Abstract
Tumor hypoxia is a common determinant of resistance to cytotoxic therapies and metastatic behavior. In rectal cancer patients receiving preoperative chemoradiotherapy, tyrosine kinase activities in tumors with poor and good treatment responses were found to differ. Given that tyrosine kinase signaling mediates hypoxic tissue adaptation, the present study examined whether tumor kinase activity might also correlate with systemic dissemination of rectal cancer. Immunomagnetic selection of disseminated tumor cells (DTC) from bone marrow aspirates was undertaken in 55 patients with locally advanced rectal cancer. Using peptide arrays with 144 tyrosine kinase substrates, phosphopeptide signatures were generated from patients’ baseline tumor biopsies, to study association between DTC and tumor tyrosine kinase activity regulated ex vivo by sunitinib. Disseminated tumor cells were detected in 60% of cases, and these patients had significantly poorer metastasis-free survival than patients without DTC. Phosphorylation of 31 array tyrosine kinase substrates by tumor samples was significantly more strongly inhibited by sunitinib in the DTC-negative patients, with a number of phosphosubstrates representing angiogenic factors. In this cohort of rectal cancer patients, tumor phenotypes defined by a subset of tyrosine kinase activities correlating with weak ex vivo inhibition by sunitinib, was associated with early systemic dissemination.
Electronic supplementary material
The online version of this article (doi:10.1007/s10456-011-9231-3) contains supplementary material, which is available to authorized users.
Keywords: Rectal cancer, Tyrosine kinase signaling, Angiogenesis, Disseminated tumor cells, Metastasis
Introduction
In order to cure rectal cancer, two therapeutic challenges must be met, namely eradication of tumor within the pelvic cavity and secondly, the prevention of systemic tumor dissemination. The natural disease course of rectal cancer makes it an ideal model system to explore the possible role of tumor hypoxia in therapy resistance and development of metastasis. Tissue hypoxia is defined by reduced oxygen levels, typically 2% oxygen or less, and occurs in a wide range of pathological conditions [1, 2]. Within classical radiobiology, hypoxia is recognized as a main mechanism involved in tumor resistance to radiation [3, 4]. Moreover, recent research supports the hypothesis that tumor hypoxia is one of the major driving forces of the metastatic process [5]. Adaptive cellular responses to hypoxia allow for processes such as proliferation, migration, and in particular angiogenesis, and involve activation of a range of kinase signaling pathways, among them signaling initiated by the receptor tyrosine kinases PDGFR, VEGFR, and EPOR [1, 5, 6].
Locally advanced rectal cancer (LARC) comprises primary tumors that grow beyond the rectal wall to an extent that precludes primary surgical removal with adequate microscopic margins. Hence, treatment of LARC is multimodal, involving preoperative chemoradiotherapy aimed at macroscopic downsizing and control of subclinical tumor extension within the pelvic cavity, to enable complete tumor removal by subsequent surgery. However, even with successful local treatment, a substantial number of patients will develop metastatic disease as result of early undetected systemic dissemination of tumor cells [7]. The phase II trial Locally Advanced Rectal Cancer—Radiation Response Prediction (LARC-RRP), registered with ClinicalTrials.gov number NCT00278694, was launched primarily to identify predictive biomarkers of tumor radiation sensitivity, and we have recently reported that this was feasible by kinase activity profiling of baseline tumor biopsies [8]. Using peptide arrays with tyrosine kinase substrates, we found that phosphopeptide levels generated by tumors with poor response to the preoperative chemoradiotherapy were significantly higher than substrate phosphorylation resulting from tumors with good treatment response. The elevated kinase activity in poor-responding tumors was suppressed by ex vivo addition of the tyrosine kinase inhibitor sunitinib, and represented signaling implicated in experimental radiation resistance.
Given that tyrosine kinase signaling is involved in adaptive responses to tumor hypoxia, the present study aimed to determine how tumor kinase activity might relate to systemic disease dissemination. Hence, in the investigation of the LARC-RPP study patients reported here, we endeavored to correlate the individual patient’s tumor tyrosine kinase activity to negative or positive status for disseminated tumor cells (DTC) to bone marrow as the clinical endpoint, using the presence of DTC as biomarker of metastatic recurrence risk [9]. Immunomagnetic selection of DTC was performed at the time of diagnosis, and by applying previously acquired ex vivo sunitinib inhibition profiles from the baseline primary tumor biopsies [8], the association between the tumor kinome and early systemic dissemination in terms of DTC status was studied.
