Abstract
Objective
To reduce stimulus transduction artifacts in EEG while using insert earphones.
Design
Reference Equivalent Threshold SPLs (RETSPLs) were assessed for Etymotic ER-4B earphones in fifteen volunteers. Auditory brainstem responses (ABRs) and middle latency responses (MLRs) – as well as long-duration complex ABRs – to click and /dɑ/ speech stimuli were recorded in a single-case design.
Results
Transduction artifacts occurred in raw EEG responses, but they were eliminated by shielding, counter-phasing (averaging across stimuli 180° out of phase) or re-referencing.
Conclusions
Clinical-grade ABRs, MLRs, and cABRs can be recorded with a standard digital EEG system and high-fidelity insert earphones, provided one or more techniques are used to remove the stimulus transduction artifact.
Keywords: ABR, cABR, ER-4B, headphones, RETSPL
Introduction
This research focuses on the measurement of neural events in the first tens of milliseconds after the onset of a sound. ABRs are used routinely for this purpose in the field of audiology. Clinical systems typically present thousands of short sounds such as 0.1-msec clicks, then average one electrode’s EEG signal within a short time-epoched segment (e.g., from −2 to 10-msec post-stimulus onset). However, the acoustical world is largely comprised of complex, long-duration sounds including both speech and music. As with traditional click-ABRs, complex ABRs (cABRs) to such sounds encode onsets, but cABRs also reflect key properties of the ongoing acoustical waveform, including envelope and fundamental frequency information (for a review, see Skoe and Kraus 2010). As such, cABR investigations have the potential to improve our understanding of hearing impairment.
Within clinical systems, it is common to permit brief epochs of only ten to twenty milliseconds. This precludes measurement of cABRs to complex sounds, as well as middle latency responses (MLRs), and Long Latency Responses (LLRs) such as the auditory N1 or mismatch negativity (Hall, 2006:pg.2, Campbell & Neuvonen 2007, Campbell, Winkler, & Kujala 2005, 2007, Campbell, Winker, Kujala et al. 2003, for a review, see Näätänen and Winkler, 1999). Note that a few extant clinical systems do permit recordings of longer epochs, though this is not a basic feature but rather requires an add-on. Furthermore, even those systems have relatively small numbers (<10) of electrodes. For instance, Intelligent Hearing Systems SmartEP appears to lead the field with an 8-channel recording system, with an optional P300/MMN module. The Natus Bio-logic auditory evoked potential system offers a similar add-on with a 2-channel recording system. This precludes the use of techniques to localize the cortical generators of auditory Long Latency Responses and therefore, as discussed below, relate peripheral activity (ABRs) to its central neural and perceptual consequences. Thus users of clinical auditory evoked potential systems, even with add-ons, often do not have these tools available to them.
Conversely, users of ordinary digital EEG systems (Fisch & Spehlmann, 1999; Swartz, 1998), which measure longer responses and signals from multiple electrodes, are largely unaware that their system might also be used to acquire ABRs. That is, there are investigators with clinical systems who cannot measure later responses, and those with ordinary digital EEG systems who do not appreciate that they may be equipped to acquire ABRs as well. The ability to measure both ascending brainstem and later cortical responses, and to characterize their relationships, would have not only clinical but also basic scientific import.
Peripheral sensorineural damage is the most common origin of hearing impairment (Yueh, Shapiro, MacLean et al, 2003). As this damage limits the information available to the brain, the auditory periphery remains the focus of most clinical efforts. However, a more integrated view of the auditory system is necessary to understand the varied central consequences of peripheral loss (Shinn-Cunningham & Best, 2008; Pichora-Fuller & Singh, 2006) and to characterize the multiple forms of hearing impairments that are not of peripheral origin, which fall under the umbrella term of Central Auditory Processing (CAP) abnormalities (Banai & Kraus 2006; 2008). CAP is a major public health problem, shown to have a prevalence of 76.4% in a population of individuals over the age of 55 years (Golding et al. 2004). Furthermore, central adaptation to peripheral impairment over the course of our lives without an aid can also affect whether rehabilitation is necessary or possible after an aid is fitted (Woods & Yund 2007).
