Abstract
Hypoxia-inducible factor (HIF-1) is the key transcription regulator for multiple angiogenic factors and is an appealing target. Ginsenoside-Rg1, a nontoxic saponin isolated from the rhizome of Panax ginseng, exhibits potent proangiogenic activity and has the potential to be developed as a new angiotherapeutic agent. However, the mechanisms by which Rg1 promotes angiogenesis are not fully understood. Here, we show that Rg1 is an effective stimulator of HIF-1α under normal cellular oxygen conditions in human umbilical vein endothelial cells. HIF-1α steady-state mRNA was not affected by Rg1. Rather, HIF-1α protein synthesis was stimulated by Rg1. This effect was associated with constitutive activation of phosphatidylinositol 3-kinase (PI3K)/Akt and its effector p70 S6 kinase (p70S6K), but not extracellular-signal regulated kinase 1/2. We further revealed that HIF-1α induction triggered the expression of target genes, including vascular endothelial growth factor (VEGF). The use of small molecule inhibitors LY294002 or rapamycin to inhibit PI3K/Akt and p70S6K activities, respectively, resulted in diminished HIF-1α activation and subsequent VEGF expression. RNA interference-mediated knockdown of HIF-1α suppressed Rg1-induced VEGF synthesis and angiogenic tube formation, confirming that the effect was HIF-1α specific. Similarly, the angiogenic phenotype could be reversed by inhibition of PI3K/Akt and p70S6K. These results define a hypoxia-independent activation of HIF-1α, uncovering a novel mechanism for Rg1 that could play a major role in angiogenesis and vascular remodeling.
Electronic supplementary material
The online version of this article (doi:10.1007/s10456-011-9235-z) contains supplementary material, which is available to authorized users.
Keywords: Ginsenoside, PI3K/Akt, Angiogenesis, HIF-1α, Signaling
Introduction
Insufficient angiogenesis is a major pathological component of diseases such as chronic wounds and ischemic heart disease. Therapeutic angiogenesis aims at solving this problem by stimulating new vessel formation [1]. Given its central role in angiogenesis, vascular endothelial growth factor (VEGF) has become a prime target for angiotherapy. However, clinical trials have not achieved satisfactory results due to aberrant functions of the overexpressed protein. Hypoxia-inducible factor-1 (HIF-1), which can stimulate the required angiogenic growth factors endogenously, has been suggested to provide an advantage over VEGF therapy [2].
Hypoxia-inducible factor is a master regulator of the transcriptional response of angiogenesis [3]. HIF-1 is a heterodimer consisting of subunits HIF-1α and HIF-1β [4]. HIF-1β is constitutively expressed, whereas HIF-1α is tightly regulated by changes in oxygen regimes [5, 6]. HIF-1α expression is regulated by multiple mechanisms. Although it primarily involves protein ubiquitination, the accumulation of HIF-1α has also been shown to depend on its rate of de novo protein synthesis [7]. Depending on the cell types and stimuli, different signaling pathways are involved in regulation of HIF-1α expression. Once activated, HIF-1 regulates a repertoire of key angiogenic genes, including VEGF, platelet-derived growth factor (PDGF), and transforming growth factor α (TGFα) [3]. Thus, HIF-1 represents an appealing drug target. Nevertheless, targeted therapy in clinical settings, in general, is not sufficient to treat angiogenic diseases, partly due to inadequate delivery strategies [8]. Plant-based small molecule activators that exhibit low toxicity and side effects hold promise as new treatment options.