Patients and methods
Patients and procedures
The patient population reported here was enrolled between October 2005 and December 2007. Patient eligibility criteria and evaluation procedures have been described previously [8]. Three patients with synchronous resectable liver metastases were also included in this study. The experimental treatment protocol, intended to intensify preoperative therapy for LARC, consisted of two cycles of neoadjuvant chemotherapy (the Nordic FLOX regimen: oxaliplatin 85 mg/m2 on day 1 and daily bolus fluorouracil 500 mg/m2 and folinic acid 100 mg on days 1 and 2 every second week) followed by chemoradiotherapy. Radiation was delivered in daily 2-Gy fractions 5 days per week over a five-week period; the initial 23 fractions to the macroscopic tumor volume and area at risk, and the two final fractions restricted to the macroscopic tumor, as determined by computed tomography-based planning. During the radiotherapy course, concomitant chemotherapy was given as oxaliplatin 50 mg/m2 once weekly and capecitabine 825 mg/m2 twice daily on days of radiotherapy. Surgery was planned 6–8 weeks after completion of the preoperative treatment. In accordance with national guidelines, the patients did not receive postoperative therapy.
The resected primary tumor specimens were histologically evaluated for response to the preoperative treatment according to standard criteria (ypTN) and histomorphologic tumor regression grade (TRG), as previously detailed [8]. Briefly, tumor response was graded within one of five TRG categories, spanning from the absence of residual tumor cells in the resected specimen (pathologic complete response; TRG 1) to the lack of morphologic signs of tissue response to treatment (TRG 5) [10]. The review procedures of patient follow-up included clinical examination, blood tests, and computed tomography scanning of the chest, abdomen, and pelvis, at three- and six-month intervals for the first and second year, respectively, and twelve months thereafter. Locally recurrent or metastatic disease and death of any cause were recorded. Thus, the study endpoints were histomorphologic tumor response to neoadjuvant therapy, disease-free survival, and overall survival. Follow-up data was obtained from the clinical database and censored on April 6th, 2011. Valid observations of the presence or absence of distant metastases or local recurrence required designated radiological examination and/or bioptic verification. The three patients with resectable liver metastases at the time of diagnosis were excluded from analysis of metastasis-free survival.
Study-specific procedures
At the time of diagnosis, baseline study-specific primary tumor biopsies (snap-frozen in liquid nitrogen and stored at −80°C) and bone marrow (15–40 ml drawn from the anterior iliac crests) were obtained from 71 patients under heavy sedation. Of these, 16 patients were excluded from the present study, as six patients had bone marrow samples that contained too few mononuclear cells for immunomagnetic selection, and ten patients had tumor biopsy specimens in which kinase activity profiling had not been performed because the patients were either ineligible after study registration (n = 3), had withdrawn consent (n = 1), had unexpectedly died during the preoperative treatment (n = 1), had developed metastatic disease progression during preoperative treatment that precluded definitive surgery (n = 1), had tumor cell content less than 20% within the biopsy specimen (n = 2), or had a biopsy specimen in which kinase activity analysis was missing of unknown reasons (n = 2). Thus, tumor kinase activity signatures based on previous array phosphosubstrate data were successfully identified for 55 patients with known DTC status, and this study population is present within the current analyses.
The tumor biopsies were sectioned using a cryostat microtome, and hematoxylin-eosin stained slides were evaluated for tumor content. The average tumor cell content in the biopsy specimens was 44%, and no difference was found between patients positive and negative for DTC (P > 0.66; two-sample t-test). Each biopsy specimen was aliquoted by cryostat sectioning into 10-μm slices, and total tissue volume was calculated by multiplying the surface area of the section with the number of sample sections. Protein lysates were prepared by adding 36 μl lysis buffer (M-PER Mammalian Extraction Reagent containing Halt Phosphatase Inhibitor Cocktail and EDTA-free Halt Protease Inhibitor Cocktail; Pierce Biotechnology, Inc., Rockford, IL) per mm3 tissue, and following vortexing and centrifugation, 5 μl of the supernatant was added to the reaction mixture, which was composed of Abl Reaction Buffer (50 mmol/l Tris–HCl pH 7.5, 10 mmol/l MgCl2, 1 mmol/l EGTA, 2 mmol/l dithiothreitol, 0.01% Brij 35; New England BioLabs, Inc., Ipswich, MA), 1 mg/ml bovine serum albumin, 100 μmol/l ATP, and 12.5 μg/ml of the monoclonal, FITC-conjugated anti-phosphotyrosine antibody (Exalpha Biologicals, Inc., Maynard, MA) to a total volume of 40 μl in each array. No significant variation was observed in protein concentration in the sample lysates. Four technical replicates were analyzed from each patient sample to generate basal phosphosubstrate data. On the same array plate, using three technical replicates for each condition, each sample was also incubated in the presence of 2.5 μmol/l sunitinib (Axxora, Lausen, Switzerland).