Understanding the peripheral and central mechanisms of hearing, as well as their relation, will be fundamental in advancing both the basic science of hearing and clinical practice, as it could lead to biomarkers that offer specificity for treatment. In this regard, clinical systems that are specialized for ABR measurement do not allow measurement of longer thalamocortically-generated MLRs. A basic purpose of the present work is thus to demonstrate if ABRs to click and speech stimuli, together with MLRs, and the long-lasting cABR responses to speech stimuli (Skoe & Kraus, 2010) can be recorded simultaneously via one ordinary digital EEG system. This feature of simultaneous recording is not a capability of the current state-of-the-art in clinical auditory evoked potential systems. A second purpose is to identify technical recommendations for presentation of complex auditory stimuli during such a measurement, to which we now turn.
Stimulus transduction artifacts occur when stimulus-producing current in the headphone wire or transducer, proximal to the body or electrode components, contaminates the EEG measurement. The acoustical waveform mirrors aspects of the stimulus-producing current. Clinical systems use several common ways of avoiding the artifact. One is to present very brief stimuli (clicks) so the artifact ends before the ABR is evident (<1-msec). However, this does not work for realistic stimuli, which overlap in time with their cABRs (Skoe & Kraus, 2010). Another method is to deliver stimuli pneumatically via plastic tubes, where the transducer is situated at a distance of up to 20 feet from the patient (Killion 1984); e.g., the Etymotic ER-3A (Henry et al. 2001, Hall 2006:pg.71). Stimulus transduction artifact has been shown to be eliminated by grounded shielding of the electrical apparatus from such a system (Akhoun, Moulin, Jeanvoine, et al. 2008; Riazi & Ferraro 2008), even when that apparatus was close to the volunteer. However, such earphones are costly and have an inferior frequency response for sounds above 6–7 kHz (Henry et al. 2001), an important range for spatial localization and speech cues (consonant stops). For instance, high frequency cues contribute to the perception of sibilants and fricative sounds by children during middle childhood (Stelmachowicz et al., 2001; 2008). An additional shortfall of tubing is that of acoustic dispersion, whereby the low frequency components of sounds travel faster than high frequency components within a tube, such that the phase relations of different frequency components becomes distorted: e.g., a click can become smeared into a frequency-modulated sweep or “chirp” (Kinsler et al. 1982). The frequency response and fidelity of the pneumatic approach thus has limitations for those interested in the responses to everyday complex stimuli such as speech or music sounds, but the problem is also relevant for click stimuli (Kinsler et al. 1982). The tubing approach does offer a … solution to the problem of stimulus transduction artifact should: i) the investigation not require high fidelity reproduction of sound and, ii) should the investigation not need the reproduction of high frequency information such as speech or location cues.
Typical lower cost solutions to the stimulus transduction artifact include “counter-phasing”, where EEG responses to two versions of the same stimulus with opposing polarity cancel out the artifact (Hall 2006: pg.248, Aiken & Picton 2008, Skoe & Kraus 2010), and referencing, where the EEG signal is compared to an equally contaminated electrode so the artifact is Common-Mode Rejected (Fisch & Spehlmann, 1999). A third approach that we explore here is electrically-shielding the headphone wire with a grounded Faraday cage of conducting mesh.
With a view to the high-fidelity reproduction of speech cues as is ideal for cABRs to speech sounds, here we used the Etymotic ER-4B earphones (Henry et al. 2001). ER-4Bs are of a very different construction from the ER-3As that are the current state-of-the-art in clinical evaluations. The ER-4B could in theory induce greater artifact, having wires right up to the ear and the transducer in the ear canal. To determine whether auditory responses to click and /dɑ/ speech stimuli during the first 60-msec can be measured with an ordinary digital EEG system (Fisch & Spehlmann, 1999; Swartz, 1998) and personal computer, here we test the effectiveness of three low cost approaches – counter-phasing, referencing and shielding – to the eliminate stimulus transduction artifact from ER4-B earphones.
Materials and Methods
Participants
15 individuals (6 males) aged 18 years 10 months to 28 years 11 months old (mean age: 22 years 6 months, standard deviation, 2 years 9 months) participated in the audiogram calibration sessions, having reporting normal hearing (Cox & Alexander 1995) without a history of i) ear infections or injury, ii) regular firearms use, or iii) working in clubs, construction, or other loud situations. A 23-year old male with self-reported normal hearing participated in the EEG measurements reported here. In accordance with the Declaration of Helsinki, all participants gave informed written consent with ethical approval by the campus Institutional Review Board.
