Abstract
BACKGROUND:
Platelet-activating factor acetylhydrolase (PAF-AH) is a circulating enzyme that has an important role in the development of coronary artery disease (CAD). The correlations between PAF-AH and CAD are controversial. Furthermore, the differences of the enzyme levels between patients with stable and unstable CAD are not fully determined. The purpose of this study was to evaluate plasma PAF-AH levels and its association with the presence of CAD and some clinical risk factors in the patients.
METHODS:
This case-control study included 50 control subjects without CAD, 50 stable CAD patients and 50 unstable CAD patients with angiographically documented CAD. Plasma PAF-AH activity was determined by a commercial kit. The inflammatory markers, high sensitivity C-reactive protein (hsCRP) and oxidized low density lipoprotein (ox-LDL), and lipid profile were also measured. Comparisons of biochemical risk factors among all groups were performed by one way ANOVA. The association of PAF-AH activity with the presence of CAD was analyzed by multiple logistic regression.
RESULTS:
Plasma PAF-AH activity levels were higher in unstable CAD patients (0.040 ± 0.012 μmol/min/ml) than in stable CAD patients (0.032 ± 0.010 μmol/min/ml) and control subjects (0.026 ± 0.009 μmol/min/ml) (p < 0.01). Plasma PAF-AH activity was also independently associated with the presence of CAD (p< 0.01).
CONCLUSIONS:
Plasma PAF-AH activity levels were highly increased in unstable and stable CAD patients as compared to control subjects and may be a useful biomarker for CAD prediction.
KEYWORDS: Platelet-Activating Factor Acetylhydrolase, Stable Coronary Artery Disease, Unstable Coronary Artery Disease
Cardiovascular disease has a high mortality and morbidity in most developing populations in the Middle East such as Iran.1 Therefore, evaluation of cardiovascular biomarkers in diagnosis and pathogenesis of CAD is a matter of priority.
Inflammation is an important factor in the development of atherosclerosis and its seque-lae.2 Lipoprotein-associated phospholipase A2 (Lp-PLA2), also known as platelet-activating factor acetylhydrolase (PAF-AH), is a new inflammatory enzyme that produces some proinflammatory mediators.3 Plasma PAF-AH is associated predominately with LDL particles and to a lesser extent to other lipoproteins.4 PAF-AH has been proposed as a useful biomarker for patients at high risk of cardiovascular disease5 and its plasma levels are associated with higher plaque burden in coronary arteries6 and may also be associated with plaque instability and rupture.7 Furthermore, PAF-AH may improve the identification of individuals at high risk for cardiovascular disease, independent of other risk factor such as cholesterol, or high sensitivity C-reactive protein (hsCRP) levels.8 Elevation of plasma PAF-AH activity may be used as a clinical biomarker for stratification and evaluation of patients with stable coronary disease.9,10 On the other hand, the results of some studies demonstrated that PAFAH is not a marker of acute cardiovascular events but may rather exhibit an increased risk of progression to coronary instability.11,12 Therefore, it is not clear that PAF-AH is simply a risk biomarker or directly promotes athero-sclerosis.13
The elevation of PAF-AH activity in stable and unstable CAD patients is not fully characterized. Also, the association of plasma PAFAH activity with the presence of CAD need further evaluation.
The purpose of the study was first to assess whether plasma PAF-AH activity was increased in patients with stable and unstable CAD patients compared with control subjects. In addition, we sought to assess the relationship of plasma PAF-AH activity with the presence of CAD along with certain inflammatory markers such as ox-LDL, high sensitivity Creactive protein (hsCRP), and conventional clinical risk factors in the patients, given the existence of mechanistic relationships between them in the atherosclerosis pathogenesis.
Methods
For this case-control study , from subjects who had consecutively undergone coronary angiography because of clinical manifestations of angina or suspected changes on electrocardiography, one hundred fifty subjects including 50 stable CAD patients, 50 unstable CAD patients and 50 control subjects were enrolled in the study at Isfahan Cardiovascular Research Center in Iran (ICRC, a WHO collaborating center). The patient's angiograms were assessed by two experienced cardiologists who were unaware of the participated patients. All CAD patients had a coronary obstruction ≥ 50% in at least one of the main coronary arteries. Unstable CAD patients had a random and unpredictable angina at rest or sleep, while stable patients had a chest pain during physical exertion that relieved by rest and the pattern or frequency of their chest pain duration was constant. In normal subjects coronary artery obstructions were less than 10%. In addition, they did not have any history and clinical symptoms of cardiovascular diseases. All subjects were randomly allocated to one of the three groups based on a simple randomization method. The subjects and researchers blinded to group assignment until all data were determined. Therefore, the study was conducted in a double-blind fashion. The extent of stenosis for 15 segments of the main coronary arteries was determined according to the method of Miller et al.14
The information about conventional clinical risk factors, medication and other necessary data were obtained through standardized questionnaire after admission.
