Abstract
BACKGROUND:
Insulin-like growth factor 1 (IGF-I) is an anabolic growth factor that affects nitrogen balance and its changing trend is not clearly understood in critically ill patients. This study was carried out to evaluate the association between serum IGF-I levels and its changing trend in critically ill patients.
METHODS:
In this nested case-control study, all consecutive patients admitted to the medical ICU of Rasoul-e-Akram and Firuzgar hospital (Tehran, Iran) from January through October 2008 were included. IGF1 concentration was measured within the first 24h of ICU admission and the fourth, seventh and tenth day since admission. Patients were followed until discharge from ICU or expiration.
RESULTS:
The study population consisted of 90 patients (mean age: 58.01 ± 22.56), 31 (34.4%) of who died and 59 (65.6%) were discharged. On admission, 43 patients (47.7%) had low IGF-I levels, whereas 47 (52.3%) had normal or high levels. The concentration of IGF-I was not significantly different in every 4 measurements between expired and discharged patients. Significant decrease was seen between first to fourth day IGF-I concentration (p = 0.005). Changing trend was not statistically different in two groups of patients.
CONCLUSIONS:
There was no relation between low IGF-I concentration on admission day and increased adverse outcome, but overall these patients had lower IGF1. No clear association was found between changing trend of IGF1 and mortality. Stress on admission time may cause decreasing pattern of IGF-I in the first 4 days of admission.
KEYWORDS: Intensive Care Units, Critical Illness, Insulin-Like Growth Factor I, Mortality
Almost all patients admitted to intensive care unit (ICU) represent metabolic states such as hyper-metabolism, substrate use alternations and negative nitrogen balance.1 If the stress level remains higher than the system capacity, pathological procedures like protein catabolism occur.2 Although liver protein synthesis increases, metabolic changes can lead to major endogenous protein break-down, associated with muscle wasting and shortness of immune system that may cause higher risk of nosocomial infection and reduced potential to repeal with additional stress.3,4 The highest rate of this situation is during the first 10 days of critical illness,5 while it is combined with malnutrition.6 This condition may lead to mortality and may be one of the most prevalent causes of death in ICU.7 This finding contributes that early detection of stress level and metabolic state allows in-time intervention to promote anabolism and reducing catabolism.8
Hormonal stress is one of the most influen-cing conditions that can also be easily evaluated. But there is no sign of considering this issue in most usable prognosis evaluation scoring like acute physiology and chronic health evaluation (APACHE).9 A vast number of hormones have been suggested for this matter, e.g. cortisol, glucagon, insulin, growth hormone (GH), etc. However, these assays are complex, expensive and may not be available or within reach.3 Furthermore, most of these hormones have pulsatile secretion and a single measurement is not reliable.
Insulin-like growth factor 1 (IGF-I) is an anabolic growth factor,10 primarily synthesized in liver and mainly stimulated by GH,11 but can be regulated by many factors such as glucose concentration and nutrition.12 IGF-I has an important role in cell proliferation, apoptosis and of course metabolism of protein, glucose, GH and insulin.11–13 On the other hand, protein catabolism in critically ill patients such as traumatic and septic patients is associated with complex alternation in GH/IGF-I axis.14,15 Un-like other hormones, IGF-I assays are less ex-pensive and easier to do.16 Thus, IGF-I mea-surements can be a better way to evaluate ni-trogen balance of patients admitted to ICU.
Studies carried out to find association be-tween IGF-I and patients prognosis have had controversial results. Kyle et al showed that IGF-I levels were lower in moderate and high stressed patients,17 while Hu et al demonstrated that plasma IGF-I levels had no association with all-cause mortality in ICU.18 Recent studies also showed no association between plasma IGF-I levels and mortality in critically ill patients with congestive heart failure19 and acute renal fail-ure.20 But Guimarγes et al showed that de-creased levels of IGF-I and cholesterol were clearly related to higher mortality.21
All previous studies have been done on small samples of patients. Furthermore, no study has investigated changes of IGF-I con-centration during ICU admission with an ap-propriate sample size.
