Abstract
Purpose:
To evaluate the ability of spectral domain optical coherence tomography (OCT) peripapillary retinal nerve fiber layer thickness (RNFLT) parameters to distinguish normal eyes from those with early glaucoma in Asian Indian eyes.
Design:
Observational cross-sectional study.
Materials and Methods:
One hundred and seventy eight eyes (83 glaucoma patients and 95 age matched healthy subjects) of subjects more than 40 years of age were included in the study. All subjects underwent RNFLT measurement with spectral OCT/ scanning laser ophthalmoscope (SLO) after dilatation. Sensitivity, specificity and area under the receiving operating characteristic curve (AROC) were calculated for various OCT peripapillary RNFL parameters.
Results:
The mean RNFLT in healthy subjects and patients with early glaucoma were 105.7 ± 5.1 μm and 90.7 ± 7.5 μm, respectively. The largest AROC was found for 12 o’clock- hour (0.98), average (0.96) and superior quadrant RNFLT (0.9). When target specificity was set at ≥ 90% and ≥ 80%, the parameters with highest sensitivity were 12 o’clock -hour (91.6%), average RNFLT (85.3%) and 12 o’ clock- hour (96.8 %), average RNFLT (94.7%) respectively.
Conclusion:
Our study showed good ability of spectral OCT/ SLO to differentiate normal eyes from patients with early glaucoma and hence it may serve as an useful adjunct for early diagnosis of glaucoma.
Keywords: Early glaucoma, retinal nerve fiber layer thickness, spectral optical coherence tomography / scanning laser ophthalmoscope
In glaucoma, structural damage to the optic nerve head (ONH) and retinal nerve fiber layer (RNFL) may precede functional loss.[1] Quantitative and objective structural measurements provided by optical coherence tomography (OCT) may help in detecting anatomic changes that precede functional decay. OCT is an optical imaging technique that provides high resolution and reproducible measurement of RNFL that discriminates normal from glaucomatous eyes.[2]
Previous studies using OCT 2000[3–5] and Stratus OCT[6,7] have shown that OCT RNFL thickness (RNFLT) parameters have good sensitivity and specificity for differentiating normal eyes from patients with early glaucoma.
The commercially available spectral OCT/ scanning laser ophthalmoscope (SLO) (OPKO/ OTI, Miami Florida, USA) is a combination of OCT and confocal SLO, designed to image retina for accurate, point-to-point registration and orientation. It uses low coherence interferometry to detect light echoes, relying on a spectrometer and high-speed camera and is based on the mathematical formula of fast Fourier transformation. This results in measurement of echoes of light simultaneously, as opposed to sequentially in case of time domain (TD) OCT, and hence there is significant reduction of motion artifact and an increase in signal-to-noise ratio as compared to TD-OCT. It has higher axial resolution of < 6 μm, transverse resolution of 20 μm and a scan velocity of 27,000 axial scans per second (user manual, spectral OCT/ SLO, international version, OPKO/OTI) when compared to TD OCT which has axial resolution of 8-10 μm and a scan speed of 400 axial scans per second and hence provides improved visualization of retinal morphologic features.
This study has been undertaken to evaluate the ability of spectral OCT/SLO peripapillary RNFLT parameters to distinguish normal eyes from those with early glaucoma in Asian Indian eyes.
Materials and Methods
This prospective cross-sectional study included 95 normal eyes of volunteers from the staff of the Institute and their relatives and 83 patients with early glaucoma who were recruited from patients attending glaucoma outpatient department of the Institute. After informed consent, participants who met the inclusion criteria were enrolled in the study. The methods described were adherent to the tenets of the Declaration of Helsinki. All subjects were Asian Indians.
Each participant underwent complete ophthalmic examination including review of medical history, best corrected visual acuity, slit lamp biomicroscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometry, gonioscopy using Sussman goniolens, dilated fundus and optic disc examination using +78 diopter (D) lens, standard automated perimetry (SAP) using 24-2 Swedish Interactive Threshold Algorithm (Carl Zeiss Meditec Inc., Dublin, California, USA) and ocular imaging with spectral OCT / SLO.