Emerging evidence shows that nonpeptide small molecules, such as saponins, can modulate angiogenesis, with great potential to be developed as angiotherapeutic agents [9]. Panax ginseng, containing saponins as the major and biological active components, is a key herb in traditional Chinese medicine reported to be efficacious in treating diabetes and cardiovascular concerns, and its consumption is safe and nontoxic, even at high doses in animals and humans [10]. A notable saponin isolated from P. ginseng is Rg1 [11]. We have previously shown strong proangiogenic efficacy of Rg1. In particular, Rg1 was found to potently promote human umbilical vein endothelial cell (HUVEC) proliferation, chemoinvasion, and tube formation in vitro, to stimulate neovascularization in vivo, and to induce outgrowth of aortic sprout ex vivo [12]. Given this potential as a new attractive modality for angiotherapy, it is of great interest to characterize the signal transduction pathway whereby Rg1 contributes to angiogenesis.
We have used HUVECs based on its ability to undergo in vitro angiogenesis in response to appropriate stimuli but also a standard line for angiogenic screening to provide understanding of the mechanisms of Rg1. Using this model, we show for the first time that Rg1 is a potent stimulator of HIF-1α under normal oxygen conditions. We also provide mechanistic insights suggesting that the activation of the translation signal, especially p70S6K through the PI3K/Akt mediated pathway in this process. This hypoxia-independent activation of HIF-1α elucidates an important mechanism, which may explain the promising effects of Rg1 on angiogenesis and provide a rationale for the development of Rg1 as a new source of small molecule angiomodulator.
Materials and methods
Cell culture and reagents
Human umbilical vein endothelial cells were obtained from Clonetics (San Diego, CA) and cultured in medium 199 supplemented with 20% fetal bovine serum, 20 μg/ml endothelial cell growth supplement, 90 U/ml heparin, and 1% penicillin–streptomycin in a humidified incubator at 37°C with 5% CO2. The fifth to eighth passages of HUVECs were used in these studies to ensure genetic stability of the culture. Ginsenoside-Rg1 is a reference compound (purity > 98%) purchased from the Division of Chinese Material Medica and Natural Products, National Institute for the Control of Pharmaceutical and Biological Products, Ministry of Public Health, China. A stock solution of Rg1 (50 mM) was prepared in sterile double distilled water.
Western blotting
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris pH 7.4, 2 mM EDTA, 0.2% SDS, and 1% Triton X-100). Lysates were cleared by centrifugation, and protein concentrations were determined using the Bradford method with reagents from Bio-Rad (Hercules, CA). Equal amounts of cell lysates were separated by SDS–PAGE and transferred to a nitrocellulose membrane. The blot was then probed with HIF-1α and HIF-1β (BD Transduction Laboratories, San Jose, CA; 1:500), VEGF (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000), phospho-Akt, total Akt, phospho-p70S6K, total p70S6K (Cell Signaling, Danvers, MA; 1:1,000), and β-actin (Sigma, St. Louis, MO; 1:1,000) followed by reaction with horseradish peroxidase-conjugated secondary antibody. The signal was detected using enhanced chemiluminescence (Amersham, Piscataway, NJ).
Small interfering RNA
The ON-TARGET plus SMARTpool small interfering RNA (siRNA) for human HIF-1α (L-040638-00) and nontargeting control (D-001810-10) were purchased from Dharmacon (Lafayette, CO). siRNA oligonucleotides (10 nM) were transfected into cells with siLentfect reagent (Bio-Rad, Hercules, CA). After 24 h of transfection, Western blotting was carried out to examine the knockdown of targeted proteins.
Reverse transcription–PCR
Total RNA isolated using Trizol (Invitrogen, Carlsbad, CA) was reverse transcribed with the SuperScript II reverse transcriptase (Invitrogen) using an oligo-dT primer according to the manufacturer’s protocol. The cDNA was subjected to PCR amplification using the following forward and reverse primer sets: HIF-1α, 5′-CGTTGTGAGTGGTATTATTCA GCA-3′ and 5′-CAGTTTCTGTGTCGTTGCTGCC-3′; HIF-1β, 5′-CTGCTCTG TTGCCTCTCTAA-3′ and 5′-TTCTCCTCTCCTCCACTCTC-3′; glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. PCR conditions were established in pilot experiments to ensure linear reaction rates. GAPDH was used as the internal standard. PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. Gels were photographed using Gel DOC 2000 (Bio-Rad, Hercules, CA).
