Abstract
BACKGROUND:
Chronic hemodialysis patients frequently require vascular access through central venous catheters (CVCs). The most significant complication of these catheters is infection. This risk can be lowered by the use of an antibiotic-Heparin lock. This study focuses on hemodialysis patients using Tunneled-cuffed catheters (TCC), to assess the rate of catheter-related infections (CRI) in catheter-restricted filling with Cefotaxime and Heparin in end stage renal disease patients.
METHODS:
A double-blind randomized study was conducted to compare 5000 U/ml Heparin plus10 mg/ml cefotaxime (CE/HS) as catheter-lock solutions, with Heparin (5000 U/ml) alone. A total of 30 patients with end-stage renal disease and different etiologies, were enrolled for chronic hemodialysis with permanent catheters from December 2009 to March 2010. These patients were randomly assigned to two groups of 15 members. Blood samples were collected for culture, sensitivity, and colony count, from the catheter lumen and the peripheral vein. CRI was considered as the end point.
RESULTS:
The rate of CRI was significantly lower in the cefotaxime group versus control group (p < 0.001). No exit site infection was occurred in both groups. Infection-free survival rates at 180 days were 100% for the CE/HS group, and 56% for the HS group.
CONCLUSIONS:
Antibiotic lock therapy using cefotaxime reduces the risk of CRI in hemodialysis patients.
Keywords: Catheter, Hemodialysis, Lock Solution Infection, Cefotaxime, Heparin
Anative arteriovenous fistula is the gold-standard vascular access for haemodialysis (HD), but venous catheter use is also widespread.1 Catheter-related infection (CRI) is a significant complication of chronic hemodialysis. CRI is associated with high morbidity and mortality and the prevention of CRI has remained a significant challenge.1 CRI constitutes a substantial component of hospital-acquired infections. Hospital admissions for vascular access infection has doubled in the last decade2,3 resulting not only in substantial patient morbidity, but also in consumption of hospital resources. Furthermore, this is also associated with increased all-cause infection-related mortality.4–6
An incidence of two to three CRI episodes per 1000 catheter-days is considered relatively low.7–10 Most studies reporting four to six episodes per 1000 catheter-days.11–16 The prevalence of Gram-positive organisms (usually Staphylococcus species) is about 60% and Gramnegative organisms 40%, depending upon local patterns of bacterial flora. The frequency of CRI depends on several factors including criteria for the definition of bacteremia and infection, duration of catheter use, type of catheter (temporary vs. permanent), frequency of manipulations and exit site care, history of bacteremia, and infection-control procedures.11,17
Although the use of tunneled cuffed hemodialysis catheters (TCC) has increased in past years, but yet the appropriate strategy for TCC management is controversial. Typically, initial treatment of CRI is administration of a systemic antibiotic.11 However, the reported efficacy of systemic antibiotics alone is only 25-32%.18,19
In order to prevent intraluminal colonization and the development of a biofilm, an approach has been used to instill an antimicrobial solution into the lumen(s) of the catheter (lock solution) at the end of each hemodialysis session. Fong suggested that antibiotic infusion followed by Vancomycin or Ciprofloxacin antibiotic-lock successfully eradicated catheter-related sepsis without catheter removal.20 To further investigate intraluminal antibiotics in the prevention of CRI in patients requiring hemodialysis, we determined the rate of catheter-related infection (CRI) of Cefotaxime and Heparin in end-stage renal disease patients.
Methods
Study Design
A hundred and fifty patients with end-stage renal disease were assessed for eligibility. The study was a prospective, randomized, controlled clinical trial (Iranian Clinical Registration Number 138811182370N6), and conducted at two tertiary-care urban medical center. Patients with end-stage renal disease from December 2009 to March 2010 were included in the study. The catheters were dual-lumen, 200-mm, and silicone based.
Inclusion criteria included age more than 18, being dialyzed with central tunneled catheter, Med Comp (only if placed in the internal jugular vein), with a maximum time of one month post catheterization, dialysis 3 times a week and having accepted to participate in the study. Exclusion criteria were allergy to Cefotaxime, antibiotic treatment within 2 weeks prior to enrollment, patients requiring a surrogate decision maker, catheters with blood flow rates less than 300 ml/min, or requiring frequent thrombolytic solution dwells in the catheter lumen because of malfunction.