For further investigation, this study was carried out to evaluate the association between serum IGF-I levels and its changing trend in ICU. The aim was to demonstrate that serum IGF-I levels may be a suitable prognostic factor for critically ill patients who may need more evaluation and care.
Methods
Patients
In this nested case-control study, 90 consecutive patients admitted to the medical ICU of the university teaching hospitals from January through October 2008 were included. The pa-tients mostly suffered from pulmonary diseases (pneumonia [n = 4, 4.4%], chronic pulmonary obstructive disease [n = 4, 4.4%]), infectious diseases (septic shock and sepsis [n = 13, 14.4%]), and cerebrovascular accidents (n = 9, 10%).
Verbal consent was obtained from all pa-tients (or from their next of kin) after detailed explanations and a letter of information was also given. This investigation has been ap-proved by ethical committee of the University.
Assessments
At admission time, baseline clinical variables including age, sex, APACHE II score, and dis-ease diagnosis were recorded. The APACHE II score incorporates acute physiologic variables and chronic health evaluation into a measure of the risk of in-hospital mortality.9 History of other diseases was considered. Length of stay in the ICU was also recorded. The clinical end-point was death or discharge from the ICU at any time during hospitalization.
IGF-I concentration was measured within the first 24 hours of ICU admission and the fourth, seventh and tenth day since admission. This was suggested by the ICU team and was based on their clinical judgment. The IGF-I concentration was evaluated using radioimmunoassay. On the basis of the company instructions, the concentration less than 107 is considered low, and 107 to 310 ng/ml is normal range of this assay, but on the basis of research results, the normal values are 95 to 249 ng/ml.22,23 For IGF-I concentration, about 2-3 cc blood were obtained and centrifuged; then the serum was separated and sent for measurement of IGF-I in a private laboratory with a kit, on the basis of double sandwich ELISA (AssayMax, Missouri, USA). Intra-assay and inter-assay coefficients of variation were 4.8% and 7.2%, respectively.
Because of a laboratory fault, 1 patient did not have IGF-I level measured at admission, but IGF-Is were measured on days 4, 7 and 10; these data were included in the analysis. Out of all patients, 44 had the first two, 28 had the first three, and 20 patients had all four serial measurements for IGF-I. Patients discharged from the ICU or patients who died were not measured for IGF-I.
Statistical Analysis
Baseline characteristics of the low and normal IGF-I groups were compared using the Pearson chi-square test. Continuous variables were compared using the Student's t-test for normally distributed variables and the Mann-Whitney U test when either of these conditions was not met. Serial measurements were compared using the paired t-test. For evaluation of trend of changes in IGF-I during ICU admission, multiple measurement ANOVA analysis was carried out. The Log-Rank method was done to compare patient survival in the Kaplan-Meier analysis. Summary data are presented as mean ± SD. P values less than 0.05 were considered significant.
Results
Baseline Characteristics
The study population consisted of 90 patients admitted to ICU over a period of 12 months. It consisted of 51 men and 39 women, with the age range of 15 to 100 years (mean ± SD = 58.01 ± 22.56). On admission, 43 patients (47.7%) had low IGF-I levels, whereas 47 (52.3%) had normal or high levels. Out of all patients, 31 (34.4%) ex-pired and 59 (65.6%) were discharged.
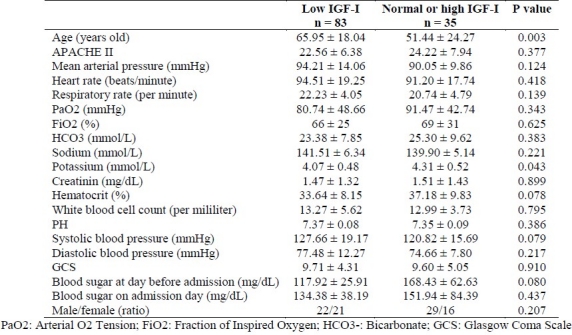
There was no significant difference between the two groups with low and normal or high IGF-I in some baseline characteristics, includ-ing Glasgow Coma Scale (GCS), PaO2 and etc. on admission. However, significant differences were found between groups in age and serum potassium level (Table 1).
