Abstract
Background:
Severe bleeding as a result of trauma frequently leads to poor outcome by means of direct or delayed mechanisms. Prehospital fluid therapy is still regarded as the main option of primary treatment in many rescue situations. Our study aimed to assess the influence of prehospital fluid replacement on the posttraumatic course of severely injured patients in a retrospective analysis of matched pairs.
Materials and Methods:
We reviewed data from 35,664 patients recorded in the Trauma Registry of the German Society for Trauma Surgery (DGU). The following patients were selected: patients having an Injury Severity Score >16 points, who were ≥16 years of age, with trauma, excluding those with craniocerebral injuries, who were admitted directly to the participating hospitals from the accident site. All patients had recorded values for replaced volume and blood pressure, hemoglobin concentration, and units of packed red blood cells given. The patients were matched based on similar blood pressure characteristics, age groups, and type of accident to create pairs. Pairs were subdivided into two groups based on the volumes infused prior to hospitalization: group 1: 0-1500 (low), group 2: ≥2000 mL (high) volume.
Results:
We identified 1351 pairs consistent with the inclusion criteria. Patients in group 2 received significantly more packed red blood cells (group 1: 6.9 units, group 2: 9.2 units; P=0.001), they had a significantly reduced capacity of blood coagulation (prothrombin ratio: group 1: 72%, group 2: 61.4%; P≤0.001), and a lower hemoglobin value on arrival at hospital (group 1: 10.6 mg/dL, group 2: 9.1 mg/dL; P≤0.001). The number of ICU-free days concerning the first 30 days after trauma was significantly higher in group 1 (group 1: 11.5 d, group 2: 10.1 d; P≤0.001). By comparison, the rate of sepsis was significantly lower in the first group (group 1: 13.8%, group 2: 18.6%; P=0.002); the same applies to organ failure (group 1: 36.0%, group 2: 39.2%; P≤0.001).
Conclusion:
The high amounts of intravenous fluid replacement was related to early traumatic coagulopathy, organ failure, and sepsis rate.
Keywords: Hemorrhagic shock, mortality, prehospital volume replacement, rescue time, trauma registry, trauma
INTRODUCTION
Bleeding, as a result of severe trauma, is correlated with high initial mortality and a high rate of secondary complications.[1–4] Blunt trauma represents the most frequent form of severe trauma in Europe (95% in Germany according to the Trauma Registry 2008 Annual Report). Blunt trauma causing bleeding into the large body cavities (thoracic and abdominal) is especially hard to access therapeutically. Furthermore, these injuries are related with an increased mortality.[5–8]
At first glance, the reasonable course of action appears to be to replace the blood lost by fluid as quickly as possible, that is, at the accident site.[9] However, this assumption has not yet been confirmed in any study with sufficient patient numbers. In contrast to blunt trauma, the influence of prehospital fluid replacement on penetrating injuries has been more thoroughly investigated. Follow-up examinations of soldiers wounded in the Falklands war reported that patients with hypotensive circulation and simultaneous hyperpyrexia prior to hospital treatment had a better outcome.[10] Further studies involving patients who suffered penetrating injuries showed that an excessive volume replacement (>2000 mL), which also resulted in longer rescue times, correlated with increased mortality after trauma in most cases.[11–13] Kreimeier et al. also showed an advantage for moderate fluid replacement and permissive hypotension (90 mmHg) in patients with penetrating injuries. This strategy has also the benefit that it reduces rescue time and is supported by several authors.[14–17]
To date definite evidence-based recommendations for the prehospital treatment of patients with hemorrhage after blunt trauma do not exist. Anecdotal reports suggest that a short rescue time and delivery directly to a level one trauma center is beneficial, as per the recommended course of action for penetrating injuries, but no recommendations exist regarding how aggressively the circulation should be supported by volume replacement. A recent study by Geeraedts et al. recommended keeping treatment at the accident site as minimal as possible, with the goal of maintaining vital signs and providing a rapid transport to a higher level trauma center.[18] On the other hand, some reports continue to recommend extensive volume replacement as the best treatment option.[19–21] Turner et al. identified no dependence of mortality or outcome on the infused volume in patients with blunt trauma.[22] However, this study focused on less severely injured patients (>75% had injury severity score (ISS) <16).
