Abstract
Context:
Preseptal cellulitis is the commonest orbital disease which frequently needs to be differentiated from orbital cellulitis. Prompt diagnosis and treatment with appropriate antibiotics can prevent vision loss and life-threatening complications of orbital cellulitis.
Aims:
To describe the clinical profile of cases with preseptal and orbital cellulitis admitted to a tertiary care hospital during a period of nine years. The causative organisms and the clinical outcome were analyzed.
Settings and Design:
Retrospective descriptive case study done in a tertiary care hospital in South India.
Material and Methods:
The in-patient records of patients with preseptal and orbital cellulitis were reviewed from 1998 to 2006. The factors reviewed included ocular findings aiding in the distinction of the two clinical conditions, the duration of symptoms, the duration of hospital stay, microbiological culture report of pus or wound swab, blood culture, drugs used for treatment, the response to therapy and complications.
Statistical Analysis Used:
Descriptive analysis.
Results:
One hundred and ten cases, 77 patients with preseptal cellulitis and 33 patients with orbital cellulitis were reviewed. Five percent of children and 21% of adults presented with cutaneous anthrax contributing to preseptal cellulitis. Thirty-nine percent cases with orbital cellulitis were caused by methicillin-resistant Staphylococcus aureus (MRSA).
Conclusions:
This study has helped in identifying organisms which cause orbital infections, especially community-acquired MRSA. It indicates the need for modifying our empirical antimicrobial therapy, especially in orbital cellulitis.
Keywords: Anthrax, community-acquired methicillin-resistant Staphyloccus aureus, orbital cellulitis, preseptal cellulitis
Orbital cellulitis can be classified as preseptal and post-septal cellulitis based on the anatomic landmark, the orbital septum. The septum forms a barrier, preventing the spread of superficial infection into the deeper orbit. Orbital infection limited anterior to the septum is called preseptal cellulitis and that posterior to the septum is termed as post-septal or orbital cellulitis. Clinical distinction between the two is important as the ocular morbidity and prognosis differs.
Preseptal cellulitis is characterized by lid edema, warmth, erythema and tenderness. The distinctive features of orbital cellulitis are proptosis and limitation of ocular movements.[1] Additional useful signs are chemosis of bulbar conjunctiva, reduced visual acuity, afferent pupillary defect and toxic systemic symptoms. Prompt diagnosis and treatment of orbital cellulitis is vital as it is associated with serious complications like cavernous venous thrombosis, visual loss, meningitis, brain abscess and sepsis.[1,2]
In this study, we reviewed the in-patient records of patients with preseptal and orbital cellulitis over nine years in a tertiary care hospital. The clinical findings, causative organism, management and complications of the two conditions are illustrated.
Materials and Methods
The in-patient records of patients with preseptal and orbital cellulitis were reviewed from 1998 to 2006. The clinical details of the patients were noted and analyzed. Subjects of age 13 years or below were considered to belong to the pediatric age group. Patients were classified as having preseptal or orbital cellulitis based on the clinical finding. Presence of lid edema, restricted ocular movements, proptosis, loss of vision and relative afferent pupillary defect were looked for. Orbital cellulitis was diagnosed in the presence of any three of the above five clinical findings. Preseptal cellulitis was diagnosed when a patient had lid edema with warmth and tenderness with no additional ocular findings.
The factors reviewed in the study included ocular findings aiding in the distinction of the two clinical conditions, the duration of symptoms at the time of presentation, the duration of hospital stay, microbiological culture report of pus or wound swab, blood culture, drugs used for treatment, response to therapy and complications. Other parameters studied were general physical examination, systemic blood counts and temperature. Wound swab was taken either from the site of infection or ulceration or conjunctival sac. This was immediately sent for culture and sensitivity. Pus was examined by Gram's staining, KOH mount and cultured on blood agar, chocolate agar and Sabouraud's dextrose agar. Radiological investigations like X-ray or computed tomography (CT) orbit and paranasal sinuses were taken in all cases of or suspected orbital cellulitis. Both clinical improvement and improvement in vision were considered in outcome measures.