For determination of DTC status, superparamagnetic sheep-antimouse IgG particles (Dynabeads M450; Invitrogen–Life Technologies, Oslo, Norway) were conjugated with the monoclonal antibody MOC-31 (IQ Products, Groningen, The Netherlands), and for each study patient, immunomagnetic selection of tumor cells in bone marrow was undertaken as previously described [11]. Briefly, mononuclear cells were isolated from the bone marrow aspirate and incubated with magnetic immunobeads with conjugated antibody, or without antibody for negative control, and subsequently exposed to a magnet field to separate bead-rosetting cells from unbound cells. A patient sample was classified as positive for DTC if a minimum of two cells rosetted at least five beads with the MOC-31 antibody and no rosetted cells were detected in the negative control.
Data adaptation and statistical analyses
The array data is available in the ArrayExpress database [12] by accession number E-TABM-913. The curated sunitinib inhibition data set that had been calculated from the signal intensity from each array peptide after background subtraction and used previously [8] was applied as input for the current statistical analysis. The data was log-transformed after handling a small number of negative data points by subtracting the 1% percentile of the data and subsequently setting all remaining data points with value less than 1 to the value 1. For each peptide, the sunitinib-induced log-fold change was calculated by subtracting the log-transformed signal in the absence of sunitinib (control) from that in the presence of this tyrosine kinase inhibitor. Peptides with sample-averaged signal less than 210 in the control condition were excluded, leaving 102 peptides above this threshold. A two-sample t-test was performed to test for different level of sunitinib inhibition in DTC-positive and DTC-negative patients (Supplementary Table 1). The sunitinib inhibition profiles were visualized as data color maps, in which clustering of peptides and samples was imposed by sorting the data according to the value of the first principal component (peptides) and the value of the scores on the first principal component (samples) of a principal component analysis, using samples as observations and spots as variables. Distribution of value of the score on the first principal component was compared to clinical parameters using correlation coefficients for continuous variables and one-way ANOVA tests for categorical data. Data processing and visualizations were performed in Matlab R2010A including the statistics toolbox (Mathworks, Natick, MA).
Disease-free and overall survival was estimated by the Kaplan–Meier method. The log-rank test was used to determine survival differences in DTC-positive and DTC-negative patients. Survival was measured from the date of bone marrow sampling to the date of recurrent disease detection or death. Distribution of parameters between different groups was compared using Pearson’s Chi-square exact two-sided test for categorical data and two-sample t-test for continuous variables. The data analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). P values less than 0.05 were considered statistically significant. Pathway connectivity of peptides was determined using UniProtKB/SwissProt database [13] and literature search.
Results
Patients
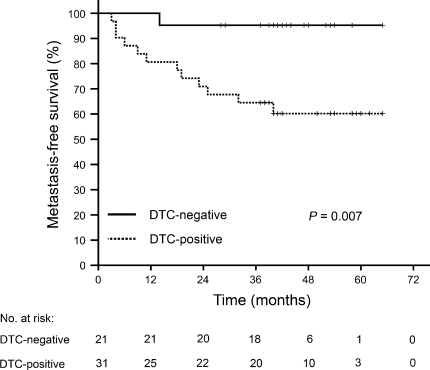
Table 1 describes characteristics of the 55 patients, in whom immunomagnetic selection of tumor cells in bone marrow aspirates as well as tyrosine kinase activity profiling of tumor biopsies at the time of study enrolment were performed. In 60% of patients, a median tumor cell count of 6 (range 2–150) was detected in the bone marrow samples (DTC-positive patients). No differences were found between DTC-positive and DTC-negative patients regarding gender, age, radiological TNM stage at diagnosis, serum carcinoembryonic antigen levels or hemoglobin count at the time of diagnosis, or histological ypTN stage or histomorphologic TRG score of the surgical specimens. Median follow-up was 42 months (range 7–65). Three patients (one DTC-negative and two DTC-positive individuals) were noted to have locally recurrent disease. Metastasis-free survival was assessed for 52 patients, as the three patients with synchronous liver metastases at the time of diagnosis were omitted from this analysis, with the DTC-positive group demonstrating significantly poorer metastasis-free survival (61%) than the DTC-negative group (95%; P = 0.007; Fig. 1). At the time of follow-up data censoring, eight patients were reported as deceased; the number of cases was not statistically different between the two groups of patients with negative and positive DTC status.
Table 1.
