Abstract
Intraoperative consultations may pose considerable diagnostic challenge to the neuropathologist in diagnosing primary and metastatic neoplasms of the central nervous system (CNS). Cytological preparations in the form of squash, touch, imprint or smears are few of the available modalities in addition to the frozen section (FS). Although the latter is superior in providing both histologic patterns and cytomorphologic details yet smears are of vital importance when tissue available is limited (stereotactic biopsy), scrutinisation of intercellular matrix (astrocytoma versus oligodendroglioma) and evaluation of discohesive cells (lymphoma, pituitary adenoma) and in inflammatory lesions. This review is intended to emphasize the value, applicability and limitations of neurocytology aiming to expedite the intraoperative smear-based diagnoses of CNS neoplasia as per the World Health Organization (WHO) classification. We recommend that whenever possible, both smears and FS should be examined concomitantly and in a correlative manner. In the unlikely event of a mismatch between the findings on smear and FS, intraoperative diagnosis is primarily based on FS, if adequate tissue is available. However, each case must be evaluated on its own merit and in difficult cases relevant differential diagnoses should be offered to facilitate surgical decisions and optimally triage patient management.
Keywords: Frozen section, intraoperative neurocytology, squash
Primary or metastatic neoplasia involving the central nervous system (CNS) is unique in being potentially life threatening due to the associated rise in intracranial pressure and involvement of critical or eloquent structures. When faced with a crucial decision to maximize the clinical benefit while keeping the therapeutic deficits to a minimum, neurosurgeon often resorts to a stereotactic biopsy as the diagnostic procedure. The neuropathologists are thus often required to make the diagnoses on very small biopsy specimens. During intraoperative consultation, neuropathologists are expected to render diagnosis that can not only suitably modify the surgical procedure, but also ensure maximal preservation of lesional tissue for definitive diagnosis on formalin-fixed paraffin-embedded (FFPE) sections. Of the two intraoperative pathologic techniques, frozen section is superior to cytologic preparations in providing both histologic patterns and cytomorphology, yet is not often suitable for small brain biopsies as it provides a limited view of the sample, wastes substantial tissue and causes freezing artefacts rendering the tissue suboptimal for FFPE evaluation and special stains. Cytological methods, particularly smear[1] or squash[2] preparations and sometimes touch preparations[1,2] are in some ways better suited for intraoperative diagnosis of tiny stereotactic brain biopsy specimens[1–9] so long as their limitations are fully understood by both neuropathologists and neurosurgeons.[1,2,5,10–13] In general, cytologic preparations are faster to prepare and provide invaluable nuclear detail but due to variation in smear thickness, cellular overlapping, and stretching take longer time to examine.[1,2,5] This review is intended to facilitate and expedite the intraoperative smear-based diagnoses of CNS neoplasia.
The importance of intra-operative pathologic diagnosis is mainly six-fold.[1,2,5] In the vast majority of cases, the neurosurgeon's main reason for intra-operative neuropathologic consultation is to ensure that a diagnostic specimen was obtained in the least invasive manner and with the fewest complications. This is especially important for lesions in brainstem or in other locations with a high-risk of hemorrhage where only a single biopsy may be possible. Second, if the smear diagnosis is an infection or lymphoma; to make sure that additional biopsies are submitted in adequate quantity for microbiologic or flow cytometric studies respectively; an infectious disease specialist or hematologist-oncologist on duty are immediately contacted to ensure institution of or modification to appropriate medical treatment. For these two purposes, an intra-operative cytological examination has indeed been reported to be an excellent method. In one study, tissue adequacy of a specimen could be confirmed with 96% sensitivity and 75% specificity rates.[11] Third, given that even a relatively broad intra-operative diagnosis, such as glioma, may likely to reflect the periphery of a high-grade glioma if suggested by imaging features, the neurosurgeon can decide to proceed with obtaining a deeper biopsy to include the more cellular and/or necrotic portion of the tumor based on which the FFPE diagnosis can be further narrowed to a glioblastoma. Fourth, in a select group of cases, intraoperative diagnosis may influence the extent of resection or adjuvant therapy of lesional brain or spinal cord parenchyma. For example, if the diagnosis of a high-grade glioma, lymphoma or infection can be ascertained intra-operatively, adjuvant therapy may be instituted at the time of the surgical procedure or in the immediate postoperative period. Similarly, detection of lymphoma in a ring-enhancing brain lesion or a pituitary adenoma in clinically suspected Cushing's disease would limit the surgery and prevent the unnecessary resection. Fifth, an accurate and specific intraoperative diagnosis may be obtained in few of the cases, who may be offered immediate medical or surgical treatment, while in most cases, the neurosurgeon has to wait for and rely on FFPE sections that is available the next day. However, an intra-operative pathologic diagnosis is nevertheless of great interest and emotional value to the anxiously waiting patient and family besides serving the professional satisfaction of neurosurgeons. Finally, smears can be readily used for diagnosis in case the facility or trained personnel for frozen sections are unavailable.
A smear or squash preparation provides a combination of detailed cytologic features and some histologic patterns, but have diagnostic limitations as compared to either frozen or FFPE sections which need to be understood and kept in mind during intra-operative consultation.[1,2,5] Cytologic details well-illustrated on smears include astrocytic processes in astrocytomas, neuropil background in neurocytic tumors, oligodendroglial/neurocytic cells with round and regular (if not anaplastic) nuclei that are smaller than an astrocyte with denser chromatin, ganglion type neurons with large nuclei with prominent nucleoli, meningothelial plump nuclei with pseudo-inclusions and pituitary lesions with rounded nuclei with punctate ‘salt and pepper’ chromatin. However, interpretation of each of these entities is benefitted by synchronous evaluation of histologic patterns which is better achieved on frozen section although to a certain extent also on smears. For example, well-spaced astrocytic cells with robust lengthy processes favors reactive gliosis over an astrocytoma in that the fibrillary processes are coarser and more intricately networked. Similarly, floating neurons and specific glioneuronal elements of dysembryoplastic neuroepithelial tumor (DNET) are different from sheets of neurocytic cells with small irregular rosettes or neuropil islands in central or extraventricular neurocytomas. Parenchymal infiltration with perineuronal satellitosis differentiates oligodendrogliomas from neurocytomas. Meningothelial whorls can be well seen on smears, but verocay bodies of schwannoma are difficult to appreciate on smears and need frozen sections. Pituitary tissue can be recognized on smears, but differentiation between hyperplasia and adenoma is facilitated by frozen section, especially in Cushing's syndrome in which both can co-exist, but best achieved on FFPE sections with reticulin stain. Lesions occurring in cauda equina include myxopapillary ependymoma and paraganglioma. Although there is some histologic overlap between the two, such as perivascular orientation, the presence of myxoid material on smears, highlighted by Diff Quick stain, aids in this differential diagnosis.[12]
Low-grade CNS neoplasms can be diagnosed on cytologic preparations within certain limitations. Smears are particularly helpful in highlighting glial fibrillary architecture.
