Abstract
BACKGROUND:
Local ethical committees in medical sciences in Iran were established in 1999 in order to assess and evaluate the observance of ethical standards throughout the universities and research centers. The purpose of this study is to analyze the situation of local ethical committees in order to develop research ethics guideline.
METHODS:
For this cross-sectional study which has been conducted with the support of WHO, 40 local ethical committees in all universities of medical sciences were evaluated by use of determined questionnaires.
RESULTS:
In this study, 40 universities of medical sciences participated; all of them have established local ethical committees. Each committee has 5 to 11 members and in more than 80% cases, written guidelines for selecting the committee's members are available. The minimum number of members for official session is at least 3 and replacement of absent members, did not take place in more than 85% of the committees. Informed consent in 95% of these local ethical committees is available. In all committees, researches regarding the use of human subjects are under ethical consideration. In half of the local ethical committees, penalties for non-compliance with the regulations are considered. The average number of research project evaluated in last session of these committees was 15.2 and the committees in 50% of cases have provided ethics training specifically for their members.
CONCLUSIONS:
Policymakers should develop a standard guideline for local ethical committees in medical science universities in Iran.
Keywords: Situation Analysis, Ethics Committees, Medical Sciences, Iran
In 1982, World Medical Association in cooperation with WHO has suggested the compilation of an international research guideline on human biomedical research. This guideline was intended to address a more precise experimentation especially on matters regarding researches implemented on the developing countries.
In 1987, the ethical evaluation on every research protocols in the whole collection of the declarations from the past years has been reviewed and has gained advancement and finally was stipulated in the documentation of clinical research in the United States and the first ethical guideline for the conservation of experimentation in medical researches was published In 1953.1
A system composed of different local ethical committees in medical research or revised councils has been fundamentally designed in order to protect interests of someone who is doing research activities by assessing all research recommendations on humans before the start of the research.2 In most developing countries, this system is either on the process of completion and or in the process of implementation.3
In Iran, the first National Ethical Committee on Research in Medical Sciences under the leadership of the Ministry of Health was established in 1998.4
Now all of the universities of medical sciences in Iran have local ethical committees. The main duty of these committees is protecting humans against possible threats as a result of research. These committees are composed of 7 members that conform to the rules and regulations and the guidelines of the National Committee.
The purpose of this study is to analyze the current situation of local ethical committees in universities of medical sciences in order to develop research ethics guideline.
Methods
This investigation is a cross sectional study (descriptive-analytical) that was conducted in Iran. In this study, 40 local ethical committees in all universities of medical sciences throughout the country were evaluated by scientific committee's members. Its members included three main investigators of the project that have been quite familiar with objectives and data collection of this study.
Data collection was performed by using determined questionnaire; its validity and reliability was confirmed by WHO and scientific committee through pilot study. The questionnaires were completed by secretaries or informed experts of ethics committees, with regard to documentation based on signed recorded minutes by members of local ethical committee and also all of the completed questionnaires were checked and signed by undersecretary for research in university of medical sciences; and so, all of them are reliable. If it was necessary, or there was ambiguity in a question, the phone numbers of two members of scientific committee were recorded in questionnaires. Each questionnaire consists of six parts; 55 quantitative and 15 qualitative questions. The six main subjects include: a) activities of local ethical committee at the university/school of medical sciences; b) the general policies of the local ethical committee; c) activities of the last session of the local ethical committee; d) ethics training; e) management's support; and f) additional meetings. Qualitative part had open questions that included problems in six categories concerning “ethics in research” and their opinion to solve them. Quantitative data analysis was performed by SPSS software version 11 (Chicago IL USA). Statistical significance level was set at p <0.05 (two tailed) and qualitative data was analyzed according to content analysis approach, but in this article, we presented the quantitative results.
In this research, permission of the ethics committee has been obtained and ethical principles in gathering information, completing surveys and data analysis is considered and results have presented for all of the stakeholders. Also confidentiality was assured and all of the respondents were free from any pressure without any conflict of interests.
Results
In this study, 40 universities of medical sciences participated. The first local ethical committee in research was established in 1985 in Shahid Beheshti University of Medical Sciences and by 2006 all of the universities of medical sciences have established local ethical committees. Each committee has 5 to 11 members and more than 50% of these committees with about 7-8 members have organized meetings.
In more than 80% cases, written guidelines for selecting the committee's members are available but in 4 medical universities, these guidelines were not available. In 11 universities, no female members were found in these committees but in most universities at least one woman has been a member and one university has 4 female members in combination with male members.
Table 1 shows the composition of local ethical committees in medical universities in Iran. Selection of committee's head in 66% of cases was done by highly experienced regular employee. In 28 committees, they impose a time limitation for membership registration. The minimum number of members for official session is at least 3 and in 28 committees, the presence of at least 4-5 members is considered official.
Table 1.
