Abstract
BACKGROUND:
Analysis of venous blood gas (VBG) can represent arterial blood gas (ABG) analysis in patients with various diseases. The effects of hypotension on differences between the results of simultaneous venous and arterial blood gas analyses were reviewed.
METHODS:
This observational, cross-sectional study was conducted from March to October 2010 in emergency departments of two university hospitals in Tehran (Iran) on consecutive adult patients for whom ABG had been indicated for diagnosis/treatment. Arterial and peripheral venous bloods were simultaneously sampled with blood pressure measurement. The VBG-ABG amount of difference regarding pH, HCO3, PCO2, PO2, SO2, and Base Excess (BE) was compared between those with and without hypotension.
RESULTS:
During the study, 192 patients (51.6 ± 23.6 years, 67.7% males) were entered into the hypotension (n = 78) and normotensive groups (n = 114). The average VBG-ABG amount of difference (95% limits of agreement) in the hypotension versus normotensive group were -0.030 (-0.09 to 0.03) vs. -0.016 (-0.1 to 0.068) for pH (p = 0.01), 1.79 (-1.91 to 5.49) vs. 1.32 (-1.94 to 4.58) mEq/L for HCO3 (p = 0.032), 2.69 (-20.43 to 25.81) vs. 2.03 (-7.75 to 11.81) mmHg for PCO2 (p = 0.295), -35.97 (-130.17 to 58.23) vs. -32.65 (-104.79 to 39.49) mmHg for PO2 (p = 0.293), -18.58 (-14.66 to 51.82) vs. -9.06 (-31.28 to 13.16) percent (p < 0.001) for SO2, and 0.25 (-3.73 to 4.23) vs. 0.79 (-2.51 to 4.09) for BE (p = 0.036).
CONCLUSIONS:
Hypotensive status is associated with an increase in the amount of difference between VBG and ABG analysis regarding pH, HCO3, and BE, though the amount of increase does not seem to be clinically important. Studying the precise effects of replacing ABG with VBG on the clinical decision-making and the following outcomes is worth-while.
KEYWORDS: Blood Gas Analysis, Arterial Blood Gas, Venous Blood Gas, Hypotension, Hypoperfusion
Every day, it is required to evaluate acid-base abnormalities and blood gas status for diagnosis and treatment of many patients with different complaints. Blood gas analysis provides valuable information on acid-base status, oxygenation, and ventilation, which plays an important role in evaluation and treatment of patient with critical conditions.1
In the blood gas analysis, pH value, PO2, PCO2, and oxygen saturation (SO2) in arterial blood samples are considered to be the gold standard. However, arterial blood sampling is an invasive and painful procedure, with (low) risk of vascular injuries, thrombosis along with ischemia of the extremities, hemorrhage, formation of aneurysm, median nerve injury, and infection. Furthermore, arterial blood sampling is, in some cases, technically difficult and fairly time-consuming which requires experience for sampling, and sometimes is accompanied with the risk of staff's needle stick exposure. In many patients, to monitor the disease trend, treatment effect, and modification of treatment regimens, frequent samplings are needed, which can cause increases in the risk of arterial puncture complications2. Considering the invasive nature and also the probable complications of this method, today non-invasive or less invasive procedures, including venous blood gas analysis have received special attention.
In Comparison with arterial blood sampling, taking venous blood sample is an easier, more rapid, and safer method with less pain. Besides, considering the lower frequency of need for puncture in venous blood sampling, the risk of needle stick exposure in the medical staff would be lower. According to the results of the studies on adults and also children with various conditions, including diabetic ketoacidosis,3 trauma,4 intoxications,5 patients in critical care units,6,7 and emergency departments,8–10 there was a proper correlation between ar-terial and venous samples regarding to the values of pH, PCO2, and HCO3. Nevertheless, in some other studies, the correlation value was lower regarding to PO2and SO2,7,9,11 but simultaneous use of pulse oximeter, made measurement of SO2 more precise.12–14 Finally, it seems that in many cases, arterial blood sampling can be replaced by venous blood sampling, although there is not any data on the clinical outcomes of such a strategy.
