Abstract
BACKGROUND:
We investigated coronary angiogenesis and serum vascular endothelial growth factor (VEGF) and its soluble receptor-1 (sFlt-1) concentrations in two-kidney one-clip (2K1C) hypertensive rats before and after reversal of hypertension.
METHODS:
The animal groups were: (i) sham-clipped for 12 weeks; (ii) 2K1C for 12 weeks; (iii) sham-clipped for 12 weeks and unclipped for 12 weeks; and (iv) 2K1C for 12 weeks and unclipped for 12 weeks. Blood samples were taken before experiments and after clipping and unclipping; capillary density was also evaluated.
RESULTS:
Our results showed that blood pressure in hypertensive animals was higher than sham group (175 ± 10 vs. 110.3 ± 11.3 mmHg; p < 0.05). Unclipping significantly reduced blood pressure in hypertensive rats (p < 0.05). Serum VEGF and sFlt-1 levels in hypertensive group were significantly lower than sham group (VEGF: 74.36 ± 5.85 vs. 104.07 ± 7.75 pg/ml; sFlt-1: 426.67 ± 25.74 vs. 690.76 ± 41.14 pg/ml, respectively; p < 0.05). Unclipping in hypertensive animals increased serum VEGF and sFlt-1 concentrations (VEGF: 93.65 ± 8.61 vs. 74.36 ± 5.85 pg/ml; sFlt-1: 742.05 ± 79.23 vs. 426.67 ± 25.74 pg/ml, respectively; p < 0.05). In hypertensive animals, capillary density in the heart was higher than sham group, non-significantly (p > 0.05) and after unclipping, it reached to sham group level.
CONCLUSIONS:
It seems that changes in capillary density and serum VEGF and sFlt-1 concentrations in renovascular hypertension are reversible by removing the cause of hypertension and it shows the importance of early diagnosis and treatment of hypertension in clinical condition.
Keywords: Hypertension, Angiogenesis Inhibitor, Coronary Vessels, Vascular Endothelial Growth Factor Receptor-1
Angiogenesis is a process by which new vessels sprout from pre-existing vessels in response to angiogenic molecules and hypoxia that result from tissue injury. Angiogenesis is an important process in development of cardiovascular system. Among the angiogenic factors, vascular endothelial growth factor (VEGF) is a key angiogenic factor in physiological and pathological vascular development.1 VEGF is a 45-kDa glycoprotein which stimulates proliferation and migration of endothelial cells and inhibits apoptosis resulting in formation of collateral vessels.2 VEGF has two tyrosine kinase receptors: VEGFR-1 and VEGFR-2. The soluble form of VEGFR-1 (sFlt-1) is found in circulation which can inhibit VEGF actions by direct sequestration.3,4
Hypertension is a well known risk factor for cardiovascular disease. It is well established that hypertension is associated with several vascular abnormalities such as endothelial dysfunction,5 microvascular rarefaction and remodeling.6,7 Hypertension is associated with a defect in blood vessel growth.7,8 Impaired angiogenesis in hypertensive subjects has been reported in some studies,9 but other data indicated enhanced angiogenic factors in serums of hypertensive subjects.10 On the other hand, impaired angiogenesis during embryo or infant development leads to underdevelopment of vascular system and predispose hypertension in later life.7
In this study, we evaluated the effect of hypertension and its reverse on coronary angiogenesis and some angiogenic factors (VEGF and sFlt-1). In the first part of the study, we used two-kidney one-clip (2K1C) hypertensive rats to study the effect of hypertension on serum VEGF and sFlt-1 levels and coronary angiogenesis. In the second part, the effect of unclipping and reversal of hypertension was evaluated.
Methods
Animals
32 male wistar rats (10-12 weeks old; weight: 200 ± 20 gr) were obtained from Pasteur Institute of Iran. The animals were kept in animal room with 12 h light/dark cycle and temperature of 20-25°C. The animals had free access to tap water and commercial rat chow throughout the experiment. Ethical committee of Isfahan University of Medical Sciences approved the animal experiment.
Experimental Protocol, Clipping and Unclipping
After one week of habituation to animal room, blood samples were taken and centrifuged at 3000 rpm for 15 minutes. Plasma and serums were stored at -70°C for further analysis. Then, the animals were randomly divided into hypertensive (n = 16) and sham-operated groups (n = 16) and each group was divided into clipped and unclipped groups. The experimental groups were as follows: (i) 2K1C (n = 8), and (ii) sham-clipped (n = 8); these groups were kept for 12 weeks. (iii) 2K1C for 12 weeks and unclipped for 12 weeks (n = 8), and (iv) sham-clipped for 12 weeks and unclipped for 12 weeks (n = 8).