The following question arises after examination of the current literature: Does the quantity of the volume replaced have consequences for hemorrhagic shock in the posttraumatic course, including multiple organ failure (MOF), sepsis, and outcome? We addressed these questions in a patient cohort selected from the DGU Trauma Registry, which had suffered severe injuries (abbreviated injury scale >3) that resulted in hemorrhage.
MATERIALS AND METHODS
The Trauma Registry of the German Society for Trauma Surgery was started in 1993, which contains prospectively collected data from 145 collaborating European trauma centers. Data were entered manually from patient records until 2001, when data input was automated for central submission via online data entry software (since 2002). About 100 data points per patient are collected, including the coding of each injury according to the abbreviated injury scale (AIS), revised version of 1998. Data are submitted to a central database that is hosted by the Institute for Research in Operative Medicine at the University of Witten/Herdecke, Cologne, Germany. Irreversible data anonymity is guaranteed for the patient and the participating hospital. Only patients from Germany and Austria were included in this study to minimize variation due to different rescue systems. All patients were attended by a physician prehospitally. Records collected between 1993 and 2007 (total: 35,664 patients) were considered for this study. The data of the Trauma Registry of the DGU has received the full approval of the ethics committee of the University of Witten/Herdecke, Cologne, Germany.
Patients were selected for this study according to the following criteria:
Primary admission to the hospital (no transfers)
ISS ≥16
Age ≥16 years
Infusion of at least one unit of packed red blood cells (pRBC)
Data available for the prehospital administered fluid volume, Hb concentration on hospital admission and blood pressure at accident site and on admission.
According to the amount of prehospitally administered volume (crystalloids plus colloids), the patients were divided into a “low-volume” (≤1500 mL) and a “high-volume” (≥2000 mL) group. This classification complies with the statistical mean value of all patients that were recorded in the Trauma Registry of the DGU (MV, 1296±1137 mL; n=35,664 patients). Values between 1501 and 1999 mL were hardly documented and excluded from this analysis, because only a small number of patients were administered a volume between 1501 and 1999 mL. The decision for a higher volume replacement always seems to be reconciled with a volume higher than 1999 mL. In order to evaluate the effect of prehospital volume administration, patients with high and low volume were then matched according to the following criteria:
Pattern of injury for the following three body regions: head, torso (thorax and abdomen), and extremities, where matching criteria were AIS severity ≥ or <3 points
Date of accident had to be within ±1 year
Systolic blood pressure at the accident site had to be at least 60 mmHg and was subdivided into three groups which had to match exactly: (1) 60-89, (2) 90-99, and (3) ≥100 mmHg.
Age categories with three subgroups had to match exactly: (1) 16-54, (2) 55-69, and (3) ≥70 years of age.
Sepsis was defined according to the criteria of bone which are close to the American College of Chest Physicians/Society of Critical Care Medicine (ACCP-SCCM) consensus conference definition.[23] Single organ failure was defined as a value ≥3, using the definition determined by the Sequential Organ Failure Assessment (SOFA) Score.[24] This value is entered as the total point value by individual hospitals. A posteriori, no conclusion about the individual patient can be drawn. MOF is listed if simultaneous organ failure was recorded for at least 2 organs. Prehospital parameters, hospital stay, and coagulation were examined separately in each group. Concerning the coagulation, the prothrombin ratio is the only value recorded in the Trauma Registry. The fibrinogen levels, for instance, are not documented. To be able to evaluate the ISS within the groups with sufficiently complete data, prognosis estimation by means of the revised injury severity classification (RISC) was performed.[25] The prognosis established in this way is contrasted with the observed mortality in the corresponding group. Prognosis was also calculated according to the Trauma and Injury Severity Score (TRISS).
Statistics
Data were analyzed with the Statistical Package for the Social Sciences (SPSS; version 15, Chicago, IL, USA). Incidences are represented as the number of cases and percentages, and measured values as mean and standard deviation. Differences between the two groups with low and high amount of prehospital volume were compared with the Chi-square test in case of categorical variables, and t test in case of continuous variables. In case of obvious deviation from normality continuous variables were tested with a nonparametric rank test (Mann-Whitney). We applied a significance level α of 5% to all statistical tests.