RETSPL Measurement
RETSPLs for Etymotic (Elk Grove Village, Illinois, USA) ER-4B earphones (Henry et al. 2001) with ER4-18 3-flange eartips were determined with an automated audiogram procedure. dB SPL values were measured using a Brüel & Kjær 4157 ear-simulator attached to a Larson Davis ½″ preamplifier. dB SPL values were calibrated to a 1000 Hz tone from a Larson Davis Cal200 acoustic calibrator. Participants were instructed to press a computer key in response to hearing a tone. Pure tones (125 to 16000-Hz; Table 1) were presented for 200-msec with a 20-msec linear onset and offset ramp with 1000 to 1500-msec jittered delay. Each frequency × ear (13 × 2) was tested in random order using an automated version of the modified Hughson-Westlake ANSI S3.21-1978 (R-1992) procedure, starting at a below threshold volume, with a 10 dB increase on misses and a 5 dB decrease for hits, requiring a 2/3 ascending hit/miss ratio to determine each threshold. Using the RETSPL values derived from the average of the calibration participants, the EEG participant’s audiometric thresholds were determined to be better than 20 dB HL at all frequencies, as tested before and after the EEG session.
Table 1.
RETSPL as a function of tone frequency for Etymotic ER-4B insert phones with ER4-18 3-flange eartips, referenced with a Brüel & Kjær 4157 ear-simulator (N= 15).
| Frequency | RETSPL |
|---|---|
| 125 | 54.08 |
| 250 | 38.25 |
| 500 | 24.04 |
| 750 | 18.85 |
| 1000 | 16.42 |
| 1500 | 18.88 |
| 2000 | 22.03 |
| 3000 | 21.37 |
| 4000 | 19.39 |
| 6000 | 18.43 |
| 8000 | 17.26 |
| 11200 | 27.20 |
| 16000 | 51.83 |
EEG recording session
A single participant was situated in a chair within an acoustically- and electrically-shielded chamber where he watched a silent subtitled movie of his choice, while EEG was measured and sound presented via the right earphone. The level of the sound was determined to be 80 dB(C) beforehand by the comparison of an acoustical recording of the stimulus to that of a 1kHz pure tone reference produced by a Larson Davis Cal200 acoustic calibrator. These recordings were made via a Brüel & Kjær 4157 ear-simulator attached to a Larson Davis ½″ preamplifier. This comparison used the root mean square of the reference recording and the peak of the stimulus recording, filtered with a C frequency-weighting as implemented in MATLAB 7.4.0(2007a); a form of filtering that approximates the sensitivity of the human auditory system at the moderate sound levels used in this study (Moore 2003: pg. 130). Neurobehavioral Systems Presentation software (Albany, California, USA) is a crucial element of the paradigm, as it controlled event timing to within 0.1msec. ER-4B wires were either unshielded or shielded, depending on the experimental condition. Shielding was comprised of tinned-copper mesh (Scotch 24 electrical shielding tape, Austin, Texas, USA) wrapped around the length of the wire, attached to the electrical ground, and insulated with low-voltage Plymouth/Plymouth-Yonshue (Canton, China) vinyl electrical tape with rubber-based adhesive.
The experimental session began with 16 blocks of EEG recording. Blocks lasting 3 minutes 3 s contained 4 trains of 1056 1-msec clicks with linear rise and abrupt offset at Stimulus Onset Asynchrony (SOA) 40-msec, while blocks lasting 6 minutes 12 s contained 8 trains of 480 40-msec /dɑ/ stimuli (SOA: 90-msec). A 1-ms duration click was used to ensure any transduction artifact, if present, would be identified in the EEG signal, collected at a sample rate of 16384 Hz. Speech stimuli were synthesized by Praat (Boersma & Weenink 2010). The SOA between the last click of train and the first click of the next train was 4.800 s and the SOA between the last /dɑ/ of a train and the first click of the next train was 3.890 s. Leading phase (condensation, rarefaction) was randomized within blocks presented in counterbalanced order.
Electrophysiological Recording Equipment
Digital EEG and Electroculogram (EOG) was acquired via a Biosemi Active Two system (Amsterdam, The Netherlands). Each scalp electrode within this system is described as “active” by virtue of containing a pre-amplifier that dramatically reduces impedance before the signal travels along the wires to the amplifier and analog-to-digital converter. These electrodes used here are described as ‘standard’, in contrast to the ultra-flat active electrode components of the Biosemi ABR option, which could provide an even lower internal input noise and higher gain in the amplification selectively within the 100–3000 Hz pass band. Within this pass band resides the EEG power of the ABR, but not that of MLR and LLR signals. Our measurements are thus a test of sufficiency for these standard electrodes – which can be used to measure MLRs and LLRs – to also measure ABRs and cABRs.