Patient characteristics
| All patients (n = 55) | DTC-negative patients (n = 22) | DTC-positive patients (n = 33) | |
|---|---|---|---|
| TNM stage at diagnosis | |||
| T2 | 3 (5.5%) | 2 (9.1%) | 1 (3.0%) |
| T3 | 33 (60.0%) | 14 (63.6%) | 19 (57.6%) |
| T4 | 19 (34.5%) | 6 (27.3%) | 13 (39.4%) |
| N0 | 6 (10.9%) | 2 (9.1%) | 4 (12.1%) |
| N1 | 8 (14.5%) | 3 (13.6%) | 5 (15.2%) |
| N2 | 41 (74.5%) | 17 (77.3%) | 24 (72.3%) |
| M0 | 52 (94.5%) | 21 (95.5%) | 31 (93.9%) |
| M1 | 3 (5.5%) | 1 (4.5%) | 2 (6.1%) |
| TN stage after chemoradiotherapy | |||
| ypT0 | 12 (21.8%) | 5 (22.7%) | 7 (21.2%) |
| ypT1 | 8 (14.5%) | 4 (18.2%) | 4 (12.1%) |
| ypT2 | 13 (23.6%) | 6 (27.3%) | 7 (21.2%) |
| ypT3 | 14 (25.5%) | 4 (18.2%) | 10 (30.3%) |
| ypT4 | 8 (14.5%) | 3 (13.6%) | 5 (15.2%) |
| ypN0 | 43 (78.2%) | 19 (86.4%) | 24 (72.7%) |
| ypN1 | 9 (16.4%) | 3 (13.6%) | 6 (18.2%) |
| ypN2 | 3 (5.5%) | 0 (0%) | 3 (9.1%) |
| TRG | |||
| 1–2, good responders | 40 (72.7%) | 16 (72.7%) | 24 (72.7%) |
| 3, intermediate responders | 9 (16.4%) | 5 (22.7%) | 4 (12.1%) |
| 4, poor responders | 6 (10.9%) | 1 (4.5%) | 5 (15.2%) |
| CEA | |||
| <5 μg/l | 33 (60.0%) | 14 (63.6%) | 19 (57.6%) |
| ≥5 μg/l | 22 (40.0%) | 8 (36.4%) | 14 (42.4%) |
| Median hemoglobin count, g/dl (range) | 13.9 (10.0–16.3) | 14.0 (10.0–16.3) | 13.9 (10.8–15.4) |
| Gender | |||
| Male | 31 (56.4%) | 13 (59.1%) | 18 (54.5%) |
| Female | 24 (43.6%) | 9 (40.9%) | 15 (45.5%) |
| Median age, years (range) | 61 (31–73) | 61 (38–73) | 59 (31–73) |
| Follow-up resultsa | |||
| Locally recurrent disease | 3 (5.5%) | 1 (4.5%) | 2 (6.1%) |
| Metastatic disease | 16 (29.1%) | 2 (9.1%) | 14 (42.4%) |
| Death | 8 (14.5%) | 2 (9.1%) | 6 (18.2%) |
TNM tumor–node–metastasis, yp histopathologic staging following chemoradiotherapy, TRG histomorphologic tumor regression grade following chemoradiotherapy, CEA carcinoembryonic antigen
aCensored at a median period of 42 months (range 7–65)
Fig. 1.
Metastasis-free survival of 52 study patients with locally advanced rectal cancer as function of negative or positive status for disseminated tumor cells (DTC) to bone marrow at the time of diagnosis
Tumor tyrosine kinase activities
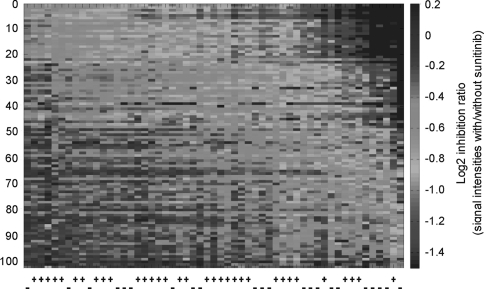
Ex vivo sunitinib inhibition profiles were derived from 102 (of 144 on the array) peptide kinase substrates that had signal intensities above the defined threshold. In Fig. 2, patients (horizontal axis) and peptides (vertical axis) were sorted according to principal component analysis. No correlation was observed between tumor kinase activity inhibition and gender, age, diagnostic TNM stage, ypTN stage, or serum carcinoembryonic antigen levels or hemoglobin count. A borderline significant association was found between inhibition of the phosphosubstrates and tumor response to preoperative treatment in terms of TRG status (P = 0.049), with the poor responders exhibiting strongest inhibition (Supplementary Fig. 1).
Fig. 2.