Capillary tube formation assay
HUVECs (1 × 105 cells) were seeded on a growth factor-reduced Matrigel-coated 24-well plate (BD Biosciences, San Jose, CA) in the presence or absence of Rg1 or with a combination of Rg1 and various small molecule inhibitors or HIF-1α siRNA. After 6 h of incubation, images were captured using a phase-contrast microscope (10×) using a CCD camera. The degree of tube formation was quantified by counting the number of tube-like structures in 4 randomly chosen fields randomly selected for each well without overlap.
Statistical analysis
Data were expressed as the mean ± SD. Comparisons were made using one-way analysis of variance, with Tukey’s least significant difference t test for post hoc analysis (GraphPad software, San Diego, CA). Differences were considered to be statistically significant at P < 0.05.
Results
Rg1 stimulates HIF-1α accumulation in HUVECs
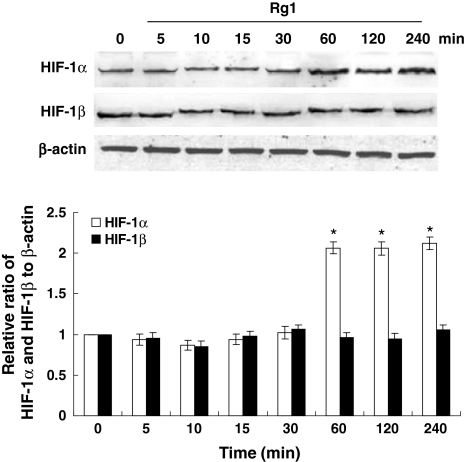
To investigate whether Rg1 could affect the activity of HIF-1, we first performed time course experiments to examine HIF-1 protein expression in the absence and presence of Rg1. As shown in Fig. 1, Rg1 induced a robust accumulation of HIF-1α protein in HUVEC under normoxic conditions, which was clearly detectable after 1 h, and this time point was used in all subsequent experiments. In contrast, Rg1 did not change levels of HIF-1β protein (Fig. 1).
Fig. 1.
Rg1 regulates HIF-1α protein. HUVECs were treated with Rg1 (150 nM) for 0, 5, 10, 15, 30, 60, 120, and 240 min. Cell lysates (50 μg) were subjected to immunoblotting with antibody against HIF-1α and HIF-1β. β-actin was used as a loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independent experiments. *P < 0.05, difference with untreated control
Rg1 does not affect HIF-1α mRNA accumulation but increases its protein synthesis
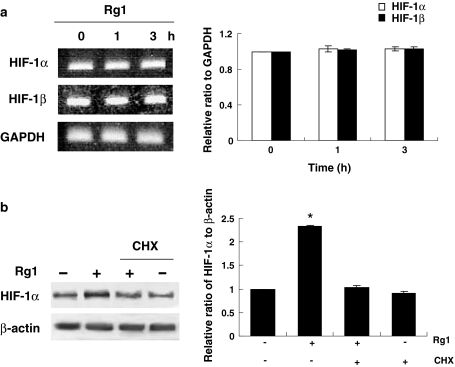
The rapid induction of HIF-1 suggests that it is likely that activation is controlled at the posttranscriptional level [5]. We asked whether Rg1 may be acting on HIF-1α production. The results of RT-PCR, however, revealed that HIF-1α mRNA levels remained unchanged by Rg1 treatment (Fig. 2a), indicating that Rg1 does not affect HIF-1α mRNA accumulation. Similar results were obtained in HIF-1β (Fig. 2a). To determine whether Rg1-mediated increase of HIF-1α was the result of increased protein synthesis, we utilized the protein synthesis inhibitor cycloheximide. Figure 2b shows that the inhibition with cycloheximide greatly decreased the accumulation of HIF-1α induced by Rg1, suggesting that protein synthesis is involved in this process.