At the beginning of the study, 150 patients were dialyzed with tunneled catheters. According to the inclusion criteria, 38 patients were eligible to participate in the study, but 30 of them were consented (20.6%). Study profile is shown in (Figure 1).
Figure 1.
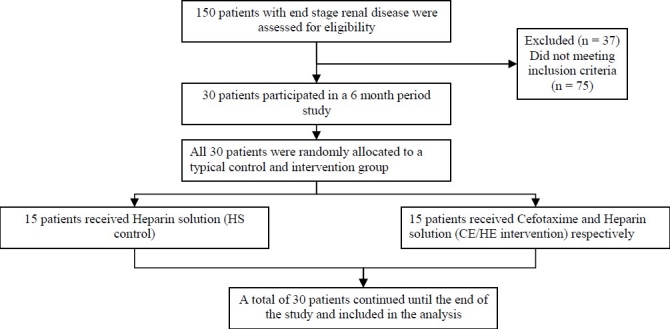
Study profile
Enrolled patients were randomly assigned by using a block computerized randomization protocol into two groups of 15 patients. In intervention group, we used Cefotaxime, at a concentration of 10 mg/ml as the experimental antibiotic lock solution.
Cefotaxime-Heparin “lock” solutions were prepared by dissolving sterile Cefotaxime sodium powder in normal saline for injection to reach a concentration of 10 mg/mL for Cefotaxime. Then mixture of 1.5 cc Cefotaxime (10 mg/ml) and 1.5 cc Heparin solution (5,000 U/ml) (CE/HS), was prepared in a syringe using aseptic precautions to fill 1.6 mL in the venous and 1.4 ml in the arterial lumen of the catheter at the end of each dialysis session (exact volume of each venous and arterial port so that Cefotaxime would not inter the blood stream, preventing its systemic effect and microbial resistance). In control group we just filled 1.6 ml and 1.4 ml of standard Heparin solution (HS; 5,000 U/ml) in the venous and arterial lumens respectively. In both groups, the catheter exit-site was washed with antiseptic solution at the initiation and termination of each session, and covered by topical mupirocin and dry sterile gauze during dialysis intervals. Antibiotic solutions were stored in the refrigerator and Nurses were trained to instill the solution into each of the two ports of the HD catheter at the end of each dialysis session, and withdraw it immediately before the next dialysis session. TCCs were inserted by experienced vascular access surgeons. In this study all the included patients received dialysis three times a week with the same catheter and the same exit site care, and they all had the same duration of catheter insertion.
Catheter-Related Infection
Enrolled patients were routinely monitored clinically for symptoms and signs of infection, during each session of dialysis. Blood cultures were drawn if patients had fever, chills, rigors, sweats, change in mental status, or exit-site infection4. Other causes of infection were ruled out. The study protocol required peripheral and catheter blood cultures and an exit site swab to be collected, if clinically indicated. If the catheter was removed, the catheter tip was cultured. Patients with positive blood culture results were treated with systemic antibiotics driven by type of organism and antibiotic susceptibility. Blood stream infections were defined by the CDC (Centers for Disease Control) criteria21.
Definite blood stream infection was defined as the same organism from a semiquantitative culture of the catheter tip (15 colony-forming units/catheter segments) or catheter blood sample versus peripheral blood sample in a symptomatic patient with no other apparent source of infection22. If colony count of catheter were 5 times more than blood sample, blood stream infection were established7,20.
Statistical Analysis
Data were analyzed using SPSS software (SPSS Inc., Chicago, IL; ver.18). Results are expressed as mean ±SD or Number (percent). Statistical analysis was performed with independent Sample T-test and Chi-square tests. P-value less than 0.05 was considered as statistically significant.
Results
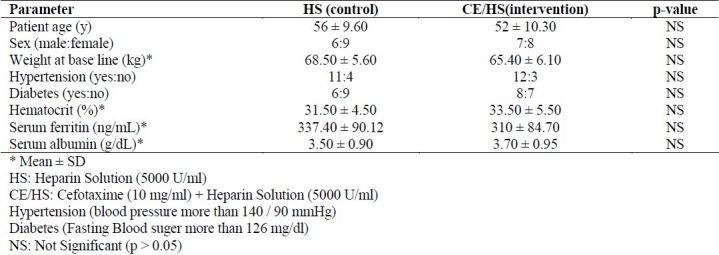
All patients were visited in the HD unit on every other daily basis, and clinical events were closely examined. No patient was lost to follow-up. Baseline demographic and clinical data including, age, male-female ratio, weight, diabetes, hypertension, lab data (complete blood count, Serum Ferritin and Serum albumin) did not have significant difference among two groups (p > 0.05) (Table 1). No fatalities occurred during the follow up.
