Abstract
Renal ischemic events open tight junctions and disrupt epithelial polarity. The purpose of this study was to examine the effects of ischemia–reperfusion (IR) injury on expression and distribution of the tight junction proteins, occludin and ZO-1, in the rat kidney. IR injury was induced by clamping both renal pedicles for 30 min and animals were killed at 6 h after the reperfusion. IR injury decreased blood bicarbonate level, but did not persistently alter pH, Na+, K+, or Cl−. In control kidneys, occludin immunoreactivity was intense in the tight junctions in the thick ascending limb, distal convoluted tubule, and collecting duct, moderate in the thin limbs of the loop of Henle, and was not detected in the proximal tubule, glomerulus, and blood vessels. ZO-1 was expressed in the same sites in which occludin was expressed, and additionally was also expressed in the proximal tubule, glomerulus, and vascular endothelial cells. IR kidneys exhibited damaged renal tubular epithelial cells in both proximal tubule and collecting duct segments in the outer medulla. In the collecting duct, the response of intercalated cells and principal cells differed. Following IR injury, intercalated cells, but not principal cells, lost their normal epithelial polarity and were frequently extruded into the tubule lumen. Occludin, instead of being localized to tight junctions, was localized diffusely in the cytoplasm in intercalated cells of IR kidneys. Principal cells, in contrast, were not detectably affected and neither occludin nor ZO-1 expression were altered in response to IR injury. The normal localization of ZO-1 expression to tight junction sites in both the proximal tubule and collecting duct was altered in response to IR, and, instead, ZO-1 expression was present diffusely in the cytoplasm. IR injury did not alter detectably either occludin or ZO-1 localization to the tight junction of the thick ascending limb cells. The abundance of total occludin protein by immunoblot analysis was not changed with IR injury. These results demonstrate that renal IR injury causes tight junction disruptions in both the proximal tubule and the collecting duct, and that altered distribution of the tight junction protein, occludin, may play a critical role in the collecting duct dysfunction which IR induces.
Keywords: Collecting duct, Ischemia/reperfusion injury, Kidney, Occludin, Tight junction
Introduction
Renal tubular cells have unique fluid and electrolyte transport capabilities across the distinct apical and basolateral membrane domains. Tight junctions maintain the functional integrity and polarity of tubular surface membrane, and regulate paracellular and transcellular transports (Fish and Molitoris 1994; Lee et al. 2006; Weinstein 2003). The tight junction complex consists of multiple adhesion proteins linking it to the cytoskeleton of the individual cells.
Occludin, ZO-1, and the claudin family are major components of the growing number of tight junction proteins (Furuse et al. 1993; Mitic et al. 2000; Schneeberger and Lynch 2004; Stevenson et al. 1986). Occludin carries out an adhesive function when interacting with ZO-1 in kidney cells (Furuse et al. 1994; Van Itallie and Anderson 1997). Although the exact roles of occludin and ZO-1 in the kidney are not completely understood, their distribution varies markedly along the tubule segments. The amount of occludin and ZO-1 expression is significantly greater in more distal tubular segments than in proximal tubules, which correlates with the greater transepithelial electrical resistance in those segments (Gonzalez-Mariscal et al. 2000). In cultured cells, overexpression of occludin dramatically increases transepithelial resistance (Balda et al. 1996; McCarthy et al. 1996). Thus, occludin and ZO-1 may play an important role in increasing the adhesive strength of the tight junction in renal tubular epithelia.
Renal ischemic injury is a commonly observed complication in hospitalized patients that results in multiple impairments of water and ion transport (Clarkson et al. 2008). The disruption of tight junctions is an important component of the pathogenesis of renal ischemic injury and causes polarity alteration of tubular epithelium (Fish and Molitoris 1994; Lee et al. 2006; Molitoris et al. 1989, 1992). Distorted distributions of the tight junction proteins occludin and ZO-1 have been observed with ATP depletion (an in vitro model of renal ischemia) in cultured cells (Bacallao et al. 1994; Gopalakrishnan et al. 2007). ZO-1 staining in renal allograft recipients with sustained acute renal failure exhibits diminished intensity and redistribution in the proximal tubule (Kwon et al. 1998). Ischemic injury also has been shown to induce glomerular podocyte effacement in association with ZO-1 dissociation (Wagner et al. 2008). However, the effects of ischemic injury on occludin and ZO-1 distribution in renal epithelial cells have not yet been fully investigated in the kidney.
In the present study, we show that renal ischemia–reperfusion injury causes cellular damages not only in the proximal tubule but also in the collecting duct and that the disruption of tight junctions is cell-specific to intercalated cells in the collecting duct. The collecting duct is composed of two histologically different cell types, the principal cells and intercalated cells. The principal cells reabsorb water, whereas the intercalated cells are involved in acid–base homeostasis (Madsen and Tisher 1986). Renal ammonia excretion is a central component of net acid excretion and ischemia–reperfusion injury decreases renal ammonia excretion (Han et al. 2007; Wagner et al. 2011; Weiner and Verlander 2011). We show that disruption of tight junctions is associated with the polarity loss or delocalization of the ammonia transporting Rhesus protein, Rhcg, as well as other acid–base transporter proteins in intercalated cells. The results may have significant value in understanding clinical manifestations of renal ischemic injury such as metabolic acidosis.
Materials and methods
Animals and surgical procedures
Male Sprague–Dawley rats weighing 200–250 g were used in all experiments. Animals were maintained in a temperature-controlled room with alternating 12:12-h light–dark cycles and had free access to water and food throughout the experiment. These studies were approved by the Ewha Womans University Institutional Animal Care and Use Committee (EMRI 07-0073, 08-0083).
