Abstract
Background:
Current guidance recommends the use of fine needle aspiration cytology (FNAC) as an essential investigation in patients presenting with a thyroid lump. Current literature suggests that the sensitivity of FNAC in thyroid nodules ranges between 80-90%. However, only very few studies have looked specifically at the sensitivity of FNAC in solely thyroid cancer patients.
Aims:
The aim of our study was to investigate the value of FNAC as a first-line investigation in patients with thyroid cancer. We aimed specifically to assess the sensitivity of FNAC within this group.
Materials and Methods:
Patients diagnosed with thyroid cancer between 2000-08 were identified from a local histopathology database. Sixty-seven case notes were retrieved, retrospectively reviewed and analyzed. Analysis included results of FNAC, ultrasound scanning and final histopathological diagnosis.
Results:
Analysis of the 56 patients who underwent FNAC revealed that a cytological grading of thy3 or greater was only given to 31 cases (55.3%).
Conclusion:
In this study, FNAC findings of thy3 or greater were reported only in 55.3% of proven thyroid cancer cases. This study highlights the greater diagnostic difficulties of thyroid cancer compared to other thyroid nodules. Our findings suggest that clinicians must interpret the results of this initial investigation with caution and consider the routine use of ultrasound scanning to help guide FNAC.
Introduction
Thyroid cancer is the most common form of endocrine malignancy.[1] Data suggests that its incidence is slowly rising. The office for national statistics (ONS) published Cancer Statistics-registrations, which revealed that there were 1475 new cases of thyroid malignancies in England during 2005.[2]
The most common presentation of thyroid cancer is a newly diagnosed palpable nodule or an increase in size to a pre-existing nodule.[1] As for most head and neck cancers, early stage diagnosis and management often lead to better outcomes.[3] It is therefore of paramount importance that these changes are investigated correctly. It has been estimated that between 5-10% of thyroid nodules are malignant.[4] Previous management would have included excision of such nodules surgically in order to obtain a diagnosis. However, with the aid of better techniques, it is now possible to further identify those nodules that are at a higher risk of being malignant in an attempt to avoid any unnecessary procedures for those which are benign. Current guidance taken from recent British Thyroid Association/Royal College of Physicians (2007) and the American Association of Clinical Endocrinologists (AACE)/Association Medici Endocrinologi (AME)(2006) publications implicate fine needle aspiration cytology (FNAC) as the early investigation of choice for thyroid cancer.[1,5] FNAC is considered as the gold standard in the investigation of thyroid nodules. Studies have suggested a high sensitivity and specificity for predicting thyroid malignancies averaging 83%[5] and 92% respectively.[6–9] Unfortunately, FNAC is complicated by a recognized false-negative rate of approximately 5%.[5] Factors implicated for this rate include technique, slide preparation and interpretation of results by a cytopathologist. It is also well recognized that certain thyroid pathologies have similar cytological features which make diagnosis extremely difficult.[10] This problem is commonly reported with follicular lesions and therefore is often responsible for incorrect FNAC diagnoses.[11] In an attempt to reduce this error, guidance suggests the use of ultrasound scanning (USS) as an aid to diagnosis.[1,5] It has been shown that USS is helpful in reducing the number of inadequate fine-needle aspirates.[1] The AACE/AME suggested that USS should be used to “detect features suggestive of malignant growth” and to select lesions that would be suitable for FNAC.[5]
USS plus FNAC has been shown to be more sensitive, specific and accurate than either technique alone and is therefore recommended in the work-up of all thyroid nodules.[1,4,5]
From the review of the literature it is evident that there are only a handful of studies looking specifically at thyroid cancer groups. The majority of the existing evidence relates to the sensitivity of these investigations in assessing a thyroid nodule or neck lump, regardless of the final histology. As a result, accuracy and sensitivity rates have been shown to differ considerably as outlined later in the article. Therefore, the main aim and significance of our study is to ascertain the diagnostic yield of FNAC in patients with proven thyroid cancer, within a local geographic area. This data would then be compared to any existing evidence pertaining to the usefulness of FNAC in the diagnosis of thyroid cancer.
Materials and Methods
Sixty-seven patients were identified from a local histopathological database with a diagnosis of thyroid cancer. The database included patients from the Pennine Acute NHS Trust during the period 2000-08.