RESULTS
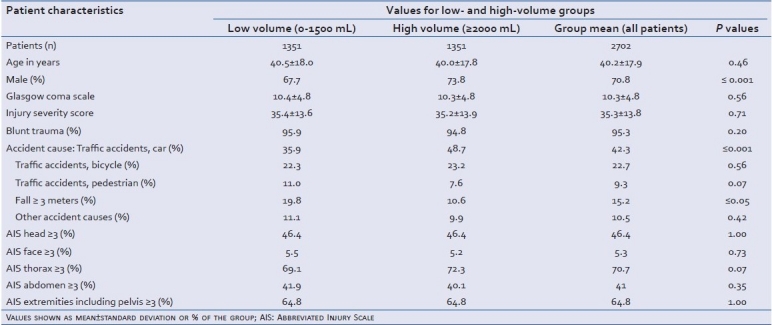
From the DGU Trauma Registry, 1351 severely injured patients with a high amount of prehospital volume could be matched with 1351 patients from the low-volume group. Patient age in the total group averaged 40.2 years (±17.9), and 70.8% were male. There were significantly more males in the high-volume than in the low-volume group (73.8% versus 67.7%, respectively; P≤0,001). The ISS was the same in both groups (low-volume: 35.4±13.6, high-volume: 35.2±13.9; P=0.71). There was a similar distribution for the cause of accident in high- and low-volume groups. However, there were proportionally more patients in the high-volume group that were injured in car accidents (high-volume: 48.7%, low-volume: 35.9%; P≤0,001), and proportionally more accidents involving falls from a height of ≥3 m in the low-volume group (low-volume: 19.8%, high-volume: 10.6%; P≤0,05). All other accident causes were distributed homogenously without differences in frequency [Table 1]. As expected, the majority of the injuries were blunt trauma (95.3%). The subdivision of the injury severity in the corresponding body regions had nearly identical distributions in the groups [Table 1], as was the Glasgow Coma Scale (GCS; low-volume: 10.4, high-volume: 10.3). The similarity of these general characteristics of the patients involved in this study support that the groups receiving low- or high-volume replacement therapy were similar, and are comparable.
Table 1.
Demographical and clinical data for bleeding, severely injured patients treated prior to hospitalization with low- and high-volume fluid replacement therapy (1351 patients per group)
Prehospital and emergency room treatment
Although significantly less fluid was infused prior to arrival at the hospital in the low-volume group (on average, 1053 mL compared with 2793 mL in the high-volume group; P≤0.001), there was no significant difference in the blood pressure measured at the accident site based on the distribution of the blood pressure categories within the groups. As shown in Table 2, the percentage of patients who had a blood pressure ≤90 mmHg was exactly the same in both groups. It is a similar situation with the heart rate. As shown in Table 2, the groups are almost the same here, too (low-volume group: 98.0 beats/min, high-volume group: 102.2 beats/min). The heart rate as well as the blood pressure recovered at arrival in the emergency room. However, fewer (24.8%) of the low-volume group patients had blood pressures ≤90 mmHg as compared with the high-volume group (26.4%, Table 2). Taken together, the clinical parameters for circulation are similar in the patient groups receiving either low- or high-volume fluid replacement therapy.
Table 2.
Patient data from treatment at the accident site, in the emergency room and during initial surgical treatment, group-specific
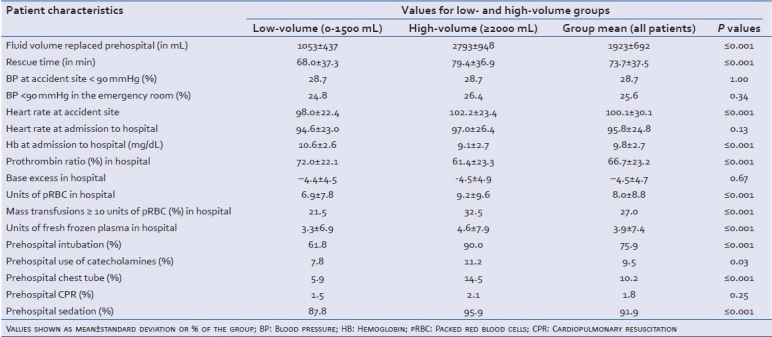
Hemoglobin, base excess and coagulation values were measured during treatment in the emergency room. Patients who received high fluid volumes showed significantly worse blood values: The hemoglobin value was significantly lower in the high-volume group (low-volume: 10.6±2.6 mg/dL, high-volume: 9.1±2.7 mg/dL; P≤0.001 [Table 2]. The same applies to the prothrombin ratio (low-volume: 72.0% ± 22.1%, high-volume: 61.4% ± 23.3%; P≤0.001). Patients in the high-volume group received more units of pRBC (low-volume: 6.9 units ±7.8 units, high-volume: 9.2 units ±9.6 units; P≤0.001). A higher percentage of patients in the high-volume group received more than 10 units pRBC (low-volume: 21.5%, high-volume: 32.5%; P≤0.001). The same holds true for the transfusion of fresh-frozen plasma (low-volume: 3.3 units, high-volume: 4.6 units; P≤0.001, Table 2).