The composition of the ethical committee in the medical universities in Iran
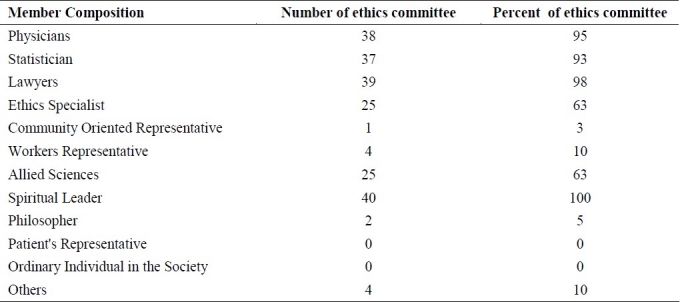
About half of these committees did not impose any regulations regarding the removal of any committee member.
Replacement of absent members, did not take place in more than 85% of the committees. 65% of the committees have written guidelines and 75% of these guidelines have been referred to in the national or international ethical regulations. Informed consent in 95% of these ethical committees is available. In all committees, researches regarding the use of human subjects are under ethical considerations. All universities are required to perform an ethical review for research involving traditional or complementary medicine. 73% of researches involving animals were under ethical reviews.
The initial evaluation of research proposals are implemented on the 34 local ethical committees established within the universities and it is being stressed to the researchers that suggestions must be presented in a written form. In 95% of the ethical committees, in order to assess the ethical standards for a research proposal, a research protocol is required from the researcher.
In half of the local ethical committees, penalties for non-compliance with the regulations are considered, and most of these penalties are implemented by suspending the research followed by a withdrawal of funding and a referral to the higher decision making committee.
Table 2 shows types of penalties.
Table 2.
The type of punishment
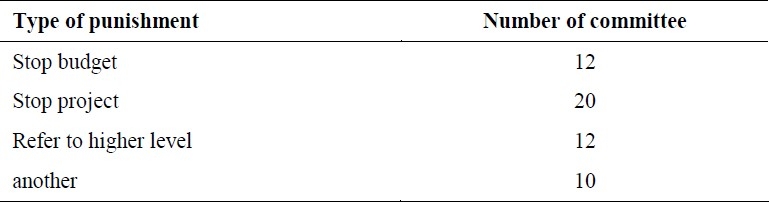
It should be mentioned that some of penalties are common.
Activities of the Last Meeting Organized By Local Ethical Committees
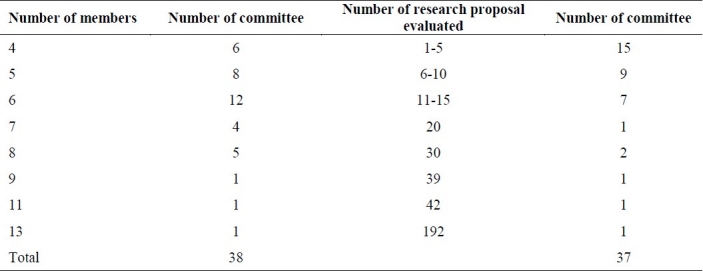
From 37 universities, the last meeting organized was planned beforehand. The number of members presented in that meeting was variable from 13 in one committee to 4 in six committees. The average number of members in that meeting was 6.
The total number of research proposals evaluated in the last meeting of local ethical committees in all universities was 563 and the average number of research projects evaluated was 15.2(Table 3)
Table 3.
Number of members and research proposal evaluated in last meeting
The most important protocols reviewed in these committees during their last meetings were as follow:
Procedures or documentation to obtain informed consent from potential subjects, including clarity and comprehensiveness of documentation and certificate of consent (80%)
Criteria for withdrawing subjects or discontinuing study including possible need for a data and safety monitoring board (68%)
Medical or health risks and benefits for study participants or community (62%)
Acceptability of level of medical care/health services provided in light of risk to subjects (55%)
Having a direct or potential benefit in the present or future, for people in the subgroup, geographical region or country in which the research is to be conducted (7%: at least)
About 40% of research procedures may not be respectful of local culture
The final reference for decision making related to research projects in majority of cases (97.6%) was the head of the committee.
Ethical Training
The committees in 50% of cases have provided ethics training specifically for their members. In 10 committees, the presence of members in the educational program was compulsory.
Management's Support
Special supports to complete the ethical forms for the researchers and members are being accepted in 12 committees; and ministerial and managerial supports in all universities are provided.
Additional Meeting or Ad Hoc Ethical Review Committees
At least in one university, there is a scientific review committee in lieu of an ethical review committee in terms of ethics evaluation. In 16 universities, the research councils reviewed the proposals in terms of ethical and scientific consideration. The main reason or driving force which leads to an ad-hoc ethical review committee, in the most cases, was request of researchers.