Results
One hundred and ten patients with preseptal and orbital cellulitis were identified. Seventy-seven patients had preseptal cellulitis and 33 patients had orbital cellulitis [Table 1]. It was noted that all cases with suspected orbital cellulitis and cases of preseptal cellulitis in the pediatric age group were admitted. Adult patients with preseptal cellulitis were admitted if there was tense swelling of the lids with inability to open the lids, lid abscess, systemic toxemia or poor response to therapy with oral antibiotics.
Table 1.
Summary of results
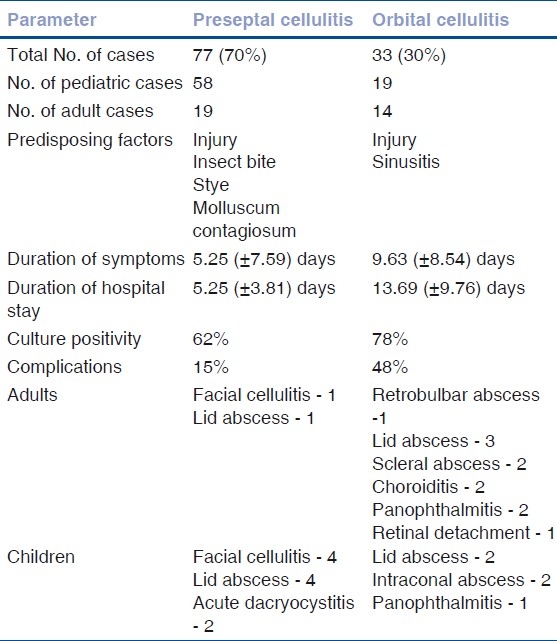
Among patients with preseptal cellulitis, 75% (n=58) were children while adults accounted for 24% (n=19) of cases. The mean age was 3.62 years and 34.2 years in the pediatric and adult group, respectively. Sex distribution was equal in adults with male preponderance in children. In patients with orbital cellulitis, 57% (n=19) were adults while children accounted for 42% (n=14) of cases. The mean age was 4 years and 45 years in the pediatric and adult group, respectively. Sex distribution was equal in children with male preponderance in adults.
An important factor predisposing to both clinical entities was injury, 21% in preseptal cellulitis and 24% in orbital cellulitis. In children, additional predisposing factors noted were insect bite (10%), hordeolum and molluscum contagiosum of the lid with secondary bacterial infection. Among adults, since most of them were laborers, injury with stick and thorn while at work was the predisposing factor in nine cases and it was sinusitis in five patients. One patient had fungal pansinusitis.
The average duration of symptoms for patients with preseptal cellulitis was 4.05 (±2.54) days in the adult group and 5.96 (±11.04) days in the pediatric group. The average duration of hospital stay was 5.25 (±3.81) days. The majority of them, 89% (n=69), were treated in 10 days or less while 10% (n=8) cases were hospitalized for a longer duration. Visual acuity at presentation was better than 20/60 in 14 patients (74%) in the adult age group.
In those with orbital cellulitis, the average duration of symptoms was 8.89 (±8.02) days in the adult group and 10.64 (±9.41) days in the pediatric group. Patients who presented late and those with associated sinusitis had increased ocular morbidity. The average hospital stay was 13.69 (±9.76) days. Nineteen patients had a prolonged hospital stay of more than 10 days. Visual acuity at presentation was less than 20/60 in 15 adult patients. Two cases however had visual acuity of 20/20. Three patients could not perceive light at presentation.
Blood was cultured only in patients with suspected septicemia; 17 patients with preseptal cellulitis and all cases with orbital cellulitis. There was no growth in the former group, while organisms (Klebsiella pneumonia and Staphylococcus aureus) were isolated in two children with orbital cellulitis.