To be included, subjects had to have best corrected visual acuity of 20/30 or better, refraction within ± 4 D of sphere and ± 2 D of cylinder, clear ocular media (nuclear opalescence, nuclear color and cortical changes up to grade 3 [NO1-3, NC1-3, C1-3], no posterior subcapsular opacity on lens opacities classification system III)[8] and open angles on gonioscopy. Participants with family history of glaucoma, uveitis, corneal, retinal or macular pathology, neurological disease or abnormal disc appearance such as tilted disc or discs with peripapillary atrophy were excluded from the study. If both eyes met the inclusion criteria, one eye was randomly selected and subjected to analysis.
Normal eyes had IOP of 21 mmHg or less, no past history of raised IOP, healthy appearing optic disc and RNFL on clinical examination (i.e. no focal or diffuse rim thinning or notching, vertical cup disc ratio > 0.7, no cup disc asymmetry of > 0.2 between 2 eyes, optic disc hemorrhage or RNFL defect) and normal visual field [defined as a mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits and glaucoma hemifield test (GHT) within normal limits].
Patients were classified as having early glaucoma if they had IOP > 21 mmHg on more than two occasions, MD better than –6 dB (Hodapp-Parrish-Anderson grading scale of severity of visual field defect)[9] and glaucomatous optic neuropathy and visual field loss consistent with optic nerve damage. Glaucomatous visual field loss was defined as the presence of a cluster of three or more adjacent points on pattern deviation plot with a probability of occurring in fewer than 5% of the normal population in a single hemifield (P < 5%) with one or more points having a probability of occurring in < 1% of normal population (P < 1%) and GHT outside normal limits. Patients required at least two reliable visual field examinations which were defined as the one with < 33% false negatives and false positives and fixation losses < 20%.[10]
OCT technique
All participants underwent RNFL scanning with spectral OCT/SLO, after dilation with 1% tropicamide eye drop and the images were acquired by a single operator . The subjects were asked to look at internal fixation target and a circular scan with a circle diameter of 3.4 mm was centered around optic disc. The location of scan was observed on the SLO image to ensure proper positioning of scan in relation to the ONH and average of three consecutive OCT images of the RNFL were obtained. The RNFL analysis uses an automated OCT software algorithm to identify anterior and posterior margins of RNFL.
The following RNFL parameters were evaluated: average peripapillary RNFLT (360°), the four quadrant average RNFLT (superior, nasal, inferior and temporal) and 12 o’clock-hours RNFLT. The sectors were defined in clockwise order for the right eye and counter clockwise order for the left eye.
Criteria for determining scan quality were the following: signal strength > 7, a clear SLO image allowing optic disc and scan circle visibility prior to and during image acquisition, even and dense color saturation throughout all retinal layers with red color visible in the retinal pigment epithelium and RNFL and a continuous scan pattern without missing or blank areas (i.e. no algorithm failure).
Statistical analysis
Statistical analysis was performed using SPSS software version 15 (SPSS Inc, Chicago, IL). Student's t test was used to evaluate differences between healthy and glaucomatous eyes. Receiver operating characteristic (ROC) curves were used to describe the ability of spectral OCT/ SLO to differentiate early glaucoma from healthy eyes for each RNFL parameter.
The ROC curve shows the trade-off between sensitivity and 1-specificity. An area under ROC (AROC) of 1.0 represents perfect discrimination, whereas an area of 0.5 represents chance discrimination. Performance of RNFL parameters were evaluated by calculating highest sensitivity values with target specificities fixed at ≥ 80% and ≥ 90%.[11]
Results
The mean age ± standard deviation (SD) of normal subjects was 56.9 ± 11 years and mean age ± SD of glaucoma subjects was 57.1 ± 6.1 years. There was no significant difference in age between two groups.
The average MD ± SD in normal subjects was −1.1 ± 0.4 decibel (dB) and −4.6 ± 0.3 dB in patients with glaucoma. The average PSD ± SD in normal subjects was 1.2 ± 0.2 dB and 5.2 ± 0.7 dB in patients with glaucoma. The MD and PSD were significantly higher in glaucoma group when compared to normal controls (P < 0.001). The clinical characteristics of study population are shown in Table 1.
Table 1.