As shown in Table 2, patients receiving high-volume replacement also required further prehospital therapeutic measures, such as catecholamine supplementation, intubation, and chest tube at the accident site. Rescue time, defined as the time until arrival in the emergency room, for the high-volume group was significantly higher than for the low-volume group, 79.4 min versus 68.0 min (P≤0.001), respectively [Table 2].
Clinical course and outcome
The number of patients treated with an operative therapy was significantly higher in the low-volume group (low-volume: 95.8%, high-volume: 94%; P≤0.036, Table 3). The days spent in the intensive care unit (ICU), the total hospital length of stay, and the total days of intubation were also similar in both groups [Table 3]. Some parameters differed significantly between low- and high-volume groups: the ventilator-free days with reference to the first 30 days (low-volume: 15.6 d, high-volume: 13.9 d; P≤0,001) and the ICU-free days with reference to the first 30 days (low-volume: 11.5 d, high-volume: 10.1 d; P≤0.001) [Table 2]. The occurrence of sepsis and organ failure was significantly higher in patients receiving high-volume replacement [Table 3]. The occurrence of multiple organ failure was not significantly different in the groups; however, there was a trend toward lower occurrence of multiple organ failure in the low-volume group [Table 3].
Table 3.
Clinical course and outcome of patients receiving either low- or high-volume prehospital replacement therapy after trauma and bleeding
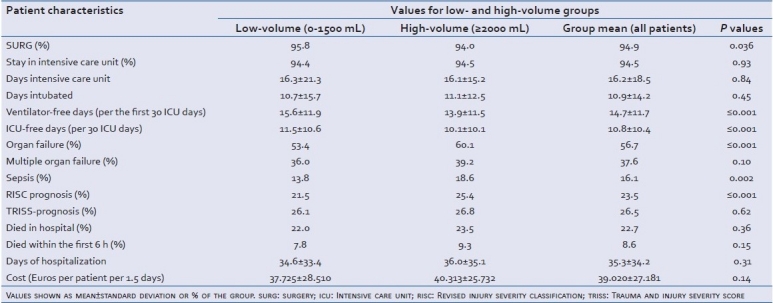
Although the TRISS prognosis showed no significant difference between low- and high-volume groups, the RISC prognosis showed a higher probability of dying for patients in the high-volume group, 25.4% compared with 21.5% in the low-volume group (P≤0.001; Table 3). In this context, it has to be pointed out that for the calculation of the TRISS prognosis, the respiratory rate has to be documented at the accident site. In the Trauma Registry of the DGU (Effective 2009), this is the case in only 60% of the documented patients. Therefore, the validity of the TRISS prognosis, in contrast to the RISC prognosis, is restricted because of the small number of cases. The percentage of mortalities was similar between low- and high-volume groups, with 22.0% versus 23.5% (P≥0.62), respectively. The percentage of patients that died within the first 6 h was also not significantly different; however, the percentage in the low-volume group was lower with 7.8% versus 9.3% in the high-volume group (P=0.15; Table 3). Treatment cost was higher for the high-volume group with treatment costing €2500 more per 1.5 days per patient [Table 3].
DISCUSSION
The aim of this study was to statistically examine relevant differences in organ failure, sepsis, clinical course, and outcome in patients receiving either low or high volumes of prehospital fluid replacement during rescue after trauma with severe hemorrhage. The study was limited to patients for whom it was possible to show an advantage or disadvantage of a fluid replacement therapy based on the severity of their injuries with related blood loss and similar regions of injury. Matched-pairs analysis was performed to distinguish between two groups having the same initial conditions but receiving different volumes of replacement therapy, thus allowing the assessment of the patient benefit from different volumes of prehospital fluid replacement. It has to be pointed out that a matched-pairs analysis based in a retrospective analysis of a trauma registry can only give clues to particular connections.