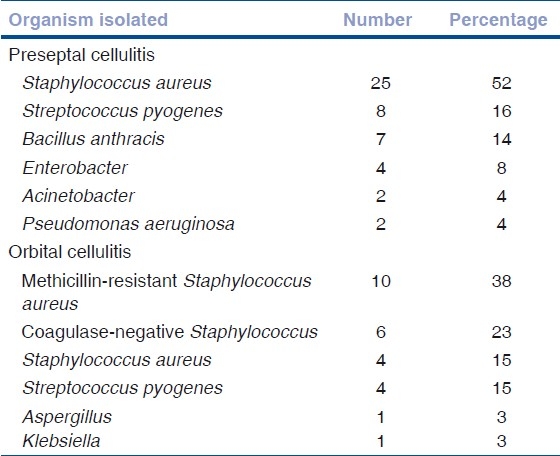
Among the culture-positive patients, Staphylococcus aureus was the most common organism isolated in both groups. Wound swab culture was positive in 78% cases (n=26) of orbital cellulitis. Methicillin-resistant Staphylococcus aureus (MRSA) was cultured from 38% cases (10 cases, four children and six adults) followed by coagulase-negative staphylococcus (23%) and Staphylococcus aureus (15%). The other organisms isolated were Streptococcus pyogenes, Klebsiella and Aspergillus [Table 2]. Conjunctival and wound swab cultures showed no growth in 37% patients of preseptal cellulitis and 21% of orbital celllulitis.
Table 2.
Organisms isolated
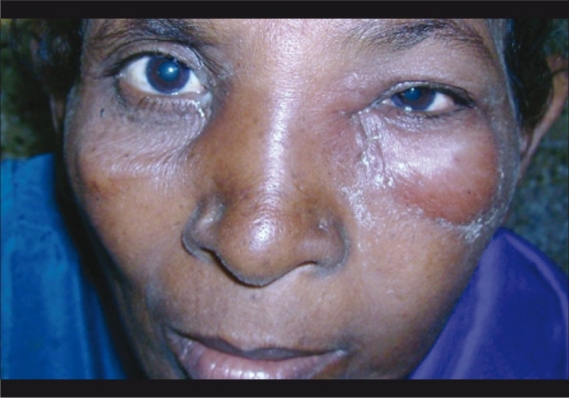
Three children and four adults presented with cutaneous anthrax contributing to preseptal cellulitis [Fig. 1]. None of the patients with orbital cellulitis had such lesions.
Figure 1.
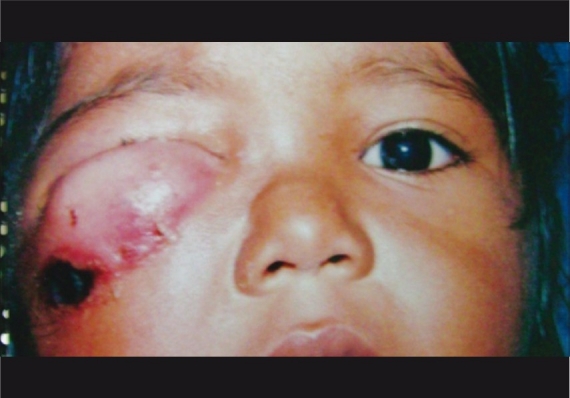
Cutaneous anthrax causing preseptal cellulitis in a child
In patients with preseptal cellulitis, radiological investigations were done only in whom there was a suspicion of spreading cellulitis, to rule out orbital cellulitis and sinusitis. No abnormality was detected in patients in whom these investigations were performed.
Investigations such as CT scan, ultrasonography (USG) orbit [Fig. 2] and X-ray orbit were done in patients with orbital cellulitis. X-ray orbit was done in all patients to rule out associated sinusitis. CT scan was done in patients with severe proptosis and in whom panophthalmitis or cavernous venous thrombosis was suspected. CT scan was done in 20 patients with orbital cellulitis and six patients with preseptal cellulitis. Evidence of haziness of one or more sinuses associated with orbital cellulitis was present in plain paranasal sinus (PNS) roentgenograms and CT scans of 15% patients. While sinusitis was the most common radiological finding in the adult group, lid abscess, intraconal abscess and panophthalmitis were the findings seen on radio-imaging in the pediatric group. Subperiosteal abscess was not reported among our patients who underwent CT imaging.
Figure 2.