Clinical characteristics of study population
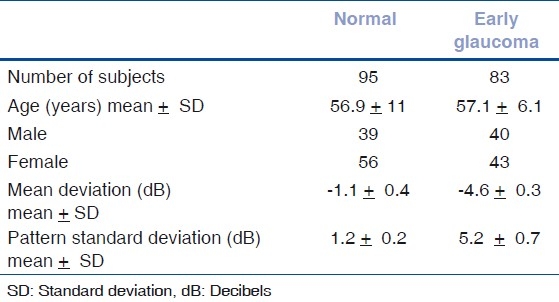
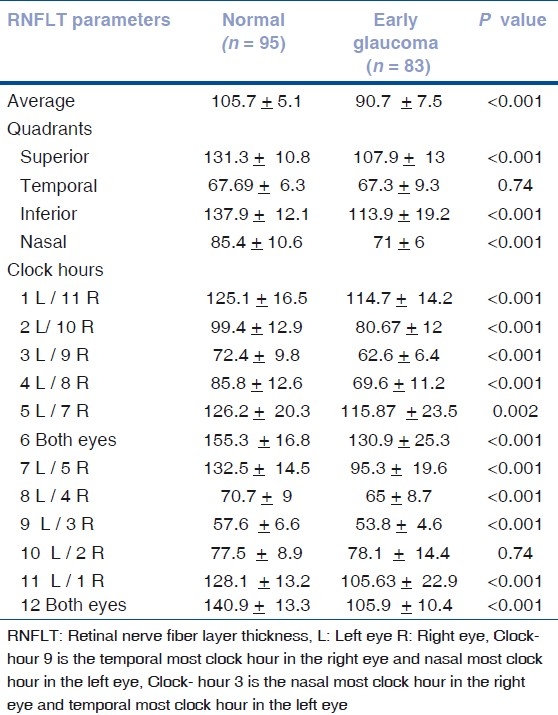
When OCT parameters in normal eyes were compared with patients with early glaucoma, all RNFLT measures were significantly thinner in early glaucoma patients except for temporal quadrant and 10 o’clock- hour [Table 2].
Table 2.
Mean ± standard deviation of retinal nerve fiber layer thickness parameters (in μm) in normal and early glaucoma patients
The largest AROC was found for 12 o’ clock- hour (0.98), average RNFL (0.96) and superior quadrant RNFL (0.9) [Table 3].
Table 3.
Area under receiver operating characteristic curve, sensitivity and specifi city of RNFLT parameters for detection of early glaucoma
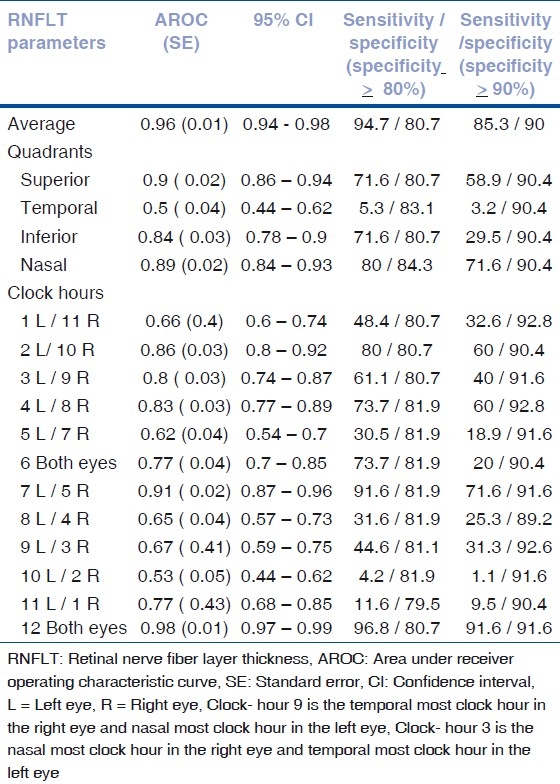
When target specificity was set at ≥ 90%, the parameters with two highest sensitivity were 12 o’clock-hour (91.6%) and average RNFLT (85.3%). When target specificity was set at ≥ 80%, the parameters with two highest sensitivity were again 12 o’clock- hour (96.8%) and average RNFLT (94.7%) [Table 3].
Discussion
Previous studies using OCT 2000[3–5] and Stratus OCT[6,7] have shown variable sensitivity and specificity for identification of early glaucomatous eyes. With the advent of new generation spectral domain (SD) OCT, it is hoped that it would be useful for early diagnosis of glaucoma as RNFLT measurements with SD OCT has better resolution because of increased number of A-scans and it is reported to be more reproducible than previous generations of OCT for different grades of glaucoma severity.[12]
In this study, the normal eyes had thickest RNFLT in inferior quadrant followed by superior, nasal and temporal quadrant. Statistically significant differences in RNFLT measurements were found for most of the parameters except temporal quadrant and 10 o’clock-hour RNFLT between glaucoma and normal eyes. For temporal sector, measurements were nearly similar in the two groups, which reflected in very low specificity for discrimination of temporal RNFLT measurement between normal and early glaucomatous eyes.