Table 1.
Characteristics of 89 critically ill patients on admission in the first IGF-I measurement
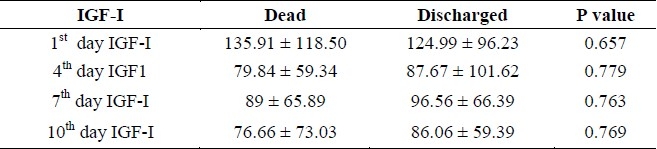
The concentration of IGF-I was not signifi-cantly different in every 4 measurements be-tween dead and discharged patients. (Table 2)
Table 2.
Blood concentration of IGF-I in dead and discharged patients (mean ± SD) (ng/ml)
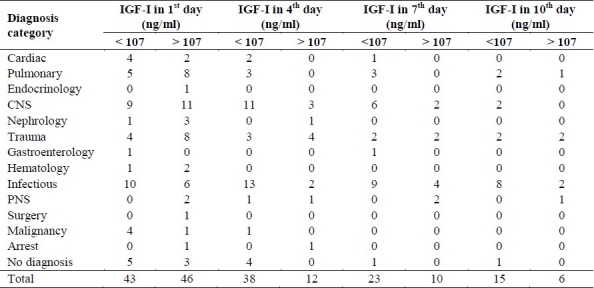
IGF-I levels did not differ significantly be-tween different diseases. Also there was no significant difference between different pa-tients’ groups in IGF-I concentration.
No difference was seen between APACHE-II scores of patients with first day IGF-I concentration lower and higher than our cut-point. (22.56 ± 6.38 ng/ml vs. 24.22 ± 7.94 ng/ml, p = 0.377).
Also, correlation between IGF-I (in all four measurements) and APACHE II score was not significant (p > 0.05).
Serial Measurements of IGF-I and Mortality In The ICU
A significant correlation between measure-ments of IGF-I in the a significant difference was seen between first and fourth day IGF-I concentration (113.91 ± 92.96 ng/ml in the first day and 85.45 ± 88.78 ng/ml in the fourth day, p = 0.005). The changing trend could be seen in figure 1 in all patients (A) and two groups with different outcome (B). No useful fact about the changes in patients could be found, nor associ-ation of IGF-I concentration in 10 days with mortality was significant (Table 3).
Figure 1.
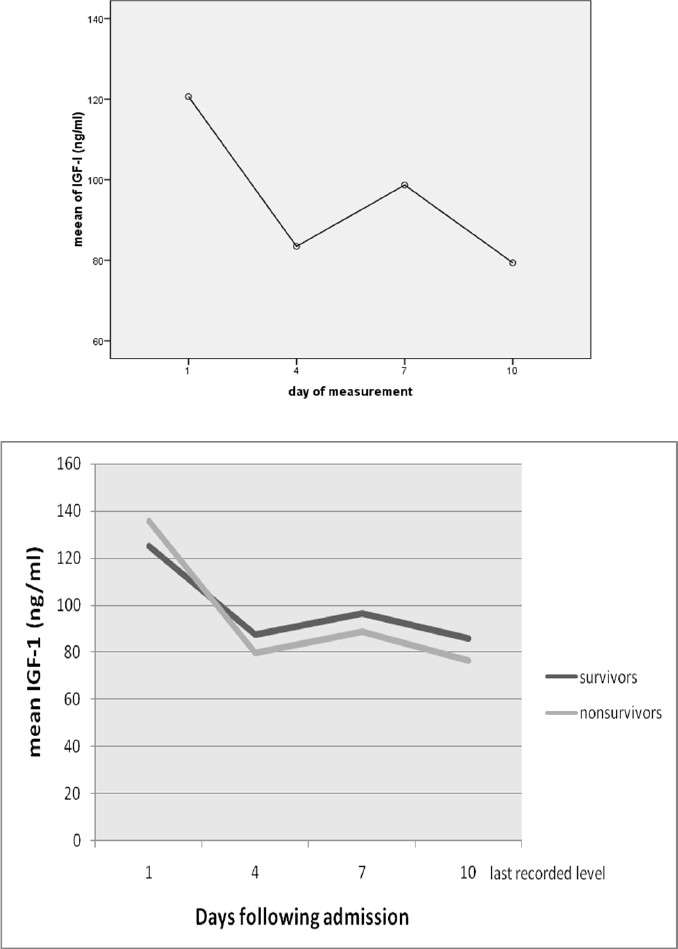
(A) Serial measurements of IGF-I for 20 patients and (B) trend in IGF-I levels over four times in two groups of patients that died or were discharged (20 patients)
Table 3.