For preparation of 2K1C hypertensive rats, the animals were anesthetized with Ketamine Hydrochloride (75 mg/kg) and Xylazine (7.5 mg/kg), injected intraperitoneally. Left kidneys were exposed via flank incision. After cleaning the connective tissue surrounding the renal artery, a silver clip (internal gap of 0.20 mm) was put around the artery. In sham-clipped group, the same procedure was done without using silver clip. After closing the wound, penicillin G 25000 IU (i.p) was injected. Then, the animals were maintained in animal care facilities. Blood pressure was measured twice a week using tail cuff method (ADinstrument, Australia). Rats were fed with standard rat chow and allowed free access to tap water throughout the study. After 12 weeks, the animals of groups 1 and 2 were anaesthetized. Direct blood pressure was measured via a catheter (PE-50) inserted into carotid artery. Blood art apex tissues were taken for subsequent determination of plasma renin activity, serum VEGF and sFlt-1 concentrations and evaluation of capillary density. To investigate the effect of reversal of hypertension, the animals of group 3 were anesthetized. Left renal arteries were exposed and silver clips gently removed without damaging renal artery or causing bleeding. In sham-unclipped group (group 4), the same procedure was done without removing the clip. Blood pressure was measured via tail cuff method every week. After 12 weeks, direct blood pressure was measured, blood samples were taken and coronary angiogenesis was evaluated.
Serum VEGF and sflt-1 Measurements
Serum VEGF and sFlt-1 assays were performed using a Sandwich Enzyme Immunoassay kits and reagents (R&D systems, USA) according to the manufacturer's instructions. The minimum sensitivity of VEGF assay is 3.9 pg/ml with an intra- and inter-assay coefficient of variation (CV) of < 10% and < 5%, respectively.
The sFlt-1 assay has lower limit of sensitivity of 3.8 pg/ml and intra- and inter-assay coefficients of variation less than 10% and 5%, respectively.
Plasma Renin Activity
Plasma renin activity (PRA) was measured using Gamma coat PRA Radioimmunoassay kit (DiaSorin Inc). PRA was measured after clipping and unclipping in 2K1C groups.
Evaluation of Capillary Density
Sample tissues were obtained from apex of the hearts after 12 weeks induction of hypertension and its reverse. Frozen tissue sections with 5 μm thickness were prepared from each sample. Endothelial cells were stained by immunohistochemical staining using rat anti-mouse CD31 monoclonal antibody (Abcam Co). Capillary density was evaluated by counting twenty random microscopic fields (magnification × 400) from three different sections in each tissue block by three blind examiners. Capillary density was expressed as the number of CD31+ cells per square millimeter (mm2).
Statistical Analysis
Data are reported as mean ± SE. One-way ANOVA was used for comparison of data between groups. The difference between two groups was analyzed by student's t-test. Data before and after experiment were compared by paired t-test. P value less than 0.05 was considered statistically significant.
Results
Blood Pressure
Systolic blood pressure measurement showed that 2K1C hypertensive rats had significantly higher blood pressure compared with sham-clipped group (p < 0.05). Unclipping procedure resulted in a significant reduction in blood pressure in 2K1C group (p < 0.05) (Figure 1).
Figure 1.
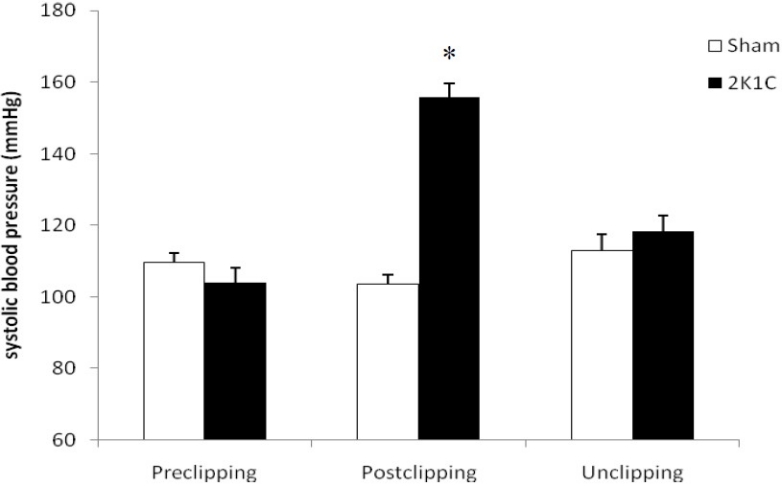
Systolic blood pressure in experimental groups. * p<0.05 when compare with other groups.