Pilocytic astrocytoma (the World Health Organization [WHO] grade I) on smears usually shows a variable admixture of piloid (hairlike) and fibrillary astrocytic cells with bland elongated nuclei and very thin processes; with/without Rosenthal fibres and eosinophilic granular bodies. In addition, at least to some extent, even the biphasic architecture typical of pilocytic astrocytomas on histology with alternating microcystic and compact cellular areas can be discerned on smears depending on the quantity and sampling of tissue and quality of smears [Figure 1]. However, there can be a lot of variation to this basic theme. They can also show a number of atypical features that are acceptable within the diagnosis and WHO grade I without their known ominous implication and high-grade designation in diffuse fibrillary astrocytoma. These include cytologic atypia, smudgy chromatin and microvascular proliferation. However, the presence of these features in a pilocytic astrocytoma that is rich in a fibrillary pattern can easily prompt a diagnosis of high-grade astrocytoma during intra-operative consultation.[1] The presence of atypical or neoplastic neurons within an otherwise unequivocal pilocytic astrocytic background suggest a ganglioglioma; however, this decision unless obvious on concomitant frozen section is best deferred to permanent sections and immunohistochemical confirmation.[14]
Figure 1.
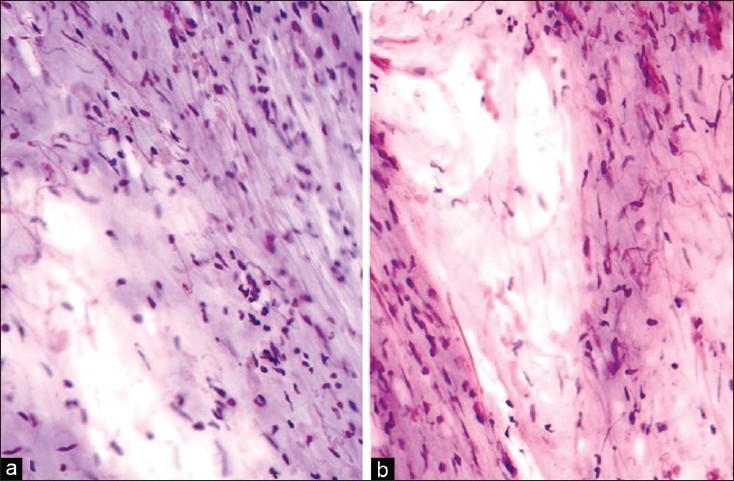
(a) Pilocytic astrocytoma: Biphasic pattern with compact areas showing bipolar cells with bland spindle nuclei, along with a loose microcystic area (H and E, ×200). (b) Pilocytic astrocytoma: Biphasic pattern with compact areas showing bipolar cells with bland spindle nuclei, along with a loose microcystic area. Many Rosenthal fibres are noted (H and E, ×200)
Diffuse astrocytoma typically infiltrates normal brain therefore shows an admixture of neoplastic and normal glial cells. Therefore, the diagnosis of diffuse astrocytoma should only be based on identification and analysis of a significant population of atypical glial cells but not by pointing at single cells.[1] Typical features include hyperchromatic nuclei with irregular often ‘sausage-shaped’ nuclear outline, coarse chromatin, with/without gemistocytic phenotype embedded in the background of a meshwork of glial fibres [Figure 2].
Figure 2.
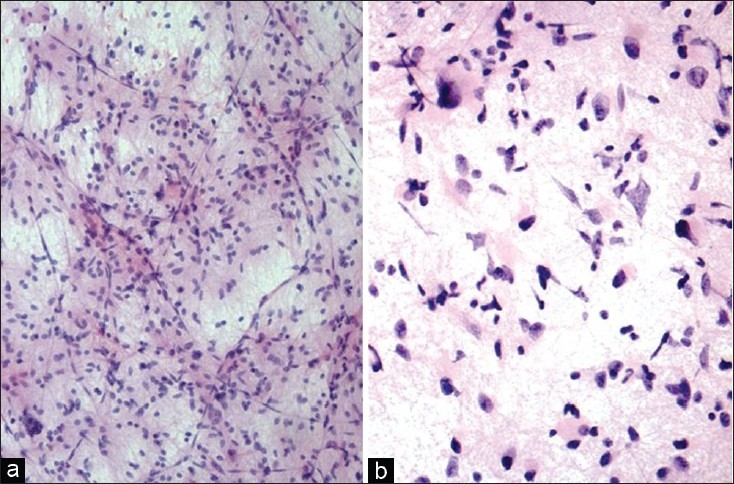
(a) Diffuse fibrillary astrocytoma: Elongated astrocytic cells in mostly dense meshwork of fibrillary processes (H and E, ×100). (b) Diffuse fibrillary astrocytoma: Astrocytic cells with irregular hyperchromatic nuclei and up to moderate pleomorphism, along with gemistocytic forms in a meshwork of fibrillary processes (H and E, ×200)
In contrast, oligodendrogliomas typically have relatively rounded, smoother and monomorphic nuclei [Figure 3]. The differences in nuclei decrease with increasing histologic grade. While many nuclei may be devoid of cytoplasm, raising the differential diagnosis of neurocytoma, lymphoma and metastatic small cell carcinoma in such cellular foci on low-power, the presence of relatively bland chromatin, mini-gemistocytes and some glial strands on high-power help ascertain the diagnosis of oligodendroglioma. Peri-nuclear halos that give oligodendroglioma the ‘fried-egg’ appearance on FFPE sections is an artefact of fixation and not present on smears. Calcification when present may be a helpful feature.
Figure 3.