Mortality rate based on IGF-I concentration in different measurements
Survival Analysis
The Survival rate of patients with low IGF-I on the first, fourth and tenth day of admission were 95%, 85% and 70%, respectively and in patients with normal or high IGF-I levels were 93%, 81% and 70%, respectively. The estimated mean survival time of patients with low IGF-I concentration was 34.25 (SE = 11.4) days and it was higher than the other group with a mean survival time of 21 (SE = 3.28) days, but this difference was not significant (p = 0.677) (Figure 2).
Figure 2.
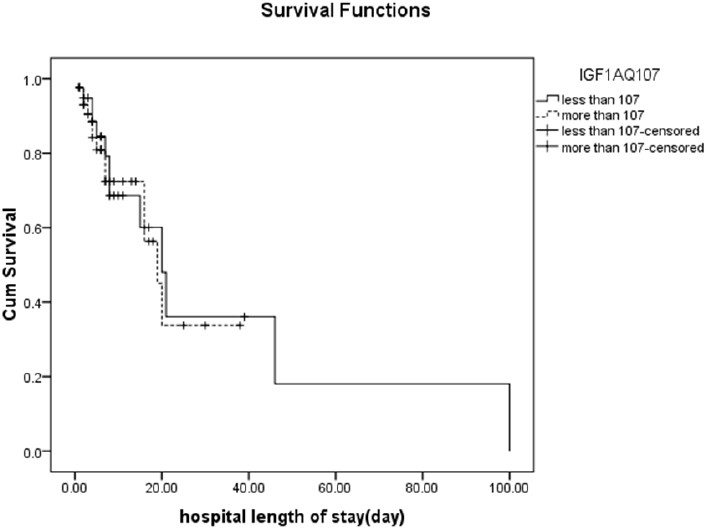
Patients’ survival (p = 0.677)
Discussion
Based on the results, an ambiguous association was found between the IGF-I and outcome. Concentration of IGF-I was low in most of the patients on admission day and later in ICU. In our study, there was no relation between low IGF-I concentration on admission day and increased adverse outcome, even a higher survival (although not significant) was found in patients with low IGF-I. The association of IGF-I on admission day and patients’ prognosis was unclear, though there are controversies in the literature.17–21
The decreasing pattern of IGF-I in the first 4 days of admission (the second measurement in comparison with the first one) could suggest effect of stress on IGF-I in the first days, when the patient may not be prepared to confront stress. Stress conditions are known to disturb the GH- IGF-I axis.