Measurement of PRA
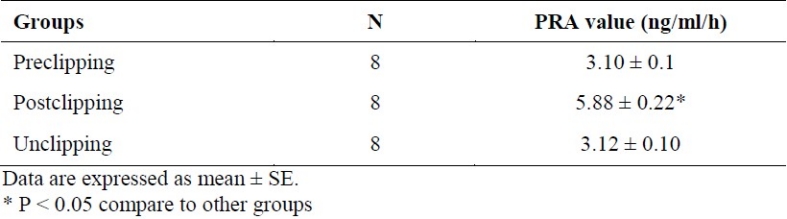
2K1C hypertensive rats had significantly higher PRA level with mean concentration of 5.70 ± 0.24 ng/ml/h compared to sham-clipped group (3.14 ± 0.18 ng/ml/h) (p < 0.05). Unclipping resulted in the decrease of plasma PRA level in hypertensive group (p < 0.05) (Table 1).
Table 1.
Plasma renin activity (PRA) values (ng/ml/h) in 2K1C hypertensive rats before and after clipping and unclipping
Serum VEGF and sflt-1 Concentrations
Hypertension was associated with significant reduction in serum VEGF levels with mean concentration of 74.36 ± 5.85 pg/ml compared with sham-clipped group (103.7 ± 7.75 pg/ml) (p < 0.05). Unclipping in hypertensive rats increased serum VEGF concentration close to sham-unclipped group (93.65 ± 8.61 vs. 95.03 ± 4.95 pg/ml, respectively) (Figure 2). Serum sFlt-1 was decreased after induction of hypertension (p < 0.05). Reversal of blood pressure increased serum sFlt-1 to sham-clipped levels (Figure 2).
Figure 2.
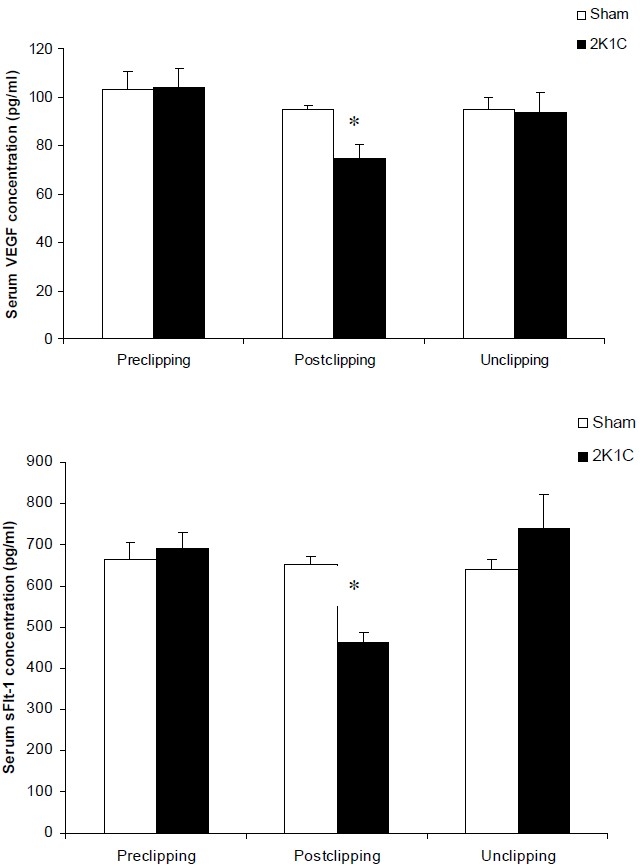
serum VEGF and sFlt-1 concentrations an all experimental groups. * p<0.05 compare with other groups.
Capillary Density
As demonstrated in figure 3, capillary density (expressed as number of capillary (CD31+) per mm2) was higher in hypertensive animals compared with normotensive group, although it was not statistically significant. Reduction of blood pressure and unclipping reduced capillary density in hypertensive animals. Samples of histological sections are shown in figure 4.
Figure 3.