Fig. 2.
Rg1 does not affect HIF-1α protein degradation but increases its protein synthesis. a HUVECs treated with Rg1 (150 nM) for 0, 1, and 3 h. Total RNA was isolated, and RT-PCR was performed using HIF-1α and HIF-1β sequence-specific primers. GAPDH was included as the internal control. b Cells treated with Rg1 were incubated in the presence of cycloheximide (CHX, 5 μg/ml) for 60 min and then subjected to immunoblotting with anti-HIF-1α. β-actin was used as a loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independent experiments. *P < 0.05, difference with untreated control
Rg1 regulates HIF-1α in a PI3K/Akt and p70S6K-dependent manner
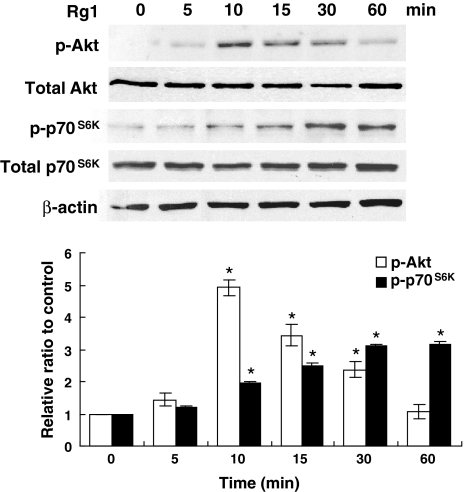
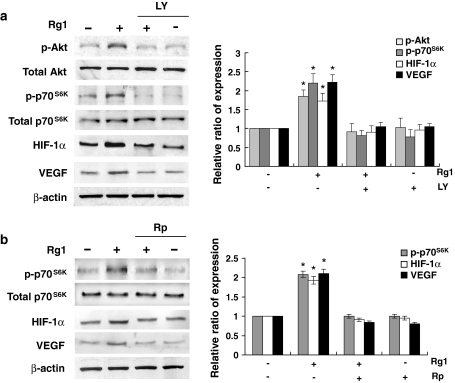
To determine the signaling pathway involved, HUVECs were treated with Rg1 for indicated times and the activation of various signaling molecules were analyzed by Western blotting using phospho-specific antibodies. The PI3K/Akt pathway is implicated in regulation of the translation of HIF-1α expression [13–15]. To address its involvement in Rg1-mediated HIF-1α accumulation, we measured the phosphorylation status of Akt and its effector p70S6K. Interestingly, treatment of Rg1 resulted in a time-dependent phosphorylation of Akt, which correlated with the phosphorylation of p70S6K, and with the induction of HIF-1α accumulation in HUVEC (Fig. 3). Rg1 did not affect the total protein levels of these kinases, indicating that this effect was specific to protein phosphorylation (Fig. 3). The activation of p-Akt by Rg1 required the activity of its upstream regulator PI3K, as the PI3K inhibitor LY294002 blocked this activation. LY294002 also blocked the stimulatory effect of Rg1 on the expression HIF-1α (Fig. 4a). We further revealed that HIF-1α induction triggered the expression of target genes, including VEGF, which one of the best characterized and most frequent responses to HIF-1α activation that may have a positive effect on angiogenesis function [16]. As shown in Fig. 4a, LY294002 repressed the stimulatory ability of Rg1 upon VEGF expression. Similarly, the mTOR/p70S6K inhibitor rapamycin that produced a rapid inhibition of p70S6K also reduced the HIF-1α expression and subsequent VEGF expression (Fig. 4b). Together, these results suggest the involvement of PI3K/Akt/p70S6K signaling in the activation of HIF-1α protein synthesis by Rg1.
Fig. 3.