The Biosemi ActiveTwo replaces the classical “ground” electrode with two electrodes. These are the Common Mode Sense active electrode that detects the effects upon the participant of current return from the Analog-to-Digital convertor via the Driven Right Leg (DRL) electrode that contains no amplifier. A CMS/DRL feedback loop equates the potential of the participant to the reference voltage of the Analog-to-Digital Conversion box. EEG was acquired at a scalp electrode in “raw” mode, that is, relative to this reference voltage. Thus the “reference” in “raw” recordings was the CMS electrode, which was situated at a right-posterior site between POz and PO4. This “raw” mode of recording did not permit the full Common Mode Rejection (Fisch & Spehlmann, 1999) of extraneous signals, such as stimulus transduction artifact that was present at both an EEG electrode and the CMS. These “raw” measurements are thus a test of the effectiveness of methods of counter-phasing and shielding without Common Mode Rejection by re-referencing.
EEG and EOG acquisition parameters
EEG was acquired in “raw” mode at CPz relative to a CMS reference with a pass band of DC to 3334Hz and sampled at 16384 Hz. Horizontal eye movements were monitored with a bipolar set-up, with two electrodes attached laterally to the outer canthi of each eye. Vertical eye movements were monitored by bipolar channels using pre-frontal electrodes (Fp1, Fp2) amplified against an electrode upon the tip of the nose. Electrodes were also attached to the mastoids.
Derivation of ABR, cABR, and MLR responses
For ABRs/cABRs, EEG/EOG was filtered offline (70- to 2000-Hz) and subsequently analyzed with EEGLAB (Delorme and Makeig, 2004)*. The “raw” EEG/EOG data was retained, but also re-referenced offline to the right mastoid to permit Common Mode Rejection (Fisch & Spehlmann, 1999) of stimulus transduction artifact. Analyzing raw and re-referenced data separately, EEG/EOG data were epoched, baseline-corrected, artifact-rejected and averaged; mathematical processes that are detailed below. Continuous time-series of EOG/EEG data were segmented or “epoched” into shorter time series of electrophysiological data. The “epochs” began 6-ms before the onset of each sound and ended 60 ms after the onset of each sound. The electrophysiological data in the first 6 ms of each 66-ms epoch was used to determine a mean pre-stimulus baseline value for each electrophysiological channel (e.g., CPz), which was then subtracted from every data point in that channel for the whole epoch; a process that is termed “baseline correction”. Epochs were then “artifact-rejected”, that is, these epochs were excluded from the subsequent averaging process if that baseline-corrected epoch contained potentials +/−50-μV within any EEG or EOG channel. Also, the first and last two sounds of a block, together with events immediately before, during or immediately after timing jitter greater than 1 sample (61.03-μsec) were excluded from the subsequent averaging process. Means of the electrophysiological data at each corresponding time point, in each corresponding channel, for each of the remaining epochs was taken to derive the averaged responses of interest. That is, for each combination of stimulus (click, /dɑ/), reference (raw, re-referenced) and shielding (shielding, unshielded), responses time-locked to stimuli were averaged from accepted epochs containing sounds of i) condensation and ii) rarefaction leading phase, as well as iii) collapsed across leading phase. To reveal MLRs, EEG/EOG was filtered (15 to 2000-Hz), re-referenced and responses similarly derived. These averaged responses derived from the CPz EEG channel were then over-plotted (Figures 1–2).
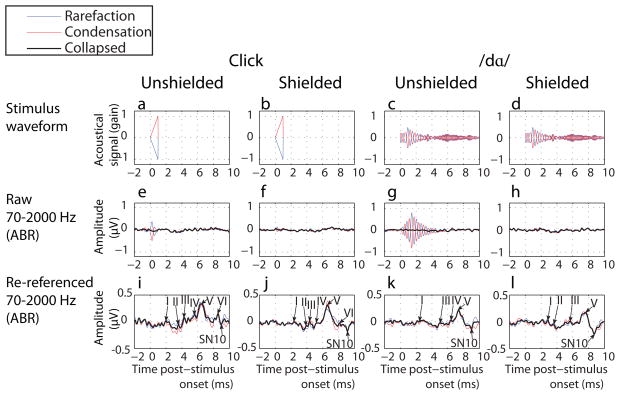
Figure 1.