(a) Oligodendroglioma: Glial cells with rounded nuclei with relatively open chromatin, in a sparse meshwork of fine fibrillary processes with a thin-walled capillary network (H and E, ×200). (b) Oligodendroglioma: Glial cells with rounded bland nuclei with relatively open chromatin, in a sparse meshwork of fine fibrillary processes (H and E, ×400)
It is important to differentiate a low-grade ependymoma from a diffuse astrocytoma as the former is typically treated with gross total resection. On cytologic preparations, a low-grade ependymoma typically shows monomorphic population of round cells with salt-and pepper-like chromatin and detectable micronucleoli in fibrillary background. Due to these glial features and sometimes eosinophilic cytoplasm, ependymomas may resemble astrocytoma on smears. Therefore, the arrangement of tumor cells encasing blood vessels variably separated by perivascular cell-free zones of fibrillary processes (perivascular pseudorosette) are essential to the diagnosis of ependymomas on both smears and frozen sections [Figure 4], especially in the clinical context of ventricular or periventricular tumors.[1,15] A delicate smear may preserve the myxoid lakes or spheres of myxopapillary ependymoma, especially on Diff-Quick stain.
Figure 4.
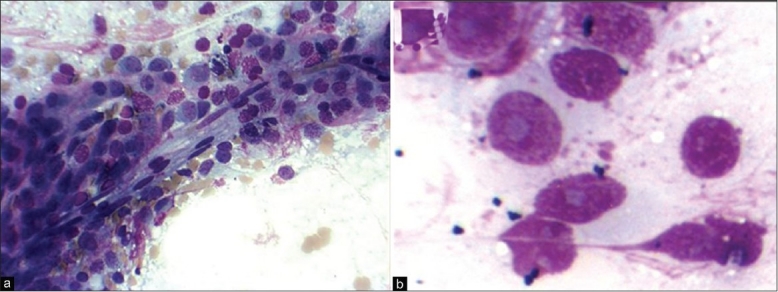
(a) Ependymoma: Arrangement of tumor cells encasing blood vessels (MGG ×200). (b) Ependymoma: Cells with prominent nucleoli (MGG ×400)
Smears of dysembryoplastic neuroepithelial tumor (DNET) usually show a diagnostic combination of cytologic features. These include small uniform neurocytic cells with delicate capillary vasculature and myxoid microcystic architecture. Admixed are large ganglion cells present variably admixed and sometimes floating within the microcysts similar to histologic sections [Figure 5]. If additional gliomatous patterns are present, the tumor is classified as complex DNET which may raise the differential diagnoses of pilocytic astrocytoma, oligodendroglioma or ganglioglioma.
Figure 5.
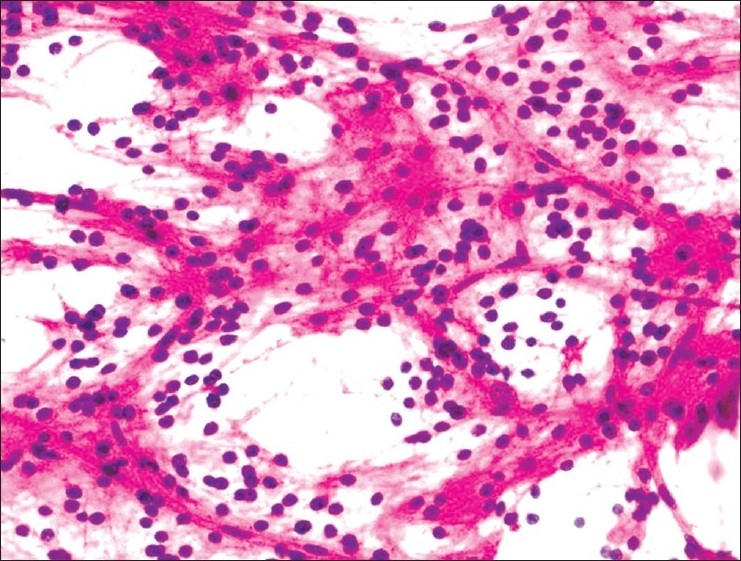
Dysembryoplastic neuroepithelial tumor: Microcysts separated by clusters of neurocytic cells with rounded bland nuclei, in a sparse meshwork of fine fibrillary neuropil-like processes. Large ganglion cells are noted embedded in the wall of microcysts (H and E, ×400)
High-grade and metastatic neoplasms can be diagnosed with fairly reasonable accuracy on intra-operative smears using the following broad approach:
(A) Cohesive tumor cells [Figure 6]:
Figure 6.
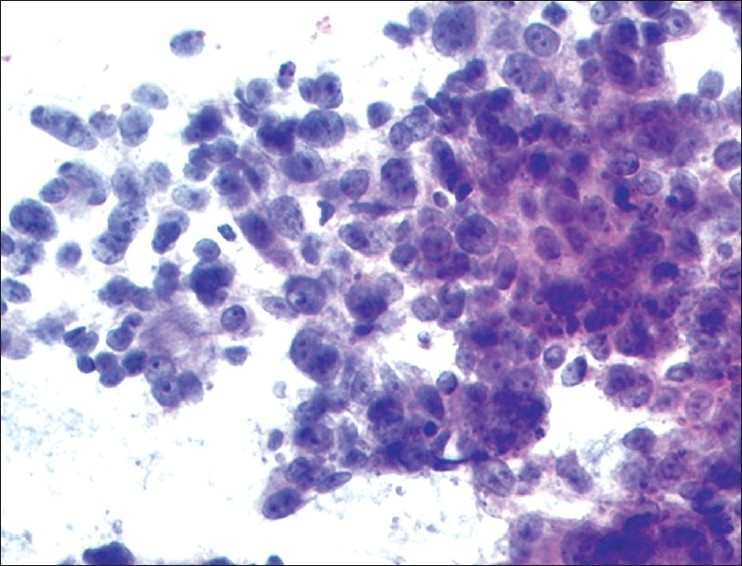
Metastatic adenocarcinoma: Cohesive cluster of malignant non-small epithelial cells forming glands with extracellular mucin (H and E, ×200)
Metastatic carcinomas display cohesive nests of atypical or overtly neoplastic cells and are often high-grade with nuclear anaplasia, prominent nucleoli, frequent mitoses and a necrotic background. The degree of squamous or glandular differentiation may be obvious or more often limited to cell junctions or focal glands/intracytoplasmic mucin respectively. The tumor differentiation is often better assessed on frozen section and the determination of site of origin while sometimes possible on smear morphology is best attempted in conjunction with immunohistochemical staining results.[12]
Non-germinomatous germ cell tumors typically occur in young adults and can have a variety of cytomorphology depending on the subtype present or preponderant. The teratomas may be mature and include mature differentiation of ectodermal, endodermal or mesenchymal patterns, or have immature, fetal or embryonic appearance. Anaplastic epithelial clusters of large cells with prominent nucleoli and necrotic debris suggest an embryonal carcinoma. The presence of mucoid microcystic matrix and anastomosing thin clusters of anaplastic cells may suggest yolk sac tumor.