Rg1 increases Akt phosphorylation and p70S6K signaling. HUVECs were untreated or treated with 150 nM Rg1 for various times as indicated. An equal amount of cell lysates (50 μg) was determined by immunoblotting. Akt and p70S6K activities were analyzed using anti-phospho-specific (p)-Akt and p-p70S6K antibodies. β-actin was also included as a loading control. The signal intensities were determined by densitometry and expressed as p-Akt and p-p70S6K relative to total Akt and p70S6K for each sample. Data are shown as mean ± SD of three independent experiments. *P < 0.05, difference with untreated control
Fig. 4.
Rg1 regulates HIF-1α expression via PI3K/Akt and p70S6K signaling. HUVECs were pretreated with a 10 μM LY294002 (LY) or b 10 nM rapamycin (Rp) before treated with Rg1 (150 nM) for 30 min. Equal amounts of protein (50 μg) were analyzed by immunoblotting using specific antibodies recognizing phospho (p)-Akt and p-p70S6K. The cells were also harvested 1 h after treatment to analyze HIF-1α expression. β-actin was included as a loading control. The signal intensity was determined by densitometry and expressed as p-Akt and p-p70S6K relative to total Akt and p70S6K, and HIF-1α and VEGF to β-actin for each sample. Data are shown as mean ± SD of three independent experiments. *P < 0.05, difference with untreated control
Rg1 effects on HIF-1 require glucocorticoid receptor
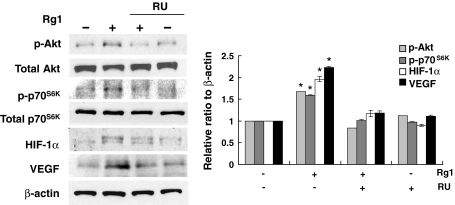
Rg1 signals have been shown to be transduced via the glucocorticoid receptor (GR) [17, 18]. RU486, an inhibitor specific to GR, significantly abrogated Rg1-induced HIF-1α as well as Akt and p70S6K phosphorylation, which is a critical step in Rg1-induced HIF-1α activation, indicating that GR mediates the action of Rg1 (Fig. 5). In support of this observation, RU486 also completely aborted the effect of Rg1 on VEGF expression (Fig. 5).
Fig. 5.
Rg1 signals via GR. Cells were pretreated with 10 μM RU486 (RU) before treated with Rg1 (150 nM) for 30 min. Equal amounts of protein (50 μg) were analyzed by immunoblotting using specific antibodies recognizing phospho (p)-Akt and p-p70S6K. The cells were also harvested 1 h after treatment to analyze HIF-1α and VEGF expression. β-actin was included as a loading control. The signal intensities were determined by densitometry and expressed as p-Akt and p-p70S6K relative to total Akt and p70S6K, or HIF-1α and VEGF to β-actin for each sample. Data are shown as mean ± SD of three independent experiments. *P < 0.05, difference with untreated control
HIF-1α is involved in Rg-1-induced angiogenic action in HUVEC
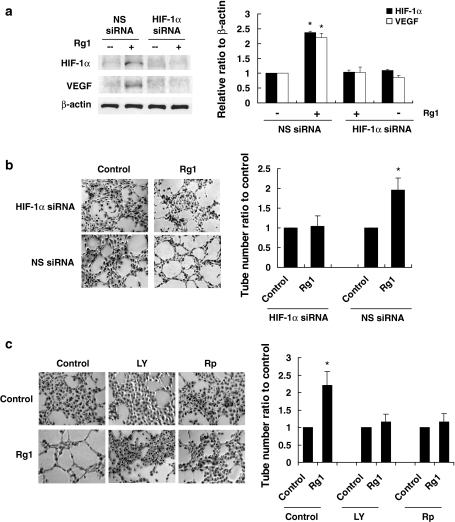
Given that HIF-1α is the key regulator of angiogenesis, we next analyzed the effect of HIF-1α on Rg1-induced angiogenic function. The tube formation assay was chosen because it has the advantage over other angiogenic assays, in which it replicates multiple key steps of the angiogenic process involving adhesion, migration, differentiation, and growth [19]. We used siRNA to ablate HIF-1α expression. Transfection with HIF-1α siRNA, but not nonspecific siRNA, revealed a significant decrease in HIF-1α protein level and subsequent VEGF upregulation (Fig. 6a). Importantly, HIF-1α siRNA-transfected cells exhibited a discernible reduction in Rg1-induced angiogenic tube formation, indicating that HIF-1α function is required for this process (Fig. 6b). No inhibitory effect on tube formation was observed for nonspecific siRNA. Similarly, inhibition of PI3K/Akt by LY294002 or inhibition of mTOR/p70S6K by rapamycin markedly attenuated Rg1-stimulated tube formation (Fig. 6c). These data indicate a role for HIF-1α in the angiogenic activity of Rg1 in HUVEC and that the effect was mediated via PI3K/Akt and p70S6K.