Discussion
In wrapping up the finals of the study done through local ethical committees, it was determined that one of the problems concerning local ethical committee composition was lack of experts in ethical course. It is clear that there are a few experts in committee considering the high number of committees, for example about 27% of committees do not have any ethicist and about 90% of committees do not have any scientist in other disciplines. It seems that holding post doctorate courses in bio-ethics field is necessary. Some researches in other countries confirmed the lack of experts in ethics. For example Oguz (2003) founded that the number of people with formal education in research ethics is too low to meet the demands of studies involving human subjects.5
In Turkey, for instance, education in research ethics is optional in part because the founders of RECs (Research Ethics Committees) were academics, self-educated and willing to participate in RECs regardless of their knowledge of ethics. Once this practice was accepted, no further mention of education in ethical evaluation was made.6
The results in our study showed that, all of the local ethical committees have spiritual leaders in our compositions but there is not any representative from patient or community and only in 2 local ethical committees, a philosopher attends the sessions. Another important issue in these committees is lack of written guidelines in 35% of them. Results showed that the regulation is vague, unclear, different in each university, and inconsistent with Iranian culture. The regulation needed to be modified and some other instructions in variety of issues need to be added to it.
Medical ethics with its methodology and practical approach, on one hand, requires sufficient knowledge of medicine and new technology, and on the other hand because of its value-laden nature, it is mutually related to religion, culture, philosophy, and law.
Also the policy makers and scientists should carry out some endeavors to prepare appropriate law, codes and guidelines based on international regulations, cultural and socioeconomic situation and religious beliefs for Iran.7
Another study in Australian Health Ethics Committee (AHEC), shows that the membership is specified in the Act and draws on expertise in philosophy, the ethics of medical research, public health and social science research, clinical medical practice and nursing, disability, law, religion, and health consumer issues.8
Molyneux and Geissler (2008) indicated that the abstract principles of existing codes are very hard to apply in practice; history, geography, culture, gender-relations and economic status can have important implications for the way in which “universal” ethical principles and guidelines are prioritized and applied in different contexts. Despite this fact, they also believed that although many guidelines emphasizes the need to adapt recommendations to local circumstances, there is a risk of guidelines being interpreted and drawn upon far more narrowly than was originally intended.9
Also there was inequality in composition of committees, because there were no female members in 26.8% of committees and there was only 1 female member in majority of them. Regarding the last session, results showed that in average, 15 proposals were reviewed in these sessions that provided additional evidence for problems concerning “session structure”. Another problem in this area was not attending members in sessions.
It is very exciting, that in one of the committees, 192 proposals were presented in the last session. It seems that all of the annually projects in one committee were evaluated in one session!
In almost half of the universities, there was one council for evaluating the projects regarding ethics and scientific matters; and it is incompatible with the ethics guidelines.
Conclusions
In summarize, the challenges faced by the committees members in Iran compared to international ethical regulations include: inappropriate session's structure, faults in regulation, lack of executive supportive rules for committee decisions, lack of financial support from committee members in local ethical committee practice, ethical review policies, training on ethics, and institutional support.
Policymakers with cooperation of other scientists should develop a standard guideline for local ethical committees in medical sciences according to international regulations, cultural and socioeconomic situation, and religious beliefs in Iran.
Authors’ Contributions
The first author was main investigator and the corresponding author was main co- investigator and the other authors were active in executive phase and data analysis.
Acknowledgments
This paper is a part of joint research project with World Health Organization as “situation analysis of local ethical committees in Iran”. The authors wish to thank a group of respected experts in universities of medical sciences and local ethical committees for assistance in data collection. This project received financial support from WHO via the Grant no: APW/08/03581.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Frewer A. Stuttgart: Steiner; 2007. History of medicine and ethics in conflict: research on National Socialism as moral problem; pp. 255–82. [Google Scholar]
- 2.Frewer A. Human rights from the Nuremberg Doctors Trial to the Geneva Declaration.Persons and institutions in medical ethics and history. Med Health Care Philos. 2010;13(3):259–68. doi: 10.1007/s11019-010-9247-2. [DOI] [PubMed] [Google Scholar]
- 3.Rwabihama JP, Girre C, Duguet AM. Ethics committees for biomedical research in some African emerging countries: which establishment for which independence? A comparison with the USA and Canada. J Med Ethics. 2010;36(4):243–9. doi: 10.1136/jme.2009.033142. [DOI] [PubMed] [Google Scholar]
- 4.Larijani B, Zahedi F. Contemporary medical ethics: an overview from Iran. Dev World Bioeth. 2008;8(3):192–6. doi: 10.1111/j.1471-8847.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 5.Oguz NY. Research ethics committees in developing countries and informed consent: with special reference to Turkey. J Lab Clin Med. 2003;141(5):292–6. doi: 10.1016/S0022-2143(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 6.Arda B. Ethical bodies: are they possible under democratic systems? The Turkish example. Med Law. 2009;28(3):531–9. [PubMed] [Google Scholar]
- 7.Zahedi F, Emami Razavi SH, Larijani B. A two-decade review of medical ethics in Iran. Iranian J Publ Health. 2009;38(Suppl 1):40–6. [Google Scholar]
- 8.Jamrozik K, Kolybaba M. Are ethics committees retarding the improvement of health services in Australia? Med J Aust. 1999;170(1):26–8. doi: 10.5694/j.1326-5377.1999.tb126862.x. [DOI] [PubMed] [Google Scholar]
- 9.Molyneux S, Geissler PW. Ethics and the ethnography of medical research in Africa. Soc Sci Med. 2008;67(5):685–95. doi: 10.1016/j.socscimed.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