An epithelial neoplasm with varying degrees of anaplasia with/without papillary pattern in a child in ventricular/periventricular region may represent a choroid plexus carcinoma.
Anaplastic meningiomas can be cohesive and show a variety of patterns and cytomorphology yet show at least some evidence of meningothelial differentiation such as focal whorls and/or typical nuclear features. However, typically meningothelial cells do not show distinct cell borders.
Rarely epithelioid glioblastomas or gliosarcomas can show cellular cohesion yet there is an evidence of at least focal glial differentiation, such as glial processes or gemistocytes, typically highlighted by immunostain for GFAP.
The smear diagnosis of atypical teratoid/rhabdoid tumor (AT/RT) is based upon identification of discohesive ‘plasmacytoid’ cells, characteristic ‘rhabdoid’ cells and sometimes admixed primitive neuroectodermal or ‘papillary-like’ appearance.[16]
(B) Discohesive or loosely cohesive tumor cells: In contrast, if a smear or touch preparation shows numerous large atypical discohesive cells, the possibilities are more diverse ([Figure 7] panel).
Figure 7.
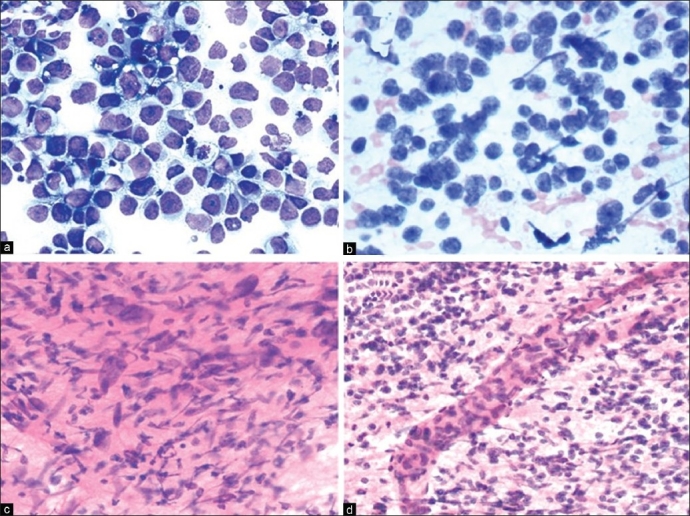
(a) Germinoma: Syncytial arrangement of loosely cohesive neoplastic cells with vacuolated cytoplasm, moderate nuclear pleomorphism, prominent nucleoli and frequent mitoses. Occasional interspersed mature lymphocytes are noted (H and E, × 200). (b) Medulloblastoma: Sheets of malignant cells with primitive appearing nuclei, attempted Homer-Wright rosettes and fine neuropil-like intercellular material (H and E, ×400). (c) GBM: Markedly pleomorphic malignant astrocytic cells in well-formed fibrillary meshwork (H and E, ×200). (d) GBM: Malignant small astrocytic cells in well-formed fibrillary meshwork with a hyperplastic blood vessel (H and E, ×100)
-
Relatively monomorphic discohesive cells: Main possibilities are as follows.
- The presence of relatively monotonous large, discohesive cells with scant to moderate bluish cytoplasm and prominent nucleoli in the background of dispersed lymphoglandular bodies on Diff quick suggests primary central nervous system lymphoma (PCNSL). Cytologic preparations are often superior to FS in this regard.[11] In such cases, the surgical procedure is brought to a halt and an additional biopsy if any required is only obtained to ensure that an adequate tissue can be submitted for FFPE sections and flow cytometry to establish the lineage, clonality and immunophenotyping; and for cytogenetics if considered necessary.
- If the discohesive cells are small rounded to spindled with hyperchromatic nuclei, frequent molding and show frequent mitoses and apoptosis/necrosis, one should consider a small cell carcinoma such as of lung primary and ask pertinent clinical questions.[12]
- Germinomas can appear relatively monomorphic on smears and are composed of large cells with distinct borders with/without cytoplasmic vacuolation, vesicular nuclei and prominent nucleoli at times with admixed mature lymphocytes.[17] The sycytiotrophoblasts may be seen admixed.
- In the context of undifferentiated ‘primitive’ cytology, differential diagnosis includes medulloblastoma (MB), primitive neuroepithelial tumor (PNET) and PNET-like areas of atypical teratoid rhabbdoid tumour (ATRT). These are densely cellular tumors composed of small round cells that are often monomorphic with hyperchromatic often molded nuclei, scant to no cytoplasm, high nuclear- cytoplasmic ratio, small nucleoli, with frequent mitoses and apoptotic bodies or necrotic debris.[1,10] Homer-Wright rosettes are usually indistinct on smears. Fibrillary processes are sparse and associated with glial differentiation.[1]
- In the context of ‘primitive’ cytology, the presence of ependymoblastic rosettes and lack of fibrillary processes may suggest ependymoblastoma, whereas the epithelial appearance with gland-like features and canals with/without glioneuronal, epithelial and mesenchymal differentiation suggest a medulloepithelioma or immature teratoma.[10] These diagnoses are seldom made on smears alone and usually need concomitant FS.
-
If discohesive cells are pleomorphic:
-
The smear should be carefully evaluated for any evidence of glial, embryonal or neuronal lineage typically in the form of fibrillary processes (not explainable by smearing artefacts). High-grade diffuse gliomas of either astrocytic or oligodendroglial lineage tend to produce clumps of cells. However, smear due to shearing effect is an insensitive method to recognise mitoses that is the most important criterion for diagnosis of high-grade in gliomas.[1] Moreover, recurrent treated tumors are often more anaplastic yet have fewer mitoses than primary tumors.
- An anaplastic astrocytoma or glioblastoma typically shows pleomorphic or gemistocytic cells, multipolar coarse fibrillary processes often forming a meshwork and suggestive of infiltration of gray matter (perineuronal satellitosis; often difficult to appreciate on smears). The distinction between anaplastic astrocytoma and glioblastoma is based on necrosis and microvascular proliferation, the recognition of both of which can be problematic on smears. Hyperplastic vessels have multilayered cells (more than expected from the degree of cellular overlap in that portion of the smear) and complex loops. Necrosis is difficult to recognize due to poor sticking to slides and reduced or absent ghost cell outlines but individual cell necrosis and apoptosis are more readily detectable.[1]
- An anaplastic oligodendroglioma typically shows relatively rounded cells, mini-gemistocytic cells, sparse fibrillary processes, suggestive of infiltration of gray matter and none to variable micro-vascular proliferation. In cases where the glial lineage is uncertain, it is reasonable to offer a diagnosis of ‘infiltrating or high-grade glioma’ on intra-operative smears as it may not alter immediate surgical management in most cases.