Patients with recent (within 6 month) myocardial infarction or cardiovascular events, surgery (within 3 month), cancer and infective or inflammatory diseases were not included in the study. Patients with myocardial infarction identified based on high levels of creatine kinase MB and troponin T were not entered in the study. Within one week after angiography, patients with any other cardiovascular events or treatments that could interfere with PAFAH activity were not included in the study. Before enrolment, written informed consent was obtained from all the subjects and approval of the study was provided by the Ethics Committee of Isfahan University of Medical Sciences and Isfahan Cardiovascular Research Center.
One week after angiography, 12 hours fasting blood samples were collected into Vacutainer EDTA-tubes (BD Vacutainer®, Brocken Bow, NE USA), centrifuged and stored at -80°C until analyses were performed. Plasma PAFAH activity was assayed using a colorimetric kit based on the manufacturer's instructions (Cayman Chemical, 0.02μmol/min/ml sensitivity). The intra- and interassay variation coefficient of the assay were 3.5% and 10%, respectively. Ox-LDL was measured with an ELISA kit (Immundiagnostik AG, Bensheim, Germany). Plasma levels of hsCRP were measured with a high sensitive latex-enhanced immunoturbidimetric assay (Randox laboratory Ltd, Belfast, United Kingdom). Plasma total lipoprotein profile (total cholesterol, triglycerides, LDL-cholesterol, and HDL-cholesterol) were determined using enzymatic test kits (Pars-Azmun, Iran).
All statistical analyses were performed with the SPSS statistical software version 15.0 (SPSS Inc., Chicago, IL, USA). Normal distribution of variables was examined by the Kolmogorov-Smirnov test. Comparison of biochemical data among CAD patients and control groups were examined by one-way ANOVA and Bonferroni post-hoc test. Multiple logistic regression analysis was performed to determine the relation of plasma PAF-AH activity levels with the presence of CAD. Data was expressed as mean ± standard deviations or counts and percentages. P value < 0.05 was considered statistically significant.
Results
The clinical characteristics and biochemical data of the patients are summarized in Table 1. Patients and control subjects were age and gender matched. Cardiovascular risk factors are seen predominantly in patients. Control subjects almost were not taking medications. Inflammatory markers such as hsCRP and ox-LDL were significantly higher in CAD patients than in control subjects, being also higher in unstable CAD patients than in stable CAD patients. Lipid profile was different among all three groups although these differences were not significant except for LDL that was high significantly in unstable CAD patients in comparison with control subjects (p = 0.009).
Table 1.
Clinical characteristic of the study participants
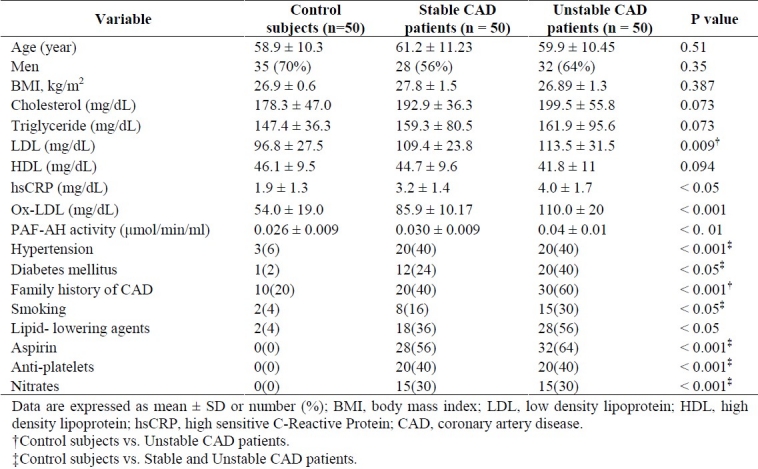
As shown in Table 1, plasma PAF-AH activity levels were significantly higher in unstable CAD patients (0.040 ± 0.012 μmol/min/ml) than in stable CAD patients (0.032 ± 0.010 μmol/min/ml) and control subjects (0.026 ± 0.009 μmol/min/ml), and also in stable CAD patients than in control subjects (p < 0.01).
The CAD patients exhibited a trend towards higher levels of cholesterol, triglyceride, hsCRP and lower levels of HDL compared with the control subjects, but these differences were not statistically significant except for hsCRP (p < 0.05) and LDL(only in unstable CAD patients, p = 0.009).