Medical records were retrieved and retrospectively analyzed. Data recorded included demographics, ultrasound findings, FNAC results and final histology. FNAC was classified according to the thy grading system which attaches a numerical coding to cytology, this in turn guides management [Table 1].
Table 1.
Diagnostic categories and proposed action after FNAC*

FNAC was performed by Ear, Nose and Throat (ENT) surgeons in the outpatient clinic; any other samples were obtained under ultrasound guidance. Cytopathology interpretation was undertaken by head and neck pathologists.
Data was then analyzed and correlation was made between the results of the FNAC with the final histological grading. The sensitivity and the false-negative rate were calculated in diagnosing thyroid neoplasms. Positive markers for FNAC were taken as thy 3 or greater which suggests a higher risk of cancer and usually necessitates surgery. USS reports were reviewed for any suspicious features of malignancy [Table 2] which may have aided diagnosis.[4,12–14]
Table 2.

Results
Of the 67 thyroid cancer patients identified, 50 were female and 17 were male (ratio 2.94:1). Mean ages were 54.1 and 57.8 for female and male patients respectively. The age range for the whole group was 27-90 years.
Table 3 correlates cytology with the final histology in the 56 patients who underwent FNAC. From these, diagnoses of thyroid cancer were represented by Papillary (n = 36), Follicular (n = 11), Anaplastic (n = 2), Medullary (n = 1) and metastatic (n = 2) subtypes.
Table 3.
Cytology versus final histology
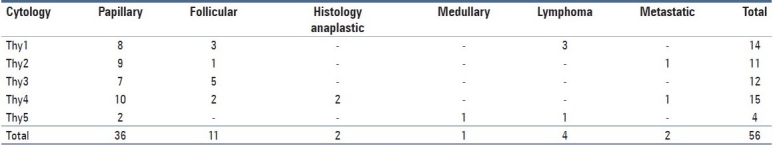
Cytology was thy3 or greater in 31/56 (55.3%) FNAC samples. As these samples were all taken from eventually proven thyroid cancer patients, this represents a sensitivity of 55.3% and therefore a false-negative rate of 44.7% for FNAC within this series.
Fifty-one patients underwent USS. Of these, USS showing suspicious features were noted in 10/51 (19.6%) patients. When correlating with cytology, these were subdivided into thy3 and greater (n = 8) and less than thy3 (n = 2).
Discussion
FNAC is recommended as the primary investigation for suspected thyroid cancer. This study aimed to evaluate the usefulness of the diagnostic modality when used in a population of proven thyroid cancer patients.
FNAC has been widely accepted as a rapid, accurate and inexpensive diagnostic method which either enhances or reduces the probability of thyroid cancer prior to definitive histology.[15,16] It is therefore used as a tool in the planning for surgery.[1] In this series, the sensitivity of FNAC was found to be 55.3%, with a false-negative rate of 44.7%. This means that from the study group of patients who underwent FNAC and would be found to have thyroid cancer, only just over half (n = 31) obtained results of thy3 or above which indicated a need for surgery. Therefore, it was shown that a significant number of patients with thyroid cancer had FNAC results which did not suggest the need for more urgent management.
There are few studies published that focus solely upon thyroid cancer patients. A study of 62 thyroid cancer patients at the University of California, Los Angeles (UCLA) revealed that FNAC had a sensitivity of 71% and a false-negative rate of 14.5%.[11] Both these values are regarded to represent relatively poor test performance and therefore may be related to thyroid cancer specifically versus other histology. One further explanation may be that these outcomes are reflective of the performance of this diagnostic modality within a non-study environment. To improve outcomes it is recommended that ultrasound-guided FNAC techniques become more commonplace as the method for obtaining cytology as it has been shown to obtain better samples hence improve diagnostic rates.[17]
The current guidance recommends that aspiration may be performed by those clinicians with “expertise and interest within thyroid disease” and that they “should perform sufficient aspirates to maintain expertise and their performance should be monitored”.[1] FNAC samples from this study group were obtained by experienced registrars and consultant ENT surgeons. This ensured that poor test performance due to inexperience was minimized.