- In pediatric, especially, infratentorial tumors with some neuropil type fibrillary processes, increased nuclear pleomorphism, in the absence of rhabdoid cells may suggest anaplastic or large cell medulloblastoma.[10]
-
Presence of plump plasmacytoid cells but without astrocytic processes and not classifiable as gemistocytes may be
- Metastatic poorly differentiated non-small cell carcinoma. Further evaluation is often not possible on smears and requires immunohistochemical work up on FFPE including cytokeratin (CK) - AE1/AE3, 7, 20, (thyroid transcription factor)TTF1, estrogen receptor (ER) and progesterone receptor (PR). Undifferentiated examples may need Ber-EP4 or MOC31 to establish the diagnosis of carcinoma.
- Metastatic or meningeal melanoma. Diverse cell morphologies and melanin pigment are useful clues to diagnosis. Immunohistochemical work up should include S-100, HMB45, Melan-A and tyrosinase (preferably a cocktail).
- Rhabdoid tumors including ATRT in children and rhabdoid meningioma in elderly. In pediatric, especially, infratentorial tumors, increased nuclear pleomorphism, large cells with open chromatin and more distinct nucleoli with or without obvious rhabdoid cells suggest ATRT.[10,16] This is especially the case in cerebellopontine angle in a child younger than two years. Rhabdoid immunohistochemical work up should include cytokeratins, epithelial cell antigen, desmin, S-100 and integrase inteactor-1. Molecular testing for both somatic and germline mutations not only confirm the diagnosis, but also provide option of family counselling.
-
There are many advantages to smear preparations in individual cases. In general, smear preparations have been found to be superior to frozen sections for soft, highly cellular lesions or very small specimens.[9] Inflammatory cell typically shed easily in smears. Therefore, finding significant quantity of neutrophils may indicate an infection, whereas abundance of macrophages (typically a reaction to brain parenchymal destruction) may indicate demyelination or an infarct.[1] Moreover, cytological preparations are faster, simpler, less expensive and require less equipment than frozen sections, thereby minimising freezing artefact and contamination in infectious specimens and maximizing availability of tissue for FFPE examination.[11]
Limitation of smears must be taken into consideration when determining the nature of the lesion. Commonly encountered limitations/pitfalls of smears are as follows:
Difficulty in the estimation of cellularity:[12,18] usually thick smears lead to overestimation of cellularity whereas underestimation is possible on touch smears. This is relevant because an erroneous diagnosis of high-grade glioma may prematurely terminate the surgery in the false belief that adequate diagnostic tissue has been obtained.[19] On the contrary, since gliomas infiltrate the brain parenchyma with decreasing cellularity, even a high-grade glioma can be only mildly hypercellular if sampled at the periphery that may result in undergrading.
-
Mistaking reactive gliosis for low-grade glioma including the periphery of diffuse (fibrillary or gemistocytic) astrocytoma, oligodendroglioma and pilocytic astrocytoma.
- Diffuse astrocytoma and oligodendrogioma can be problematic because parenchymal infiltration (a defining feature of diffuse glioma), well illustrated by perineuronal satellitosis and gradient of cellularity on histology, are difficult to appreciate on smears due to the lack of tumor-brain interface definition. Moreover, it is common to find at least some degree of gliosis adjacent to and associated with a tumor. Typically, in gliosis, reactive astrocytes tend to be evenly distributed, with slightly enlarged and eccentric nuclei, abundant, eosinophilic cytoplasm with stellate longer, tapering processes and low nucleus/cytoplasmic (N/C) ratio. In contrast, low-grade fibrillary astrocytomas have uneven distribution of neoplastic cells with increased N/C ratios, irregular hyperchromatic nuclei with coarse chromatin, shorter, thinner and more variably intersecting processes, mitotic figures especially atypical ones, and at times microcystic change.[19]
- Pilocytic astrocytomas (PA) can be problematic since Rosenthal fibres are often abundant in gliosis in both the common locations of PA, namely cerebellum and hypothalamus, such as adjacent to hemangioblastoma and craniopharyngioma, respectively. The presence of well-spaced astrocytes with bland nuclei and uniform robust fibrillary processes strongly favor reactive gliosis on smears. Frozen sections and FFPE sections are needed for definitive diagnosis.
A cytologic smear with foamy and mitotically active macrophages can be hypercellular. When associated with atypical (Creutzfeldt) astrocytes, such a smear can raise the possibility of a glioma. Alternatively, if stripped of cytoplasm during smearing, the near-naked nuclei of activated macrophages can mimic lymphoma.[1] In such cases, differential diagnosis must include demyelination, an infarct and infection. In difficult cases, it is best to give a broad intra-operative diagnosis such as ‘necrotic and gliotic tissue’ or ‘inflammatory and gliotic tissue and defer the diagnosis to evaluation of FFPE sections in correlation with clinical picture.[1]
On the contrary, one must look for evidence of neoplasia in an inflammatory lesion. For example, PCNSL often have an accompanying component of demyelination which may become more obvious following corticosteroid induced tumor regression.[1] Demyelinating features can also be seen in melanoma and post-radiation gliomas.
On occasions, an anaplastic oligodendroglioma or even anaplastic astrocytoma may resemble lymphoma on smears with mostly discohesive cells. Such a mistake can have significant treatment implications, as a diagnosis of lymphoma will abort the surgery, whereas high-grade glioma may undergo maximal safe resection or intracavity radiation.[20]
Although rare, a major pitfall on smears can be mistaking pilocytic astrocytoma (grade I) for anaplastic astrocytoma when it has significant nuclear atypia, vascular proliferation and perivascular infiltration. If present, Rosenthal fibres and eosinophilic granular bodies can be diagnostic in favour of pilocytic astrocytoma.
Mistaking granular cells of normal cerebellar cortex for a small round cell tumor such as medulloblastoma or lymphoma. Lack of anaplasia, mitosis, apoptosis, and presence of a regular fine neuropil and occasional Purkinje cells are helpful clues. Concomitant frozen section helps resolve this dilemma in most cases.
It is important to consider the differential diagnosis of low-grade neoplasms with monomorphic rounded nuclei. In addition to oligodendrogliomas, ependymoma, central neurocytoma (CN) and dysembryoplastic neuroepithelial tumor (DNET) can produce these features on smears.[21] Besides tumor location and imaging features, concomitant frozen sections and FFPE sections are needed for diagnosis.