Animals were anesthetized with an intraperitoneal injection of tiletamine/zolazepam (10 mg/kg; Zoletil 50, Virbac Laboratories, Carros, France) and 2% xylazine hydrochloride (2 mg/kg; Rumpun, Bayer Korea, Ansan, Korea). After the abdomen was opened through a midline incision, both renal pedicles were exposed and cleaned by blunt dissection. Microvascular clamps (RS-5422, Roboz Surgical Instrument Co., Inc., Gaithersburg, MD, USA) were placed on both renal arteries to completely block renal blood flow. After 30 min, the clamps were removed and blood flow returned to the kidneys. Control animals underwent sham operation. The animals were killed 6 h after reperfusion. Arterial blood samples of the animals were collected from the abdominal aorta. The collected blood sample was placed on ice, and the blood gas was immediately measured (Beckman Instruments, Palo Alto, CA, USA).
Tissue preservation
The kidneys were first perfused briefly through the abdominal aorta with cold PBS and subsequently perfused with the fixative solution, periodate-lysine-2% paraformaldehyde (PLP), for 10 min. The kidneys were cut transversely into 1- to 2-mm thick slices and immersed in PLP overnight at 4°C. The tissue was rinsed in PBS, dehydrated in a series of ethanol, and embedded in wax (Polysciences, Inc., Warrington, PA, USA) for light microscopy.
Antibodies
Mouse anti-occludin monoclonal antibodies (Zymed Laboratories, San Francisco, CA, USA; catalog No 33-1500) and rabbit anti-ZO-1 polyclonal antibodies (Zymed Laboratories, San Francisco, CA, USA; catalog No 40-2300) were used. Aquaporin 1 (Chemicon Inc., Temecula, CA, USA; catalog No AB3065), bumetanide-sensitive Na+/K+/2Cl− cotransporter (NKCC2; Jeon et al. 2007), aquaporin 2 (Chemicon Inc., Temecula, CA, USA; catalog No AB3066), Rh C glycoprotein (Rhcg; Han et al. 2007), and anion exchanger 1 (AE1; Alpha Diagnostics, San Antonio, TX, USA; catalog No AE11-A) were used to identify the proximal tubule and descending thin limb, thick ascending limb, collecting duct principal cells, collecting duct intercalated cells, and type A intercalated cells, respectively.
Light microscopic immunohistochemistry
Four micrometer-thick wax sections were processed for immunohistochemistry using immunoperoxidase procedures, as described previously (Han et al. 2003, 2004, 2005). The sections were dewaxed with xylene and ethanol and rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 for 30 min. The sections were treated with proteinase K (DakoCytomation, Carpinteria, CA, USA) for 10 min and blocked with the blocking solution (DakoCytomation, Carpinteria, CA, USA) for 30 min before incubation with primary antibody (occludin 1:200; aquaporin 1:1000; NKCC2 1:500) overnight at 4°C. The sections were washed in PBS and incubated for 1 h with peroxidase-conjugated secondary antibodies against mouse or rabbit IgG (Jackson ImmunoResearch laboratories, West Grove, PA, USA) diluted 1:100 in PBS. The sections were again washed with PBS and then exposed to a mixture of 0.05% 3,3′-diaminobenzidine and 0.01% H2O2. The sections were washed in Tris–HCl buffer, dehydrated with graded ethanol and xylene, mounted, and observed by light microscopy.
Double immunohistochemistry
Colocalization was accomplished using sequential immunoperoxidase procedures, as described previously (Han et al. 2007). Briefly, the first immunoperoxidase procedure used the protocol for single labeling described above. After the diaminobenzidine reaction, the sections were washed in PBS and incubated in 3% H2O2 for 30 min and blocked with the blocking solution. The sections were treated for 1 h with the second primary antibodies (aquaporin 1 1:1000; NKCC2 1:500; Rhcg 1:1000), washed in PBS, incubated with the secondary antibodies, and then washed with PBS. Vector SG (Vector Laboratories, Burlingame, CA, USA) was used as the chromogen to produce a blue label easily distinguishable from the brown label produced by the diaminobenzidine used for detection of the first protein. The sections were washed in Tris–HCl buffer, dehydrated with graded ethanol and xylene, mounted, and observed by light microscopy.
Confocal microscopy
Kidney sections were dewaxed and rehydrated in a graded series of ethanol. The sections were treated with proteinase K (DakoCytomation, Carpinteria, CA, USA) for 10 min and blocked with the blocking solution (DakoCytomation, Carpinteria, CA, USA) for 30 min before incubation with a mixture of primary antibodies (occludin 1:100; AE1 1:100) overnight at 4°C. The sections were washed in PBS and incubated for 1 h with a mixture of Cy3-conjugated donkey anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch laboratories, West Grove, PA) diluted 1:100 in PBS, respectively. Coverslips were mounted with VectaShield mounting media. Images were captured on a Zeiss LSM5 PASCAL.