Ex vivo sunitinib inhibition profiles from 102 kinase substrates. Patient tumor samples along horizontal axis, annotated by negative (−) or positive (+) status for disseminated tumor cells to bone marrow, and phosphosubstrates along vertical axis. Red corresponds to stronger and blue to weaker inhibition of substrate phosphorylation. (Color figure online)
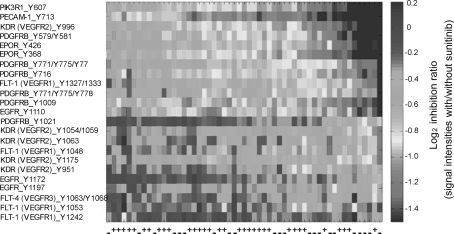
Based on the scores of the principal component analysis, ex vivo sunitinib inhibition of tumor kinase activity in DTC-negative patients was stronger than in patients with positive DTC status (P = 0.042; Supplementary Fig. 2). Of the 102 peptides constituting the tyrosine kinase inhibition profile, phosphorylation of 31 kinase substrates was significantly more strongly inhibited in the DTC-negative patients than in the DTC-positive individuals (Table 2). The 31 discriminating phosphopeptides represented proteins derived from signaling pathways implicated in various cellular processes, such as proliferation, angiogenesis, and invasion. Of these, 13 peptides, mainly representing PDGFR, VEGFR, and EPOR, were proteins involved in angiogenesis-related pathways. Within the entire 102-peptide panel, 23 angiogenesis-related substrates were identified (Fig. 3), and sunitinib inhibition of these phosphosubstrates was stronger in patients with negative DTC status than in DTC-positive patients (P = 0.019; Supplementary Fig. 3). Additionally, a significantly larger portion of angiogenesis-related substrates (13 peptides) appeared among the 31 phosphopeptides discriminating DTC status than within the remaining group of substrates (ten angiogenesis-related among a total of 71; P = 0.002).
Table 2.
Array phosphopeptides (generated by tumors from patients with and without disseminated tumor cells to bone marrow) with different levels of ex vivo sunitinib inhibition (P < 0.05), listed according to signaling pathway connectivity
| Peptide substratea | Position of peptide sequenceb | Phosphorylationb | Common namea |
|---|---|---|---|
| Angiogenesis | |||
| PDGFRB | 1002–1014 | Y1009 | Beta platelet-derived growth factor receptor |
| PDGFRB | 709–721 | Y716 | Beta platelet-derived growth factor receptor |
| PDGFRB | 771–783 | Y771, Y775, Y778 | Beta platelet-derived growth factor receptor |
| PDGFRB | 768–780 | Y771, Y775, Y778 | Beta platelet-derived growth factor receptor |
| PDGFRB | 572–584 | Y579, Y581 | Beta platelet-derived growth factor receptor |
| FLT-1 (VEGFR1) | 1326–1338 | Y1327, Y1333 | Vascular endothelial growth factor receptor 1 |
| KDR (VEGFR2) | 1168–1180 | Y1175 | Vascular endothelial growth factor receptor 2 |
| KDR (VEGFR2) | 989–1001 | Y996 | Vascular endothelial growth factor receptor 2 |
| EPOR | 361–373 | Y368 | Erythropoietin receptor |
| EPOR | 419–431 | Y426 | Erythropoietin receptor |
| PECAM-1 | 706–718 | Y713 | Platelet endothelial cell adhesion molecule |
| PIK3R1 | 600–612 | Y607 | Phosphatidylinositol 3-kinase regulatory alpha subunit |
| EGFR | 1190–1202 | Y1197 | Epidermal growth factor receptor |
| Cell adhesion, migration, and invasion | |||
| CALM1 | 95–107 | Y100 | Calmodulin |
| FES | 706–718 | Y713 | Proto-oncogene tyrosine-protein kinase Fes/Fps |
| FER | 707–719 | Y714 | Proto-oncogene tyrosine-protein kinase FER |
| LCK | 387–399 | Y394 | Proto-oncogene tyrosine-protein kinase LCK |
| PXN | 111–123 | Y118 | Paxillin |
| PXN | 24–36 | Y31/33 | Paxillin |
| MST1R | 1353–1365 | Y1353, Y1360 | Macrophage-stimulating protein receptor |
| CTTN | 476–488 | Y477, Y483 | Src substrate protein p85 |
| Cell survival and proliferation | |||
| CTNNB1 | 79–91 | Y86 | Beta-catenin |
| JAK1 | 1015–1027 | Y1022, Y1023 | Tyrosine-protein kinase JAK1 |
| PDPK1 | 2–14 | Y9 | 3-phosphoinositide dependent protein kinase 1 |
| Other | |||
| CD247 | 116–128 | Y123 | T-cell surface glycoprotein CD3 zeta chain |
| CDK2 | 8–20 | Y15, Y19 | Cell division protein kinase 2 |
| EPHA7 | 607–619 | Y608, Y614 | Ephrin type-A receptor 7 |
| EPHB1 | 771–783 | Y778 | Ephrin type-B receptor 1 |
| FRK | 380–392 | Y387 | Tyrosine-protein kinase FRK |
| KRT6E | 53–65 | Y62 | Keratin, type II cytoskeletal 6E |
| RET | 1022–1034 | Y1029 | Proto-oncogene tyrosine-protein kinase receptor ret |
aSubstrate identities and common names are retrieved from UniProtKB/SwissProt [13]
bFor each substrate, positions of the peptide sequence and the phosphorylation sites within the protein are indicated
Fig. 3.