Fig. 6.
Rg1 regulation of angiogenic activity requires HIF-1α. a HUVECs were transfected with 10 nM HIF-1α siRNA or nonspecific (NS) siRNA in the presence or absence of 150 nM Rg1 for 1 h. Total cell lysates (50 μg) were subjected to immunoblotting with antibodies against HIF-1α, VEGF, and β-actin. b Angiogenesis was assessed by tube formation 6 h after treatment. 1 × 105 cells/well were seeded in growth factor-reduced Matrigel-coated 24-well plate in medium 199 containing 1% fetal bovine serum. c Cells were pretreated with 10 μM LY294002 (LY) or 10 nM rapamycin (Rp) and then treated with or without 150 nM Rg1 for 6 h and assessed by angiogenic tube formation. Data are shown as mean ± SD of the number of tube-like structure relative to the control, which was set to be 100%, counted in 12 microscopic fields of three independent experiments. Bar 50 μm. *P < 0.05, difference with untreated control
Discussion
Hypoxia-inducible factor is one of the few genes in which its genetic deletion is embryonic lethal due to severe vascular abnormalities and thus held great promise as a therapeutic target [20–22]. These studies reveal that expression of HIF-1 is required for normal development of the heart, blood vessels, and blood cells, i.e., all three components of the circulatory system are dependent on HIF-1. Thus, factors that modulate HIF-1 are attractive modalities for angiotherapy. Ginseng is listed in the pharmacopoeias of several countries, including the United States and Europe and has been widely used throughout the world for medicinal purposes due to its rich content of saponins [9, 10]. The present study for the first time reported the functional effect of the naturally occurring saponin Rg1 from P. ginseng as a potent regulator of HIF-1α.
Although it is generally thought that HIF-1 is regulated mainly by oxygen tension, there is increasing evidence that a number of nonhypoxic factors also modulate HIF-1 expression and consequent function, for example, growth factors, cytokines and oncogenic signals [13, 14]. Previous work has demonstrated that the wound fluid environment is not hypoxic [23], suggesting that angiogenic growth factor production at the wound site may be initiated or potentiated by a factor other than hypoxia. Intriguingly, our studies suggest that Rg1 may be such a factor for regulating HIF-1. To our knowledge, such property possessed by Rg1 has not been reported for other saponins. Thus, it is rational to explore Rg1 as a source of novel angiogenic modulator.