Immunoblot analysis
Kidneys were divided into the cortex and medulla and processed for immunoblot analysis, as described previously (Han et al. 2005). Tissues were homogenized in lysis buffer containing 20 mM Tris–HCI, 1% Triton X-100, 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide, 1 mM EDTA, 10 μM leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 3,000g for 20 min at 4°C. After determination of protein concentration in the supernatant by the Coomassie method (Pierce, Rockford, IL, USA), samples were loaded (20 μg/lane) and underwent electrophoresis on sodium dodecyl sulfate-polyacrylamide gels under reducing conditions. Proteins were transferred to nitrocellulose membranes by electroblotting. To reduce nonspecific antibody binding, the membranes were blocked with 5% nonfat dried milk for 30 min at room temperature, and then incubated for 24 h at 4°C with anti-occludin antibody (1:1,000). After three washes, the blot was incubated with a peroxidase conjugated donkey anti-mouse IgG (1:1,000) for 2 h at room temperature. Samples were visualized using an enhanced chemiluminescence system (Amersham Life Science, Buckinghamshire, UK) after a 5- to 30-min exposure at room temperature. Densitometric analysis was performed using the Zero-Dscan software of the Eagle EYETMII Still Video System (Stratagene, La Jolla, CA, USA).
Statistics
Results are presented as mean ± SD. Statistical analyses were performed using Student’s unpaired t test, and P < 0.05 was taken as statistical significance.
Results
Physiological parameters
The results of the arterial blood gas are summarized in Table 1. Renal ischemia–reperfusion injury decreased significantly blood bicarbonate level compared with control animals (20.6 ± 2.36 vs. 25.1 ± 2.02 mmol/L). Arterial carbon dioxide tension (PaCO2) was slightly reduced indicating a respiratory compensation in ischemic rats. There were no significant differences in serum sodium, potassium, and chloride concentrations.
Table 1.
Effects of ischemia–reperfusion injury on blood pH and electrolyte concentration
| Control | IR | |
|---|---|---|
| pH | 7.38 ± 0.04 | 7.37 ± 0.05 |
| HCO3 − (mmol/L) | 25.1 ± 2.0 | 20.6 ± 2.4* |
| pCO2 (mmHg) | 44.1 ± 7.1 | 37.1 ± 6.3 |
| Plasma Na+ (mmol/L) | 131 ± 3.0 | 132 ± 2.1 |
| Plasma K+ (mmol/L) | 4.6 ± 0.6 | 5.1 ± 0.4 |
| Plasma Cl− (mmol/L) | 108 ± 2 | 111 ± 1 |
Values are mean ± SD of five rats (*P < 0.05)
Localization of occludin and ZO-1 in the normal rat kidney
Occludin was expressed from the cortex to the tip of the inner medulla. In both normal and sham-operated control kidneys, strong occludin immunoreactivity was observed in the distal nephron segments, including the thick ascending limb, distal convoluted tubule, and collecting duct. High-magnification microscopic examination demonstrated a classic dot or thread-like expression pattern at the apical end of the lateral membrane in the tubular epithelial cells, as expected for the location of the tight junction (Fig. 1a, b). Occludin immunolabeling was not detected in the glomerulus, proximal tubule, and blood vessels in the rat kidney (Fig. 1a, b). The thin limbs of the loop of Henle showed moderate occludin immunoreactivity. To identify the descending thin limb, we used aquaporin 1 as a marker. Occasionally, the transition point from the descending thin limb to the ascending thin limb was observed in the inner medulla, and occludin was expressed in both thin limbs (Fig. 1f–h).
Fig. 1.
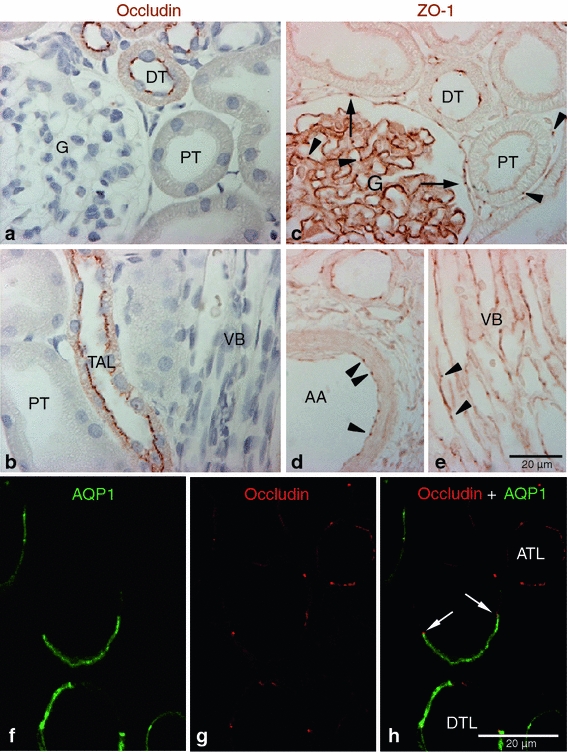
Occludin and ZO-1 expression in control kidney. Representative of occludin (a, b), ZO-1(c–e), and occludin-AQP1 confocal (f–h) immunostaining images obtained from sham-operated control kidneys. Occludin (brown) immunoreactivity was observed as dot-and-line patterns at the apical end of the lateral membrane in tubular epithelial cells in the cortex (a) and outer medulla (b). Occludin was expressed primarily in the distal tubule (DT) including the thick ascending limb (TAL). No occludin immunolabel was detected in glomeruli (G), proximal tubule (PT), or blood vessels, including the vascular bundles (VB). ZO-1 (brown) immunoreactivity was observed in all tubular segments, including the proximal tubule (PT) and distal tubule (DT) (c–e). ZO-1 labeling also appeared in the glomerular parietal epithelial cells (arrows) and in endothelial cells (arrowheads) of all blood vessels including the glomerular capillary, peritubular capillary, arcuate artery (AA), and vascular bundle (VB). To identify the thin limbs of the loop of Henle, confocal immunofluorescence staining for occludin (red) and AQP1 (green) were performed (f–h). Occasionally, transition points (white arrows) from the AQP1-positive descending thin limb (DTL) to AQP1-negative ascending thin limb (ATL) were observed and occludin immunoreactivity appeared in both thin DTL and ATL segments (g, h). Results are representative of findings in the five control rat kidneys
ZO-1 immunoreactivity was strong in the thick ascending limb, distal convoluted tubules, and collecting duct and was moderate in thin limbs of the loop of Henle in the normal kidney (Fig. 1c–e). In contrast to occludin, ZO-1 expression was also observed in the parietal epithelium of Bowman’s capsule and in vascular endothelial cells, including the arcuate artery, interlobular artery, glomerular capillaries, peritubular capillaries, and vascular bundles (Fig. 1c–e). Also in contrast to occludin, ZO-1 immunolabel was observed in the proximal tubule, although the intensity of immunolabel was less than that observed in distal nephron segments (Fig. 1c).