The high-frequency of bare nuclei in oligodendroglioma on smears can raise the differential diagnosis of lymphoma or small cell carcinoma at low-power. Such a potential error can be prevented by a careful evaluation at high-power recognising the bland chromatin, mini-gemistocytes and at least some glial strands in oligodendroglioma.
Finally, gray matter intermixed with a glioma can be mistaken for ganglioglioma. The latter diagnosis is best made on FFPE sections with supportive immunoprofile.[14] Cytologic preparations have provided an effective platform for intra-operative diagnosis of CNS lesions in published studies. Among studies comparing intra-operative and FFPE diagnoses, frozen section and smear diagnoses matched the final diagnoses in 88% and 91.4% versus 76% and 81.4%, respectively.[9,22] The reported accuracy of cytological diagnosis in comparison with final diagnosis ranged from 75 to 94% in series that included patients with both open and stereotactic biopsies depending on the breadth of definition of accuracy and amount of tissue submitted for pathologic evaluation.[9,11] On the other hand, in studies that have examined the diagnostic accuracy of cytological investigation only for stereotactic brain biopsies, the accuracy rates have reportedly ranged from 69% to 87.5%[7,8,23] with a correlation rate of 77% in the largest series of 600 biopsies.[23] In another large study using three categories of correlation between intraoperative and final diagnoses: Complete, partial and no correlation, only 11% of cytological diagnoses were found to be incorrect.[11]
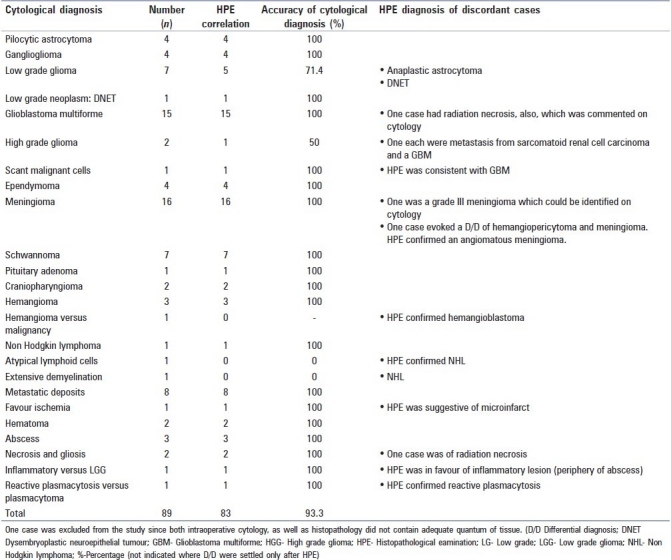
A review of our independent experiences based on recent one year's statistics retrieved from our combined databases revealed a total of 90 cases of CNS neoplasm with intraoperative as well as subsequent FFPE diagnosis [Table 1]. The quantum of tissue received in one case was suboptimal for both methods and hence excluded from the evaluation. Glial neoplasms with 38 cases (42.69%) constituted the majority, followed by 16 cases (17.97%) of meningeal tumors and 8 cases (8.98%) with metastatic deposits. Lymphomas, schwannomas, sellar/suprasellar and vascular tumors accounted for another 17 cases (19.10%). Remaining were vascular/inflammatory/reactive plasmacytosis and reactive gliosis lesions. Concordance between intra-operative cytological and subsequent histopathological diagnosis was noted in 83 out of 89 cases (93.26%). Two cases reported as low grade glioma were subsequently diagnosed as anaplastic astrocytoma and dysmbryoplastic neuroepithelial tumor (DNET). There were two cases of non-Hodgkin lymphoma in which one showed atypical lymphoid cells while the other showed features of extensive demyelination. A case with metastatic deposits from sarcomatoid renal cell carcinoma was signed out as high grade glioma on cytology. A hemangioblastoma was considered to be a hemangioma/low grade malignant tumor in cytology.
Table 1.
Correlation of intra-operative cytology with histopathology (n = 90)
Conversely, very few studies have systematically addressed the diagnostic inaccuracies of intraoperative consultation in CNS lesions. Discrepancies at intra-operative consultation have typically been reported because of sampling error, incorrect assignation of tumor grade or error in recognition of the histologic cell type.[6,19] A retrospective study of intraoperative cytologic preparations of over 4100 cases in which 95% accuracy in diagnosis (excluding grading deviations) was obtained found that ependymomas, glioblastomas, metastatic carcinomas, oligodendrogliomas, meningiomas and astrocytomas were the most frequently misdiagnosed lesions in a decreasing order.[24] Regarding discrepancy between frozen and FFPE diagnosis, a large study[19] found 57 (2.7%) discrepant diagnoses out of 2156 cases studied. About 80% of these could be classified into five categories: Spindle cell lesions including schwannomas and meningiomas (21%); astrocytoma versus oligodendroglioma (21%); differential diagnosis of CNS lymphoma (15.8%); reactive versus neoplastic process (14.0%); and tumor overgrading (7.0%). In a study of 1315 FS cases, the most frequent errors were in histologic typing of gliomas, hemangioblastomas and metastases.[25] In another study, the most common diagnostic difficulty was in differentiating low-grade glioma from gliosis.[26] Awareness of these pitfalls may help in further increasing diagnostic accuracy. The accuracy of cytologic diagnosis can be maximised by a clear understanding of the lesions that have propensity to occur in a particular location, characteristic radiologic patterns and that occur in a particular age groups.[12] It has also been suggested that adding smear to frozen section improves the overall accuracy of intraoperative diagnosis.[20]
Finally, neuropathologists should be mindful that they might have to face the challenge of ‘unexpected findings’ during intra-operative consultations from time to time. It must be kept in mind that the intraoperative pathologic diagnosis may not match the clinical picture at all. We would like to quote two simple examples from our personal experiences. In one case, stereotactic biopsy from an irregular ring-enhancing lesion (radiologically suggestive of glioblastoma) in an elderly showed numerous gemistocytes with mild atypia on smears and frozen section [Figure 8]. On further request, additional biopsies were obtained all of which showed the same features. A differential diagnosis of reactive gliosis versus the periphery of a glioma was considered and final diagnosis deferred to FFPE sections. One FFPE section additionally showed a few neutrophils focally. The final diagnosis favored a reactive inflammatory lesion such as an abscess. However, given the discrepancy with clinical findings, the treating team was only convinced of the diagnosis after the appearance of diffusion restriction on imaging a few days later and after the repeat biopsy showed obvious features of an abscess. In the second example, smear and frozen section from a patient in his fifties with similar radiologic picture showed necrosis and one toxoplasma microcyst. An additional biopsy was sent to microbiology lab and infectious work up began which showed serologic evidence of recent toxoplasma infection but also discovered unsuspected acquired immune deficiency syndrome (AIDS).