The stimulus waveform (a-d) used to elicit ABRs (e-l) that are plotted as a function of shielding, reference and leading phase. Please note the different scale for the referenced waveform where ABRs are visible and the large artifact eliminated. ABR peaks are labeled by Jewett-nomenclature.
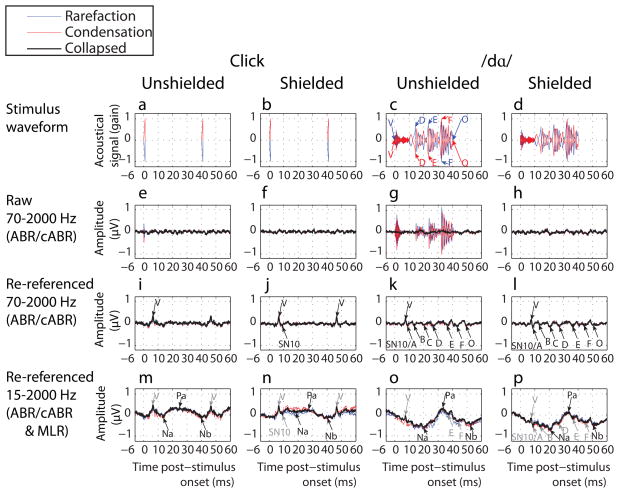
Figure 2.
The stimulus waveform (a-d) used to elicited cABRs (e-l) that are plotted as a function of shielding, reference and leading phase, together with the effect of a more open filter for referenced data that yields overlapping MLRs (m-p). ABR and MLRs labels follow Jewett- and Picton-conventions; cABRs are labeled according to Skoe and Kraus (2010), as are corresponding landmarks in the stimulus waveform.
Results
RETSPL values for the Etymotic ER-4B insert phones are depicted in Table 1.
As illustrated below, comparison of the stimulus waveform (Figures 1a-d) to this individual’s ‘raw’ ABRs to stimuli revealed that a stimulus transduction artifact was superimposed upon the ABR; an artifact that corresponded to whether the sound had a condensation or rarefaction leading phase. This correspondence was seen when headphone wires were unshielded (Figures 1e and 1g) during the interval that the sound was presented. This artifact was no longer superimposed upon the analogous ABRs following counter-phasing (Figures 1e & 1g; “collapsed” in black), or referencing (Figures 1i and 1k). Shielding (Figures 1b, 1f, 1j, 1d, 1h, 1l) also removed this superimposed artifact. Referencing revealed the ABR deflections denoted upon Figure 1i-1l.
The responses depicted over a longer time range in Figure 2e-p again revealed that artifacts superimposed onto these longer responses, in a manner that was confined to raw waveforms without counter-phasing and shielding. The artifact for /dɑ/ (Figure 2g) resembled the longer stimulus waveform (Figures 2c-d). Whether counter-phased or not, shielded or not, re-referenced waveforms (Figure 2i-l) revealed deflections of the cABR (Figure 2k and l) that did not reflect the timing and polarity of the transduction artifact, but rather corresponded to landmarks (Figures 2c-d) within the stimulus waveform; albeit time-shifted by a ca. 8-msec delay (Skoe and Kraus, 2010). These cABR deflections were not seen in the click ABRs, which instead contained an ABR to the subsequent click (Figure 2i-j). MLRs were seen with an open filter (Figure 2m-p) for clicks and /dɑ/ stimuli (black labels) onto which were superimposed the ABR and cABR deflections (grey labels).
Discussion
The results showed that a transduction artifact occurred only when both the headphone wire was not shielded, and the data was not re-referenced. In this case, counter-phasing still proved effective in eliminating transduction artifact. Accordingly, counter-phasing, shielding, and referencing were all effective in removing the transduction artifact from ABRs and cABRs. Re-referenced data revealed ABRs (Figure 1) and MLRs (Figure 2) to click and /dɑ/, and a cABR to /dɑ/ (Figure 2). Without these methods for removing transduction artifact, e.g., without grounded-shielding, ‘raw’ uncollapsed ABRs (Figures 1e and 1g) to stimulus onset and cABRs (Figure 2e and 2g) to aspects of the ongoing speech waveform were contaminated by superimposed stimulus-producing currents. The problem is more serious when the cABR responses of interest are to landmarks in that ongoing stimulus waveform (Skoe and Kraus, 2010), such that artifact could be mistaken for a cABR response. That these cABRs were elicited in the same time range as MLRs yet followed a different additive time course indicated that cABRs are not MLRs. In this investigation, one ordinary digital EEG system was thus used to simultaneously measure artifact-free ABRs, MLRs and cABRs to long-lasting speech sounds. The novel aspect of this investigation is that the established techniques of counter-phasing, shielding, and referencing proved effective when stimuli were presented via a device capable of the high-fidelity reproduction of speech cues for ABR and cABR investigations: the ER4-B. This allows us to make technical recommendations for artifact-free recordings upon presentation of complex auditory stimuli via such earphones for similar investigations. Below we consider the generality and nature of each approach to artifact elimination.