Ex vivo sunitinib inhibition profiles from 23 angiogenesis-related kinase substrates. Patient tumor samples along horizontal axis, annotated by negative (−) or positive (+) status for disseminated tumor cells to bone marrow, and phosphosubstrates along vertical axis. Red corresponds to stronger and blue to weaker inhibition of substrate phosphorylation. Left panel Substrate identities. For each substrate, the position of phosphorylation sites within the protein is indicated. (Color figure online)
Discussion
In this cohort of 55 LARC patients, tumor kinase activity signatures associated with early systemic dissemination were identified. For 31 peptides on the tyrosine kinase substrate array, ex vivo sunitinib inhibition of phosphorylation generated by tumor biopsy specimens was significantly stronger for DTC-negative patients than for patients with tumor cells identified in bone marrow, as assessed by immunomagnetic selection at the time of diagnosis. Many of the discriminating peptide substrates represented signaling pathways that are activated by tissue hypoxia, such as signaling mediated by PDGFR, VEGFR, and EPOR [1, 6]. Accordingly, tumor-generated phosphorylation of 23 angiogenesis-related peptides was weakly inhibited in DTC-positive patients, who had significantly poorer metastasis-free survival than patients without evidence of early systemic tumor dissemination.
Various reports have demonstrated that the presence of tumor cells in bone marrow is a prognostic biomarker associated with metastatic recurrence [14], including in colorectal cancer [9]. In this study, hypothesizing that hypoxic tumor signaling mediates both radiation resistance and metastatic progression in rectal cancer, and using previously acquired data [8], we endeavored to correlate the individual patient’s tumor tyrosine kinase activity to the DTC status. In two previous works applying the array technology with tyrosine kinase substrates, we were able to calculate basal kinase activity data and correlate with the biological parameters of interest [8, 15]. In the present study, however, after normalization of basal phosphosubstrate level read-outs, no difference was found among the study patients when comparing those with and without DTC (data not shown). Since a reasonable explanation might be technical variation among 96-well array plates, the analytical strategy of including a tyrosine kinase inhibitor was attempted, to enable direct comparison of substrate phosphorylation in its presence and absence on the same array plate, and possibly diminishing plate-to-plate variation [8]. Sunitinib is thoroughly characterized in vitro and in vivo for inhibiting tyrosine kinase signaling related to tumor hypoxia [16]. Whether other tyrosine kinase inhibitors might have worked equally well for normalization of basal kinase activity data in the clinical setting of interest (primary tumor signaling and DTC status), is not known.
Using this strategy, phosphorylation of 31 kinase substrates by tumor sample lysates was found to be significantly more strongly inhibited by sunitinib in the DTC-negative patients than in patients with positive DTC status. As tumors outgrow their blood supply or are otherwise deprived of oxygen, adaptive responses to the resulting hypoxic conditions are initiated [17]. In this context, the transcription factors hypoxia-inducible factor types 1α and 2α have emerged as key regulators of a range of target genes that induce angiogenesis, including genes encoding platelet-derived growth factor, vascular endothelial growth factor, and erythropoietin [1]. In this study, components of hypoxia-driven signaling were identified in the 31-peptide panel demonstrating differential response to sunitinib inhibition in patients with negative and positive DTC status, which included receptors for these ligands; five of six PDGFR type β substrates, three of ten VEGFR substrates (one VEGFR1 and two VEGFR2), and both EPOR substrates on the array. The observed ex vivo regulation of phosphorylation of these particular substrates was not unexpected, since sunitinib has been noted to inhibit several receptor tyrosine kinases, among which members of the VEGFR and PDGFR families are predominant targets [16].
Signaling initiated by VEGFR and PDGFR is fundamental for the angiogenic response [1, 5, 6], which comprises proliferation and invasion of endothelial cells and also formation of pericyte coverage of vascular sprouts for stabilization of the newly formed vessel walls. In this process, PDGFR-dependent signaling is required for pericyte differentiation directed by the tissue stroma [18]. It is tempting to speculate that the strong ex vivo sunitinib inhibition of the PDGFR array substrate phosphorylation generated by tumor samples from DTC-negative patients in this study reflects high pericyte signaling activity of mature tumor vessel that are less permeable for metastasizing tumor cells [19].