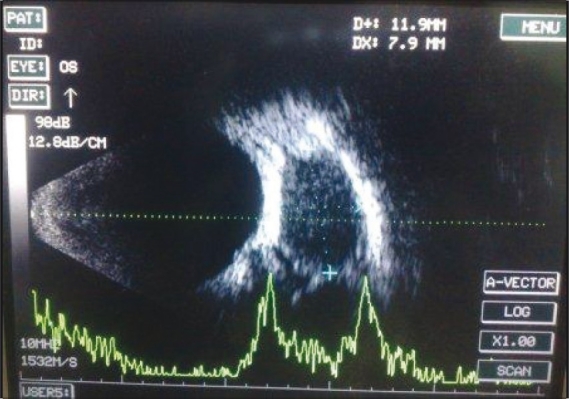
Ultrasound B-scan image of orbital abscess. Well-defi ned encapsulated lesion measuring 11.9 ×7.9 mm fi lled with low echogenic content
All patients were treated with parenteral antibiotics. Crystalline penicillin and gentamicin were the most frequently used antibiotics in both groups of patients. Other antibiotics were substituted or added for some patients based on the culture sensitivity reports and in whom response was poor even after four days to one week of therapy [Table 3]. Surgical treatment in the form of incision and drainage of abscess was done in patients with lid or orbital abscess [Fig. 3].
Table 3.
Drugs used for treatment
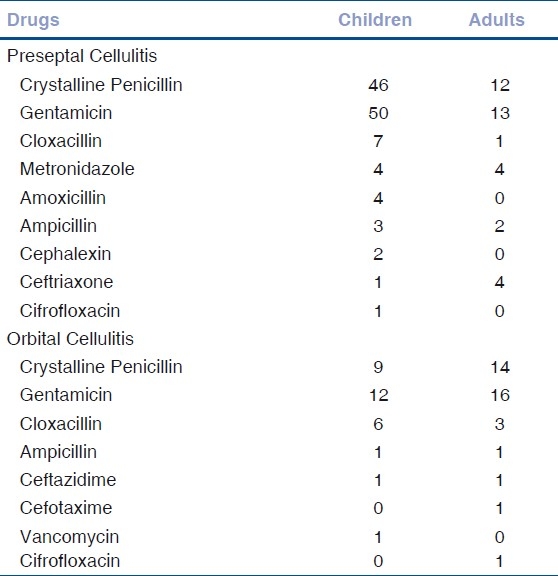
Figure 3.
Orbital cellulitis with orbital abscess with multiple discharging sinuses
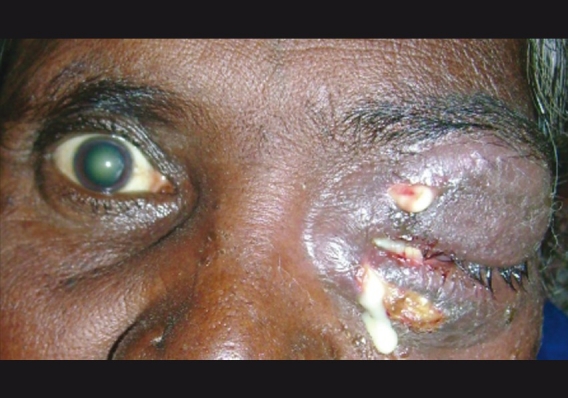
In preseptal cellulitis patients, associated complications in the form of facial cellulitis [Fig. 4] and lid abscess and acute dacryocystitis were seen in 10 children and two adults (15.58% cases). In children two cases each of acute dacryocystitis, lid abscess and four cases of facial cellulitis were noted. Complications were more frequent in the orbital group (48%), in adults, in the form of retrobulbar abscess, lid and scleral abscess, choroiditis, panophthalmitis, papillitis and retinal detachment. Children with orbital cellulitis had lid abscess, intraconal abscess and panophthalmitis as associated complications.
Figure 4.
Preseptal cellulitis with spreading facial cellulitis
A majority of patients in the preseptal group showed clinical improvement with treatment. At initial presentation itself, visual acuity remained unaffected in most of these patients. In the orbital group, improved outcome, either clinical or visual was seen in 60% (n=20) cases. Adults had slightly better outcome; 63% improved, while in children, improvement was seen only in 57% cases. The causes for poor outcome in cases with orbital cellulitis were panophthalmitis (n=2) [Fig. 5], perforated scleral abscess (n=1), phthisis bulbi (n=2), choroiditis (n=2), orbital abscess (n=4) and retinal detachment (n=1). The first three patients were treated with evisceration. Six patients lost vision due to these complications of orbital cellulitis.