One of the limitations of this study group is that it comprised FNAC samples taken by different operators, hence with varying skill levels and experience. In addition, independent monitoring of successful FNAC rates did not take place. Previous studies have shown that between 10-20% of aspirates are unsatisfactory.[5] Those which are labeled as unsatisfactory have an inadequate number of cells for analysis or may be due to cystic fluid, bloody smears or poor technique in obtaining the sample and/or preparing the slides. The Papanicolaou Society of Cytopathology task force on Standards of Practice recommends that “aspirators who persistently produce a high rate of unsatisfactory aspirates (>15%) should be identified and given remedial training.[18] Despite this, operators within this study group have been shown to be well experienced. These results therefore pose the question as to why FNAC has been found to perform so poorly within this group of thyroid cancer patients when compared to publicized rates for sensitivity and false-negatives.
Once performed, cytology should be reported by a “cytopathologist with a special interest in thyroid disease who should be a member of a multidisciplinary team (MDT)”.[1] The cytopathologist will be required to provide a descriptive report which guides management. National guidance recommends the use of numerical coding such as the “thy” grading system which provides a consistent framework to the management of cytological results. Thy grading of 3 and above often necessitates surgical management and is therefore significant. The cytology within this series was assessed by cytopathologists who were members of the head and neck cancer MDT. However, each specimen was not analyzed by the same cytopathologist as patients were investigated at different centres across the Trust. Despite the experience of the reporters in our study, there would inevitably be discrepancies within cytology reporting, thus proving to be a further limitation to the study group.
As mentioned earlier, certain pathologies of thyroid cancer have been shown to be notoriously difficult to diagnose cytologically, and therefore may explain the poor test performance despite the use of operators with a high degree of expertise. For certain lesions, cytology alone may not be enough to give a diagnosis. It has been shown that differentiation between a cellular colloid goiter and follicular lesion is very difficult.[10] Distinguishing between follicular adenoma and carcinoma is not possible via FNAC as it is requires demonstration of invasion of the capsule, lymphatics or blood vessels.[11] Results from our study show that 25% (n = 14) of thy1 cytology were operated upon. This cytology represented repeat FNAC samples. Additional information from ultrasound scanning showed features of malignancy in two cases of thy1 cytology. In the thy1 cases without suspicious USS features, the decision to operate was based upon clinical suspicion and patient preference.
Literature suggests that diagnosing papillary carcinoma is less of a problem. Interestingly, our series shows that 4/11 (36%) follicular carcinomas were graded as thy 2 or less on FNAC compared to 17/36 (47%) papillary carcinomas. These results indicate that there is a greater degree of difficulty in diagnosing the papillary form of cancer.
A possible reason for the unacceptably high false-negative rates for papillary cancer may be the variability of the cytopathologists reporting the cytology and the surgeons performing the FNAC. Although the majority of the reporters and aspirators were experienced, some were still developing their expertise within this field. However, due to the relatively few numbers, further study on a greater number of cases is needed in order to gain a more accurate picture.
These results may be explained by problems with the thy grading system with regards to under-grading cytology. An alternative system for classifying thyroid cytology is the Bethesda system. This system classifies the data into six subgroups and is shown in Table 4. The significant difference between this and the thy classification is the Atypia sub-group. This allows cytology to be categorized at possibly a more appropriate level when compared to the “thy” grading system. Within the thy grading system for example, there may be cases that fall into the thy3(ii) sub-group, notably cases where samples contain very scanty cells, and therefore cannot be included in thy2 or thy4 group. This may help to avoid more invasive procedures such as a hemi-thyroidectomy, and often necessitates a repeat FNAC. From our study data, a large number (n = 7) of final papillary cancers were placed in the thy3(ii) category due to the above reasons. These would all be classified as Bethesda III, thus warranting a repeat FNAC. However, given that the final histology was of papillary carcinoma, the Bethesda system somewhat under-classified this cytology. Consequently, this represents an alarming number of papillary carcinomas which were not detected upon the first FNAC. As already noted, this finding appears significant to our study when compared with similar series.
Table 4.
Bethesda classification for thyroid cytology

From analyzing the histological data, it is apparent that a number (n = 2) of the papillary carcinomas that were initially graded thy1 and thy2 exhibited cystic properties. This may be one explanation of the poor test performance as these properties can make diagnosis of malignancy difficult. Other reasons for such poor performance may be associated with areas already discussed such as technique, cytological reporting and operator variability.