It is interesting that the mechanisms for HIF-1α activation under hypoxic and nonhypoxic conditions are strikingly different. Unlike hypoxia, nonhypoxic stimuli often do not induce HIF-1α stabilization [24]. We show that Rg1 is able to increase the rate of HIF-1α protein synthesis. The PI3K/Akt/mTOR pathway is a key regulator of protein synthesis [13–15]. p70S6K, a central serine/threonine kinase downstream of mTOR, controls protein translation by enhancing the translation of mRNAs containing 5′-terminal oligopyrimidine tract (TOP) sequences in their 5′-UTR [25]. Interestingly, the 5′-UTR of the mRNA encoding HIF-1α contains 5′-TOP sequences that can be regulated by p70S6K activation. We have shown that treatment of HUVECs with Rg1 increased the phosphorylation of p70S6K, which concomitant with the increase of HIF-1α expression. A key role of p70S6K in the regulation of translation further suggests that Rg1-induced activation of PI3K/Akt/p70S6K pathway might be involved in HIF-1α translation. In this context, it is also interesting to note that although the effect of Rg1 could also be mediated through a MAPK pathway and there is evidence that ERK1/2 regulates HIF-1α function in certain cells [26], we ruled out this possibility in HUVEC, since treatment of cells with the ERK1/2 inhibitor PD98059 had no effect on Rg1-mediated activation of HIF-1α (Supplementary Fig. 1).
The mechanism by which Rg1 activates PI3K/Akt/p70S6K signaling is not yet fully understood, but Rg1 has been shown to be a functional ligand of GR [17, 18]. The rapid nongenomic effects of GR have been attracted increasing attention in recent years. Although the underlying mechanism of action is not clear, our recent studies show that Rg1 can exert its nongenomic effects by cross-regulation of receptor tyrosine kinases through a GR-dependent, ligand-independent mechanism [27]. The presence of membrane-bound GR is highly consistent with this hypothesis [28, 29]. Together with our previous studies [27], our data demonstrate that this new mechanism applies to more genes and can have direct and indirect effects on gene expression responsible for triggering the rapid onset of angiogenesis. This notion is consistent with the obvious protective effects of Rg1 in acute conditions, such as endothelial dysfunction or myocardial ischemia, where rapid endothelialization and/or angiogenesis are desirable [9]. Moreover, given the undesirable side effects from genomic actions, it is becoming increasingly evident that a nongenomic action is more beneficial in clinical use to maximize efficacy and minimize adverse effects or toxicity.
The implication of a dietary saponin affecting the activity of a pivotal protein such as HIF-1α is intriguing. While prolonged HIF-1α activation is important, a rapid, transient stabilization of HIF-1α might produce similar results on the induction of a stable and healthy vasculature [30]. HIF-1α has been shown to be required for the expression of multiple angiogenic genes, including VEGF, PDGF, and TGFα [3]. This might explain why it is possible to achieve profound effect in angiogenesis with a low dose of Rg1. Indeed, the extract from Sanqi ginseng, which as a predominance of Rg1, is considered the key ingredient for the treatment of trauma injuries and for promoting microcirculation [31]. Since the concentration of Rg1 (150 nM; 0.12 g/l) used in our experiments is within the physiological range of the plasma level of Rg1, based on the oral bioavailability of 3.29–18.4% for Rg1 in animal studies [32, 33], our findings are also particularly relevant to pharmacological approaches.
