of endothelial barrier function.
Upon ligation of PAR1 by thrombin, the Gα subunit of the heterotrimeric G protein dissociates from Gβγ. Gαq increases intracellular Ca2+ concentration, which increases endothelial permeability by activating MLCK and RhoA. RhoA also induces FAK activation. FAK negatively regulates RhoA-GTP by activating p190RhoGAP, thus turning off endothelial cell contraction. Gβγ mediates activation of FAK through Fyn kinase leading to interaction of FAK with p120-catenin that facilitates re-annealing of adherens junction and thereby restores normal barrier function. Inset shows the release of Gβγ from RACK1/Gα complex upon thrombin stimulation.
1.4 Relationship between small G proteins and FAK and their role in PAR1 regulation of endothelial permeability
The small GTPases RhoA, Rac1, and Cdc42 have fundamental roles in regulating endothelial barrier function by differentially regulating actin dynamics and cytoskeletal arrangement (Wojciak-Stothard et al., 2001; Wojciak-Stothard and Ridley, 2002; Mehta and Malik, 2006). Upon activation, RhoA induces stress fiber formation and Rac leads to lamellipodia formation, whereas Cdc42 induces filopodia formation (Nobes and Hall, 1999). Rac1 and Cdc42 induced actin filament organization may facilitate the formation of interendothelial junctions as the edges of the plasma membrane are propelled forward (Mehta and Malik, 2006). In addition, Cdc42 and Rac1 control cadherin-mediated cell-cell adhesion and formation of adherens junction complexes via modulation of interactions between α-catenin and the cadherin-catenin complex (Kouklis et al., 2004; Broman et al., 2006). Consistently, thrombin leads to transient activation of RhoA in association with modest decreases in Rac1 activity which leads to rapid increase in endothelial permeability (Mehta and Malik, 2006). However, thrombin leads to delayed activation of Cdc42 that has been shown to restore basal endothelial permeability in endothelial cells (Kouklis et al., 2004) and mouse lungs transducing constitutive Cdc42 under the control of VE-cadherin promoter (Ramchandran et al., 2008). Thus, Rac1 and Cdc42 appear to mitigate the permeability increase by thrombin thereby restoring the basal endothelial monolayer permeability. Evidence indicates that FAK may serve as the basis of initiating cross-talk between RhoA, Rac1 and Cdc42 to reverse the increases in endothelial permeability (Mehta and Malik, 2006). For example, RhoA was shown to be required for inducing FAK activation (Carbajal et al., 2000; Mehta et al., 2002; Torsoni et al., 2005). However, inhibition of FAK in endothelial cells markedly increased RhoA activity which leads to a persistent increase in endothelial permeability (Holinstat et al., 2006), indicating FAK may be needed to suppress RhoA activity and to facilitate the activities of Cdc42 and Rac1. In fact, FAK negatively suppressed RhoA activity by inducing p190RhoGAP activity (Holinstat et al., 2006) which specifically targets RhoA (Figure 5). Additionally, FAK was shown to be required for the translocation of Rac1 to the cell periphery by phosphorylating β-PIX (PAK-interacting exchange factor) or GIT-1, the G protein-coupled receptor kinase interactor-1 (Chang et al., 2007). Evidence also points to N-WASP, Arp2/3, and WASP-related SCAR (like WASP an activator of actin nucleation/polymerization by Arp2/3) as possible targets of Cdc42 and Rac1 because these proteins regulate lamellipodia and filopodia formation and thus might facilitate adherens junction reformation (Millard et al., 2004). FAK, through its N-terminal FERM domain, binds N-WASP and induces its tyrosine phosphorylation at a conserved Y256 (Wu et al., 2004). Additionally, N-WASP and SCAR are downstream effectors of Cdc42 and Rac1 (Millard et al., 2004). Therefore, FAK through its effectors p190RhoGAP, N-WASP and GIT-1 may influence RhoA, Rac and Cdc42 activities to maintain endothelial barrier function.
2. S1PR and endothelial barrier function
Sphingosine-1-phosphate (S1P) is a bioactive lipid that mediates a myriad of cellular responses in different tissues. S1P is synthesized from membrane phospholipids through sphingosine kinases as part of the sphingomyelin metabolic cycle that can be influenced by several physiological and pathophysiological stimuli (Spiegel and Milstien, 2003; Hla, 2004; McVerry and Garcia, 2004; Wang and Dudek, 2009). S1P circulates in the blood at high concentrations (400-700 nM, primarily due to release from platelets), but is also produced by a variety of cell types to strongly influence organ and tissue function at a local level (Spiegel and Milstien, 2003; Hla, 2004; McVerry and Garcia, 2004; Wang and Dudek, 2009).