Table 1.
Demographic and basic clinical characteristics of patients at the beginning of study
Exit site infection was occurred in neither of groups but eleven patients developed CRI in the HS (control) group at the time of the first interim analysis, whereas no patient had CRI in the CE/HS group. Clinical examination and follow-up of the patients with infection did not show any other sources except the catheters. Infection rate per 1000 catheter-days was zero in the CE/HS group versus 6.84 in the HS group (p < 0.001). The bacteria isolated from peripheral and catheter blood were gram-negative Bacilli in 64%, while 36% of them were grampositive Cocci. Among gram-positive Cocci, 75% were Staph Aureus. Infection-free survival rates at 180 days were 100% for the (CE/HS) group, and 56% for the HS group (p < 0.001, Pearson chi-square value = 17.368).
Discussion
CRI results from migration of skin organisms along the catheter into the bloodstream or contamination and colonization of catheter lumens.23 All indwelling vascular catheters develop a biofilm on internal and external surfaces. Subsequent colonization of this biofilm occurs in a high percentage of catheters and precedes bacteremia and septic symptoms.24 Through reduction in CRI rate, we can reach dramatic reduction in morbidity and mortality of hemodialysis patients. Antibiotic lock is one of the methods which can help to prevent CRI, but according to its importance and financial cost, prospective randomized clinical trials are necessary to confirm its efficacy. The aim of this study was to determine the efficacy of catheter restricted filling with Cefotaxime and Heparin in preventing tunneled catheter related infections (CRI) in hemodialysis patients.
Patients with confirmed bacteremia may benefit from administration of intravenous antibiotics and instillation of the antibiotic-Heparin lock between dialysis sessions without catheter removal. Preventing bacteremia and treating without catheter removal would be optimal. A lower concentration of the antibiotic-Heparin lock was investigated for preventing gram-positive central venous catheter related infections in 57 neutropenic patients with cancer.25 Antiseptic and antibiotic coatings on central venous catheters showed promising results in intensive care populations.26,27 A 79% reduction in the rate of catheter-related blood stream infections (relative risk [RR] 0.21, 95% CI 0.03-0.95) was reported with use of Chlorhexidine-silver sulfadiazine-coated catheters. Application of these results to patients receiving chronic hemodialysis is limited as catheters used in intensive care unit are in situ for approximately one week, versus hemodialysis catheters which may be in situ for several weeks or even months.26 Cefotaxime is active against most gram-negative bacilli (except Pseudomonas) and gram-positive cocci (except enterococcus) and is also active against many penicillin-resistant pneumococci. This study suggested that infection rates per 1000 catheter-days were significantly lower in the CE/HS group versus HS (control) group (0.000 vs. 6.84/1000 catheter days). Saxena showed a lower CRI incidence in the CE/HS group versus HS (control) group in non-tunneled HD catheters (1.65 vs. 3.13/1000 catheter days). In tunneled HD catheters, rate of CRI is less than non-tunneled HD catheters.4 Furthermore, he showed a lower CRI incidence in the CE/HS group versus HS (control) group (1.67 vs. 3.60/1000 catheter days) in TCC.28 CRI rates were significantly lower with the use of antibiotic locking solution (ALSs). Pooled data from several studies show that CRI is 7.72 (95% confidence interval, 5.1 to 10.3) times less likely with ALS than with Heparin.29 Six of these studies used Heparin, 5000 U/ml, as the control group, while the study by Kim et al26 used 1000 U/ml. Antibiotics tested include gentamicin,22,30,31 gentamicin/citrate combination,32 cefotaxime,4 minocycline,22 cefazolin/ gentamicin combination26 and chlorhexidine.33
In this study, the choice of Cefotaxime was based on its broad spectrum effect on gramnegative organisms in addition to being most effective amongst third generation Cephalosporins against Staphylococcus Aureus. Cefotaxime was preferred over Gentamicin for locking TCC, which can be potentially ototoxic, specially in elderly patients who often have preexisting hearing disabilities.28,34,35
Conclusions
The present study shows that catheter-restricted filling with a solution containing Cefotaxime and Heparin may significantly reduce the incidence of Catheter-related infection among end stage renal disease patients.