Figure 5.
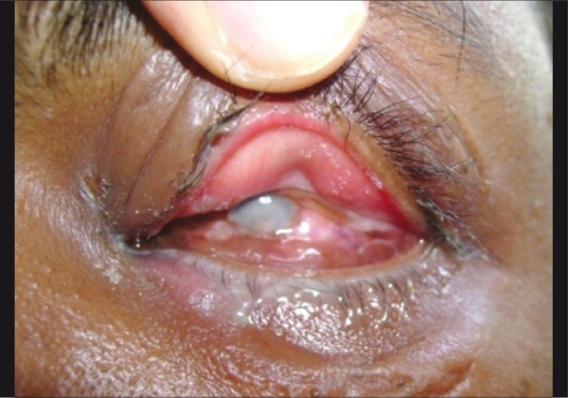
Panophthalmitis secondary to orbital cellulitis
Discussion
Amongst the cases of orbital cellulitis, preseptal celullitis constituted 70% and postseptal cellulitis 30%. Children constituted the majority of cases with preseptal cellulitis while the more serious orbital cellulitis was more frequently seen in the adult population. Staphylococci followed by streptococci were the leading causative organisms in our series which is similar to other previous reports.[1,3] Thirty-eight percent cases with orbital cellulitis were caused by MRSA. But none of these patients had recent hospitalization implying that the infection was community-acquired. Another study has shown that community-acquired (CA)-MRSA is emerging as a common cause of preseptal cellulitis.[4] The prevalence of CA-MRSA infection is increasing worldwide over the last two decades.[5,6] Though CA-MRSA is more virulent than hospital-acquired MRSA, due to the secretion of cytotoxin Panton-Valentine Leucocidin (PVL), it is more susceptible to non-β-lactam antibiotics like tetracyclines, trimethoprim/ sulfamethoxazole, fluoroquinolones and clindamycin.[5–7] For potentially severe clinical forms, linezolid, tigecycline, daptomycin, teociplamine and vancomycin are effective. Guidelines suggest the use of non-β-lactam antibiotics in places where the prevalence of CA-MRSA exceeds 15%, though in many patients with CA-MRSA infection, β-lactam antibiotics continue to be prescribed.[1] In a study done in Taiwan, most of the soft-tissue infections caused by CA-MRSA resolved irrespective of the antimicrobial sensitivity pattern of the organism.[8] A drug which may not be effective against an organism in vitro can be efficient in vivo by eliminating the susceptible subpopulation of the bacteria and helping the patient's immune system to overcome the remaining infection.[8]
Hemophilus influenzae has been one of the most common organisms causing orbital cellulitis in the pediatric age group prior to 1990.[9,10] There has been a decline in the number of invasive diseases and periocular infections caused by this pathogen.[9,10] The reduction in the number of cases of orbital cellulitis caused by it cannot be solely attributed to the introduction of HiB vaccination as non-typable Hemophilus influenzae is the common cause of sinusitis.[9,10] Introduction of better antibiotics, use of antibiotics prior to hospitalization, herd immunity and the cyclical nature of the disease would have led to its downfall.[9,10]
Bacillus anthracis was isolated from seven cases with preseptal cellulitis Fig. 4. The significance is that anthrax of the eyelid can lead to cicatrisation and ectropion.[11–13] In our series all patients responded well to intravenous penicillin and did not develop complications.
In older series, CT helped in the diagnosis when clinical features were not yet marked, aided in localizing the pathology to the anatomical spaces in the orbit and ruling out any associated sinusitis.[2,14] In our series, CT scan helped to diagnose intraconal and extraconal orbital abscess in two patients and to diagnose panophthalmitis in two cases with orbital cellulitis.
All patients with preseptal cellulitis resolved without any sequelae. Ocular complications occurred in the orbital group. Six patients lost vision due to orbital cellulitis.
All cases were empirically treated with intravenous penicillin and gentamicin to cover both Gram-positive and -negative organisms. Cephalosporins, vancomycin and other antibiotics were given based on the sensitivity pattern or if there was no clinical improvement with empirical therapy. The sixteen patients with orbital cellulitis and 16 patients with preseptal cellulitis didn’t respond to empirical antibiotics and required higher generation antibiotics. This may be due to the change in the sensitivity pattern of the organisms.