Figure 8.
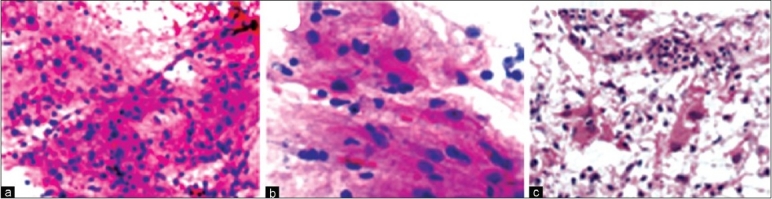
(a) Sparsely cellular glial tissue with a few capillaries (H and E, ×200). (b) Sparsely cellular glial tissue with a few mildly atypical gemistocytes (H and E, ×400) (c) Gemistocytes in tiny loose clusters with mild atypia admixed with neutrophils (H and E, ×400)
Based on our experiences, suggested algorithmic approaches to neurocytology are described in Figures 9–12.
Figure 9.
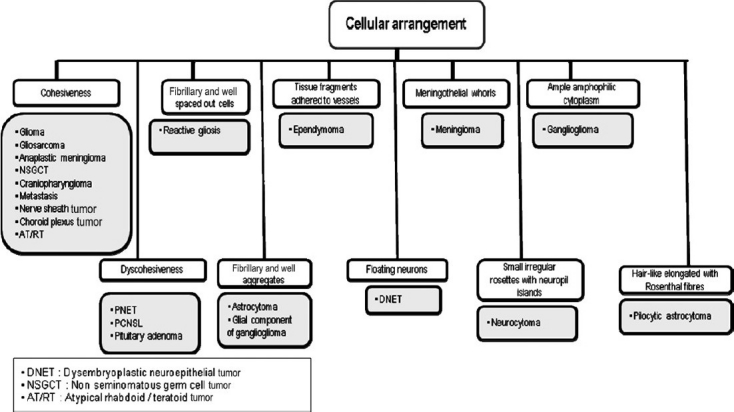
Algorithmic approach to neurocytology based on cellular arrangement
Figure 12.
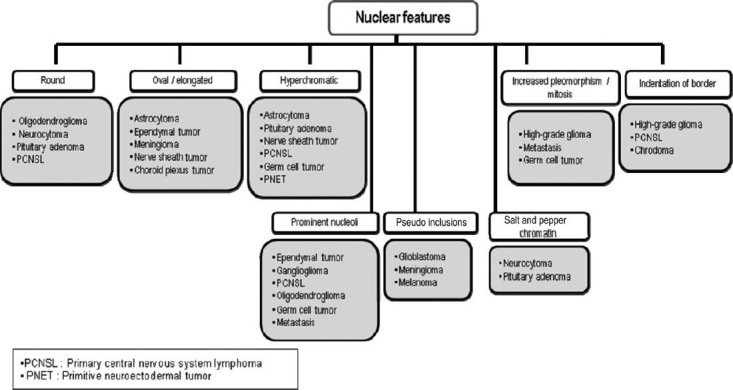
Algorithmic approach to neurocytology based on nuclear features
Figure 10.
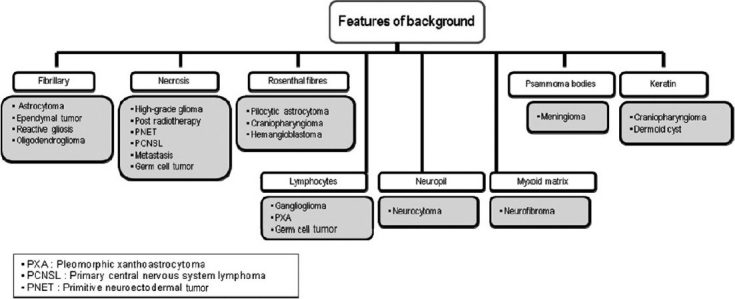
Algorithmic approach to neurocytology based on the background appearance
Figure 11.
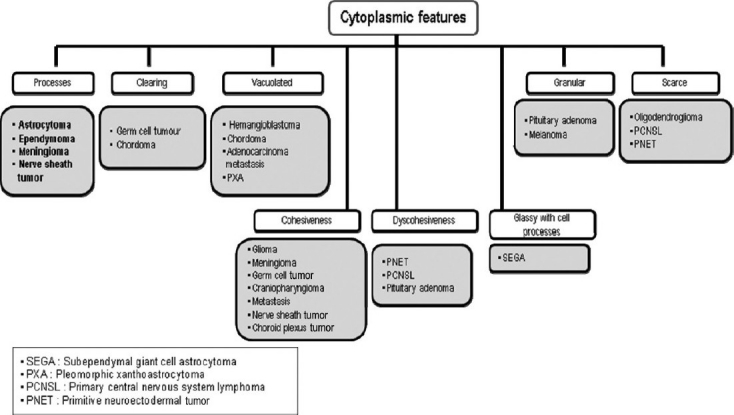
Algorithmic approach to neurocytology based on the cytoplasmic appearance
In summary, intraoperative cytologic evaluation constitutes an effective diagnostic modality, particularly on small samples with the caveat that its limitations must be clearly understood by both neuropathologists and neurosurgeons. It is important to evaluate intra-operative smears in the context of clinical and radiologic findings. This review is expected to provide a simplified approach to intra-operative neurocytologic diagnosis intended to improve the accuracy of surgical and neuropathologists. It is neither intended for beginners nor is it inclusive of the numerous details of individual entities and exceptions which are beyond the scope of this review. For details, the reader should refer to the standardized literature such as the WHO classification of the tumors of CNS,[27] listed references, review articles on recently described entities in CNS neoplasia and individual reports on rare CNS tumors. Therefore, this approach should only be used as guidelines and each case ultimately treated on its own merit.
Footnotes
Source of Support: Research incentive from the Dean of Medical College of Georgia to Suash Sharma
Conflict of Interest: None declared.