The applicability of counter-phasing depends upon the research question and subsequent analyses. That is, counter-phasing offers no solution to the problem of transduction artifact for those interested in the effects of leading phase upon early waves of the ABR (Schoonhoven 1992), for instance with inter-trial phase coherence (Delorme and Makeig 2004), which may bear perceptually-relevant information.
The effectiveness of referencing depends upon numerical equivalence of the amplitude of artifact at data and reference electrodes. Such equivalence is likely when the transduction artifact reflects mainly a current induced within the human body that reaches the sensors by volume conduction, as could be the case in the reported data. Were transduction artifact due to electromagnetic interference from the earphone wire’s field, within which the scalp and reference electrode wires are situated, the spatial proximity or orientation of each electrode wire to the earphone wire would be critical to the effectiveness of referencing. The best choices of reference electrode(s) depends upon the research question (Fisch & Spehlmann 1999), so if such electromagnetic interference were a key source of transduction artifact (Hall 2006), it could prove impossible to maximize the EEG signal of interest while eliminating the artifact via referencing alone.
Grounded shielding proved effective here, and this is probably due to the containment of the electromagnetic field produced by earphone wire currents. Without shielding, this field is thought to have induced a stimulus transduction artifact in the volunteer’s body that reached the electrodes via volume conduction. The transducer itself remained unshielded throughout, yet the transduction artifact was basically eliminated by shielding the wires alone. The primary origin of stimulus transduction artifact is thus the current in the earphone wire, rather than the balanced-armature transducer of the ER-4B. As noted in the Introduction, grounded shielding of the electrical apparatus has proved successful in eliminating transduction artifact with systems that deliver stimuli via pneumatic tubes (Akhoun et al. 2008; Riazi & Ferraro 2008). Grounded shielding approaches could have considerable potential in the investigation of the cochlear microphonic and ABRs in early life, as they could improve approaches to the early diagnosis of auditory neuropathy (Riazi & Ferraro 2008). However, earphones that deliver stimuli via pneumatic tubes suffer from acoustic dispersion (Kinsler et al. 1983) and offer an inferior representation of high frequencies (Henry et al. 2001); information that is important for speech recognition and spatial localization. A distinct approach, shielding the wiring yet not the transducer, was shown here to eliminate transduction artifact from lower cost ER4-B headphones that are viable for presentation of speech content for ABR and cABR investigations, and the presentation of high frequency auditory localization cues. While the ER3-A is the current state-of-the-art in routine clinical use, the ER4-B would show further clinical promise, should aspects of the neural processing of speech stimuli or auditory localization cues, as reflected by ABRs, cABRs or MLRs, become established as biomarkers that offer specificity for treatment. In the absence of such biomarkers, the additional clinical utility of the ER4-B is still necessarily an empirical question.
Other empirical challenges also remain. One limitation of simultaneously recording responses from several levels of the auditory system is the difficulty in finding a single stimulus and set of stimulus parameters that are optimal for all the responses. For instance, different evoked potential components exhibit, in a different manner, the property of “refractoriness”, where components become progressively attenuated upon repeated stimulation, recovering after a period of silence (Alciani et al, 1995; Campbell et al. 2003). In cortex, the optimal SOA for recording the N1 component is 1.5 seconds (Campbell et al. 2005). By contrast, the refractory period of wave V of the ABRs to a repeated click is 30 ms (Picton et al. 1981) and the optimal SOA somewhat shorter. Thus when simultaneously recording components from several levels, some degree of compromise is inevitable. However, we show here that ABRs and cABRs may be recorded alongside MLRs. Indeed, it is also possible to measure LLRs simultaneously with ABRs and MLRs (Woldorff et al. 1987; Woldoff et al. 1991), provided SOAs are jittered to allow techniques such as Adjar correction when necessary (Woldorff, 1993). Whether ABRs, cABRs, MLRs and LLRs can be simultaneously recorded will therefore depend on the experimental constraints. When they can, the potentially clinically-relevant interaction of responses at different levels of the auditory system can then be assessed.