Cefotaxime as an antibiotic lock solution appears to be effective and safe; although there is some concern about the allergy to its leaked solution from lumens. Therefore we excluded the patients who had allergy to Cefotaxime. In order to omit biases in our study, all the patients had the same frequency of manipulation, they all received dialysis three times a week with the same catheter and they all had the same duration of catheter insertion. With the use of antibiotic lock method for reduction in CRI rate, we may reach dramatic reduction in morbidity and mortality of hemodialysis patients.
Since this study was a small sample size and short-term follow-up study, and according to its importance and financial cost associated, there is a need for additional studies with long-term follow-up on larger population to evaluate the efficacy of Cefotaxime lock protocol and its probable adverse effects and its correlation with using topical agents.
Authors’ Contributions
MM, SA, BP, and AA selected and managed patients, FS referred eligible patients after Catheter placement, MA was our statistician, RS, AHDJ, and NS were the major contributors in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgments
No financial support received in support of the study. This study was an experimental investigation on human subjects. Ethics committee of Isfahan University of Medical Sciences approved in 2009, reference number 38833.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Bristol: UK Renal Registry; 2005. UK Renal Registry. New Adult Patients Starting Renal Replacement Therapy in the UK in 2004; pp. 12–26. [Google Scholar]
- 2.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, et al. The spectrum of infection-related morbid-ity in hospitalized haemodialysis patients. Nephrol Dial Transplant. 2005;20(6):1180–6. doi: 10.1093/ndt/gfh729. [DOI] [PubMed] [Google Scholar]
- 3.Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. U.S. Renal Data System, USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 4.Saxena AK, Panhotra BR. The impact of catheter-restricted filling with cefotaxime and heparin on the lifespan of temporary hemodialysis catheters: a case controlled study. J Nephrol. 2005;18(6):755–63. [PubMed] [Google Scholar]
- 5.Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62(2):620–6. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 6.Al Hwiesh AK. Tunneled catheter-antibiotic lock therapy for prevention of dialysis catheter-related infections: a single center experience. Saudi J Kidney Dis Transpl. 2008;19(4):593–602. [PubMed] [Google Scholar]
- 7.Betjes MG, van Agteren M. Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution. Nephrol Dial Transplant. 2004;19(6):1546–51. doi: 10.1093/ndt/gfh014. [DOI] [PubMed] [Google Scholar]
- 8.Develter W, De Cubber A, Van Biesen W, Vanholder R, Lameire N. Survival and complications of indwelling venous catheters for permanent use in hemodialysis patients. Artif Organs. 2005;29(5):399–405. doi: 10.1111/j.1525-1594.2005.29067.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869–76. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 10.Dopirak M, Hill C, Oleksiw M, Dumigan D, Arvai J, English E, et al. Surveillance of hemodialysis-associated primary bloodstream infections: the experience of ten hospital-based centers. Infect Control Hosp Epidemiol. 2002;23(12):721–4. doi: 10.1086/502000. [DOI] [PubMed] [Google Scholar]
- 11.Battistella M, Bhola C, Lok CE. Long-term Follow-up of the Hemodialysis Infection Prevention With Polysporin Ointment (HIPPO) Study: A Quality Improvement Report. Am J Kidney Dis. 2011;57(3):432–41. doi: 10.1053/j.ajkd.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Saad TF. Bacteremia associated with tunneled, cuffed hemodialysis catheters. Am J Kidney Dis. 1999;34(6):1114–24. doi: 10.1016/S0272-6386(99)70018-1. [DOI] [PubMed] [Google Scholar]
- 13.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, et al. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002;61(3):1136–42. doi: 10.1046/j.1523-1755.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 14.Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant. 2004;19(5):1237–44. doi: 10.1093/ndt/gfh041. [DOI] [PubMed] [Google Scholar]
- 15.Allon M. Prophylaxis against dialysis catheter-related bacteremia with a novel antimicrobial lock solution. Clin Infect Dis. 2003;36(12):1539–44. doi: 10.1086/375234. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW, MacGinley R, Kay TD, Hawley CM, Campbell SB, Isbel NM, et al. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant. 2002;17(10):1802–7. doi: 10.1093/ndt/17.10.1802. [DOI] [PubMed] [Google Scholar]