Nouri-Mahdavi et al,[3] using OCT 2000, reported that the best discriminating parameters to distinguish normal and 59 early glaucoma patients (defined by SAP, mean MD of -2.9 ± 1.7 dB) were RNFLT at 11 o’clock position, 7 o’clock position and the superior quadrant. The RNFLT at the 7 o’clock position showed 85% sensitivity at 90% specificity and 89% sensitivity at 80% specificity.
Kanamori et al,[4] examined 89 patients with early glaucoma (defined by glaucomatous optic neuropathy and associated visual field loss in the corresponding visual field location, mean MD of –3.1 ± 1.8 dB) using OCT 2000 and found that RNFLT at the 7 o’clock inferotemporal segment had the widest AROC in all parameters for early glaucoma (0.873).
Bowd et al,[5] used OCT 2000 to differentiate normal from early glaucoma patients defined by SAP, mean MD of –4.0 ± 4.2 dB (42 patients) and optic disc appearance (51 patients). For OCT parameters, highest AROC for diagnosis based on SAP was 0.91 for inferior quadrant thickness. When target specificity was set at ≥ 90%, the parameter with the highest sensitivity was inferior quadrant thickness at 79% and when target specificity was set at ≥ 70%, the parameter with the highest sensitivity was inferior quadrant thickness at 88%. Highest AROC was 0.89 for inferior quadrant thickness when the diagnosis was based on optic disc appearance. When target specificity was set at ≥ 90%, the parameter with the highest sensitivity was 6 o’clock hour thickness at 75% and when target specificity was set at ≥ 70%, the parameter with the highest sensitivity was inferior quadrant thickness at 88%. Diagnostic ability of most of OCT parameters increased slightly (although not significantly) when glaucoma was based on functional criteria as these patients have more RNFL damage when compared to those who have not yet developed visual field defect.
Budenz et al,[6] reported sensitivity and specificity of the RNFL parameters for 18 patients with early glaucoma (defined by SAP and optic disc appearance) using Stratus OCT. Average RNFLT (P < 5%) had sensitivity of 78% with specificity of 98%. One or more quadrant with average RNFLT (P < 5%) had sensitivity of 89% with specificity of 95%. One or more clock hours with average RNFLT (P < 5%) had sensitivity of 83% with specificity of 92%. However in this study, the control group was relatively younger and as the RNFLT is known to become thinner with age, it can result in better specificity of OCT.
Parikh et al,[7] analyzed 72 Asian Indian eyes with early glaucoma (defined by SAP, mean MD of -3.57 ± 1.45 dB and optic disc appearance) with Stratus OCT. The highest AROC was 0.82 for inferior quadrant and 6 o’clock hour thickness. They found that the inferior maximum parameter had the best combination of sensitivity and specificity, 75% and 89.6% respectively to differentiate between normal and early glaucoma patients. The 6 o’clock parameter had a sensitivity of 61.1% and specificity of 99%, for an assumed prevalence of 5%.
Sihota et al,[13] analyzed 61 Asian Indian patients with early glaucoma (defined by SAP and glaucomatous optic neuropathy) using Stratus OCT and reported that the average RNFLT had the highest AROC of 0.905, 89.4% sensitivity and 80.3% specificity. Our results show a better performance for most of the RNFLT parameters (with specificity set at 90%). However for some of the parameters (i.e. temporal quadrant and 10 o’clock- hour RNFLT), our values were considerably lower than those previously reported. Large inter-individual variation in RNFLT[14] may result in considerable overlap in RNFLT among study groups which may be responsible for variation in RNFLT measurements and hence may prove difficult to acquire correct judgment of glaucoma using any imaging device. From the statistical point of view, sample size and characteristics of study subjects have influence on the results of the study. Differences in OCT performance among various studies could be due to differences in software, severity of glaucoma, sample size and methodology of study design.