Abnormal localization of occludin and ZO-1 after renal ischemia–reperfusion injury
After ischemia–reperfusion injury, abnormal localization of occludin was observed in the outer medulla region (Fig. 2). Typical dot or thread-like patterns of occludin immunoreactivity was disrupted. Instead, occludin was distributed diffusely in the cytoplasm in a subset of tubular epithelial cells in the outer medulla of IR injury kidneys (Fig. 2d).
Fig. 2.
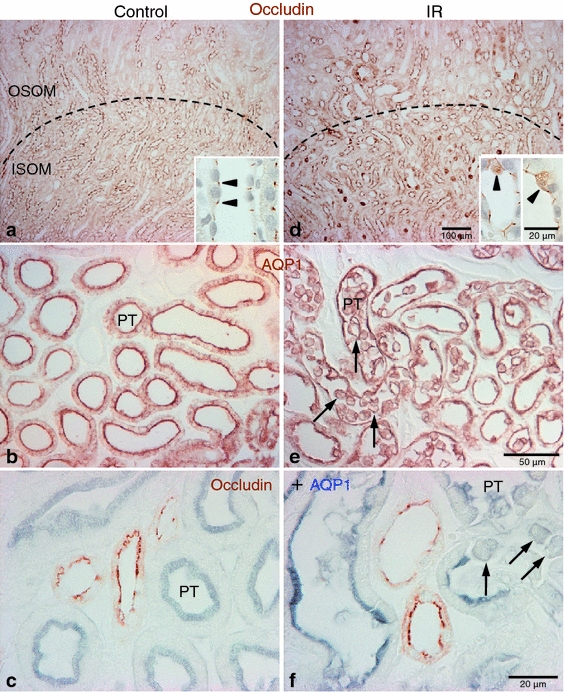
Effects of IR injury on occludin immunolabel in the rat kidney. Representative micrographs showing occludin (a, d), AQP1 (b, e) and occludin-AQP1 double immunostaining (c, f) images in the outer medulla in sham-operated control (a–c, n = 5) and IR (d–f, n = 5) kidneys. Dashed lines indicate boundary between the outer stripe of the outer medulla (OSOM) and inner stripe of the outer medulla (ISOM). Compared with control kidneys, abnormal localization of occludin (brown) immunoreactivity was observed in the outer medullary region in ischemic kidneys (d). In a subpopulation of cells, occludin diffusely localized in the cytoplasm (inset, arrowheads) in the ischemic kidneys (d). Many AQP1 (brown in b, e; blue in c, f)-positive proximal tubular (PT) cells lost polarity, detached from basement membrane, and were present in the tubule lumen (arrows) in ischemic kidneys (e, f). Occludin (brown in c, f) immunoreactivity was not observed in the proximal tubule either under control or following IR injury
Because there are three different tubular segments (the proximal straight tubule, thick ascending limb, and collecting duct) in the outer medulla, we used specific marker proteins to identify the different epithelial segments and cells affected by IR injury. We used AQP1 to identify proximal tubule segments, NKCC-2 to identify the thick ascending limb of the loop of Henle and Rhcg, and AQP2 and AE1 to identify collecting duct segments.
Many aquaporin 1-positive proximal tubule cells were damaged and detached into the tubular lumen in IR kidneys (Fig. 2e). However, occludin immunoreactivity was not detected in the proximal tubule cells in either control or ischemic kidneys (Fig. 2c, f).
NKCC2-positive thick ascending limb cells were relatively intact and did not show detectable morphological damage in this model of ischemia–reperfusion injury (Fig. 3). Occludin expression was strong in the thick ascending limb in both control and IR kidney, and there was no change in occludin distribution in the thick ascending limb in response to IR injury (Fig. 3b, d).
Fig. 3.