Although many studies have been carried out on the correlation between the analysis of blood gases in arterial and venous blood samples, there is a few information on those conditions, which can weaken the desired correlation. For instance, hypoperfusion following hypotension is a condition which may influence the correlation of analysis of blood gases in peripheral arterial and venous blood samples. The results of a study on 116 simultaneous obtained samples of peripheral arterial, venous, and capillary blood samples from children hospitalized in critical care unit showed that there was a good correlation between the results obtained from the samples with respect to the measurement of pH, PCO2, base excess (BE), and HCO3 under normotensive, hypotension, hypothermia, and hyperthermia conditions. However, under hypotensive condition, there was a weak relationship between the PO2 values of venous and arterial samples.7 In another study, Umeda et al. compared the difference between arterial and venous PCO2 and pH values in normal volunteers and volunteers under hyperventilation. The results indicated that the differences between PCO2 and pH values of venous and arterial blood samples increased by finger exercise and hyperventilation and this increase in differences was associated with a decrease in peripheral blood flow according to the findings of Dopp-ler ultrasonography.15
Since there are very few studies in this field, definite conclusions cannot be obtained yet. Regarding the importance of identifying the conditions that affect the validity of using analysis of blood gases in peripheral venous blood samples (VBG) for assessment of acid-base and respiratory status of the patient, the current study was carried out to evaluate the results of analyses of blood samples in peripheral venous and arterial blood samples and compare the results between normotensive and hypotension conditions.
Methods
Patients and setting. This observational cross-sectional study was carried out on consecutive patients referred to the emergency departments of Rasul-e Akram, and Shohaday-e haft-e Tir hospitals in Tehran (Iran) from March to October 2010. The inclusion criteria included the age 18 years old or more, having the symptoms for analysis of arterial blood gases (ABG) according to the order of emergency medicine specialist, and patient's consent form for arterial blood sampling. The exclusion criteria also included existence of contraindications for arterial blood sampling including impalpable or negative Allen's test in the upper extremities, infection or fistula at the desired site of puncture, or having severe coagulation disorders, interval more than three minutes between arterial and venous sampling, inappropriate transferring of the sample to the laboratory, and sampling errors. By considering power of the test as 80% and determining the first type error = 0.05(alpha), and also considering the standard deviation and changes of values of ABG components according to the previous studies,15 the sample size was calculated to be at least 63 cases in each group; with and without hypotension. The ethics committee of Iran University of Medical Sciences approved the study (grant # 4838) and informed consent form was obtained from all the patients or their guardians.
Assessments. After considering the study, venous and arterial blood sampling was performed simultaneously. Venous blood sample was obtained by a nurse, from the cubital or dorsal hand veins. Provided that emergency medicine specialist ordered ABG after the insertion of intravenous line, venous blood sampling had been carried out again, but provided that physician ordered ABG before insertion of IV line (intravenous line) and venous blood sampling, the analysis had performed on the same venous blood sample. Data included pH, HCO3, PO2, PCO2, SO2, and Base Excess (BE) were extracted from the blood gas analysis. Coincident with the sampling or with the least possible interval (Max. three minutes), blood pressure were measured and also blood oxygen saturation was determined with pulse oximeter. Hypotension was defined as systolic/diastolic blood pressure less than 90/60 mmHg.1
Immediately after the sampling, the arterial and venous blood samples were placed on ice, transferred to the hospital laboratory, and then analyzed with automatic blood gas analyzer. To prevent interference of the results obtained by analysis of venous blood samples with the treatment course, only the results of the arterial blood samples were reported to the attending physician. If during the course of diagnosis or treatment of a single patient, repeated ABG was indicated, the patient was considered as a new participant.
Data analysis was carried out using SPSS software16 (SPSS Inc., Chicago, IL). Intraclass correlation coefficient (ICC) was applied to evaluate the correlation of blood gas variables between arterial and venous samples of each case. Independent sample t-test was applied to compare VBG-ABG amount of differences regarding blood gas variables between the hypotensive and normotensive groups. The 95% limits of agreement were also reported based on Bland-Altman analysis. P value of < 0.05 was considered statistically significant for all the analyses.
Results
During the study, 192 patients with the mean age of 51.6 ± 23.6 years were entered into the study. The patients consisted of 130 males and 62 females, out of which 78 and 114 patients were in the hypotension and normotensive groups respectively. The intraclass correlation coefficients (ICC) between the results of ABG and VBG analyses in patients with and without hypotension are provided in Table 1.