This consist a biphasic pattern. The initial stress response consists of activated GH release whereas circulating level of GH-dependent IGF-I fall. ‘In contrast, in the chronic intensive care-dependent phase of severe illness, pulsa-tile GH secretion substantially decreases whe-reas the non-pulsatile fraction remains relative-ly elevated, resulting in an abnormal flat GH secreting pattern and low-normal mean noc-turnal GH serum concentrations. Specifically the reduced amount of GH released in pulses is found to be related to low circulating levels of IGF-I, IGF Bonding Protein-3 and acid-labile subunit, which suggests that a relative hypo-somatotropism may participate in the patho-genesis of the wasting syndrome distinctively in the chronic phase of critical illness.’24
Wasting syndrome “results from both an increase in muscle protein degradation as well as a decreased rate of both myofibrillar and sacroplasmic protein synthesis. This protein imbalance may be caused by an increased presence or activity of various catabolic agents, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), or glucocorticoids" other than mentioned low concentration and enhanced resistance of GH, IGF1 and other anabolic factors.7,25,26 These re-sults in “increased protein turnover and negative nitrogen balance. Severe catabolism leads to end-organ dysfunction and muscular weakness and make the need for mechanical ventilation” longer than usual.27
Protein loss in wasting process “leading to reduced lean body mass is recognized to contribute to the high levels of morbidity and mortality, seen in critical illness.”7
This study was designed to evaluate the IGF-I changes during ICU admission in addi-tion to baseline concentration of IGF-I.
The changing trend of IGF-I levels in this study was not significant in patients admitted to ICU. Furthermore, difference in the IGF-I changing trend was not significant between the two groups with different outcome. In previous studies, recovery in survived patients has been associated with increasing IGF-I and IGF bind-ing protein 3 concentrations and cessation of protease activity and increased levels of anabol-ic hormones,25,28 but a recent study indicated that the patient with the best anabolic parame-ters may not necessarily be the most favored to survive in ICU.29 Association of IGF-I changes in critically ill patients is not well known.
Catabolism cannot be prevented with standard parenteral or enteral nutrition formulas. In some studies, prescription of GH is suggested for critically ill patients to prevent catabolism. A recent multinational randomized controlled trial revealed that increased mortality of critically ill patients following administration of recombinant growth hormone could be caused by the negative effect of GH on IGF-I secretion.27
The primary disease on admission did not have significant association with the concentration of IGF-I, or the everyday blood sugar in this investigation. There are a few studies on catabolic response and pituitary axis dysfunction and low concentration of IGF-I in different patients admitted to ICU. Low IGF-I concentration has been reported in the patients with severe trauma, infection, severe brain trauma, heart surgery and abdominal surgery but the real significance of these findings still needs to be elucidated.27,30,31
Based on our findings, no difference was seen in the concentration of IGF-I between the two groups of patients with different outcome. Moreover, no association was seen between IGF-I and predictors of outcome like APACHE. Based on the calculated power, these could be due to small sample size (power = 0.5).
There is some evidence about better out-come in females that is associated with higher anabolic hormones and more stable pulsatility and regularity pattern of GH and concomitantly IGF-I levels.25,32
There are some limitations in the present study and the most important one is the high number of lost patients. The other limitation is that other hormones like insulin and GH and the drugs that affect their levels has not been evaluated to find the relation between these hormones in this situation. Further practical and detailed studies are recommended to clarify these issues. It is also recommended to perform other studies with a higher sample size of ICU-patients focusing on a subgroup of baseline disease and checking for other hormonal biomarkers.
Conclusions
Despite the low concentration of IGF-I in most patients admitted to ICU, no clear association was seen between IGF-I levels with outcome and other associated factors of outcome. Several reviewed studies have shown controversy in the relation of IGF-I with adverse outcome and diseases. Designing a multi-center investigation to evaluate the association of these variables is suggested.
Authors’ Contributions
SH was the chief supervisor, idea creator and wrote the primary protocol. MEK was the second supervisor and helped improving the data and the protocol. SG and SRJK were responsible for writing the protocol, data collection, data analysis, writing the manuscript and submission follow-up. NSM and ASS were responsible for writing the protocol, data collection and writing the manuscript. GG, MM, RM, MN and MRK helped in developing the protocol, data collection, writing, and revising the manuscript. All authors have read and approved the content of the manuscript.
Acknowledgments
This Research was funded by Research Center of Endocrinology and Metabolism Institute of Iran University of Medical Sciences and conducted by Medical Students Research Commit-tee (IUMS-MSRC) (Grant no. 222/MT).