A further challenge is that studies of the electrical auditory brainstem response (EABR) and the electrical auditory middle latency response (EAMLR) to electrical stimulation via various implanted arrays of electrodes (Starr & Brackmann, 1979; Waring et al. 1998; Firszt et al. 2002) are often fraught with technical difficulties due to stimulus transduction artifact. The applicability of variants of the techniques outlined here to the electrically-evoked responses of cochlear and brainstem implants remains both a technical challenge and an empirical question.
Conclusions
ABRs to click and speech stimuli, MLRs, and long-lasting cABR responses can be recorded simultaneously via one ordinary digital EEG system. Such equipment thus has promise for investigations that intend a more integrated approach to the auditory system in improving our understanding of hearing and hearing disorders, particularly when long-lasting responses such as MLRs and cABRs to long-duration artifact-producing stimuli are of interest. Counter-phasing, referencing and shielding each eliminated stimulus transduction artifact that originated primarily from stimulus-related current in the earphone wire. A combination of all three techniques is recommended, where appropriate, to permit the measurement of auditory responses including ABR/cABR and MLR with ordinary digital EEG hardware using ER-4B earphones.
Acknowledgments
The authors thank Nazanin Nooraei at Starkey Hearing Research Center (Starkey Laboratories, Inc.) for audiogram validation. Tom Campbell is now at the Center for Visual and Cognitive Neuroscience, Department of Psychology, North Dakota State University. This work was supported by grant R01-DC8171 (to L. M.) and F31-DC011429 (to C. B.) from the National Institutes of Health: National Institute on Deafness and other Communication Disorders.
Footnotes
ERPs can be derived using the ERPLAB plugin (http://erpinfo.org/erplab/) to EEGLAB.
References
- Akhoun I, Moulin A, Jeanvoine A, et al. Speech auditory brainstem response (speech ABR) characteristics depending on recording conditions, and hearing status: An experimental parametric study. J Neurosci Methods. 2008;175(2):196–205. doi: 10.1016/j.jneumeth.2008.07.026. [DOI] [PubMed] [Google Scholar]
- ANSI S3.21–1978. American National Standard for Manual Audiometry. R-1992. [Google Scholar]
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res. 2008 Nov;245(1–2):35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Banai K, Kraus N. Neurobiology of (central) auditory processing disorder and language-based learning disability. In: Chermak GD, Musiek FE, editors. Handbook of Central Auditory Processing Disorder: Volume I: Auditory Neuroscience and Diagnosis. San Diego, California: Plural Publishing Inc; 2006. pp. 89–116. [Google Scholar]
- Banai K, Kraus N. The dynamic brainstem: implications for APD. In: McFarland D, Cacace A, editors. Current controversies in central auditory processing disorder. San Diego, California: Plural Publishing Inc; 2008. pp. 269–289. [Google Scholar]
- Boersma P, Weenink D. Praat: doing phonetics by computer [Computer program] Version 5.1.43. 2010 retrieved 4 August 2010 from http://www.praat.org/
- Campbell T, Neuvonen T. Adaptation of neuromagnetic N1 without shifts in dipolar orientation. Neuroreport. 2007;18(4):377–380. doi: 10.1097/WNR.0b013e32801b3ce8. [DOI] [PubMed] [Google Scholar]
- Campbell T, Winkler I, Kujala T. Disruption of immediate memory and brain processes: an auditory ERP protocol. Brain Research Protocols. 2005;14(2):77–86. doi: 10.1016/j.brainresprot.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Campbell T, Winkler I, Kujala T. N1 and the mismatch negativity are spatiotemporally distinct ERP components: Disruption of immediate memory by auditory distraction can be related to N1. Psychophysiology. 2007;44(4):530–540. doi: 10.1111/j.1469-8986.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Campbell T, Winkler I, Kujala T, Näätänen R. The N1 hypothesis and irrelevant sound: Evidence from token set effects. Brain Res Cogn Brain Res. 2003;18(1):39–47. doi: 10.1016/j.cogbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Cox RM, Alexander GC. The Abbreviated Profile of Hearing-Aid Benefit. Ear Hear. 1995;16(2):176–186. doi: 10.1097/00003446-199504000-00005. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Firszt J, Chambers R, Kraus N, Reeder R. Neurophysiology of cochlear implant users I: Effects of stimulus current level and electrode site on the electrical ABR, MLR and N1-P2 response. Ear Hear. 2002;23(6):502–515. doi: 10.1097/00003446-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Fisch BJ, Spehlmann R. EEG primer: basic principles of digital and analog EEG. New York: Elsevier; 1999. [Google Scholar]
