Abstract
In this study, 60 fungal isolates from 60 patients with fungal keratitis were tested in vitro for their susceptibility to natamycin and the mean minimum inhibitory concentrations of natamycin (MICn) were correlated with clinical outcome. The mean MICn for various groups of fungi from patients with either early (<10 days) or late (≥10 days) presentation was correlated with the outcome. Aspergillus flavus showed resistance to natamycin with a high mean MICn (>16 μg/ml). While the clinical response in all patients with early A. flavus keratitis was good it was poor in late cases (5/8 patients, 62.5%). Fusarium species, Acremonium species and dematiaceous fungi were sensitive with low mean MICn (Fusarium: 5.7-7.2 μg/ml, Acremonium: 5.7-6.8 μg/ml, dematiaceous: (1.6-4 μg/ml). However, 46.6% (7/15) patients in Fusarium and 57.1% (4/7) in Acremonium group needed keratoplasty. We conclude that despite susceptibility of most fungal species causing keratitis to natamycin, the treatment outcome is poor in advanced fungal keratitis.
Keywords: Keratomycosis, minimum inhibitory concentration, natamycin, susceptibility, treatment outcome
Keratomycosis is the leading cause of ocular morbidity throughout the world and fungi are the principal etiological agents of corneal ulceration in India.[1–3] Different classes of antifungal drugs used for treatment of fungal keratitis include polyenes, triazoles, and echinocandins.[2] Of these, natamycin is the mainstay of treatment and is widely used in India. However, treatment outcome in fungal ulcers tends to be poorer compared to bacterial and Acanthamoeba keratitis.[1,3]
In the non-ophthalmic literature a fairly wide range of antifungal susceptibility test results are available for prediction of clinical outcome in fungal infections.[4] In contrast, such information is lacking in keratomycosis. Only a limited number of antifungal susceptibility testing of ocular isolates has been reported[5,6] and except for one none have correlated with response to treatment in the patients.[6] In the present study 60 fungal isolates from 60 patients with fungal keratitis were tested in vitro for their susceptibility to natamycin eye drop and the mean minimum inhibitory concentrations (MIC) were correlated with the clinical outcome with topical 5% natamycin eye drops.
Materials and Methods
In this prospective, non randomized interventional study corneal scrapings from patients with microbial keratitis were processed and interpreted as per our institutional protocol.[3] Consent was obtained from all patients. Taking appropriate laboratory precautions all significant fungal growth were tested for their susceptibility to natamycin eye drop (Sun pharmaceutical Ind. Ltd, Mumbai, India) by the previously described microbroth dilution method.[7]
All patients received intensive treatment with one hourly 5% natamycin eye drops round the clock in the affected eye for the first three days with subsequent modification as per response. Twenty five patients were given oral ketoconazole (200 mg twice daily) and two patients received oral fluconazole (150 mg twice daily). Clinical outcome at one month was considered for analysis. An ulcer was defined either completely healed or resolving (reduction in infiltrate size) or worsened (increase in infiltrate size, keratoplasty). Since the treatment outcome is known to be affected by severity of disease the mean MIC of natamycin for different groups of fungi for patients presenting with in 10 days (early onset) or after 10 days (late onset) of symptoms were correlated with the clinical outcome.[8]
Results
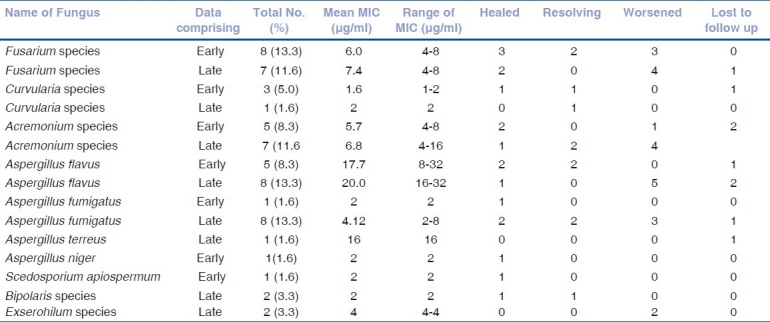
Fungal species wise comparison of mean MIC of natamycin with treatment outcome was made among the patients with early and late onset disease [Table 1]. A. flavus (n = 13) showed the highest mean MIC (early-17.7 μg/ml, late-20.0 μg/ml) suggesting lowest susceptibility to natamycin. Excluding the patients lost to follow up five out of 6 patients (83.3%) had poor clinical response in the late group (A. flavus) while none had worsened in the early group (A. flavus). A. fumigatus, in contrast, showed high susceptibility to natamycin in vitro with mean MIC of 4.12 μg/ml. The clinical outcome, however, was poor in the cases that presented late. MIC of natamycin was relatively low for Fusarium species with mean MIC between 5.7-8 μg/ml. Nevertheless, 7 out of 14 patients (50.0%, 1 lost to follow up) needed penetrating keratoplasty to control the infection suggesting poor clinical response to natamycin. Among the dematiaceous fungi, Curvularia species were isolated in four patients and natamycin showed low MIC against these isolates (mean MIC in early - 1.6 μg/ml, late - 2 μg/ml).