References
- 1.Adesina AM. Intraoperative consultation in the diagnosis of pediatric brain tumors. Arch Pathol Lab Med. 2005;129:1653–60. doi: 10.5858/2005-129-1653-ICITDO. [DOI] [PubMed] [Google Scholar]
- 2.Brainard JA, Prayson RA, Barnett GH. Frozen section evaluation of stereotactic brain biopsies: diagnostic yield at the stereotactic target position in 188 cases. Arch Pathol Lab Med. 1997;121:481–4. [PubMed] [Google Scholar]
- 3.Brommeland T, Lindal S, Straume B, Dahl IL, Hennig R. Does imprint cytology of brain tumours improve intraoperative diagnoses? Acta Neurol Scand. 2003;108:153–6. doi: 10.1034/j.1600-0404.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 4.Burger PC, Nelson JS. Stereotactic brain biopsies: specimen preparation and evaluation. Arch Pathol Lab Med. 1997;121:477–80. [PubMed] [Google Scholar]
- 5.Burger PC. Smears and frozen sections in surgical neuropathology. Baltimore: PB Medical Publishing; 2009. pp. 3–10. (163-287, 335-47, 359-99). [Google Scholar]
- 6.Firlik KS, Martinez AJ, Lunsford LD. Use of cytological preparations for the intraoperative diagnosis of stereotactically obtained brain biopsies: a 19-year experience and survey of neuropathologists. J Neurosurg. 1999;91:454–8. doi: 10.3171/jns.1999.91.3.0454. [DOI] [PubMed] [Google Scholar]
- 7.Folkerth RD. Smears and frozen sections in the intraoperative diagnosis of central nervous system lesions. Neurosurg Clin N Am. 1994;5:1–18. [PubMed] [Google Scholar]
- 8.Hayden R, Cajulis RS, Frias-Hidvegi D, Brody BA, Yu G, Levy R. Intraoperative diagnostic techniques for stereotactic brain biopsy: cytology versus frozen-section histopathology. Stereotact Funct Neurosurg. 1995;65:187–93. doi: 10.1159/000098693. [DOI] [PubMed] [Google Scholar]
- 9.Joseph JT. Diagnostic neuropathology smears. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 1–234. [Google Scholar]
- 10.Kitchen ND, Bradford R, McLaughlin JE. The value of per-operative smear examination during stereotactic biopsy. Acta Neurochir. 1993;121:196–8. doi: 10.1007/BF01809275. [DOI] [PubMed] [Google Scholar]
- 11.Kleihues P, Volk B, Anagnostopoulos J, Kiessling M. Morphologic evaluation of stereotactic brain tumor biopsies. Acta Neurochir Suppl. 1984;33:171–81. doi: 10.1007/978-3-7091-8726-5_26. [DOI] [PubMed] [Google Scholar]
- 12.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of tumors of the central nervous system - Lyon: IARC. (131-50, 163-204).2007:10–110. [Google Scholar]
- 13.Martinez AJ, Pollack I, Hall WA, Lunsford LD. Touch preparations in the rapid intraoperative diagnosis of central nervous system lesions.A comparison with frozen sections and paraffin-embedded sections. Mod Pathol. 1988;1:378–84. [PubMed] [Google Scholar]
- 14.Miller DC, Lang FF, Epstein FJ. Central nervous system gangliogliomas.Part 1: Pathology. J Neurosurg. 1993;79:859–66. doi: 10.3171/jns.1993.79.6.0859. [DOI] [PubMed] [Google Scholar]
- 15.Miller DC. Modern surgical neuropathology. New York: Cambridge University Press; 2009. pp. 5–9. (24-273, 363-98). [Google Scholar]
- 16.Ng HK. Cytologic features of ependymomas in smear preparations. Acta Cytol. 1994;38:331–4. [PubMed] [Google Scholar]
- 17.Ng HK. Cytologic diagnosis of intracranial germinomas in smear preparations. Acta Cytol. 1995;39:693–7. [PubMed] [Google Scholar]
- 18.Olasode BJ, Ironside JW. The brain smear, a rapid affordable intraoperative diagnostic technique for brain tumours appropriate for Africa. Trop Doct. 2004;34:223–5. doi: 10.1177/004947550403400412. [DOI] [PubMed] [Google Scholar]
- 19.Oneson RH, Minke JA, Silverberg SG. Intraoperative pathologic consultation: An audit of 1,000 recent consecutive cases. Am J Surg Pathol. 1989;13:237–43. [PubMed] [Google Scholar]
- 20.Parvani AV, Stelow EB, Pambuccian SE, Burger PC, Ali SZ. Atypical teratoid/rhabdoid tumor of the brain: cytopathologic characteristics and differential diagnosis. Cancer. 2005;105:65–70. doi: 10.1002/cncr.20872. [DOI] [PubMed] [Google Scholar]
- 21.Plesec TP, Prayson RA. Frozen section discrepancy in the evaluation of central nervous system tumors. Arch Pathol Lab Med. 2007;131:1532–40. doi: 10.5858/2007-131-1532-FSDITE. [DOI] [PubMed] [Google Scholar]
- 22.Powell SZ. Intraoperative consultation, cytologic preparations, and frozen section in the central nervous system. Arch Pathol Lab Med. 2005;129:1635–52. doi: 10.5858/2005-129-1635-ICCPAF. [DOI] [PubMed] [Google Scholar]
- 23.Regragui A, Amarti Riffi A, Maher M, El Khamlichi A, Saidi A. Accuracy of intraoperative diagnosis in central nervous system tumors: report of 1315 cases. Neurochirurgie. 2003;49:67–72. [PubMed] [Google Scholar]
- 24.Robbins PD, Yu LL, Lee M, Stokes BA, Thomas GW, Watson P, et al. Stereotactic biopsy of 100 intracerebral lesions at Sir Charles Gairdner Hospital. Pathology. 1994;26:410–3. doi: 10.1080/00313029400169092. [DOI] [PubMed] [Google Scholar]
- 25.Roessler K, Dietrich W, Kitz K. High diagnostic accuracy of cytologic smears of central nervous system tumors: A 15-year experience based on 4,172 patients. Acta Cytol. 2002;46:667–74. doi: 10.1159/000326973. [DOI] [PubMed] [Google Scholar]
- 26.Savargaonkar P, Farmer PM. Utility of intra-operative consultations for the diagnosis of central nervous system lesions. Ann Clin Lab Sci. 2001;31:133–9. [PubMed] [Google Scholar]
- 27.Sugita Y, Tokunaga O, Morimatsu M, Abe H. Cytodiagnosis of central neurocytoma in intraoperative preparations. Acta Cytol. 2004;48:194–8. doi: 10.1159/000326315. [DOI] [PubMed] [Google Scholar]