This series revealed a false-negative rate of 44.7% for FNAC. As mentioned earlier, published ranges for false-negative FNAC are far more modest (range 0-29%).[15] However, a study by Jarvi et al.,[19] reported false-negative rates of 50%, which is similar to our series. Their study focused upon 20 patients with thyroid malignancy. The authors attributed the poor results to inexperienced operators. However, results from our series most likely reflect a combination of operator variability, low numbers and the diagnostic difficulty of using FNAC in certain thyroid pathologies. Given the high rate of false-negative FNAC results, it is recommended that solitary nodules are subject to close follow-up. It has been shown that 7% of repeat FNAC are reported as positive for malignancy, where initial FNAC was negative.[20] This implies that repeat FNACs have an important role in the non-surgical management of thyroid nodules.
This study was a retrospective case note review of patients who were histologically diagnosed with thyroid cancer. This posed several limitations pertaining to the statistical analysis of the data obtained as there was no data within the database with regards to benign histology. This series would therefore not provide figures for specificity, accuracy, false-positive and true-negative rates for FNAC in thyroid cancer. Sensitivity is defined as “the likelihood that the patient with a disease has positive test results” and therefore could be reflected from the series.[5] Likewise, false-negative rates could be calculated as it is defined as the proportion of patients where investigation is negative despite having the disease.
Although this series reported high false-negative rates for individual modalities, many patients progressed to surgery as a result of a combination of a high index of clinical suspicion, FNAC and USS. This reinforces the importance of MDT discussion in the correct management of thyroid cancer patients.
FNAC has been shown to have a good diagnostic yield when used correctly. The British Thyroid Association/Royal College of Physicians and AACE/AME recommend that USS and FNAC are used in the initial work-up of thyroid nodules, as studies have shown higher rates for sensitivity, specificity and accuracy when combined.
When reviewing our data retrospectively, we found that a surprisingly low number of USS (19.6%) showed suspicious features as listed in Table 2. In the 25 cases of thy1 or thy2 cytology, only two showed suspicious features on USS. It may be the case that there are certain features related to this group which explain why both USS and cytology did not identify the tumor. For example, the tumor may have been small or posteriorly placed. In addition, patient factors such as a large neck may have made sampling more technically challenging as well as imaging less accurate. Despite this, USS has been shown in the literature to yield acceptable sensitivity rates, such as 84.9% by Moon et al.,[17] in diagnosing thyroid nodules greater and lesser than 1 cm in size. USS has been shown to be useful as a result of the combination of the technique being a cheap, non-invasive method of assessing the characteristics of a thyroid lump. In addition, it can be a vital tool in aiding adequate cytological sampling. These reasons combined with evidence from the literature on sensitivity rates as well as the authors’ own findings/experience makes USS a useful tool in the workup for thyroid cancer. A review of the literature yielded very little evidence regarding the relationship between the histological subtypes of thyroid cancer and diagnosis with USS. Therefore, as USS can only infer malignancy depending on the radiologists’ interpretation, it is often used as an adjuvant (with FNAC) in the diagnosis of thyroid malignancy.
We have shown that in a series of ‘thyroid cancer only’ patients the sensitivity rates of FNAC were below those commonly quoted in the literature, which for thyroid nodules are between 80-90%.[5] A low rate was also reported in a similar sample size group of ‘thyroid cancer only’ patients from the UCLA study.[11] These studies therefore highlight the greater diagnostic difficulty of thyroid cancer compared to thyroid nodules, using FNAC and USS. Greater consideration should be given to the inherent diagnostic difficulties associated with performing FNAC on thyroid malignancies as opposed to other pathologies. This can have serious implications upon the management of patients with thyroid lumps. We therefore suggest that routine use of USS guidance when obtaining FNAC samples is important in reducing false-negative rates for this modality when dealing with thyroid cancer. If this is not possible, the clinician must keep a high index of suspicion when dealing with these patients in order to avoid missing a diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Perros P, editor. Report of the Thyroid Cancer Guidelines Update Group. 2nd ed. London: Royal College of Physicians; 2007. British Thyroid Association, Royal College of Physicians. Guidelines for the management of thyroid cancer. [Google Scholar]
- 2.Cancer Statistics; Registrations [internet] The Office for National Statistics. [Last updated on 2005; Last cited on 2009 June 24]. Available from: http://www.statistics.gov.uk .
- 3.Guidance on cancer services: Improving outcomes in head and neck cancers - the manual [internet] National Institute of Clinical Excellence. [Last Updated on 2004; Last cited on 2008 Aug 24]. Available from http://www.nice.org.uk/guidance/index.jsp%action = byIDando = 10897 .