Table 1.
Intraclass correlation coefficients between the ABG and VBG Results in the two groups
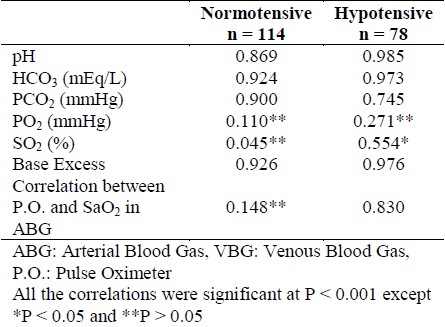
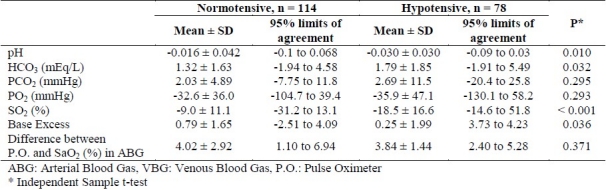
Comparison the VBG-ABG amount of difference between patients with and without hypotension and also and 95% limits of the agreement are presented in Table 2. The VBG-ABG amount of difference regarding pH (p = 0.01), HCO3 (p = 0.032), PO2 (p < 0.001), and BE (p = 0.036) was increased under hypotensive compared with normotensive status. The amount of difference between VBG and ABG regarding PCO2 and the difference between oxygen saturation by pulse oximetry versus arterial sample was not significantly change under hypotensive compared with normotensive status (p > 0.05).
Table 2.
Comparison of ABG-VBG amount of difference between the two groups
Discussion
According to available evidences in different diseases, VBG can reflect ABG analysis with a reasonable degree of confidence.16 However, some researchers believed that under some conditions such as hypotension, the validity of venous blood gas analysis was decreased. Thus, this study aimed to compare the results of VBG analysis with those obtained by ABG analysis in patients referred to the emergency department under hypotensive and normotensive conditions. Then, the effect of hypotension on the amount of difference between the results of VBG and ABG analysis could be assessed. We found that difference in the VBG-ABG amount regarding pH, HCO3, PO2, and BE was increased under hypotensive status.
Few studies are available on the effect of different factors such as hemodynamic changes on the relationship and difference of results of ABG and VBG analyses. In an animal experiment, Theusinger et al17 assessed the effect of hemorrhage on the correlation between the results obtained from ABG and VBG analyses. In their study, 24 pigs underwent experimental hemorrhage and until the animal died, 195 blood sampling was performed from those 24 pigs. According to the results, considering the BE, the difference and correlation values were similar under hemorrhage and hemodilution for arterial and venous blood samples. But, while regarding lactate the difference between arterial and venous blood samples increased under hemorrhage17 which was similar to our study considering HCO3.
The results of another study which was done on 116 simultaneous obtained arterial, venous, and capillary samples from children hospitalized in intensive care unit showed that under normotensive, hypotension, hypothermia, and hyperthermia conditions, there was a reasonable correlation among the samples with regard to values of pH, PCO2, BE, and HCO3. With respect to PO2, under hypotensive condition, there was a weak relationship between the results of venous and arterial blood samples.7 It should be noted that in the mentioned study, the sample size was small and there were only 24 cases of hypotension.
In the study of Umeda et al,15 the difference between arterial and venous PCO2 and pH values in normal volunteers and volunteers under hyperventilation was compared. Their results indicated that the amount of difference between venous and arterial PCO2 and pH increased by finger exercise and hyperventilation and based upon Doppler ultrasonography findings, the increase was due to the decrease in blood flow. They concluded that during exercise of the extremity or under hypoventilation the VBG analysis can lead to overestimation of respiratory acidosis and underestimation of alkalosis.15 The results of this study were in accordance with the findings of the present study, since in our study the amount of difference between venous and arterial blood with respect to pH increased in hypotensive conditions; in which peripheral blood flow decreases.