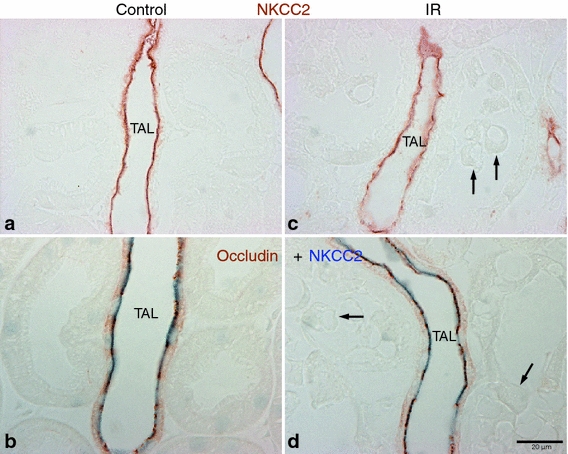
Effects of IR injury on TAL segments. Representative images of NKCC2 (a, c) and occludin-NKCC2 double immunostaining (b, d) in the outer medulla of sham-operated control (a, b, n = 5) and IR (c, d, n = 5) kidneys. Apical NKCC2 (brown in a, c; blue in b, d) immunoreactivity was used to identify the thick ascending limb (TAL). No detectable morphological damages were observed in the TAL, although many neighboring tubular cells (arrows) were severely damaged after IR injury. Therefore, there were no changes in occludin (brown in b, d) localization in the TAL after IR injury
Ischemia–reperfusion injury caused considerable damage to a subset of cells in the outer medullary collecting duct (OMCD). To determine whether the damage is specific to either intercalated cells or principal cells, the two primary cell types present in the collecting duct, we double-immunolabel with Rhcg, to identify intercalated cells, and AQP2, to identify principal cells. We observed that Rhcg-positive intercalated cells were detached frequently from the basement membrane, whereas principal cells, in contrast, appeared intact and undamaged (Fig. 4a, f). To identify whether disturbances in occludin localization were present and whether changes in its localization were specific to intercalated cells, we performed double-immunolabel fluorescent microscopy using antibodies to occludin and the intercalated cell marker, AE1. Confocal fluorescent microscopy confirmed that occludin disruption in the OMCD was specific to damaged intercalated cells and that occludin lost its typical apical tight junction localization and was localized diffusely in detached intercalated cells. Occludin expression was only slightly delocalized in cells remaining in the OMCD (Fig. 4b–e, g–j).
Fig. 4.
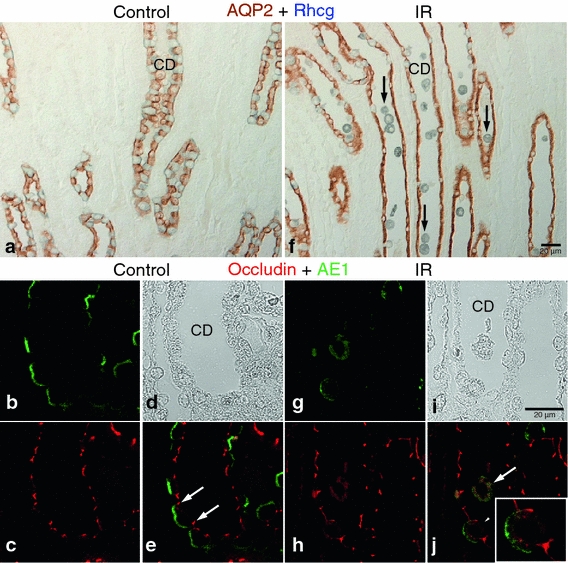
Effects of IR injury in the OMCD. Representative of AQP2-Rhcg double immunohistochemistry (a, f) and occludin-AE1 confocal fluorescent microscopy (b–e, g–j) images in the outer medulla in sham-operated control (a–e, n = 5) and IR (f–j, n = 5) kidneys. Compared with control, many Rhcg (blue)-positive collecting duct (CD) cells detached from the basement membrane and were present in the tubule lumen (arrows) in ischemic kidneys (f). However, AQP2 (brown)-positive cells were not damaged after IR injury. Confocal images confirmed the intercalated cell-specific damage in the CD. In control kidneys, occludin (red) labeling was observed at the apical end of the lateral membrane in both principal and intercalated cells (e, white arrows). In IR injury kidneys, both occludin and AE1 (green) lost their normal polarity and were localized diffusely in the cytoplasm in cells released into the lumen (j, white arrow). Partial delocalization or internalization of occludin and AE1 was also observed in cells still remaining in the tubule wall (white arrowheads, inset) in ischemic kidneys (j)
IR injury also resulted in significant changes in ZO-1 immunolabel in the outer medulla. However, in contrast to occludin, ZO-1 distribution was disrupted in both the proximal tubule and the collecting duct, but not in the thick ascending limb of the loop of Henle (Fig. 5). In both the proximal tubule and the OMCD, ZO-1 immunolabel intensity was decreased in the tight junction regions and its localization was often diffuse in damaged cells, rather than being limited to the tight junction regions (Fig. 5c, d).
Fig. 5.
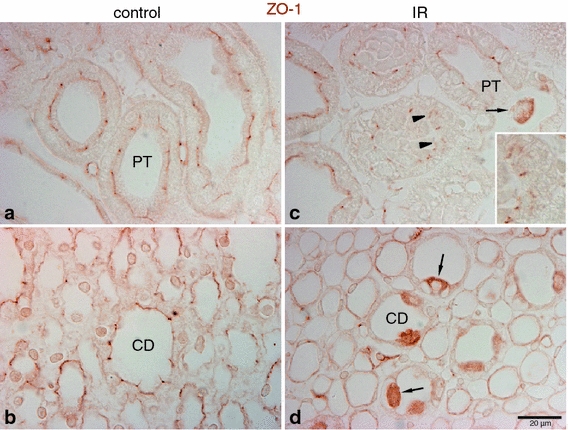
Effects of IR injury on ZO-1 expression in the outer medulla. Representative micrographs of ZO-1 localization in the outer medulla in control (n = 5) and IR (n = 5) kidneys. In control kidneys (a, b), ZO-1 (brown), immunoreactivity was observed in the proximal tubule (PT) as well as in the collecting duct (CD). In ischemic kidneys (c, d), ZO-1 localization was distorted and abnormally localized in the cytoplasm (arrowheads, inset). Occasionally, a combination of both delocalization and upregulation of ZO-1 expression was observed in some cells (arrows) in the PT and CD
Western blot analysis
Occludin bands were detected at ~60 kDa. There was no significant change in occludin protein expression after ischemia–reperfusion injury in the cortex (not shown) and medulla (Fig. 6).