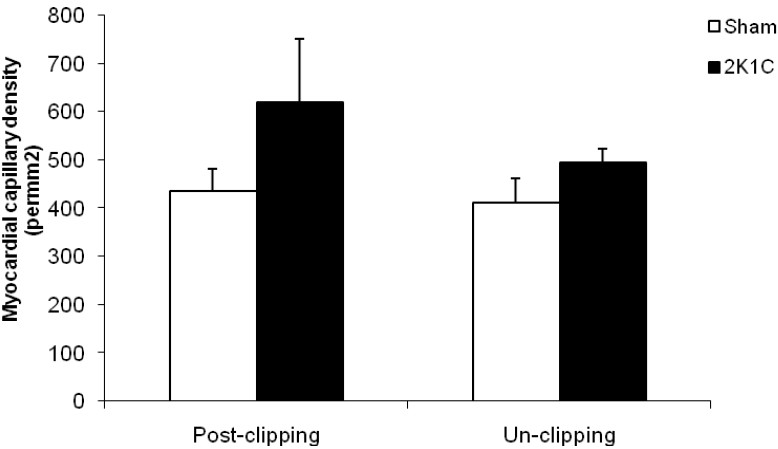
capillary density (expressed as number of capillary/mm2) of heart (apex) during hypertension and after unclipping.
Figure 4.

representative photograph of the cross section of apex of the hearts in experimental groups. Original magnification: × 400. (A: Sham-clipped; B:2K1C; C:Sham-unclipped; D:2K1C-unclipped).
Discussion
Our results showed higher blood pressure and PRA level in 2K1C rats compared with sham-clipped group. Studies indicated that in early phase of 2K1C hypertensive model, increased activity of renin-angiotensin-aldosterone is responsible for rising blood pressure11,12; however, after 6 weeks of clipping, changes of vascular structure is important in maintenance of hypertension.11 Reduced blood pressure after unclipping may be due to reduced plasma renin activity11 and/or reduced activity of sympathetic system.13 In this study, we found that unclipping reduced PRA and blood pressure in hypertensive animals.
Hypertension is associated with several vascular abnormalities including endothelial dysfunction and vascular rarefaction14,15 which could contribute to increased vascular resistance and hypertension. Our results showed that coronary angiogenesis was increased in hypertensive rats non-significantly. Changes of microvascular density during development of hypertension has been documented in several experimental studies.14,16,17 A study on SHR rats showed higher vessel density in mesenterium compared with similar area in normal rats.17 Another study in hypertensive rats showed lower capillary and arteriolar density in the heart of young animals.16 In a study on human, no changes were found in capillary number of quadriceps muscle of hypertensive subjects.18 It seems that effect of hypertension on microcirculation depends on tissue, time course of developing of hypertension and model of hypertension.7,17
VEGF is essential in endothelial and vascular function. It stimulates endothelial migration, proliferation, and survival, and enhances nitric oxide production.2 It also inhibits endothelial apoptosis. VEGF-R1 is a tyrosin kinase receptor which expressed primarily on endothelial cells and involves in angiogenesis, endothelial cell migration and proliferation.2 First time, in 1995, Fong et al revealed that VEGF-R1 plays a negative role in angiogenesis.19 VEGF-R1 has high affinity for VEGF but has weak tyrosine kinase activity.4,20 It is indicated that soluble VEGF-R1 (sFlt-1) has anti angiogenic properties by dampening angiogenic VEGF-VEGFR-2 signalling.4,20 In this study, we found that serum VEGF and sFlt-1 levels were decreased during hypertension and reversal of hypertension returned them to normotensive levels. There are contradictory reports regarding the changes of plasma angiogenic factors during hypertension. For the first time, Belgore et al showed that plasma VEGF and sFlt-1 concentrations were significantly higher in uncomplicated essential hypertensive patients compared with normotensive controls and treatment of hypertension significantly reduced plasma VEGF and sFlt-1 levels.10 Other clinical studies demonstrated a positive association of VEGF and HGF with hypertension.3,21 They suggested that VEGF may be a marker for hypertension with a response to high blood pressure. In contrast, Felmeden et al reported higher VEGF and lower sFlt-1 in plasma of hypertensive patients.15 A recent study indicated that plasma VEGF and sFlt-1 levels have no correlation with blood pressure.22 These data variability can be attributable to methodological differences, sampling preparation and method of analysis of these factors.4 It should be considered that method using to determine free VEGF, total VEGF or total VEGF-VEGFR-1 complex (bound or unbound proteins) makes differences in quantification of circulating VEGF or sFlt-1 levels in studies.4
Conclusions
In conclusion, it seems that changes in capillary density and serum VEGF and sFlt-1 concentrations in renovascular hypertension are reversible by removing the cause of hypertension and it shows the importance of early diagnosis and treatment of hypertension in clinical condition.