Similar to previous evidence,16 our study results showed a significant and strong correlation between arterial and venous samples regarding pH, HCO3, and BE in both hypotensive and normotensive conditions, but the correlation was weaker for PCO2, and there was not a reasonable correlation for PO2. In previous studies, the correlation between the results of pulse oximetry and SaO2 was not considered, which was determined to be 0.148 and 0.830 for our normotensive and hypotensive samples respectively. However, the clinical value for this correlation relies on the difference between the saturation percentage in pulse oximetry and arterial blood sample which is discussed in the following.
Clinical significance of the findings:
The strong correlation and also the small difference between VBG and ABG results with respect to pH values, which was mentioned in almost all the previous studies, indicated the possibility of replacement of ABG with VBG in this regard. In our study, hypotension led to an increase in the mean difference of pH value from -0.016 to -0.030. Nevertheless, it should be noted that 95% limits of agreement in the two groups of normotensive and hypotensive were -0.1 to 0.068 and -0.09 to 0.03 respectively. Therefore, the largest decrease in pH value in venous sample was similar to that in the arterial blood sample (0.1 vs. 0.09) respectively. As a result, hypotension may not have a clinically important effect on the pH value difference between venous and arterial blood samples. There was also a strong relationship and a relatively small difference between VBG and ABG with regard to HCO3. Again, this finding increased the possibility of replacement of ABG results with those of VBG. In our study, hypotension led to an increase in the average of this difference from 1.32 to 1.79 mEq/L, which may not be clinically important. Considering PCO2 values, the correlation between the values in VBG and ABG results was lower than those of the pH and HCO3. Although under hypotensive condition, the VBG-ABG amount of difference regarding PCO2 did not change compared with normotensive condition, 95% limits of agreement under hypotensive condition (-20.4 to 25.8) had a higher degree of dispersion compared with normotensive condition (-7.75 to 11.8). The observed degree of dispersion made a doubt about the possibility of replacement of PCO2 from arterial blood sample with that of the venous sample, particularly under hypotensive conditions.
Considering PO2 and SO2, the possibility of replacement of arterial values with venous values cannot be expected. Nonetheless, the new finding of our study was about the results of pulse oximetry versus arterial SO2 (SaO2). In normotensive patients, oxygen saturation in pulse oximetry was 4.02% (95% limits of agreement 1.10 to 6.94) higher than that of the arterial sample and hypotension did not influence the amount of difference nor its dispersion. These finding showed the possibility of replacement of SaO2 with pulse oximetry.
With respect to BE, the change in the amount of difference was significant under hypotensive condition and venous blood samples tended to have higher acidity value, compared with arterial blood samples. However, similar to pH and HCO3, the effect of hypotension on increasing the acidity of the venous sample was small; highlighting the possibility of substituting the VBG for ABG in this respect.
The current study had some limitations. Although compared with previous studies, our study was done with a larger sample size and in two separate centers; the sample size was not adequate for investigation of interactive effects of factors such as body temperature, respiratory rate, heart rate, blood pressure, underlying diseases, and cause of referral in multivariate analysis. According to complementary calculations, for precise analysis of all the predictive factors in this respect, the sample size of 450 patients (samples) was required. Also, timing of the blood sampling, transferring the samples to laboratory, and other measurements were precisely observed; however, the clinical conditions of emergency department did not provide the required conditions for carrying out an accurate research work and perhaps an animal experiment would help to better control for covariate factors.
Conclusions
The results of this study indicated a very strong correlation and small difference between the venous and arterial blood samples with respect to pH, HCO3, and BE and it seems that venous blood sample can be substituted for arterial blood sample in this regard, and the effect of hypotension on the amount of difference may not be clinically significant. It should be noted that venous blood sample has higher acidity value compared with arterial blood sample and the hypotensive status increases its acidity. Considering PO2 and SO2, venous blood sample cannot be used instead of arterial sample, but by using pulse oximetry results, the value of SaO2 can be well estimated. In spite of all the abovementioned items, until now, no study has been carried out to evaluate the clinical effects of replacement of ABG with VBG in clinical decision-making and also its positive and negative clinical outcomes. Before suggesting the replacement of ABG with VBG, performing accurate studies in this regard with respect to different causes of hypotension may be required.
Authors’ Contributions
All authors were participated in generating the idea and designing the study protocol. FSh and RS managed the study and data gathering. AGh analyzed the data and wrote the draft of the report. All authors studied, edited, and approved the final report.