- 17.Chazan JA, London MR, Pono LM. Long-term survival of vascular accesses in a large chronic hemodialysis population. Nephron. 1995;69(3):228–33. doi: 10.1159/000188461. [DOI] [PubMed] [Google Scholar]
- 18.Huraib S, Askar A, Abu-Aisha H, al Wakeel J. Prevalence of infection from subclavian dialysis catheters with two different postinsertion catheter cares: a randomized comparative study. Angiology. 1994;45(12):1047–51. doi: 10.1177/000331979404501208. [DOI] [PubMed] [Google Scholar]
- 19.Hoen B, Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant. 1995;10(3):377–81. [PubMed] [Google Scholar]
- 20.Fong IW. Prevention of haemodialysis and peritoneal dialysis catheter related infection by topical povidoneiodine. Postgrad Med J. 1993;69(Suppl 3):S15–7. [PubMed] [Google Scholar]
- 21.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections.Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-10):1–29. [PubMed] [Google Scholar]
- 22.Nori US, Manoharan A, Yee J, Besarab A. Comparison of low-dose gentamicin with minocycline as catheter lock solutions in the prevention of catheter-related bacteremia. Am J Kidney Dis. 2006;48(4):596–605. doi: 10.1053/j.ajkd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Taal MW, Fluck RJ, McIntyre CW. Preventing catheter related infections in haemodialysis patients. Curr Opin Nephrol Hypertens. 2006;15(6):599–602. doi: 10.1097/01.mnh.0000250685.51717.53. [DOI] [PubMed] [Google Scholar]
- 24.Dittmer ID, Sharp D, McNulty CA, Williams AJ, Banks RA. A prospective study of central venous hemodialysis catheter colonization and peripheral bacteremia. Clin Nephrol. 1999;51(1):34–9. [PubMed] [Google Scholar]
- 25.Carratala J, Niubo J, Fernandez-Sevilla A, Juve E, Castellsague X, Berlanga J, et al. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob Agents Chemother. 1999;43(9):2200–4. doi: 10.1128/aac.43.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Song KI, Chang JW, Kim SB, Sung SA, Jo SK, et al. Prevention of uncuffed hemodialysis catheter-related bacteremia using an antibiotic lock technique: a prospective, randomized clinical trial. Kidney Int. 2006;69(1):161–4. doi: 10.1038/sj.ki.5000012. [DOI] [PubMed] [Google Scholar]
- 27.Saxena AK, Panhotra BR, Sundaram DS, Al Hafiz A, Naguib M, Venkateshappa CK, et al. Tunneled catheters’ outcome optimization among diabetics on dialysis through antibiotic-lock placement. Kidney Int. 2006;70(9):1629–35. doi: 10.1038/sj.ki.5001776. [DOI] [PubMed] [Google Scholar]
- 28.Saxena AK, Panhotra BR, Sundaram DS, Morsy MN, Al Ghamdi AM. Enhancing the survival of tunneled haemodialysis catheters using an antibiotic lock in the elderly: a randomised, double-blind clinical trial. Nephrology (Carlton ) 2006;11(4):299–305. doi: 10.1111/j.1440-1797.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 29.Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis. 2008;51(2):233–41. doi: 10.1053/j.ajkd.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre CW, Hulme LJ, Taal M, Fluck RJ. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int. 2004;66(2):801–5. doi: 10.1111/j.1523-1755.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 31.Chow KM, Poon YL, Lam MP, Poon KL, Szeto CC, Li PK. Antibiotic lock solutions for the prevention of catheter-related bacteraemia in haemodialysis patients. Hong Kong Med J. 2010;16(4):269–74. [PubMed] [Google Scholar]
- 32.Dogra GK, Herson H, Hutchison B, Irish AB, Heath CH, Golledge C, et al. Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: a randomized controlled study. J Am Soc Nephrol. 2002;13(8):2133–9. doi: 10.1097/01.asn.0000022890.29656.22. [DOI] [PubMed] [Google Scholar]
- 33.Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Org, Pease S, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–41. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 34.Allon M. Prophylaxis against dialysis catheter-related bacteremia: a glimmer of hope. Am J Kidney Dis. 2008;51(2):165–8. doi: 10.1053/j.ajkd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Lerma F, Palomar M, Olaechea P, Sierra R, Cerda E. [Cefotaxime, twenty years later.Observational study in critically ill patients] Enferm Infecc Microbiol Clin. 2001;19(5):211–8. doi: 10.1016/s0213-005x(01)72615-8. [DOI] [PubMed] [Google Scholar]