Fig. 6.
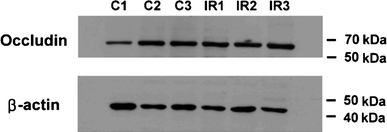
Effects of IR injury on occludin protein expression. Total occludin protein expression in the medulla of control (C1-3) and ischemic kidneys (IR1-3) was quantified using immunoblot analysis. There was no significant difference between the two groups
Discussion
The present study provides important new information regarding both the distribution of the tight junction proteins, occludin and ZO-1, and the effect of IR injury on their localization. In the rat kidney, occludin is localized mainly in distal nephron segments, whereas ZO-1 is widely expressed both in epithelial and endothelial cells. IR injury causes cell-specific changes in occludin localization, with development of diffuse intracellular localization in collecting duct intercalated cells, but not principal cells, and no detectable change in the thick ascending limb. ZO-1 localization was altered in both proximal tubule and collecting duct cells, but not in the thick ascending limb. Thus, the distribution of occludin differs from that of ZO-1, and ischemia–reperfusion injury causes cell-specific disruption of tight junction proteins in the collecting duct.
The current study provides the first examination of occludin expression in the rat kidney. In particular, the distinct axial expression pattern, collecting duct and distal tubule > loop of Henle >> proximal tubule, parallels both transepithelial resistance and the complexity of junctional intramembrane strands (Claude and Goodenough 1973). Axial variations in occludin expression also parallel axial variations in the expression of other tight junction proteins, specifically claudins, which exhibit differences in specific claudin family member expression in different epithelial segments (Kiuchi-Saishin et al. 2002).
Occludin localization may differ, but only slightly, between the rat, rabbit, and mouse kidney. In the rabbit kidney, the highest level of occludin expression is in the distal nephron segments similar to that observed in the current study in the rat kidney (Gonzalez-Mariscal et al. 2000). Proximal tubule cells in the rabbit kidney also express low level occludin expression, less than 1% of its expression in the collecting duct (recalculation of data from Gonzalez-Mariscal et al. 2000), which contrasts with the findings in the rat kidney (current study), where occludin was not detected in the rat proximal tubule despite the use of multiple technical approaches. Thus, in the rat kidney, occludin is either not expressed in the proximal tubule or expressed at very low levels. In the mouse kidney, occludin immunolabel appears to colocalize with claudin-8, suggesting collecting duct and distal tubule expression, and with claudin-2 in the thin descending limb of the loop of Henle (Kiuchi-Saishin et al. 2002). Whether occludin immunolabel was present in the mouse thick ascending limb of the loop of Henle, as observed in the rat kidney in the current study, was not reported (Kiuchi-Saishin et al. 2002). Thus, in the rat, rabbit, and mouse kidney, occludin immunolabel expression appears to exhibit substantial axial heterogeneity, paralleling axial variations in transepithelial resistance, with substantially less proximal tubule expression than collecting duct expression.
Although occludin is a critical component of the tight junction and thus likely to play a central role in paracellular permeability and transepithelial resistance and in epithelial cell polarity biogenesis, its specific role cannot be determined definitively at present. Expression of occludin mutants lacking the C-terminal cytoplasmic domain, which binds to the submembrane cytoskeleton, alters size-selective paracellular permeability, disrupts apical junctional ring formation, and prevents normal localization of proteins to specific apical and basolateral plasma membrane domains (Balda et al. 1996). In contrast, occludin-deficient ES cells are able to generate apparently normal cystic embryoid bodies, normal appearing tight junction strands, and normal concentration of ZO-1 at tight junctions (Saitou et al. 1998). Occludin-deficient mice exhibit a number of abnormalities, including chronic inflammation and hyperplasia of the gastric epithelium, calcification in the brain, testicular atrophy, loss of cytoplasmic granules in striated duct cells of the salivary gland, and thinning of the compact bone (Saitou et al. 2000). In occludin-deficient mice, renal epithelial cell tight junctions were normal by immunofluorescence microscopy and electron microscopy, but whether there were differences in fluid and electrolyte homeostasis or in epithelial polarity have not been reported (Saitou et al. 2000). These observations suggest that although occludin has an important role in paracellular permeability and epithelial polarity, in its absence adaptive changes in other proteins, possibly by claudin family members that coexist in tight junctions, can substitute for occludin.
ZO-1, another member of the tight junction protein complex, exhibited a different epithelial cell distribution than observed for occludin, suggesting a different functional role. ZO-1 was present in all renal epithelial cells. In contrast to occludin, an integral membrane protein, ZO-1 is a membrane-associated protein that appears to function as a scaffolding protein for many tight junction proteins. In addition to interacting with occludin, ZO-1 also interacts with multiple claudin family members, ZO-2, ZO-3, the ZO-1-associated nucleic acid-binding proteins (ZONAB), the coxsackievirus and adenovirus receptor (CAR), and myosin and actin filaments. Current models suggest that ZO-1 functions primarily as a scaffolding protein involved in tight junction biogenesis, providing a link to the actin cytoskeleton, and transducing regulatory signals, both to and from the tight junction proteins, occludin and claudins (Fanning and Anderson 2009; Landau 2006).