S1P acts as an extracellular ligand, in either a paracrine or autocrine manner, for five closely related S1P receptors; S1P1-5 (formerly termed the endothelial differentiation gene (Edg) receptors (Spiegel and Milstien, 2003; Hla, 2004; McVerry and Garcia, 2004; Wang and Dudek, 2009). S1P1-5 is differentially expressed on a variety of cell types including endothelial cells, neurons, leukocytes and smooth muscle cells. These receptors initiate intracellular signaling cascades including adherens junction assembly, cell migration, cytoskeletal changes, proliferation, and apoptosis by stimulating downstream heterotrimeric G proteins. The different activities triggered by S1P are dependent on the pattern of expression of S1P receptors in the respective cell type. Global and endothelial specific deletion of S1P1 in mice induces embryonic lethality at d8.5 due to massive embryonic hemorrhage (Liu et al., 2000; Allende et al., 2003), suggesting S1P1 is required for blood vessel development. Human pulmonary endothelial cells typically express the S1P1, S1P2, and S1P3 receptors (Lin et al., 2007; Tauseef et al., 2008). In several studies, S1P has been shown to induce increases in transendothelial electrical resistances, indicating S1P enhances endothelial barrier function (Garcia et al., 2001; Mehta et al., 2005). Suppression of S1P1 receptor prevented S1P-induced strengthening of endothelial barrier function (Lee et al., 2006; Tauseef et al., 2008), thus suggesting that barrier protective effect of S1P occurs through S1P1. Likewise, in both rodent and dog models of lung injury, infusion of S1P into the vasculature protected their lungs from edema formation (Szczepaniak et al., 2008; Lee et al., 2009). Furthermore, S1P analog FTY720 significantly decreased LPS-induced pulmonary microvascular leakage (Peng et al., 2004), showing that S1P signaling resists increases in vascular permeability. Interestingly, S1P was shown to induce more increases in transendothelial resistance in pulmonary microvascular cells compared to pulmonary arterial endothelial cells (Liu et al., 2001; Allende et al., 2003), indicating S1P1 expression profile may differ between endothelial cells from various vascular beds. However, subsegmental expression profiling of S1P1 in vivo and whether it contributes in differences in endothelial permeability in the vascular bed is unknown.
In contrast to S1P1, targeted disruption of the S1P2 gene is not embryonic lethal, but causes decreased vascular tone and blunted responses to vasoconstrictor agents (Lorenz et al., 2007), indicating that S1P2 maintains normal cardiovascular function. Hla’s group showed that S1P2 induces phosphatase and tensin homolog (PTEN) phosphatase by a Rho-GTPase dependent pathway which in turn inhibit endothelial cell migration and chemotaxis (Sanchez et al., 2007). Additionally, S1P via S1P3 activated eNOS (endothelial nitric oxide synthase) in mouse arteries that induced vasodilation (Tolle et al., 2005). Lee et al. (2009) compared the effects of S1P1 and S1P2 agonism and antagonism in regulating endothelial barrier function in response to histamine. They showed that S1P1 agonists SEW2871 and FTY720 significantly inhibited histamine-induced microvascular leakage. However, antagonizing S1P1 signaling using VPC 23019 markedly enhanced the venular leakage in response to histamine while inhibition of S1P2 signaling by JTE-013, a specific antagonist of S1P2 protected histamine-induced microvascular permeability responses. They further demonstrated that S1P1- and S1P2-mediated signaling altered endothelial barrier function by affecting endothelial tight junctions (Lee et al., 2009). Thus, balance between S1P1 and S1P2 signaling may play a critical role in regulating vascular fluid homeostasis. As S1P1 is arguably the S1P receptor which signals cues to enhance barrier function, the following section will review the role of S1P1 in regulating FAK activity and how it modulates endothelial barrier function.
2.1 G proteins involved in S1P1 regulation of endothelial barrier function
Unlike PAR1 which couples to several heterotrimeric G proteins, it has been shown that S1P1 couples exclusively to Gi (Garcia et al., 2001; Lee et al., 2001; Schaphorst et al., 2003). Consistently, studies have shown that S1P-mediated endothelial cell chemotaxis and angiogenic responses were Ptx-sensitive (Lee et al., 1999; Lee et al., 2001; Liu et al., 2001). Additionally, S1P failed to enhance transendothelial electrical resistance in Ptx pretreated endothelial monolayers (Garcia et al., 2001; Liu et al., 2001; Mehta et al., 2005) (Figure 6A). Evidence indicates that transient transfection of CT-βARK-1 peptide, which inhibits Gβγ, attenuated S1P induced transendothelial electrical resistances (Garcia et al., 2001; Gonzalez et al., 2006), indicating that Gβγ may also serve as a barrier enhancing mechanism in the case of S1P1. Interestingly, S1P at high concentrations (5 μM) can activate RhoA and actin stress fiber formation in human pulmonary arterial endothelial cells possibly via Gαq and Gα12/Gα13 (Garcia et al., 2001). Thus endothelial barrier-promoting effect of S1P may be concentration dependent and occur in a narrow physiological range. It is likely that at higher concentrations S1P may disrupt the endothelial barrier by activating other S1P receptors (i.e. S1P2/3) via Gαq or Gα12/Gα13-mediated signaling, however, whether such a level of S1P occurs naturally in the circulation is doubtful.
Figure 6. S1P enhances endothelial barrier function.
A. Gi increases transendothelial electrical resistance in response to S1P. HPAEC seeded on TER electrodes were stimulated with 1μM S1P in the absence or presence of pertussis toxin (Ptx), which inhibits Gi. S1P alone caused a rapid, sustained increase in TER values, which was prevented in cells pre-treated with Ptx, indicating S1P strengthens endothelial barrier function through at Gi dependent pathway for (This research was originally published in the Journal of Biological Chemistry. Mehta et al. Sphingosine 1-Phosphate-induced Mobilization of Intracellular Ca2+ Mediates Rac Activation and Adherens Junction Assembly in Endothelial Cells. JBC. 2002; Vol: 280, NO.17, pp17321-17328. © the American Society for Biochemistry and Molecular Biology.) B. S1P induced tyrosine phosphorylation of FAK is sensitive to inhibitors of Gi and PLC. HUVECs were preincubated with various inhibitors for 30 min after which these cells were stimulated with 5 μM S1P for 2 min. Cell lysates were immunoprecipitated after which they were immunoblotted with anti-phosphotyrosine antibody to assess FAK activation. Results showed that S1P-induced FAK phosphorylation via Gi-PLC pathway independent of PKC activity (Reprinted from Lee et al., Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway, 5;268, 47-53, 2000 with permission from Elsevier).