We did not find a significant difference in plasma PAF-AH activity levels between patients with and without drug therapy.
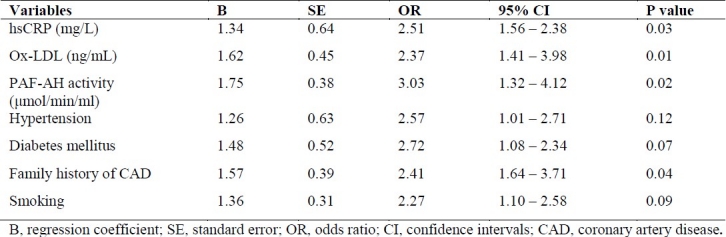
To evaluate the independent association of plasma PAF-AH activity levels with the presence of CAD, a multiple logistic regression analysis was performed adjusting for some of the most important risk factors such as ox-LDL, hsCRP, hypertension, diabetes mellitus, smoking and family history of CAD. As shown in Table 2, plasma PAF-AH activity levels were positively correlated with CAD (OR = 3.03, p = 0.02). Other predictors for the presence of CAD were hsCRP (OR = 2.51, p = 0.03), ox-LDL (OR = 2.34, p = 0.01), and family history of CAD (OR = 2.41, p = 0.04).
Table 2.
Multiple logistic regression analysis for the presence of CAD in patients
Discussion
The findings of the study demonstrated that PAF-AH activity levels were increased in patients with stable and unstable CAD as compared with control subjects and could identify the patients. Also, the study demonstrated that plasma PAF-AH activity levels were positively associated with the presence of CAD.
In plasma, about 80% of PAF-AH is attached to LDL, and the remaining 20% is bound to other lipoproteins.15 Although the exact role of PAF-AH in atherogenic process is incomplete,16 its plasma level has been proposed as a potent and independent marker of cardiovascular disease.17 In fact, the relationship between PAF-AH levels and the risk of cardiovascular disease is inconsistent. Some studies have demonstrated beneficial effects of the enzyme18–20 but others have proposed an opposite view.21,22 The reason of these controversies may be the small sample size, the characteristics of the population studied, and the underlying effects of hormonal therapy, which has been correlated with lower levels of PAFAH in the former studies.
In the present study, plasma PAF-AH activity levels were higher in unstable CAD patients than in stable CAD patients, being also higher in stable CAD patients than in control subjects (Figure 1). These findings are in accordance with the deleterious effects of PAFAH on CAD. On the other hand, the independent correlation between plasma PAF-AH activity levels and CAD in the present study further corroborated the possible antiatherogenic effects of the enzyme.
PAF-AH activity levels are also associated with plaque instability, a characteristic of unstable CAD.23,24 The higher levels of PAFAH activity in unstable CAD patients than in stable CAD patients in our study are in agreement with this finding.
We did not find a significant association between PAF-AH activity levels and plasma lipid profile. This reflected that the atherogenic effects of PAF-AH, in part, may be related to its inflammatory properties.
In multiple logistic regression analysis adjusting for some important cardiovascular risk factors, PAF-AH was an independent positive predictor of the presence of CAD in the present study. In this regard, again our results are in line of the findings of other studies.25,26
Some limitations of the study should be mentioned. From the findings, it could not be determined the causal association between PAFAH and CAD although some evidence have been addressed. Another limitation was sample size, however all variables had a normal distribution.
The findings of the study exhibited that plasma PAF-AH activity levels were increased in unstable and stable CAD patients compared with control subjects. Increases in PAF-AH activity levels may be helpful in identification of stable from unstable CAD patients. The correlation between PAF-AH activity levels and ox-LDL as well as hsCRP indirectly corroborated the atherogenic effects of PAF-AH in the cardiovascular diseases and therefore, elucidated some informative evidence with respect to the mechanisms of atherosclerosis progression in vivo that may be implicated in the disease assessment.
Authors’ Contributions
SS directed the study. GB provided the design of the study, coordinated and carried out all the experiments and prepared the manuscript. AM and MP provided assistance for some experiments. NS participated in clinical identification of CAD patients and control subjects. All authors have read and approved the content of the manuscript.