Renal ischemia–reperfusion causes cellular and morphologic damage primarily in cells in the outer medullary region (Heyman et al. 1988, 2010; Lieberthal and Nigam 1998). Presumably, this is due to altered tissue oxygenation in this region, making it more susceptible to ischemia–reperfusion injury. There are three different tubular segments in the outer medulla: the proximal tubule (S3 segment), the thick ascending limb, and the collecting duct. Although the proximal tubule is an important locus of ischemia–reperfusion injury in the IR injury experimental model, the current study adds to accumulating evidence that the collecting duct, in general, and OMCD intercalated cells, in particular, are also susceptible to ischemia–reperfusion injury (Han et al. 2007; Heyman et al. 2010). The current study adds to these previous studies, both by confirming intercalated cell-specific cellular damage in response to IR injury and by showing that the tight junction proteins, occludin and ZO-1, undergo specific alterations in their cellular localization, with removal from tight junction sites and instead diffuse cellular localization.
Ischemia–reperfusion injury leads to altered glomerular filtration and solute excretion because of cellular extrusion from the basement membrane, leading to intra-tubular obstruction and increased intraluminal pressures, filtrate backleak, and altered epithelial cell ion transport. At least in part this involves altered structure and function of the slit diaphragm proteins, neph1 and ZO-1, as well as the tubular adherens junctional complex proteins, E-cadherin and catenin (Bush et al. 2000; Wagner et al. 2008). The current study adds to this information by showing that IR injury also alters expression of the tight junction proteins, occludin and ZO-1, in specific renal tubular cells. Ischemia–reperfusion caused abnormal ZO-1 distribution in both the proximal tubule and collecting duct, indicating tight junction disruptions in both segments and alterations in occludin expression in OMCD intercalated cells.
Occludin may play a critical role in the pathogenesis of collecting duct injury during renal ischemia–reperfusion insults. Occludin expression in the collecting duct was very strong and abnormally distributed after ischemia–reperfusion, and these changes were limited to intercalated cells, one of the two morphologically and functionally different cell types present in the collecting duct. These differential effects on intercalated cell, but not principal cell, and occludin expression parallel differential sensitivity of these two cell types to injury. Recently, we reported that renal ischemia–reperfusion injury caused intercalated cell-specific cell detachment and apoptosis (Han et al. 2007). Butt et al. demonstrated that intercalated cells preferentially lose polarity during fetal urinary tract obstruction (Butt et al. 2007). Thus, abnormal localization of occludin observed in intercalated cells after ischemia–reperfusion injury may contribute to intercalated cell-specific injury.
The cell-specific change of occludin distribution in the collecting duct may be important to fluid and electrolyte derangements that result from renal ischemia–reperfusion injury. Renal ischemic injury frequently induces metabolic acidosis and impairs ammonia excretion (Clarkson et al. 2008; Han et al. 2007; Lenhart et al. 1988). We demonstrated recently that renal ischemia–reperfusion injury decreased urinary ammonia excretion and induced loss of epithelial polarity of multiple proteins involved in renal ammonia secretion, including the ammonia transporter family members, Rh B and Rh C glycoprotein, the vacuolar H+-pump, H+-ATPase, and the basolateral anion exchange AE1 (Han et al. 2007). The current study also showed that renal ischemia–reperfusion significantly decreased blood bicarbonate concentration (Table 1). Because renal ammonia excretion requires generation of steep transepithelial ammonia and pH gradients across the collecting duct, intact tight junction function is necessary. Disruption of tight junctions may enable a backleak of H+, ammonia, and bicarbonate, thereby impairing net acid excretion and contributing to development of metabolic acidosis (Fig. 7). In the present study, abnormal localization of occludin was closely related to the polarity loss or partial internalization of intercalated cell-specific transporters in the collecting duct, which we demonstrated recently in response to IR injury (Han et al. 2007). Intercalated cells play an important role in acid–base homeostasis and the ischemia-induced disruption of tight junction proteins may contribute to the development of collecting duct dysfunction.
Fig. 7.
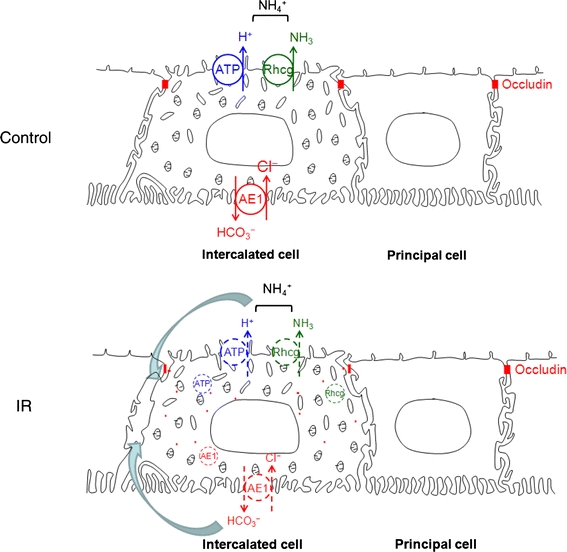
Effects of renal ischemia–reperfusion injury on occludin and acid–base transporter proteins. Intercalated cell-specific delocalization of occludin may cause the polarity loss or partial internalization of H+-ATPase, Rhcg, and AE1. Abnormal occludin distribution may also lead to backleak (curved arrows) of secreted ions through the paracellular pathways
In summary, the current studies demonstrate the expression of occludin and ZO-1 in the normal rat kidney and in response to ischemia–reperfusion injury. ZO-1 is widely expressed in renal epithelial and endothelial cells, consistent with ZO-1 mediating a central role as a membrane-associated protein involved in scaffolding functions in cell–cell interaction. Occludin expression is localized to distal epithelial segments. Ischemia–reperfusion injury induces specific changes in occludin expression in collecting duct intercalated cells, which parallels damage to intercalated cell polarity and integrity, and likely contributes to the altered acid–base homeostasis which occurs.