2.1.1 Gi signaling regulating S1P1-induced enhancement of endothelial barrier function
S1P rapidly activates the release of Ca2+ from the ER and subsequent Ca2+ entry via SOCs (Muraki and Imaizumi, 2001; Mehta et al., 2005). Inhibiting Gαi, PLC, or the IP3 receptor prevented S1P induced junction assembly and barrier enhancement (Mehta et al., 2005). Additionally, Ptx treatment alone was shown to induce a leaky endothelial barrier indicating that the basal activity of Gαi-coupled to S1P1 may itself be required for barrier function (Patterson et al., 1995). However, inhibition of SOC channels did not alter S1P-induced enhancement of endothelial barrier function demonstrating S1P induces endothelial barrier enhancement via Gi→PLC-dependent release of Ca2+ from the ER stores via IP3-sensitive channels (Mehta et al., 2005). Based on the findings that inhibition of Gβγ subunits partially attenuated S1P induced strengthening of the endothelial barrier (Gonzalez et al., 2006; Garcia et al., 2001) it can be speculated that Gβγ-induced increase in Ca2+ is required for S1P1-induced enhancement of barrier function.
2.1.2 G-proteins involved in S1P1-induced FAK activation
S1P induces a rapid increase in tyrosine phosphorylation of FAK in endothelial cells at Y397, but more robust phosphorylation at Y576 (Lee et al., 2000). Suppression of endogenous FAK expression by siRNA transfection significantly attenuated S1P-induced increases in transendothelial electrical resistance (Thennes et al., 2008), indicating FAK plays a key role in S1P-induced barrier enhancement. S1P failed to induce FAK activity in endothelial cells pretreated with Ptx (Figure 6B) (Lee et al., 2000), suggesting that S1P-mediated FAK phosphorylation occurs downstream of Gαi. S1P is known to activate ERK and p38 MAPK activation in a Ptx-sensitive manner (Lee et al., 1999). However, treatment of human umbilical vein endothelial cells with either MEK (mitogen-activated protein kinase/extracellular signal-regulated kinase) inhibitor, U0126, or the p38 MAPK inhibitor, SB203580, did not influence the S1P-induced FAK tyrosine phosphorylation (Lee et al., 2000), indicating that MEK or MAPKs do not participate in Gαi-induced FAK phosphorylation. FAK phosphorylation by S1P was blocked by U73122, a PLC inhibitor, indicating S1P activated FAK via PLC (Lee et al., 2000). Since PLC-mediated increase in intracellular Ca2+ can stimulate PKC a possibility exists that PKC may induce FAK activity. However, studies showed that S1P-induced FAK phosphorylation was not affected following inhibition of PKC with GF109203X (Lee et al., 2000). In contrast, FAK phosphorylation was completely blocked by the intracellular Ca2+ chelator, BAPTA-AM, suggesting PLC→ Ca2+ increase plays an important role in S1P-induced FAK tyrosine phosphorylation (Lee et al., 2000). These studies show that FAK tyrosine phosphorylation is mediated by Gi-coupled receptors and subsequent downstream signaling involved PLC→Ca2+ pathway, but not PKC. Because Fyn induced FAK activation plays a critical role in maintaining endothelial barrier function (Knezevic et al., 2009), it is possible that an increase in intracellular Ca2+ may induce the activity of these SFKs. Since Gβγ contributed in Gαi-induced barrier enhancement by S1P it is probable that similar to PAR1, Gβγ→ Fyn axis may be required for inducing FAK activity downstream of S1P1.
2.2 Role and relationship between small G proteins and FAK downstream of S1P1 in strengthening endothelial barrier function
At physiological concentrations S1P1 rapidly induces Rac1 activity but had no effect on RhoA and Cdc42 activity (Garcia et al., 2001; Lee et al., 2001; Mehta et al., 2005). Rac1 activity was required for S1P1 strengthening of the endothelial barrier since S1P failed to enhance endothelial barrier function in endothelial cells transducing dominant negative-Rac1 (Mehta et al., 2005). Using live cell imaging of sub-confluent endothelial cells, Mehta et al. (2005) showed that S1P stimulated Rac1 translocation to intercellular junctions which sealed the gaps between endothelial cells. They also demonstrated that Rac1 was activated by PLC-dependent intracellular Ca2+ release. Rac1 affects actin remodeling through ADP-ribosylation factor GTPase activating proteins (ARF GAPs) (Turner et al., 2001). ARF GAPs, G-protein-coupled receptor kinase-interacting proteins GIT1 and GIT2 redistribute to the cell periphery following S1P stimulation (Shikata et al., 2003b), suggesting their involvement in Rac1 mediated cortical actin ring formation and focal adhesion redistribution. As previously mentioned, FAK modulates changes in barrier permeability by promoting the assembly of adherens junctions which may occur through Rac1 and Cdc42-dependent mechanisms (Wu et al., 2004; Holinstat et al., 2006; Chang et al., 2007). Rac1 activation requires the activation of upstream regulators which convert Rac1-GDP to Rac1-GTP (Takai et al., 2001). Rac1 is held inactive by GDI-1, thus dissociation of GDI-1 is required for the GTP exchange by Rac- guanine exchange factor (GEF)s, such as Tiam-1 and Vav2 (Crespo et al., 1997; Mertens et al., 2003). FAK regulates Rac-GEF Tiam1 (Elias et al., 2010). Activated FAK associates with GIT1 and GIT2 following focal adhesion redistribution (Shikata et al., 2003a). GIT1 is tyrosine phosphorylated following agonist stimulation in a Rho Kinase, Src, and FAK dependent manner (van Nieuw Amerongen et al., 2004). Therefore, FAK and Rac1 signaling may interact downstream of S1P1 to enhance endothelial barrier function (Figure 7).