In summary, the results of this study reveal an important new mechanism of the proangiogenic activity of Rg1. This mechanism involves Rg1 activates HIF-1α activity, which is the starting step of the angiogenic reaction. These data highlight the importance of developing Rg1 as a new nonpeptide small molecule-based prototype for therapeutic angiogenesis, such as in wounding healing, cardiovascular, and ischemic disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1: No effect of ERK1/2 inhibitor on HIF-1α. a HUVECs were untreated or treated with 150 nM Rg1 for various times as indicated. b Cells were pretreated with 10 M PD98059 (PD) before treated with Rg1 (150 nM) for 15 min. Equal amounts of protein (50 g) were analyzed by immunoblotting using specific antibodies recognizing phospho (p)-ERK1/2. The cells were also harvested 1 h after treatment to analyze HIF-1α and VEGF expression. β-actin was included as a loading control. The signal intensity was determined by densitometry and expressed as p-ERK1/2 relative to total ERK1/2, and HIF-1α and VEGF to β-actin for each sample. Data are shown as mean ± S.D. of three independent experiments. *, P < 0.05, difference with untreated control. Supplementary material 1 (PPT 210 kb)
Acknowledgments
This study was supported by grants from the Hong Kong Research Grant Council HKBU 1/06C and the HKU Outstanding Young Researcher Award to A.S.T.W.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Pajusola K, Kunnapuu J, Vuorikoski S, Soronen J, Andre H, Pereira T, Korpisalo P, Yla-Herttuala S, Poellinger L, Alitalo K. Stabilized HIF-1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 2005;19:1365–1367. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- 3.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 5.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 7.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2005;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 8.Khan TA, Sellke FW, Laham RJ. Gene therapy progress and prospects: therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther. 2003;10:285–291. doi: 10.1038/sj.gt.3301969. [DOI] [PubMed] [Google Scholar]
- 9.Leung KW, Yung KK, Mak NK, Yue PY, Luo HB, Cheng YK, Fan TP, Yeung HW, Ng TB, Wong RN. Angiomodulatory and neurological effects of ginsenosides. Curr Med Chem. 2007;14:1371–1380. doi: 10.2174/092986707780597916. [DOI] [PubMed] [Google Scholar]
- 10.Chang YS, Seo EK, Gyllenhaal C. Panax ginseng: a role in cancer therapy? Integr Cancer Ther. 2003;2:13–33. doi: 10.1177/1534735403251167. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Chiou W, Zhang J. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- 13.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 14.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/S0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 18.Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 19.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:269–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 20.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 23.Howdieshell TR, Riegner C, Gupta V, Callaway D, Grembowicz K, Sathyanarayana, McNeil PL (1998) Normoxic wound fluid contains high levels of vascular endothelial growth factor. Ann Surg 228:707–715 [DOI] [PMC free article] [PubMed]
- 24.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible factor-1alpha stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas G. The S6 kinase signaling pathway in the control of development and growth. Biol Res. 2002;35:305–313. doi: 10.4067/S0716-97602002000200022. [DOI] [PubMed] [Google Scholar]
- 26.Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/S0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 27.Cheung LW, Leung KW, Wong CK, Wong RN, Wong AS. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res. 2011;89:419–425. doi: 10.1093/cvr/cvq300. [DOI] [PubMed] [Google Scholar]
- 28.Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis of new drug developments. Mol Cell Endocrinol. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruick RK, McKnight SL. Building better vasculature. Genes Dev. 2001;15:2497–2502. doi: 10.1101/gad.931601. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Wang H, Itokawa H, Morris-Natschke SL. Current perspective on Chinese medicines and dietary supplements in China, Japan, and the United States. J Food Drug Anal. 2000;8:219–228. [Google Scholar]
- 32.Xu QF, Fand XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmcol. 2003;84:187–192. doi: 10.1016/S0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 33.Han M, Fang X. Difference in oral absorption of ginsenoside Rg1 between in vitro and in vivo models. Acta Pharmacol Sin. 2006;27:499–505. doi: 10.1111/j.1745-7254.2006.00303.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: No effect of ERK1/2 inhibitor on HIF-1α. a HUVECs were untreated or treated with 150 nM Rg1 for various times as indicated. b Cells were pretreated with 10 M PD98059 (PD) before treated with Rg1 (150 nM) for 15 min. Equal amounts of protein (50 g) were analyzed by immunoblotting using specific antibodies recognizing phospho (p)-ERK1/2. The cells were also harvested 1 h after treatment to analyze HIF-1α and VEGF expression. β-actin was included as a loading control. The signal intensity was determined by densitometry and expressed as p-ERK1/2 relative to total ERK1/2, and HIF-1α and VEGF to β-actin for each sample. Data are shown as mean ± S.D. of three independent experiments. *, P < 0.05, difference with untreated control. Supplementary material 1 (PPT 210 kb)