Compared to penetrating trauma, definite recommendations concerning the quantity of prehospital fluid replacement therapy after blunt trauma did not previously exist in the literature. The data records for blood pressure-related penetrating trauma are well documented, and recommend, in addition to fast transport to a level one trauma center, a permissive hypotension and only moderate fluid replacement.[15–17]
Our study shows that rescue time has an influence on the hemorrhage volume prior to hospitalization, and thus, must be regarded as a factor in future studies making recommendations for prehospital therapy. The question regarding the appropriate volume for prehospital therapy cannot be answered without also considering the rescue time. Our results show a possible connection between a long rescue time, a related increased volume replacement and, as a consequence, an impairment of the coagulation system and the hemoglobin values upon arrival at the hospital. This is also illustrated by the number of units of transfused pRBC necessary in patients receiving either low or high volumes of prehospital replacement therapy, and is especially apparent in those patients who received more than 10 units of pRBC. A connection supported in studies assessing blunt trauma patients in England by Turner et al., Trunkey, and a current study by Geeraedts et al.[18,22,26] In addition, our study shows an association between a high number of therapeutic activities during rescue, such as intubation and chest tube, and a longer rescue time as well as a higher volume of fluid replacement in case of similar injury severity of the pairs. The decision of extending therapy conducted at the accident site must be made individually on a case-by-case basis. A comprehensive set protocol can certainly not be established for this situation. However, this study supports that recommendations for the prehospital treatment of patients with penetrating trauma also apply to patient with blunt traumas. That is, to limit prehospital therapy to only the bare necessities of stabilizing the cardiovascular and pulmonary systems, and make rapid transport to a level one trauma center the priority.[18]
The question why the patients of the high-volume group on average received so much more volume (~1500 mL) remains speculative. The classification was made in a way that not only the hemodynamic initial conditions were approximately identical, but also the injury severity per body region (AIS). Apart from the thoracic injuries, of which 3.2% more were found in the high-volume group, the remaining injuries of the body regions were almost exactly the same. A possible bias by means of the distribution of dissimilar severity injuries was thus minimized. In addition, the difference of the mean ISS between both the groups is not significant. As already mentioned at the outset, the individual decision on the volume to be administered rests with the attending personnel. However, it has to be emphasized that in a retrospective statistical analysis, no conclusions about the individual decisions can be drawn. Therefore, it is only to be assumed, and this is supported by the present data, that once a team opts for an extended therapy, this is related to an elevated volume replacement. The injury severity does not always seem to be determining in this context. The significant higher number of chest tubes can certainly not be explained by the slightly higher number of thoracic injuries in the high-volume group. It may be that here rather the standard of education and the experience of the medical personnel matter. This assumption is supported by a review from Oestern concerning the medical assistance of severely injured patients in the emergency trauma room.[27] On the other hand, it has to be considered that an extended rescue time and the related increased volume replacement can be induced by the fact that during an extended rescue time (eg, due to a patient stuck in a car), more volume is given. In this context, it has to be pointed out that an average difference of 11.4 min between the two groups does not explain an elevated volume replacement of 1740 mL on average. But in individual cases, this may be of importance. Unfortunately, this connection cannot be discussed due to missing studies in the literature. However, this should be considered in future studies.