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsisand major trauma: a basis for clinical care. Crit Care Med. 1999;27(7):1295–302. doi: 10.1097/00003246-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CL. Taber's cyclopedic medical dictionary. Philadelphia: F.A. Davis Company; 1977. [Google Scholar]
- 3.Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition. 1996;12(6):456–8. doi: 10.1016/s0899-9007(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G. Endocrine evaluation of patients with critical illness. Endocrinol Metab Clin North Am. 2003;32(2):385–410. doi: 10.1016/s0889-8529(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 5.Plank LD, Hill GL. Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann N Y Acad Sci. 2000;904:592–602. doi: 10.1111/j.1749-6632.2000.tb06521.x. [DOI] [PubMed] [Google Scholar]
- 6.Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91(3):447–52. doi: 10.1038/sj.bjc.6601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll PV, Jackson NC, Russell-Jones DL, Treacher DF, Sonksen PH, Umpleby AM. Combined growth hor-mone/insulin-like growth factor I in addition to glutamine-supplemented TPN results in net protein anabolism in critical illness. Am J Physiol Endocrinol Metab. 2004;286(1):E151–7. doi: 10.1152/ajpendo.00122.2003. [DOI] [PubMed] [Google Scholar]
- 8.Bérard MP, Zazzo JF, Condat P, Vasson MP, Cynober L. Total parenteral nutrition enriched with arginine and glu-tamate generates glutamine and limits protein catabolism in surgical patients hospitalized in intensive care units. Crit Care Med. 2000;28(11):3637–44. doi: 10.1097/00003246-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 10.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13(4):113–70. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 11.Yakar S, Kim H, Zhao H, Toyoshima Y, Pennisi P, Gavrilova O, et al. The growth hormone-insulin like growth factor axis revisited: lessons from IGF-I and IGF-I receptor gene targeting. Pediatr Nephrol. 2005;20(3):251–4. doi: 10.1007/s00467-004-1613-y. [DOI] [PubMed] [Google Scholar]
- 12.Hambrecht R, Schulze PC, Gielen S, Linke A, Möbius-Winkler S, Yu J, et al. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39(7):1175–81. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 13.Pavelić J, Matijević T, Knezević J. Biological and physiological aspects of action of insulin-like growth factor peptide family. Indian J Med Res. 2007;125(4):511–22. [PubMed] [Google Scholar]
- 14.Roth J, Glick SM, Yalow RS, Berson SA. Secretion of human growth hormone: physiologic and experimental mod-ification. Metabolism. 1963;12:577–9. [PubMed] [Google Scholar]
- 15.Osterziel KJ, Ranke MB, Strohm O, Dietz R. The somatotrophic system in patients with dilated cardiomyopathy: relation of insulin-like growth factor-1 and its alterations during growth hormone therapy to cardiac function. Clin Endocrinol (Oxf) 2000;53(1):61–8. doi: 10.1046/j.1365-2265.2000.01029.x. [DOI] [PubMed] [Google Scholar]
- 16.Palsgaard J, Brown AE, Jensen M, Borup R, Walker M, De Meyts P. Insulin-like growth factor I (IGF-I) is a more potent regulator of gene expression than insulin in primary human myoblasts and myotubes. Growth Horm IGF Res. 2009;19(2):168–78. doi: 10.1016/j.ghir.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Kyle UG, Jolliet P, Genton L, Meier CA, Mensi N, Graf JD, et al. Clinical evaluation of hormonal stress state in medical ICU patients: a prospective blinded observational study. Intensive Care Med. 2005;31(12):1669–75. doi: 10.1007/s00134-005-2832-9. [DOI] [PubMed] [Google Scholar]