However, it cannot be ignored that among the 23 peptides identified as angiogenesis-related in this study, ten substrates were not correlated with the patients’ DTC status, including seven of ten VEGFR substrates. Regulation of tumor angiogenesis is a complex phenomenon. In colorectal cancer, this complexity has recently been highlighted by the observation that anti-angiogenic therapy (bevacizumab) that has proven efficacious in metastatic colorectal cancer, failed to meet the endpoint of prolonged disease-free survival in randomized phase III trials in the adjuvant setting [20, 21]. Interestingly, studies in experimental models have indicated that mature pericytes protect endothelial cells against VEGFR-directed therapies [22, 23]. The recent demonstration that tumor cells are able to induce pericyte maturation of the neovasculature during early formation of micrometastatic foci [24] might provide one explanation for the lack of efficacy of bevacizumab in eradicating occult metastatic disease in colorectal cancer.
The panel of 31 differentially inhibited phosphopeptides also included EPOR, the receptor for erythropoietin, which is expressed in many non-hematopoietic tissues, including endothelial cells and colorectal cancer [25], and has been associated with angiogenic responses in experimental tumor models [26]. Furthermore, this panel comprised other candidate angiogenic regulators, such as PECAM-1 (platelet endothelial cell adhesion molecule-1), PIK3R1 (the PI3 K regulatory subunit α), and EGFR [27–29], as well as the ephrin receptor types A7 and B1. Although experimental studies suggest that several types of ephrin receptors are activated in tumor vascularization [30], the function of many subgroups is incompletely understood, and in the current analyses, we therefore chose to exclude ephrin receptors from the angiogenesis-related 23-peptide panel.
The bone marrow compartment represents an important site for hematogenous micrometastatic spread in breast and prostate cancer, and clinical data has provided evidence for an association between tumor cells detected in bone marrow at the time of tumor resection and postoperative metastatic relapse in these cancer types [14]. The presence of systemically disseminated tumor cells has also been proposed as biomarker of metastatic recurrence risk in colorectal cancer [31]. In a study by Flatmark and co-workers [11], the immunomagnetic selection method was used to determine DTC status in 275 patients with primarily resectable colorectal cancer, and recent update of the clinical data shows that the presence of DTC was also associated with poor long-term outcome in this patient cohort [9]. In the present LARC-RRP study, using the same method to examine bone marrow aspirates, absence of DTC at the time of diagnosis was predictive of good short-term metastasis-free survival after radical treatment of the pelvic cavity. At the present stage of follow-up (median of 42 months), a non-significant trend towards the same association was found with overall survival. The overall frequency of DTC-positive samples was higher in the present study (60%) than in Flatmark’s study (17%), which might be anticipated from the more locally advanced disease stage.
In summary, within a cohort of LARC patients, tumor phenotypes defined by tyrosine kinase activities that appeared to correlate with weak ex vivo inhibition by sunitinib, particularly related to angiogenic signaling, were associated with early systemic dissemination. These patients were also noted to have heightened risk of developing metastatic disease following the course of radical treatment of the pelvic cavity. This novel strategy for studying the functional tumor kinome in early metastatic progression of rectal cancer may be used to improve our understanding of the angiogenic response in metastasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the European Union 7th Framework Programme Grant 222741 – METOXIA, the South-Eastern Norway Regional Health Authority Grant 20100014, the Norwegian Cancer Society Grant 0910106, and Astri and Birger Torsted’s Legacy. M. G. Saelen is Research Fellow 2010–2012 of the South-Eastern Norway Regional Health Authority.
Ethics
The LARC-RRP study protocol was approved by the Institutional Review Board and the Regional Committee for Medical and Health Research Ethics, and was in accordance with the Helsinki Declaration. The study was conducted according to national and local law and regulations. Written informed consent was required for participation.