Acknowledgments
This study was funded by grant number 388417 for PhD thesis from Isfahan University of Medical Sciences, Isfahan, Iran. We profoundly appreciate Isfahan Cardiovascular Research Centre laboratory personals for their assistances in patients’ management.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Hadaegh F, Harati H, Ghanbarian A, Azizi F. Prevalence of coronary heart disease among Tehran adults: Tehran Lipid and Glucose Study. East Mediterr Health J. 2009;15(1):157–66. [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25(5):923–31. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 4.Karabina SA, Liapikos TA, Grekas G, Goudevenos J, Tselepis AD. Distribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochim Biophys Acta. 1994;1213(1):34–8. doi: 10.1016/0005-2760(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 5.Madjid M, Ali M, Willerson JT. Lipoprotein-associated phospholipase A2 as a novel risk marker for cardiovascular disease: a systematic review of the literature. Tex Heart Inst J. 2010;37(1):25–39. [PMC free article] [PubMed] [Google Scholar]
- 6.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19(12):2909–17. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 7.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(11):2523–9. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 8.Khakpour H, Frishman WH. Lipoprotein-associated phospholipase A2: an independent predictor of cardiovascular risk and a novel target for immunomodulation therapy. Cardiol Rev. 2009;17(5):222–9. doi: 10.1097/CRD.0b013e3181b2434e. [DOI] [PubMed] [Google Scholar]
- 9.Robins SJ, Collins D, Nelson JJ, Bloomfield HE, Asztalos BF. Cardiovascular events with increased lipoprotein-associated phospholipase A2 and low high-density lipoprotein-cholesterol: the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2008;28(6):1172–8. doi: 10.1161/ATVBAHA.107.160739. [DOI] [PubMed] [Google Scholar]
- 10.Tanaseanu C, Moldoveanu E, Kosaka T, Tanaseanu S, Neagu M, Popescu LM. The significance of human platelet-activating factor-acetylhydrolase in patients with chronic stable angina. Eur J Intern Med. 2004;15(5):291–7. doi: 10.1016/j.ejim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Oldgren J, James SK, Siegbahn A, Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events inacute coronary syndrome patients. EurHeart J. 2007;28(6):699–704. doi: 10.1093/eurheartj/ehl565. [DOI] [PubMed] [Google Scholar]
- 12.O’Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation. 2006;113(14):1745–52. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 13.Blankenberg S, Stengel D, Rupprecht HJ, Bickel C, Meyer J, Cambien F, et al. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res. 2003;44(7):1381–6. doi: 10.1194/jlr.M300086-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Miller M, Mead LA, Kwiterovich PO, Jr, Pearson TA. Dyslipidemias with desirable plasma total cholesterol levels and angiographically demonstrated coronary artery disease. Am J Cardiol. 1990;65(1):1–5. doi: 10.1016/0002-9149(90)90017-u. [DOI] [PubMed] [Google Scholar]
- 15.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150(2):413–9. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 16.Bhatti S, Hakeem A, Cilingiroglu M. Lp-PLA2 as a marker of cardiovascular diseases. Curr Atheroscler Rep. 2010;12(2):140–4. doi: 10.1007/s11883-010-0095-6. [DOI] [PubMed] [Google Scholar]
- 17.Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82(2):159–65. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Ichihara S, Fujimura T, Yokota M. Identification of the G994--> T missense in exon 9 of the plasma platelet-activating factor acetylhydrolase gene as an independent risk factor for coronary artery disease in Japanese men. Metabolism. 1998;47(2):177–81. doi: 10.1016/s0026-0495(98)90216-5. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Yoshida H, Ichihara S, Imaizumi T, Satoh K, Yokota M. Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Atherosclerosis. 2000;150(1):209–16. doi: 10.1016/s0021-9150(99)00385-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto I, Fujitsu J, Nohnen S, Igarashi T, Motomura T, Inaba M, et al. Association of plasma PAF acetylhydrolase gene polymorphism with IMT of carotid arteries in Japanese type 2 diabetic patients. Diabetes Res Clin Pract. 2003;59(3):219–24. doi: 10.1016/s0168-8227(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter KL, Dennis IF, Challis IR, Osborn DP, Macphee CH, Leake DS, et al. Inhibition of lipoprotein-associated phospholipase A2 diminishes the death-inducing effects of oxidised LDL on human monocyte-macrophages. FEBS Lett. 2001;505(3):357–63. doi: 10.1016/s0014-5793(01)02840-x. [DOI] [PubMed] [Google Scholar]
- 22.Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn. 2002;2(1):17–22. doi: 10.1586/14737159.2.1.17. [DOI] [PubMed] [Google Scholar]
- 23.Toth PP, McCullough PA, Wegner MS, Colley KJ. Lipoprotein-associated phospholipase A2: role in atherosclerosis and utility as a cardiovascular biomarker. Expert Rev Cardiovasc Ther. 2010;8(3):425–38. doi: 10.1586/erc.10.18. [DOI] [PubMed] [Google Scholar]
- 24.White H. Darapladib and its potential for plaque stabilization and prevention of cardiac events. Clinical Lipidology. 2010;5(4):465–76. [Google Scholar]
- 25.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110(14):1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]