- 18.Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, et al. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. J Am Ge-riatr Soc. 2009;57(7):1213–8. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreassen M, Kistorp C, Raymond I, Hildebrandt P, Gustafsson F, Kristensen LØ, et al. Plasma insulin-like growth factor I as predictor of progression and all cause mortality in chronic heart failure. Growth Horm IGF Res. 2009;19(6):486–90. doi: 10.1016/j.ghir.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Basi S, Pupim LB, Simmons EM, Sezer MT, Shyr Y, Freedman S, et al. Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol. 2005;289(2):F259–64. doi: 10.1152/ajprenal.00002.2005. [DOI] [PubMed] [Google Scholar]
- 21.Guimarães SM, Lima EQ, Cipullo JP, Lobo SM, Burdmann EA. Low insulin-like growth factor-1 and hypocholesterolemia as mortality predictors in acute kidney injury in the intensive care unit. Crit Care Med. 2008;36(12):3165–70. doi: 10.1097/CCM.0b013e318186ab70. [DOI] [PubMed] [Google Scholar]
- 22.Rosario PW. Normal values of serum IGF-I in adults: results from a Brazilian population. Arq Bras Endocrinol Metabol. 2010;54(5):477–81. doi: 10.1590/s0004-27302010000500008. [DOI] [PubMed] [Google Scholar]
- 23.Kong AP, Wong GW, Choi KC, Ho CS, Chan MH, Lam CW, et al. Reference values for serum levels of insulin-like growth factor (IGF-I) and IGF-binding protein 3 (IGFBP-3) and their ratio in Chinese adolescents. Clin Biochem. 2007;40(15):1093–9. doi: 10.1016/j.clinbiochem.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berghe G. Growth hormone secretagogues in critical illness. Horm Res. 1999;51(Suppl 3):21–8. doi: 10.1159/000053158. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke MG, Barrow RE, Mlcak RP, Herndon DN. Endogenous anabolic hormones and hypermetabolism: effect of trauma and gender differences. Ann Surg. 2005;241(5):759–67. doi: 10.1097/01.sla.0000161028.43338.cd. discussion 767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang CH, Frost RA. Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. Curr Opin Clin Nutr Metab Care. 2002;5(3):271–9. doi: 10.1097/00075197-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ruokonen E, Takala J. Dangers of growth hormone therapy in critically ill patients. Ann Med. 2000;32(5):317–22. doi: 10.3109/07853890008995933. [DOI] [PubMed] [Google Scholar]
- 28.Timmins AC, Cotterill AM, Cwyfan Hughes SC, Holly JMP, Ross RJM, Blum W, et al. Critical illness is associated with low circulating concentrations of insulin-like growth factors-I and -II, alterations in insulin-like growth factor binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med. 1996;24(9):1460–6. doi: 10.1097/00003246-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mesotten D, Van den Berghe G. Changes within the GH/IGF-I/IGFBP axis in critical illness. Crit Care Clin. 2006;22(1):17–28. doi: 10.1016/j.ccc.2005.09.002. v. [DOI] [PubMed] [Google Scholar]
- 30.Nakhjavani M, Esteghamati A, Hamidi S, Esfahanian F, Nabavi H, Abbasi M, et al. Changes in growth hormone and insulin-like growth factor-I levels in the acute stage after open heart surgery and at the time of discharge. Exp Clin Endocrinol Diabetes. 2009;117(8):413–6. doi: 10.1055/s-2008-1080924. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Alé M, Flores-Cordero JM, Rincón-Ferrari MD, García-Gómez S, Sánchez-Olmedo JI, Murillo-Cabezas F, et al. Assessment of hypothalamo-pituitary axis in early phase of severe cranioencephalic brain injury. Med Intensiva. 2008;32(9):411–8. doi: 10.1016/s0210-5691(08)75717-6. (Spanish) [DOI] [PubMed] [Google Scholar]
- 32.Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Veldhuis JD. A paradoxical gender dissociation within the growth hormone/insulin-like growth factor I axis during protracted critical illness. J Clin Endocrinol Metab. 2000;85(1):183–92. doi: 10.1210/jcem.85.1.6316. [DOI] [PubMed] [Google Scholar]