Park et al,[15] used Stratus OCT and spectral domain Cirrus HD OCT (Carl Zeiss Meditec, Inc) in 52 early glaucoma (defined by SAP) and 72 normal subjects and showed that the Cirrus OCT showed better glaucoma diagnostic capability (AROC of 0.94 for inferior quadrant and 0.937 for average RNFLT) than Stratus OCT (AROC of 0.898 for inferior quadrant and 0.896 for average RNFLT). This may be explained by difference in measurement techniques, higher scan resolution and more accurate data registration by Cirrus HD OCT.
A recent study[16] has reported that the diagnostic performances of different SD-OCT devices i.e. Spectralis (Heidelberg engineering, Dossenheim, Germany), Cirrus HD OCT, RTVue (Optovue Inc., Fremont, CA) were equivalent for detecting patients with glaucomatous VF loss (average MD -5.85 dB).
Previous studies have reported that the inferior region RNFLT[4,5,7] and average RNFLT[13] often have the best performance to discriminate healthy eyes from eyes with early glaucoma. This can be explained by the fact that visual field defects associated with glaucoma usually occur initially in the superior visual field which corresponds to inferior pole defects.[17] In our study, the parameters with the best discriminating power to differentiate normal from early glaucoma mostly belonged to superior regions of the optic disc (i.e. superior clock-hour and superior quadrants) and global RNFLT, similar findings have been reported by Nouri-Mahdavi et al,[3] (OCT 2000) and Leite et al,[16] (Spectralis OCT) who reported the superior sector and global thickness to have the greatest AROC to distinguish normal from glaucomatous eyes. The finding of preferential loss in superior segment may be due to the selection or inclusion of the patients studied, as, in our study there were more patients with visual field damage and RNFL loss in the superior hemifield than inferior hemifield.
To conclude, although the quantitative estimation of some of the parameters did not provide sensitivities and specificities that justify implementing SD OCT as a population screening tool for identification of early glaucoma, it may prove to be a useful imaging modality to monitor and follow up patients with early glaucoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 2.Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 3.Nouri-Mahdavi K, Hoffman D, Tannenbaum DP, Law SK, Caprioli J. Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol. 2004;137:228–35. doi: 10.1016/j.ajo.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Kanamori A, Nakamura M, Escano MF, Seya R, Maeda H, Negi A. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–20. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 5.Bowd C, Zangwill LM, Berry CC, Blumenthal EZ, Vasile C, Sanchez-Galeana C, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 6.Budenz DL, Michael A, Chang RT, McSoley J, Katz J. Sensitivity and specificity of the Stratus OCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 7.Parikh RS, Parikh S, Sekhar GC, Kumar RS, Prabakaran S, Babu JG, et al. Diagnostic capability of optical coherence tomography (Stratus OCT 3) in early glaucoma. Ophthalmology. 2007;114:2238–43. doi: 10.1016/j.ophtha.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The longitudinal study of cataract study group: The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 9.Hodapp E, Parrish RK, Anderson DR. St. Louis: Mosby Year Book; 1993. Clinical decisions in glaucoma. [Google Scholar]
- 10.Anderson DR, Patella VM. 2nd ed. St. Louis: Mosby; 1999. Automated static perimetry; pp. 121–36. [Google Scholar]
- 11.Quigley HA. Current and future approaches to glaucoma screening. J Glaucoma. 1988;7:210–20. [PubMed] [Google Scholar]
- 12.Garas A, Vargha P, Hollo G. Reproducibility of retinal nerve fiber layer and macular thickness measurement with the RTVue-100 optical coherence tomograph. Ophthalmology. 2010;117:738–46. doi: 10.1016/j.ophtha.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–10. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 14.Poinoosawmy D, Fontana L, Wu JX, Fitzke FW, Hitchings RA. Variation of nerve fiber layer thickness measurements with age and ethnicity by scanning laser polarimetry. Br J Ophthalmol. 1997;81:350–4. doi: 10.1136/bjo.81.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SB, Sung KR, Kang SY, Kim KR, Kook MS. Comparison of glaucoma diagnostic capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol. 2009;127:1603–9. doi: 10.1001/archophthalmol.2009.296. [DOI] [PubMed] [Google Scholar]
- 16.Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118:1334–9. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner EB, Drance SM. Early visual field disturbances in glaucoma. Arch Ophthalmol. 1977;95:1173–5. doi: 10.1001/archopht.1977.04450070071002. [DOI] [PubMed] [Google Scholar]