Acknowledgments
This study was supported by Iran University of Medical Sciences (Thesis # 335). Appreciation and thanks go to Mojtaba Akbari (MSc, Isfahan University of Medical Sciences) for statistical advices and staff of Rasul-e Akram and Shohaday-e hafte Tir hospitals emergency departments for helping us to conduct the study.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Tintinalli JE, Kelen GD, Stapczynski JS. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York: McGraw-Hill Professional; 2003. [Google Scholar]
- 2.Leeuwen V, Kranpitz TR, Smith LS. Davis's Comprehensive Handbook of Laboratory and Diagnostic Tests. 2nd ed. Philadelphia: Davis Company; 2006. [Google Scholar]
- 3.Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31(4):459–65. doi: 10.1016/s0196-0644(98)70254-9. [DOI] [PubMed] [Google Scholar]
- 4.Malinoski DJ, Todd SR, Slone S, Mullins RJ, Schreiber MA. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg. 2005;140(11):1122–5. doi: 10.1001/archsurg.140.11.1122. [DOI] [PubMed] [Google Scholar]
- 5.Eizadi-Mood N, Moein N, Saghaei M. Evaluation of relationship between arterial and venous blood gas values in the patients with tricyclic antidepressant poisoning. Clin Toxicol (Phila) 2005;43(5):357–60. doi: 10.1081/clt-200066071. [DOI] [PubMed] [Google Scholar]
- 6.Bilan N, Behbahan AG, Khosroshahi AJ. Validity of venous blood gas analysis for diagnosis of acid-base imbalance in children admitted to pediatric intensive care unit. World J Pediatr. 2008;4(2):114–7. doi: 10.1007/s12519-008-0022-x. [DOI] [PubMed] [Google Scholar]
- 7.Yildizdas D, Yapicioglu H, Yilmaz HL, Sertdemir Y. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child. 2004;89(2):176–80. doi: 10.1136/adc.2002.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J. 2001;18(5):340–2. doi: 10.1136/emj.18.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24(8):569–71. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rang LC, Murray HE, Wells GA, Macgougan CK. Can peripheral venous blood gases replace arterial blood gases in emergency department patients? CJEM. 2002;4(1):7–15. doi: 10.1017/s1481803500006011. [DOI] [PubMed] [Google Scholar]
- 11.Ak A, Ogun CO, Bayir A, Kayis SA, Koylu R. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2006;210(4):285–90. doi: 10.1620/tjem.210.285. [DOI] [PubMed] [Google Scholar]
- 12.Kirubakaran C, Gnananayagam JE, Sundaravalli EK. Comparison of blood gas values in arterial and venous blood. Indian J Pediatr. 2003;70(10):781–5. doi: 10.1007/BF02723794. [DOI] [PubMed] [Google Scholar]
- 13.Rees SE, Toftegaard M, Andreassen S. A method for calculation of arterial acid-base and blood gas status from measurements in the peripheral venous blood. Comput Methods Programs Biomed. 2006;81(1):18–25. doi: 10.1016/j.cmpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Toftegaard M, Rees SE, Andreassen S. Evaluation of a method for converting venous values of acid-base and oxy-genation status to arterial values. Emerg Med J. 2009;26(4):268–72. doi: 10.1136/emj.2007.052571. [DOI] [PubMed] [Google Scholar]
- 15.Umeda A, Kawasaki K, Abe T, Watanabe M, Ishizaka A, Okada Y. Hyperventilation and finger exercise increase venous-arterial Pco2 and pH differences. Am J Emerg Med. 2008;26(9):975–80. doi: 10.1016/j.ajem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Lim BL, Kelly AM. A meta-analysis on the utility of peripheral venous blood gas analyses in exacerbations of chronic obstructive pulmonary disease in the emergency department. Eur J Emerg Med. 2010;17(5):246–8. doi: 10.1097/MEJ.0b013e328335622a. [DOI] [PubMed] [Google Scholar]
- 17.Theusinger OM, Thyes C, Frascarolo P, Schramm S, Seifert B, Spahn DR. Mismatch of arterial and central venous blood gas analysis during haemorrhage. Eur J Anaesthesiol. 2010;27(10):890–6. doi: 10.1097/EJA.0b013e32833adea8. [DOI] [PubMed] [Google Scholar]