Table 1.
Treatment outcome of patients with early (<10 days) and late (>10 days) onset of fungal keratitis caused by various groups of fungi and their correlation with MIC of natamycin
Discussion
A notable publication by Prajna et al., has convincingly shown that eye drop preparations are an alternative to pharmaceutical grade natamycin (not available to most laboratories) for testing antifungal susceptibility.[7] This study applied the same technique to evaluate the MIC of natamycin against various isolates of fungi from corneal scrapings of patients with mycotic keratitis. Owing to a small sample size in various groups of fungi, the mean MIC[9] was considered for comparison rather than MIC90 or MIC50. Susceptibility breakpoints for natamycin have not been described so far in CLSI guidelines, however, MIC of 16 μg/ml or less is considered to indicate susceptibility of a fungal isolate.[7] This study reassuringly found low MIC of natamycin (≤16 μg/ml) in all fungal isolates tested except A. flavus. A. flavus associated with keratitis is a particularly virulent fungus and is known to be associated with poor outcome in keratomycosis.[8] Apart from the toxins,[10] its perilous status is compounded by its resistance to natamycin. It is not known whether this resistance is inherent to this species.
All isolates of Fusarium species were sensitive to natamycin but that did not translate to good clinical outcome in patients with Fusarium keratitis irrespective of early or late presentation. This probably points to the well known fact of poor penetration of natamycin especially in presence of advanced fungal keratitis affecting deeper layers of the cornea. Most encouraging results were obtained with respect to dematiaceous fungi [Table 1]. Scedosporium apiospermum and Bipolaris species showed susceptibility to natamycin with low MIC as well as favourable response to treatment with natamycin. However, both patients with Exerohilum species infection had presented late and showed poor response. Good treatment outcome in keratitis caused by dematiaceous fungi can be attributed to low virulence of dematiaceous fungi and their tendency to remain in the superficial tissues of cornea for long.[11] This study shows that a low MIC of natamycin (susceptible fungi) was not always associated with good treatment outcome with natamycin in fungal keratitis. As is well known, a large number of factors affect success or failure of medical therapy of fungal keratitis, severity of infection being one of them.[8] Our treatment protocol for fungal keratitis includes systemic antifungals for patients with the ulcer more than 6 mm in diameter or extending beyond anterior half of the stroma or when there is hypopyon. Systemic antifungal therapy given in some of the patients in this study may be a confounding factor which was not analyzed and is one of the limitations of this study. Using logistic regression Shapiro et al., showed that a two fold increase in MIC results in 47% reduction in the odds of healing.[6] However, results of the present study suggest that such a conclusion may be too simplistic and a considerably large study addressing multiple factors would be necessary to determine the effect of various parameters on the treatment outcome of fungal keratitis.
Despite the limitation of small sample size in each group of fungi and statistically non significant results, we believe that our results are clinically significant. The susceptibility of A. flavus to natamycin is particularly poor and patients with this infection may require aggressive treatment, closer follow up and early surgical intervention. In vitro susceptibility of most other species of fungi, such as Fusarium spp., A. fumigatus, Acremonium spp., S. apiospermum, Bipolaris spp., Curvularia spp. etc., to natamycin may account for good clinical response when the intervention occurs early in the disease.
References
- 1.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–7. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PA. Current perspective in ophthalmic mycosis. Clin Microbiol Rev. 2003;16:730–97. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J Ophthalmol. 2009;57:273–9. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35:982–9. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 5.Gopinathan U, Garg P, Fernandes M, Sharma S, Sreedharan A, Rao GN. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in south India. Cornea. 2002;21:555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro BL, Lalitha P, Loh AR, Fothergill AW, Prajna NV, Srinivasan M, et al. Susceptibility testing and clinicaloutcome in fungal keratitis. Br J Ophthalmol. 2010;94:383–4. doi: 10.1136/bjo.2009.158675. [DOI] [PubMed] [Google Scholar]
- 7.Lalitha P, Vijaykumar R, Prajna NV, Fothergill AW. In vitro natamycin susceptibility of ocular isolates of Fusarium and Aspergillus species: Comparison of commercially formulated natamycin eye drops to pharmaceutical-grade powder. J Clin Microbiol. 2008;46:3477–8. doi: 10.1128/JCM.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113:526–30. doi: 10.1016/j.ophtha.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Tavanti A, Hensgens LA, Mogavero S, Majoros L, Senesi S, Campa M. Genotypic and phenotypic properties of Candida parapsilosis sensu strictu strains isolated from different geographic regions and body sites. BMC Microbiol. 2010;10:203. doi: 10.1186/1471-2180-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leema G, Kaliamurthy J, Geraldine P, Thomas PA. Keratitis due to Aspergillus flavus: Clinical profile, molecular identification of fungal strains and detection of aflatoxin production. Mol Vis. 2010;16:843–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Rajeev B, et al. Evaluation of agent and host factors in progression of mycotic keratitis: A histological and microbiological study of 167 buttons. Ophthalmology. 2002;109:1538–46. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]