Authors’ Contributions
MZ and MK involved in study design, conducting experiments and writing the manuscript. MRS and AAP contributed in the study design. All authors have read and approved the content of the manuscript.
Acknowledgments
This study was supported by a grant from Isfahan University of Medical Sciences (Grant No. 187008).
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Ylä-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49(10):1015–26. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 3.Lieb W, Safa R, Benjamin EJ, Xanthakis V, Yin X, Sullivan LM, et al. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J. 2009;30(9):1121–7. doi: 10.1093/eurheartj/ehp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14(3):528–52. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291(3):H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 6.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90(4):449–55. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 7.Humar R, Zimmerli L, Battegay E. Angiogenesis and hypertension: an update. J Hum Hypertens. 2009;23(12):773–82. doi: 10.1038/jhh.2009.63. [DOI] [PubMed] [Google Scholar]
- 8.le Noble FA, Stassen FR, Hacking WJ, Struijker Boudier HA. Angiogenesis and hypertension. J Hypertens. 1998;16(11):1563–72. doi: 10.1097/00004872-199816110-00001. [DOI] [PubMed] [Google Scholar]
- 9.Emanueli C, Salis MB, Stacca T, Gaspa L, Chao J, Chao L, et al. Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension. 2001;38(1):136–41. doi: 10.1161/01.hyp.38.1.136. [DOI] [PubMed] [Google Scholar]
- 10.Belgore FM, Lip GY, Bareford D, Wadley M, Stonelake P, Blann AD. Plasma levels of vascular endothelial growth factor (VEGF) and its receptor, Flt-1, in haematological cancers: a comparison with breast cancer. Am J Hematol. 2001;66(1):59–61. doi: 10.1002/1096-8652(200101)66:1<59::AID-AJH1011>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17(5):707–19. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- 12.Nyström HC, Jia J, Johansson M, Lambert G, Bergström G. Neurohormonal influences on maintenance and reversal of two-kidney one-clip renal hypertension. Acta Physiol Scand. 2002;175(3):245–51. doi: 10.1046/j.1365-201X.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 13.Göthberg G, Thorén P. Sympathetic inhibition after surgical reversal of renovascular hypertension in rats.Role of vagal nerves. Acta Physiol Scand. 1986;126(3):397–404. doi: 10.1111/j.1748-1716.1986.tb07833.x. [DOI] [PubMed] [Google Scholar]
- 14.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7(3):193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 15.Felmeden DC, Spencer CG, Belgore FM, Blann AD, Beevers DG, Lip GY. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens. 2003;16(1):11–20. doi: 10.1016/s0895-7061(02)03149-7. [DOI] [PubMed] [Google Scholar]
- 16.le Noble JL, Tangelder GJ, Slaaf DW, van Essen H, Reneman RS, Struyker-Boudier HA. A functional morphometric study of the cremaster muscle microcirculation in young spontaneously hypertensive rats. J Hypertens. 1990;8(8):741–8. doi: 10.1097/00004872-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Murfee WL, Schmid-Schönbein GW. Chapter 12.Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271–84. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández N, Torres SH, Finol HJ, Vera O. Capillary changes in skeletal muscle of patients with essential hypertension. Anat Rec. 1999;256(4):425–32. doi: 10.1002/(SICI)1097-0185(19991201)256:4<425::AID-AR9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9(4):225–30. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 21.Felmeden DC, Spencer CG, Chung NA, Belgore FM, Blann AD, Beevers DG, et al. Relation of thrombogenesis in systemic hypertension to angiogenesis and endothelial damage/dysfunction (a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial [ASCOT]) Am J Cardiol. 2003;92(4):400–5. doi: 10.1016/s0002-9149(03)00657-x. [DOI] [PubMed] [Google Scholar]
- 22.Sandhofer A, Tatarczyk T, Kirchmair R, Iglseder B, Paulweber B, Patsch JR, et al. Are plasma VEGF and its soluble receptor sFlt-1 atherogenic risk factors? Cross-sectional data from the SAPHIR study. Atherosclerosis. 2009;206(1):265–9. doi: 10.1016/j.atherosclerosis.2009.01.031. [DOI] [PubMed] [Google Scholar]