Figure 7. S1P1 signaling leading to FAK activation and enhancement of endothelial barrier function.
Upon ligation of S1P1 by S1P, the Gαi (or) Gβγ subunit mediates an increase in intracellular Ca2+ leading to FAK activation. FAK regulates Rac1 to modulate adherens junction assembly which is required for strengthening of the endothelial barrier.
3.0 Integration between PAR1 and S1P1-induced FAK activity and endothelial barrier function
Thrombin and S1P induce FAK activity but produce divergent effects on vascular endothelial cell barrier function. However, both PAR1 and S1P1 require FAK activity to strengthen or restore basal endothelial barrier function (Figure 8). There are several facets of FAK signaling that could allow it to modulate endothelial barrier function downstream of PAR1 and S1P1. These include differential FAK phosphorylation, distinct spatial/temporal activation and redistribution of FAK, or the ability of FAK to act as a kinase or scaffold protein.
Figure 8. FAK serves as an essential node between PAR1 and S1P1 signaling that maintains normal endothelial permeability.
Thrombin activation of PAR1 causes the dissociation of the dissociation of the Gα subunit of the heterotrimeric G protein dissociates from Gβγ. Gαq-mediated increase in intracellular Ca2+ concentration increases endothelial permeability by activating MLCK and RhoA. RhoA also induces FAK activation. FAK negatively regulates RhoA-GTP by activating p190RhoGAP to turn off endothelial cell contraction. P190RhoGAP also interacts with p120-catenin to facilitate re-annealing of adherens junctions thereby restoring normal barrier function downstream of PAR1. S1P activation of S1P1 stimulates Gαi (Gβγ) -mediated PLC activation and increase in intracellular Ca2+ that activates Rac1 and FAK to facilitate strengthening of adherens junctions and the endothelial barrier integrity. Importantly, PAR1 by inducing SPHK1 activity increases S1P levels that in a paracrine manner activate S1P1 signaling cascade to reverse the endothelial barrier permeability increase by thrombin.
FAK localizes differentially in endothelial cells following thrombin and S1P stimulation (Lee et al., 2000; Knezevic et al., 2009; Sun et al., 2009). For example, thrombin within minutes induces FAK translocation to the focal adhesions (Mehta et al., 2002; Knezevic et al., 2009). However, FAK was also found to be located at the intercellular junctions during the later phases of thrombin challenge (Mehta et al., 2002; Shikata et al., 2003a; Knezevic et al., 2009). S1P predominantly induces FAK redistribution at the cell periphery (Sun et al., 2009). Moreover, thrombin and S1P differentially phosphorylate FAK. Thrombin induces robust phosphorylation of FAK at three tyrosine residues namely, Y397, Y576, and Y925, whereas S1P induces more robust phosphorylation at Y576 (Shikata et al., 2003a). Selective SFK inhibitor PP2 abolished S1P-induced FAK phosphorylation at Y576 and its redistribution at cell periphery in association with inhibition of cortical actin ring formation (Shikata et al., 2003a). However, PP2 abrogated thrombin-induced phosphorylation only at Y397 but not at Y576 or Y925, and did not influence thrombin-induced actin stress fiber formation, suggesting that SFKs may be needed for S1P-induced FAK phosphorylation (Shikata et al., 2003a). We showed that Fyn was required for thrombin-induced FAK phosphorylation since Fyn, but not cSrc knockdown, prevented FAK phosphorylation and restoration of endothelial barrier permeability following thrombin challenge (Knezevic et al., 2009). Furthermore, this study showed Fyn null lungs were basally leaky and could not resolve edema formation following PAR1 activation. Expression of a FAK mutant where Y397 and Y576 were replaced with D397/D576 to mimic a phosphorylation charge (phosphomimicking mutant or constitutive phosphorylated FAK mutant) in Fyn knockout vasculature restored basal lung barrier function (Knezevic et al., 2009). Thus, differences in the phosphorylation of FAK by thrombin and S1P highlight a potentially important role for FAK in the mechanisms that underlie differential actin dynamics and cytoskeletal rearrangement needed for regulation of endothelial barrier function.
FAK cellular localization may have an important influence in regulating endothelial barrier function. FAK is historically known to localize at focal adhesions, where it regulates focal adhesion formation and turnover by modulating phosphorylation of several focal adhesion proteins, including vinculin and paxillin (Schaller, 2001). However, more stringent analysis of FAK localization in endothelial cells has shown that FAK is also localized at the cell periphery (Knezevic et al., 2009; Sun et al., 2009). Thrombin stimulation initially increases FAK presence at focal adhesion and at stress fibers, but during barrier strengthening FAK localizes to adherens junctions complexes where it interacts with β- and p-120 catenin (Knezevic et al., 2009; Sun et al., 2009), which may allow FAK to facilitate cell-cell contacts formation.