- 4.Morris LF, Ragavendra N, Yeh MW. Evidence-based assessment of the role of ultrasonography in the management of benign thyroid nodules. World J Surg. 2008;32:1253–63. doi: 10.1007/s00268-008-9494-z. [DOI] [PubMed] [Google Scholar]
- 5.Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma.American Association of Clinical Endocrinologists. American College of Endocrinology. Endocr Pract. 2001;7:202–20. [PubMed] [Google Scholar]
- 6.Gharib H. Fine-needle aspiration biopsy of thyroid nodules: Advantages, limitations, and effect. Mayo Clin Proc. 1994;69:44–9. doi: 10.1016/s0025-6196(12)61611-5. [DOI] [PubMed] [Google Scholar]
- 7.Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: Progress, practice, and pitfalls. Endocr Pract. 2003;9:128–36. doi: 10.4158/EP.9.2.128. [DOI] [PubMed] [Google Scholar]
- 8.Gharib H, Goellner JR. Fine-needle aspiration biopsy of thyroid nodules. Endocr Pract. 1995;1:410–17. doi: 10.4158/EP.1.6.410. [DOI] [PubMed] [Google Scholar]
- 9.Jeffrey PB, Miller TR. Fine-needle aspiration cytology of the thyroid. Pathology (Phila) 1996;4:319–35. [PubMed] [Google Scholar]
- 10.Howlett DC, Harper B, Quante M, Berresford A, Morley M, Grant J, et al. Diagnostic adequacy and accuracy of fine needle aspiration cytology in neck lump assessment: results from a regional cancer network over a one year period. J Laryngol Otol. 2007;121:571–9. doi: 10.1017/S0022215106004944. [DOI] [PubMed] [Google Scholar]
- 11.Kopald KH, Layfield LJ, Mohrmann R, Foshaq LJ, Giuliano AE. Clarifying the role of fine-needle aspiration cytologic evaluation and frozen section examination in the operative management of thyroid cancer. Arch Surg. 1989;124:1201–5. doi: 10.1001/archsurg.1989.01410100107018. [DOI] [PubMed] [Google Scholar]
- 12.Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of US findings in the management of thyroid nodules. QJM. 2006;100:29–35. doi: 10.1093/qjmed/hcl121. [DOI] [PubMed] [Google Scholar]
- 13.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001;136:334–7. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- 14.Leenhardt L, Menegaux F, Franc B, Delbot T, Mansour G, Hoang C, et al. Selection of patients with solitary thyroid nodules for operation. Eur J Surg. 2002;168:236–41. doi: 10.1080/11024150260102852. [DOI] [PubMed] [Google Scholar]
- 15.Morgan JL, Serpell W, Cheng MS. Fine-needle aspiration cytology of thyroid nodules: how useful is it? ANZ J Surg. 2003;73:480–3. doi: 10.1046/j.1445-1433.2003.02670.x. [DOI] [PubMed] [Google Scholar]
- 16.Handa U, Garg S, Mohan H, Nagarkar N. Role of fine needle aspiration cytology in the diagnosis and management of thyroid lesions: A study on 434 patients. J Cytol. 2008;25:13–7. [Google Scholar]
- 17.Moon HG, Jung EJ, Park ST, Ha WS, Choi SK, Hong SC, et al. Role of ultrasonography in predicting malignancy in patients with thyroid nodules. World J Surg. 2007;31:1410–6. doi: 10.1007/s00268-007-9013-7. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines of the Papanicolaou Society of Cytopathology for fineneedle aspiration procedure and reporting. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol. 1997;17:239–47. doi: 10.1002/(sici)1097-0339(199710)17:4<239::aid-dc1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Jarvi K, Mokka R, Vorne M. Problems of cancer diagnosis in thyroid nodules. Acta Chir Scand. 1988;154:93–6. [PubMed] [Google Scholar]
- 20.Dwarakanathan AA, Staren ED, D’Amore MJ, Kluskens LF, Martirano M, Economou SG. Importance of repeat fine-needle biopsy in the management of thyroid nodules. Am J Surg. 1993;166:350–2. doi: 10.1016/s0002-9610(05)80330-7. [DOI] [PubMed] [Google Scholar]