Since almost 50% of our patients with orbital cellulitis required a switch in the empirical antibiotics (penicillin and gentamicin) used, we recommend the combination of cloxacillin and third-generation cephalosporin or a fluroquinolone as the empiric therapy for the management of orbital cellulitis in our population. Larger studies are required to resolve the role of ancillary antimicrobial agents in CA-MRSA infections.
Based on this nine-year review, it can be concluded that preseptal cellulitis remains the commonest among orbital infections, of which Staphylococci and Streptococci are the most common causative organisms. Community-acquired MRSA is often implicated in orbital cellulitis, which is associated with more ocular morbidity and prolonged hospital stay. It indicates the need for modifying our empirical antimicrobial therapy, especially in orbital cellulitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: A 10-year review of hospitalised patients. J Chin Med Assoc. 2006;69:415–22. doi: 10.1016/S1726-4901(09)70284-9. [DOI] [PubMed] [Google Scholar]
- 2.Bergin DJ, Wright JE. Orbital cellulitis. Br J Ophthalmol. 1986;70:174–8. doi: 10.1136/bjo.70.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodges E, Tabbara KF. Orbital cellulitis: Review of 23 cases from Saudi Arabia. Br J Ophthalmol. 1989;73:205–208. doi: 10.1136/bjo.73.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit. Trans Am Ophthalmol Soc. 2006;104:322–45. [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho KS, Mamizuka EM, Filho PPG. Methicillin/ oxacillin resistant Staphylococcus aureus as a hospital and public health threat in Brazil. Braz J Infect Dis. 2010;14:71–6. doi: 10.1590/s1413-86702010000100014. [DOI] [PubMed] [Google Scholar]
- 6.Milstone AM, Canoll KC, Ross T, Shangraw KA, Perl TM. Community associated methicillin resistant Staphylococcus aureus strains in paediatric intensive care unit. Emerging Infect Dis. 2010;16:647–55. doi: 10.3201/eid1604.090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelatti LC, Bonamijo RR, Becker AP, d’Azevedo PA. Methicillin resistant Staphylococcus aureus: Emerging community dissemination. An Bras Dermatol. 2009;84:501–6. doi: 10.1590/s0365-05962009000500009. [DOI] [PubMed] [Google Scholar]
- 8.Teng CS, Lo WT, Wang SR, Tseng MH, Chu ML, Wang CC. The role of antimicrobial therapy for treatment of uncomplicated skin and soft tissue infection from community associated methicillin resistant Staphylococcus aureus in children. J Microbiol Immunol Infect. 2009;42:324–8. [PubMed] [Google Scholar]
- 9.Ambati BK, Ambati J, Azar N, Stratton L, Schmidt EV. Periorbital and orbital cellulitis before and after the advent of haemophilus influenzae type B vaccination. Ophthalmology. 2000;107:1450–3. doi: 10.1016/s0161-6420(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 10.Donahue SP, Schwartz G. Preseptal and orbital cellulitis in childhood: A changing microbiological spectrum. Ophthalmology. 1998;105:1902–6. doi: 10.1016/S0161-6420(98)91038-7. [DOI] [PubMed] [Google Scholar]
- 11.Thappa DM, Karthikeyan K, Rao VA. Cutaneous anthrax of the eyelid. Indian J Dermatol Venereol Leprol. 2003;69:55. [PubMed] [Google Scholar]
- 12.Soysal HG, Kirath H, Recep OF. Anthrax as the cause of preseptal cellulitis and cicatricial ectropion. Acta Ophthalmol Scand. 2001;79:208–9. doi: 10.1034/j.1600-0420.2001.079002208.x. [DOI] [PubMed] [Google Scholar]
- 13.Amraoui A, Tabbara KF, Zaghloul K. Anthrax of the eyelids. Br J Ophthalmol. 1992;76:753–4. doi: 10.1136/bjo.76.12.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman RA, Bilaniuk LT. CT of orbital infection and its cerebral complications. AJR Am J Roentgenol. 1980;134:45–50. doi: 10.2214/ajr.134.1.45. [DOI] [PubMed] [Google Scholar]