During barrier strengthening processes VE-cadherin is shown to accumulate at cell-cell borders to seal the intercellular gaps (Mehta and Malik, 2006). FAK has been shown to phosphorylate E-cadherin and promote E-cadherin enrichment at the cell periphery that enhances barrier function following stimulation of endothelial monolayer with hyperosmolar stress (Quadri et al., 2003). What dictates FAK redistribution to focal adhesions or to adherens junctions remains to be clarified? Persistent FAK activation and localization at the intercellular junctions following thrombin stimulation may be in part due to delayed activation of sphingosine kinase 1 (SPHK1) activity. SPHK1 synthesizes S1P from sphingosine, which in turn has been proposed to induce S1P1 signaling via a paracrine manner (Spiegel and Milstien, 2003). Depletion of SPHK1 in endothelial cells disrupted basal barrier function and induced a persistent increase in endothelial permeability after thrombin challenge (Tauseef et al., 2008), reciprocating the findings in FAK depleted endothelial cells (Mehta et al., 2002; Holinstat et al., 2006). Consistently, SPHK1 null mice had the similar phenotype as the Fyn null mice; i.e. the basal lung vascular permeability was more in SPHK1 null mice and these mice developed profound edema in response to PAR1 activation and endotoxin exposure (Tauseef et al., 2008; Knezevic et al., 2009). Whether PAR1 and S1P1 pathways converge at the level of Gβγ and/or SPHK1 to induce FAK activity and to correct endothelial barrier dysfunction needs to be determined.
3.1 Contribution of kinase or scaffold function of FAK in regulating endothelial barrier function
Due to the presence of several domains in FAK, including a FERM domain, proline rich domains, SH2 domains and the focal adhesion targeting (FAT) domain, FAK may regulate the endothelial cell barrier function by serving as a scaffold rather than exclusively as a kinase. Guan’s group generated endothelial cell-specific FAK kinase dead knockin mice to assess a kinase-independent role of FAK in vascular development (Zhao et al., 2010). These authors showed that FAK kinase dead knockin embryos developed normally till d13.5 as compared to endothelial cell-FAK null mice which did not survived at this stage (Shen et al., 2005; Braren et al., 2006). However, FAK-kinase dead knockin embryos exhibited vessel dilation and defective angiogenesis (Zhao et al., 2010). They further showed that FAK kinase activity was required for VE-cadherin expression thereby maintaining endothelial barrier function but not for endothelial cell survival. At the same time, Schlaeper’s group, using catalytic defective FAK knock-in mice (R454-FAK), showed that FAK activity was required for blood vessel morphogenesis and cell motility but not proliferation (Lim et al., 2010). These authors also demonstrated that FAK regulated these responses by regulating p190RhoGAP activity (Lim et al., 2010). Thus, FAK kinase activity plays a key role in endothelial barrier formation. FAK also binds to N-WASP, ASAP1, GRAF, Grb2 and p190RhoGEF that may additionally orchestrate cross-talk between focal adhesion complexes, actin cytoskeleton and intercellular adhesion (Mehta and Malik, 2006). However, neither GRAF nor ASAP1 are known to be tyrosine phosphorylated although ASAP1, a GAP for the ARF family of GTPases, binds FAK (Liu et al., 2002). The FAK/ASAP1 interaction is required for cell spreading, as it is required for the recruitment of FAK and paxillin at focal contacts. Thus, although FAK kinase activity is required for regulating endothelial barrier function FAK may also maintain barrier by kinase-independent mechanisms.
Summary
In summary, PAR1 rapidly increases endothelial permeability which is followed by a recovery phase during which intercellular junctions are re-annealed, thereby restoring basal barrier function (Mehta and Malik, 2006). S1P1 activation, on the other hand, enhances endothelial barrier function and limits the increase in endothelial permeability by PAR1 and other inflammatory agonists (Hla, 2004; McVerry et al., 2004; Tauseef et al., 2008). There is considerable evidence indicating that impairment of FAK function can lead to persistent increase in endothelial permeability by PAR1 and abrogates S1P-enhancement of barrier function (Shikata et al., 2003a; Shikata et al., 2003b; Thennes et al., 2008; Sun et al., 2009). It appears that PAR1-coupled Gα signaling mediates FAK activation which serves to oppose contraction limiting cell detachment. PAR1-coupled Gβγ induces sustained FAK activation via a Fyn-dependent pathway that re-anneals intercellular adhesion and restores permeability (Knezevic et al., 2009). Growing evidence indicates that Gαi proteins signal FAK activation and enhance barrier function downstream of S1P1. Findings from knock-in mouse models and Fyn null mice transiently expressing FAK Y397D/Y576D mutants (constitutively active FAK) (Knezevic et al., 2009) suggest that FAK kinase activity may play a predominant role in regulating endothelial barrier function. However, it is likely that protein-protein interactions mediated by the FAK FERM and FAT domains may also contribute in inducing cross-talk between the cell-matrix and cell-cell junctions crucial in maintaining endothelial barrier function. Thus, upregulation of FAK function could be beneficial in mitigating or reversing syndromes caused by leaky vessels.
Future Perspective
As we have discussed in this review, the regulation of endothelial permeability by PAR1 and S1P1 is still incompletely understood. The binding affinity of various Gα and Gβγ subunits with these receptors remains to be teased out using powerful imaging techniques such as fluorescence resonance energy transfer (FRET) analysis. It will be of interest to determine whether S1P1 signaling differs from PAR1 due to differences in the binding affinity of S1P1 with Gi (perhaps Gβγ) or due to a differential role of RACK1 in PAR1 signaling. Studies using endothelial cells and transient transfection of dn-FAK or Y397/Y576 FAK mutants in the mouse vasculature have clearly demonstrated that FAK is required for maintaining endothelial barrier integrity and for reversing PAR1 induced endothelial barrier disruption. Moreover, the FAK kinase dead knockin mouse model has also defined the developmental role of FAK in inducing vessel formation by regulating VE-cadherin expression and p190RhoGAP function. However, whether endothelial specific deletion of FAK in adult mice compromises endothelial barrier formation needs investigation. Identification of FAK as the point of demarcation and convergence between PAR1 and S1P1 signaling will lead to the discovery of signaling cues that “switch off” Gα signaling while turning on Gβγ signaling, thereby maintaining normal barrier function.