- Golding M, Carter N, Mitchell P, et al. Prevalence of central auditory processing (CAP) abnormality in an older Australian population: the Blue Mountains Hearing Study. Journal of the American Academy of Audiology. 2004;15(9):633–642. doi: 10.3766/jaaa.15.9.4. [DOI] [PubMed] [Google Scholar]
- Henry JA, Flick CL, Gilbert A, et al. Reliability of hearing thresholds: Computer-automated testing with ER-4B canal Phone (TM) earphones. J Rehabil Res Dev. 2001;38(5):567–581. [PubMed] [Google Scholar]
- Hall JW. New handbook of auditory evoked responses. Boston, Mass: Pearson; 2006. [Google Scholar]
- Kinsler LE, Frey AR, Coppens AB, Saunders JV. Fundamentals of Acoustics. San Diego: Academic Press; 1982. [Google Scholar]
- Killion MC. New insert headphones for audiometry. Hear Instrum. 1984;35(28):46. [Google Scholar]
- Moore BCJ. An introduction to the psychology of hearing. 5. Bingley, UK: Emerald, Academic Press; 2003. [Google Scholar]
- Näätänen R, Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull. 1999;125(6):826–859. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stapells DR, Campbell KB. Auditory evoked potentials from the human cochlea and brainstem. J Otolaryngol. 1981;10:1–41. [PubMed] [Google Scholar]
- Pichora-Fuller MK, Singh G. Effects of Age on Auditory and Cognitive Processing: Implications for Hearing Aid Fitting and Audiologic Rehabilitation. Trends in Amplification. 2006;10(1):29–59. doi: 10.1177/108471380601000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi M, Ferraro JA. Observations on mastoid versus ear canal recorded cochlear microphonic in newborns and adults. Journal of the American Academy of Audiology. 2008;19(1):46–55. doi: 10.3766/jaaa.19.1.5. [DOI] [PubMed] [Google Scholar]
- Schoonhoven R. Dependence of auditory brain-stem response on click polarity and high-frequency sensorineural hearing-loss. Audiology. 1992;31(2):72–86. doi: 10.3109/00206099209072903. [DOI] [PubMed] [Google Scholar]
- Shinn-Cunningham B, Best V. Selective attention in normal and impaired hearing. Trends in Amplification. 2008;12(4):283–299. doi: 10.1177/1084713808325306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory Brain Stem Response to complex sounds: A Tutorial. Ear Hear. 2010;31(3):302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Brackmann DE. Brainstem potentials evoked by electrical stimulation of the cochlea in human subjects. Annals of Otology, Rhinology and Laryngology. 1979;88(4 Pt 1):550–556. doi: 10.1177/000348947908800419. [DOI] [PubMed] [Google Scholar]
- Stelmachowicz PG, Nishi K, Choi S, Lewis DE, Hoover BM, Dierking D, Lotto A. 2008 Effects of stimulus bandwidth on the imitation of English fricatives by normal hearing children. J Speech Lang Hear Res. 2008;51:1369–1380. doi: 10.1044/1092-4388(2008/07-0115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmachowicz PG, Pittman AL, Hoover BM, Lewis DE. The effect of stimulus bandwidth on the perception of /s/ in normal and hearing-impaired children and adults. J Acoust Soc Am. 2001;110:2183–2190. doi: 10.1121/1.1400757. [DOI] [PubMed] [Google Scholar]
- Swartz BE. The advantages of digital over analog recording techniques. Electroencephalography and Clinical Neurophysiology. 1998;106(2):113–117. [PubMed] [Google Scholar]
- Waring MD, Ponton CW, Don M. Activating separate ascending auditory pathways produces different human thalamic/cortical responses. Hear Res. 1999;130(1–2):219–229. doi: 10.1016/s0378-5955(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30(1):98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Woldorff M, Hansen JC, Hillyard SA. Evidence for effects of selective attention in the mid-latency range of the human auditory event-related potential. Electroencephalogr Clin Neurophysiol. 1987;(Suppl 40):146–54. [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr Clin Neurophysiol. 1991;79(3):170–91. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- Woods DL, Yund EW. Perceptual training of phoneme identification for hearing loss. Seminars in Hearing. 2007;28(2):110–119. [Google Scholar]
- Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003;289(15):1976–1985. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]