Conflicts of interest
R de Wijn is PamGene International B.V. employee. The other authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebbesen P, Pettersen EO, Gorr TA, Jobst G, Williams K, Kieninger J, Wenger RH, Pastorekova S, Dubois L, Lambin P, Wouters BG, Van Den Beucken T, Supuran CT, Poellinger L, Ratcliffe P, Kanopka A, Görlach A, Gasmann M, Harris AL, Maxwell P, Scozzafava A. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem. 2009;24:S1–S39. doi: 10.1080/14756360902784425. [DOI] [PubMed] [Google Scholar]
- 3.Harrington K, Jankowska P, Hingorani M. Molecular biology for the radiation oncologist: the 5Rs of radiobiology meet the hallmarks of cancer. Clin Oncol (R Coll Radiol) 2007;19:561–571. doi: 10.1016/j.clon.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Folkvord S, Flatmark K, Dueland S, de Wijn R, Grøholt KK, Hole KH, Nesland JM, Ruijtenbeek R, Boender PJ, Johansen M, Giercksky KE, Ree AH. Prediction of response to preoperative chemoradiotherapy in rectal cancer by multiplex kinase activity profiling. Int J Radiat Oncol Biol Phys. 2010;78:555–562. doi: 10.1016/j.ijrobp.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Flatmark K, Borgen E, Nesland JM, Rasmussen H, Johannessen HO, Bukholm I, Rosales R, Hårklau L, Jacobsen HJ, Sandstad B, Boye K, Fodstad Ø. Disseminated tumour cells as a prognostic biomarker in colorectal cancer. Br J Cancer. 2011;104:1434–1439. doi: 10.1038/bjc.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–1130. doi: 10.1002/cncr.10327. [DOI] [PubMed] [Google Scholar]
- 11.Flatmark K, Bjornland K, Johannessen HO, Hegstad E, Rosales R, Hårklau L, Solhaug JH, Faye RS, Søreide O, Fodstad Ø. Immunomagnetic detection of micrometastatic cells in bone marrow of colorectal cancer patients. Clin Cancer Res. 2002;8:444–449. [PubMed] [Google Scholar]
- 12.European Bioinformatics Institute: ArrayExpress Experiments Archive. http://www.ebi.ac.uk/microarray-as/ae/. Accessed 26 February 2010
- 13.European Bioinformatics Institute/Swiss Institute of Bioinformatics/Protein Information Resource: Universal Protein Knowledgebase, UniProtKB/Swiss-Prot. http://au.expasy.org/sprot. Accessed 11 August 2010
- 14.Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Bratland Å, Boender PJ, Høifødt HK, Østensen IHG, Ruijtenbeek R, Wang M, Berg JP, Lilleby W, Fodstad Ø, Ree AH. Osteoblast-induced EGFR/ERBB2 signaling in androgen-sensitive prostate carcinoma cells characterized by multiplex kinase activity profiling. Clin Exp Metastasis. 2009;26:485–496. doi: 10.1007/s10585-009-9248-9. [DOI] [PubMed] [Google Scholar]
- 16.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 17.Bicknell R, Harris AL. Novel angiogenic signaling pathways and vascular targets. Annu Rev Pharmacol Toxicol. 2004;44:219–238. doi: 10.1146/annurev.pharmtox.44.101802.121650. [DOI] [PubMed] [Google Scholar]
- 18.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, O’Reilly S, Chu L, Azar CA, Lopa S, Wolmark N. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:1–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Gramont A, Van Cutsem E, Tabernero J, Moore MJ, Cunningham D, Rivera F, Im SA, Makrutzki M, Shang A, Hoff PM (2011) AVANT: results from a randomized, three-arm multinational phase III study to investigate Bevacizumab with either XELOX or FOLFOX4 versus FOLFOX4 alone as adjuvant treatment for colon cancer. In: Proceedings of 2011 Gastrointestinal Cancers Symposium, San Francisco, CA, January 20–22, 2011
- 22.Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, Kato M, Ergün S, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hlushchuk R, Baum O, Gruber G, Wood J, Djonov V. The synergistic action of a VEGF-receptor tyrosine-kinase inhibitor and a sensitizing PDGF-receptor blocker depends upon the stage of vascular maturation. Microcirculation. 2007;14:813–825. doi: 10.1080/10739680701370021. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Stewart KS, Yu L, Kleinerman ES. Bone marrow cells participate in tumor vessel formation that supports the growth of Ewing’s sarcoma in the lung. Angiogenesis. 2011;14:125–133. doi: 10.1007/s10456-010-9196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chabowska AM, Sulkowska M, Chabowski A, Wincewicz A, Koda M, Sulkowski S. Erythropoietin and erythropoietin receptor in colorectal cancer. Int J Surg Pathol. 2008;16:269–276. doi: 10.1177/1066896908315796. [DOI] [PubMed] [Google Scholar]
- 26.Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, Vujaskovic Z, Dewhirst MW, Arcasoy MO. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS One. 2007;2:e549. doi: 10.1371/journal.pone.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, Delisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–915. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 29.Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010;138:2163–2176. doi: 10.1053/j.gastro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Mosch B, Reissenweber B, Neuber C, Pietzsch J. Eph receptors and ephrin ligands: important players in angiogenesis and tumor angiogenesis. J Oncol. 2010;2010:135285. doi: 10.1155/2010/135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.