Research Highlights.
We discuss the role of the G protein subunits downstream of PAR1 in regulating FAK activity and endothelial barrier function.
We describe the role of RACK1 in negatively suppressing endothelial recovery following disruption of endothelial barrier function by PAR1
We discuss the role of the G protein subunits downstream of S1P1 in regulating FAK activity and endothelial barrier function.
We highlight FAK as the point of convergence downstream of PAR1 and S1P1 signaling that maintains barrier function.
Acknowledgments
This work was supported by National Institutes of Health grants HL71794, HL84153 and HL060678 as well as by an American Heart Association pre-doctoral fellowship to Tracy Thennes (10PRE2610268).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–51. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Adam AP, et al. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem. 2010;285:7045–55. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–42. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- Alessandro R, et al. Endothelial cell spreading on type IV collagen and spreading-induced FAK phosphorylation is regulated by Ca2+ influx. Biochem Biophys Res Commun. 1998;248:635–40. doi: 10.1006/bbrc.1998.8705. [DOI] [PubMed] [Google Scholar]
- Allende ML, et al. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–7. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- Andreeva AV, et al. G alpha12 interaction with alphaSNAP induces VE-cadherin localization at endothelial junctions and regulates barrier function. J Biol Chem. 2005;280:30376–83. doi: 10.1074/jbc.M502844200. [DOI] [PubMed] [Google Scholar]
- Andreeva AV, et al. Novel mechanisms of G protein-dependent regulation of endothelial nitric-oxide synthase. Mol Pharmacol. 2006;69:975–82. doi: 10.1124/mol.105.018846. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, et al. Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB J. 2010;24:3522–35. doi: 10.1096/fj.10-154997. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya R, et al. Differences in Galpha12- and Galpha13-mediated plasma membrane recruitment of p115-RhoGEF. Cell Signal. 2009;21:996–1006. doi: 10.1016/j.cellsig.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Blackmer T, et al. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–5. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- Blackmer T, et al. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–7. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Braren R, et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–62. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman MT, et al. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res. 2006;98:73–80. doi: 10.1161/01.RES.0000198387.44395.e9. [DOI] [PubMed] [Google Scholar]
- Brown MV, et al. PDGF receptor activation induces p120-catenin phosphorylation at serine 879 via a PKCalpha-dependent pathway. Exp Cell Res. 2009;315:39–49. doi: 10.1016/j.yexcr.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundey RA, Insel PA. Adenylyl cyclase 6 overexpression decreases the permeability of endothelial monolayers via preferential enhancement of prostacyclin receptor function. Mol Pharmacol. 2006;70:1700–7. doi: 10.1124/mol.106.028035. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, et al. ROCK mediates thrombin’s endothelial barrier dysfunction. Am J Physiol Cell Physiol. 2000;279:C195–204. doi: 10.1152/ajpcell.2000.279.1.C195. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC., Jr. RhoA inactivation enhances endothelial barrier function. Am J Physiol. 1999;277:C955–64. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- Chang BY, et al. The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem. 2001;276:20346–56. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- Chang BY, et al. RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol. 1998;18:3245–56. doi: 10.1128/mcb.18.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, et al. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell. 2007;18:253–64. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, et al. RACK1 regulates specific functions of Gbetagamma. J Biol Chem. 2004a;279:17861–8. doi: 10.1074/jbc.M313727200. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. RACK1 binds to a signal transfer region of G betagamma and inhibits phospholipase C beta2 activation. J Biol Chem. 2005;280:33445–52. doi: 10.1074/jbc.M505422200. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. Interaction of Gbetagamma with RACK1 and other WD40 repeat proteins. J Mol Cell Cardiol. 2004b;37:399–406. doi: 10.1016/j.yjmcc.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Chikumi H, et al. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002a;277:12463–73. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- Chikumi H, et al. Potent activation of RhoA by Galpha q and Gq-coupled receptors. J Biol Chem. 2002b;277:27130–4. doi: 10.1074/jbc.M204715200. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, et al. Store-operated calcium entry channels in pulmonary endothelium: the emerging story of TRPCS and Orai1. Adv Exp Med Biol. 2010;661:137–54. doi: 10.1007/978-1-60761-500-2_9. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Stevens T. Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation. 2006;13:709–23. doi: 10.1080/10739680600930354. [DOI] [PubMed] [Google Scholar]
- Cohen LA, Guan JL. Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J Biol Chem. 2005;280:8197–207. doi: 10.1074/jbc.M412021200. [DOI] [PubMed] [Google Scholar]
- Cooper LA, et al. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23:8030–41. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- Cox BD, et al. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- Crespo P, et al. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–72. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Cullere X, et al. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–5. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Del Vecchio PJ, et al. Culture and characterization of pulmonary microvascular endothelial cells. In Vitro Cell Dev Biol. 1992;28A:711–5. doi: 10.1007/BF02631058. [DOI] [PubMed] [Google Scholar]
- Drazner MH, et al. Potentiation of beta-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J Clin Invest. 1997;99:288–96. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Elias BC, et al. Polyamine-dependent activation of Rac1 is stimulated by focal adhesion-mediated Tiam1 activation. Cell Adh Migr. 2010;4:419–30. doi: 10.4161/cam.4.3.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CA, et al. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J Biol Chem. 1999;274:13718–27. doi: 10.1074/jbc.274.19.13718. [DOI] [PubMed] [Google Scholar]
- English D, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8:627–34. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- Finigan JH, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–93. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, et al. Differential expression of protease-activated receptors 1, 2, and 4 on human endothelial cells from different vascular sites. Pathobiology. 2004;71:52–8. doi: 10.1159/000072962. [DOI] [PubMed] [Google Scholar]
- Garcia JG, et al. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–94. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- Garcia JG, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, et al. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22:309–15. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem. 2008;283:29888–96. doi: 10.1074/jbc.M803880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, et al. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–6. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- Gu JL, et al. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci U S A. 2002;99:9352–7. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudermann T, et al. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found Symp. 2004;258:103–18. discussion 118-22, 155-9, 263-6. [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–72. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Herlitze S, et al. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–62. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–20. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Holinstat M, et al. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- Holinstat M, et al. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–8. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Hurowitz EH, et al. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000;7:111–20. doi: 10.1093/dnares/7.2.111. [DOI] [PubMed] [Google Scholar]
- Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jacamo RO, Rozengurt E. A truncated FAK lacking the FERM domain displays high catalytic activity but retains responsiveness to adhesion-mediated signals. Biochem Biophys Res Commun. 2005;334:1299–304. doi: 10.1016/j.bbrc.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Jin T, et al. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–6. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- Kaneider NC, et al. ’Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–12. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthou C, et al. Involvement of pertussis toxin-sensitive and -insensitive G proteins in alpha-thrombin signalling on cultured human vascular smooth muscle cells. Cell Signal. 1996;8:59–66. doi: 10.1016/0898-6568(95)02018-7. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, et al. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol. 1998;274:L810–9. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- Kini V, et al. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem. 2010;285:33082–91. doi: 10.1074/jbc.M110.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, et al. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–92. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic N, et al. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol. 2007;27:6323–33. doi: 10.1128/MCB.00523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic N, et al. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med. 2009;206:2761–77. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantoulaki M, et al. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285:L434–42. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- Korhonen H, et al. Anaphylactic shock depends on endothelial Gq/G11. J Exp Med. 2009;206:411–20. doi: 10.1084/jem.20082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouklis P, et al. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94:159–66. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- Kozasa T, et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–11. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Laird AD, et al. Src family kinase activity is required for signal tranducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2:461–9. [PubMed] [Google Scholar]
- Landis RC. Protease activated receptors: clinical relevance to hemostasis and inflammation. Hematol Oncol Clin North Am. 2007;21:103–13. doi: 10.1016/j.hoc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lee JF, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296:H33–42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- Lee MJ, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- Lee OH, et al. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–50. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- Lee OH, et al. Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway. Biochem Biophys Res Commun. 2000;268:47–53. doi: 10.1006/bbrc.2000.2087. [DOI] [PubMed] [Google Scholar]
- Li S, et al. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A. 2002;99:3546–51. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–87. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, et al. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–36. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CI, et al. Sphingosine 1-phosphate regulates inflammation-related genes in human endothelial cells through S1P1 and S1P3. Biochem Biophys Res Commun. 2007;355:895–901. doi: 10.1016/j.bbrc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. Differential regulation of sphingosine-1-phosphate- and VEGF-induced endothelial cell chemotaxis. Involvement of G(ialpha2)-linked Rho kinase activity. Am J Respir Cell Mol Biol. 2001;24:711–9. doi: 10.1165/ajrcmb.24.6.4323. [DOI] [PubMed] [Google Scholar]
- Liu G, et al. Galpha13 regulates MEF2-dependent gene transcription in endothelial cells: role in angiogenesis. Angiogenesis. 2009;12:1–15. doi: 10.1007/s10456-008-9123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell. 2002;13:2147–56. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JN, et al. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–6. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- Ludeman MJ, et al. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–8. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, et al. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- Malik AB, Siflinger-Birnboim A, editors. Vascular Endothelial Barrier Function and Its Regulation. Plenum; New York: 1993. [Google Scholar]
- Manolopoulos VG, et al. The thrombin receptor in adrenal medullary microvascular endothelial cells is negatively coupled to adenylyl cyclase through a Gi protein. Biochim Biophys Acta. 1997;1356:321–32. doi: 10.1016/s0167-4889(97)00002-5. [DOI] [PubMed] [Google Scholar]
- McCudden CR, et al. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–77. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JN, et al. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–59. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–85. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, et al. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170:987–93. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- Mehta D, et al. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–8. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mehta D, et al. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol. 2002;539:779–89. doi: 10.1113/jphysiol.2001.013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, et al. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–6. doi: 10.1016/s0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- Millard TH, et al. Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem J. 2004;380:1–17. doi: 10.1042/BJ20040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall RD, et al. Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res. 2010;80:240–9. doi: 10.1016/j.mvr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza H, et al. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–14. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, et al. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–40. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- Muraki K, Imaizumi Y. A novel function of sphingosine-1-phosphate to activate a non-selective cation channel in human endothelial cells. J Physiol. 2001;537:431–41. doi: 10.1111/j.1469-7793.2001.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, et al. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–13. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94:14489–94. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–59. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Niu J, et al. G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res. 2003;93:848–56. doi: 10.1161/01.RES.0000097607.14733.0C. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S, et al. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–5. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ, et al. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–9. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Conditional mutagenesis of G-protein coupled receptors and G-proteins. Handb Exp Pharmacol. 2007:491–509. doi: 10.1007/978-3-540-35109-2_20. [DOI] [PubMed] [Google Scholar]
- Offermanns S, et al. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science. 1997;275:533–6. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Patterson CE, et al. Mechanisms of pertussis toxin-induced barrier dysfunction in bovine pulmonary artery endothelial cell monolayers. Am J Physiol. 1995;268:L926–34. doi: 10.1152/ajplung.1995.268.6.L926. [DOI] [PubMed] [Google Scholar]