Acknowledgments
This work was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0073733, 2011-0016068) to KH Han and by NIH (R01-DK045788, R21-047624) to ID Weiner. We thank Dr. Tae-Hwan Kwon for discussion and advice, Nam-Sik Kim and Jung-Mi Han for technical assistance, and Sun Han for proofreading. A part of this work has been published in abstract form (J Am Soc Nephrol: SA-PO2161, 2009).
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci. 1994;107:3301–3313. doi: 10.1242/jcs.107.12.3301. [DOI] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, Tsukamoto T, Nigam SK. Selective degradation of E-cadherin and dissolution of E-cadherin–catenin complexes in epithelial ischemia. Am J Physiol Renal Physiol. 2000;278:F847–F852. doi: 10.1152/ajprenal.2000.278.5.F847. [DOI] [PubMed] [Google Scholar]
- Butt MJ, Tarantal AF, Jimenez DF, Matsell DG. Collecting duct epithelial–mesenchymal transition in fetal urinary tract obstruction. Kidney Int. 2007;72:936–944. doi: 10.1038/sj.ki.5002457. [DOI] [PubMed] [Google Scholar]
- Clarkson MR, Friedewald JJ, Eustace JA, Rabb H. Acute kidney injury. In: Brenner BM, editor. Brenner and Rector’s The kidney. 8. Philadelphia: Saunders and Elsevier; 2008. pp. 943–986. [Google Scholar]
- Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2652–2653. doi: 10.1046/j.1523-1755.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Hallett MA, Atkinson SJ, Marrs JA. aPKC–PAR complex dysfunction and tight junction disassembly in renal epithelial cells during ATP depletion. Am J Physiol Cell Physiol. 2007;292:C1094–C1102. doi: 10.1152/ajpcell.00099.2006. [DOI] [PubMed] [Google Scholar]
- Han KH, Jung JY, Cha JH, Kim H, Madsen KM, Kim J. 1, 25-Dihydroxyvitamin D3 stimulates osteopontin expression in rat kidney. Nephron Physiol. 2003;93:76–86. doi: 10.1159/000069556. [DOI] [PubMed] [Google Scholar]
- Han KH, Woo SK, Kim WY, Park SH, Cha JH, Kim J, Kwon HM. Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol. 2004;287:F878–F885. doi: 10.1152/ajprenal.00047.2004. [DOI] [PubMed] [Google Scholar]
- Han KH, Lim JM, Kim WY, Kim H, Madsen KM, Kim J. Expression of endothelial nitric oxide synthase in developing rat kidney. Am J Physiol Renal Physiol. 2005;288:F694–F702. doi: 10.1152/ajprenal.00085.2004. [DOI] [PubMed] [Google Scholar]
- Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia–reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol. 2007;293:F1342–F1354. doi: 10.1152/ajprenal.00437.2006. [DOI] [PubMed] [Google Scholar]
- Heyman SN, Brezis M, Reubinoff CA, Greenfeld Z, Lechene C, Epstein FH, Rosen S. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman SN, Rosenberger C, Rosen S. Experimental ischemia–reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- Jeon US, Han KH, Park SH, Lee SD, Sheen MR, Jung JY, Kim WY, Sands JM, Kim J, Kwon HM. Downregulation of renal TonEBP in hypokalemic rats. Am J Physiol Renal Physiol. 2007;293:F408–F415. doi: 10.1152/ajprenal.00502.2006. [DOI] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Dafoe D, Alfrey E, Myers BD. Backleak, tight junctions, and cell–cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau D. Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens. 2006;15:425–429. doi: 10.1097/01.mnh.0000232883.43093.76. [DOI] [PubMed] [Google Scholar]
- Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol. 2006;290:F20–F34. doi: 10.1152/ajprenal.00052.2005. [DOI] [PubMed] [Google Scholar]
- Lenhart M, Skapars J, Areas J, Razavi MH, Garcia C, Preuss HG. Role of renal ammonium accumulation in ischemic acute renal failure and acute tubular necrosis of rats. Contrib Nephrol. 1988;63:28–32. doi: 10.1159/000415694. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998;275:F623–F631. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- Madsen KM, Tisher CC. Structural–functional relationship along the distal nephron. Am J Physiol. 1986;250:F1–F15. doi: 10.1152/ajprenal.1986.250.1.F1. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest. 1989;84:1334–1339. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Dahl R, Geerdes A. Cytoskeleton disruption and apical redistribution of proximal tubule Na+-K+-ATPase during ischemia. Am J Physiol. 1992;263:F488–F495. doi: 10.1152/ajprenal.1992.263.3.F488. [DOI] [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem. 2008;283:35579–35589. doi: 10.1074/jbc.M805507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CA, Devuyst O, Belge H, Bourgeois S, Houillier P. The rhesus protein RhCG: a new perspective in ammonium transport and distal urinary acidification. Kidney Int. 2011;79:154–161. doi: 10.1038/ki.2010.386. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid–base transport. Am J Physiol Renal Physiol. 2011;300:F11–F23. doi: 10.1152/ajprenal.00554.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM. Mathematical models of renal fluid and electrolyte transport: acknowledging our uncertainty. Am J Physiol Renal Physiol. 2003;284:F871–F884. doi: 10.1152/ajprenal.00330.2002. [DOI] [PubMed] [Google Scholar]