- Peng X, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–51. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, et al. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–7. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Qiao J, et al. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–80. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- Quadri SK, et al. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. 2003;278:13342–9. doi: 10.1074/jbc.M209922200. [DOI] [PubMed] [Google Scholar]
- Quadri SK, Bhattacharya J. Resealing of endothelial junctions by focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol. 2007;292:L334–42. doi: 10.1152/ajplung.00228.2006. [DOI] [PubMed] [Google Scholar]
- Ramchandran R, et al. Critical role of Cdc42 in mediating endothelial barrier protection in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;295:L363–9. doi: 10.1152/ajplung.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–63. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–8. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Rojas RJ, et al. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–10. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LH, et al. Focal adhesions: paradigm for a signaling nexus. Circ Res. 2006;98:606–16. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- Sanchez T, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via alpha1beta1- and alpha2beta1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G547–57. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- Sandoval R, et al. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2001;280:L239–47. doi: 10.1152/ajplung.2001.280.2.L239. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schaphorst KL, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–67. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- Schaphorst KL, et al. Thrombin-mediated focal adhesion plaque reorganization in endothelium: role of protein phosphorylation. Am J Respir Cell Mol Biol. 1997;17:443–55. doi: 10.1165/ajrcmb.17.4.2502. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, et al. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–78. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–33. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Schneider T, et al. G protein interaction with K+ and Ca2+ channels. Trends Pharmacol Sci. 1997;18:8–11. doi: 10.1016/s0165-6147(96)01001-2. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, et al. Segmental differentiation of permeability, protein glycosylation, and morphology of cultured bovine lung vascular endothelium. Biochem Biophys Res Commun. 1994;199:11–9. doi: 10.1006/bbrc.1994.1185. [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 2004;279:48055–4806. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- Shajahan AN, et al. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:48055–62. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- Shen TL, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata Y, et al. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003a;17:2240–9. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- Shikata Y, et al. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol. 2003b;94:1193–203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- Simon MI, et al. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Singh I, et al. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–43. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Sun X, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77:304–13. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Y. J, Kaziro Y, Itoh H. Activation of c-fos promoter by Gbetagamma-mediated signaling: involvement of Rho adn c-Ju. J Biochem. 1999;125:515–21. doi: 10.1093/oxfordjournals.jbchem.a022315. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, et al. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–80. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Suzuki N, et al. Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals. 2009;17:55–70. doi: 10.1159/000186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak WS, et al. Sphingosine 1-phosphate rescues canine LPS-induced acute lung injury and alters systemic inflammatory cytokine production in vivo. Transl Res. 2008;152:213–24. doi: 10.1016/j.trsl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, et al. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. Adenylyl cyclases. Cell. 1992;70:869–72. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Taurin S, et al. Gbetagamma-mediated prostacyclin production and cAMP-dependent protein kinase activation by endothelin-1 promotes vascular smooth muscle cell hypertrophy through inhibition of glycogen synthase kinase-3. J Biol Chem. 2007;282:19518–25. doi: 10.1074/jbc.M702655200. [DOI] [PubMed] [Google Scholar]
- Tauseef M, et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–72. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thennes T, et al. The role of Focal Adhesion Kinase in Sphingosine-1-Phosphate induced endothelial barrier enhancement. FASEB J. 2008;22:1178.1. [Google Scholar]
- Thennes T, et al. Loss of endothelial Focal adhesion kinase in mice causes lung vascular leak, alveolar destruction and inflammation. FASEB J. 2010;24:956.4. [Google Scholar]
- Thornton C, et al. Spatial and temporal regulation of RACK1 function and N-methyl-D-aspartate receptor activity through WD40 motif-mediated dimerization. J Biol Chem. 2004;279:31357–64. doi: 10.1074/jbc.M402316200. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, et al. Impairment of store-operated Ca2+ entry in TRPC4(-/-) mice interferes with increase in lung microvascular permeability. Circ Res. 2002a;91:70–6. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, et al. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002b;39:173–85. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Tolle M, et al. Immunomodulator FTY720 Induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96:913–20. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- Torsoni AS, et al. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H1488–96. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- Turner CE, et al. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593–9. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, et al. Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem. 2000;275:26206–12. doi: 10.1074/jbc.M001863200. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, et al. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res. 2004;94:1041–9. doi: 10.1161/01.RES.0000125627.77235.0C. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, et al. PKCα-mediated signaling in regulating adherens junction integrity. FASEB J. 2009;7:121–1. [Google Scholar]
- Vanhauwe JF, et al. Thrombin receptors activate G(o) proteins in endothelial cells to regulate intracellular calcium and cell shape changes. J Biol Chem. 2002;277:34143–9. doi: 10.1074/jbc.M204477200. [DOI] [PubMed] [Google Scholar]
- Vepa S, et al. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:L150–8. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- Verin AD, et al. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–76. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- Vogel SM, et al. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics. 2000;4:137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
- Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther. 2003;97:95–115. doi: 10.1016/s0163-7258(02)00326-1. [DOI] [PubMed] [Google Scholar]
- Wildenberg GA, et al. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–39. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–66. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, et al. Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J Physiol. 2003;552:691–9. doi: 10.1113/jphysiol.2003.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, et al. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279:9565–76. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- Yaka R, et al. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99:5710–5. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189:955–65. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]