One remarkable finding of this study is that higher replacement volumes were related to a significantly more frequent occurrence of organ failure and sepsis. However, mortality during the entire period of care was not significantly different in the low- and high-volume replacement groups. We assume that better treatment possibilities in the hospital can potentially compensate for worse condition prior to hospitalization if the patient survives the first posttraumatic stage and an early intensive medical care was given. Based on the results of the study presented here, the patient group receiving high-volume replacement therapy required more extensive therapeutic care (more units of pRBC, longer intubation per first 30 ICU days, and so on) for equivalent occurrence of mortality as the low-volume patient group. To reduce the cost of patient care, the recommendations concerning a moderate volume replacement with the least possible additional impairment of the coagulation, the dilution of oxygen carriers and a fast transport in a level one trauma center for a definitive surgical and intensive medical therapy appear to be the best course of action. As already mentioned above, Geeraedts et al. and Turner et al. drew similar conclusions.[18,22] This assumption is furthermore supported by the fact that the total days spent in ICU or days of intubation do not differ significantly in patients who received either low- or high-replacement volumes, but the ICU-free days and the ventilator-free days concerning the first 30 days differed significantly. It is presumable that especially the direct therapy time after trauma was of essential importance. However, it has to be pointed out again that the mortality within the first 6 h and the total mortality did not differ significantly between the two groups, but both mortality values were elevated in the high-volume group. A reference to a maybe higher total severity of the injuries cannot be reconstructed. The study was designed in a way that the injury severity per body region (AIS) was approximately the same and the total ISS was the same in all groups. Also the elevated RISC score that the high-volume group had received does not serve as a clue to a more severely injured group, because it has to be considered that the data (eg, prothrombin ratio) included in the calculation of the RISC score had already been influenced prehospital by the volume replacement. In total, our study does not fully support the conclusion drawn by Turner et al. that the volume replacement does not influence mortality.[22]
The study presented here does not support an aggressive volume replacement after blunt trauma and bleeding. Some authors have recommended this in past studies to improve the tissue perfusion pressure.[19–21] These recommendations were mostly made for injuries of the extremities. Interestingly, patients with blood pressure less than 90 mmHg at the accident site, recovered better after a low-volume replacement than after a high-volume replacement. The same held true for the heart rates at the accident site and in the emergency room. Similar results for penetrating and blunt trauma in America were shown by Dutton et al.[28] Based on these results, low-volume replacement also appears to achieve the required perfusion pressure. Permissive hypotension could represent the better therapeutic option as has been shown for penetrating trauma patients. Geeraedts et al. drew a similar conclusion in their review.[18] Catecholamines should not substitute a volume therapy in the treatment of a hemorrhagic shock. A catecholamine therapy to maintain the mean arterial pressure is only recommended as a last-chance scenario.[19]
Another interesting aspect influenced by the choice of volume in prehospital replacement therapy became apparent when the cost of patient care was examined. In the study presented here, additional expenses amounting to €2,500 per 1.5 days per patient was concurred in the high-volume replacement therapy group. This reflects the intensified therapeutic effort in the high-volume group. In the health system with limited resources, this is a factor that should not be ignored.
There are several limitations to our retrospective study:
TRISS calculation could be performed only in 46% of the participating trauma centers, whereas RISC methodology was available in 88% of the cases. Thus, the data might be biased as TRISS could not be calculated for the majority of the trauma cases. But this also indicates that RISC is much easier to maintain than TRISS, which might be because RISC does not compute the respiratory rate prehospitally. The respiratory rate is documented by the physicians on site only in 60% of the cases
Regarding the coagulation analysis, in the Trauma Registry of the DGU, only the prothrombin ratio is documented, and therefore available for analysis. Other laboratory values, which might be of interest with respect to coagulation (eg, fibrinogen, protein C), are not documented at all in the Trauma Registry.
All the patients were treated by a physician at the accident site. However, it remains unclear which specialty (eg, trauma surgeon, anesthetist) the physician practicing at the accident site represents and which education is underlying here. In German-speaking countries, for example, a certificate in “emergency medicine” is required for working as an emergency doctor at the accident site. This certificate is independent of being a medical specialist (surgeon, anesthetist, or others).
Finally, we conducted a retrospective analysis and only associations but no causalities can be ascribed to the given data. In the future, a prospective randomized study is indispensable to clarify the advantage or disadvantage of a volume therapy in the bleeding, most severely injured patient at the accident site.
CONCLUSION
Moderate prehospital volume replacement therapy after severe trauma was related with a better clinical course in the patient content to a high amount of fluid replacement. Aggressive volume therapy is related with early traumatic coagulopathy and increased rescue time. From these analyses, rapid transport to a hospital appears to be the more important factor. The results of this study suggest recommending permissive hypotension and a restrained volume replacement during rescue of patients suffering from trauma and severe bleeding.
ACKNOWLEDGMENTS
Special thanks go to the IFOM Institute under the direction of Prof. Neugebauer and his associate PD Dr. Rolf Lefering for their outstanding support. Great thanks go to Kathy Astrahantseff for critical reading. The authors also thank the members of the emergency care, intensive care, and treatment teams of severely injured patients of the DGU for their long-standing intense involvement in the Trauma Registry. The following members have decisively contributed to the success of the Trauma Registry of the DGU: F. Barth, A. Bonk, B. Bouillon, K. Grimme, S. Grote, M. Grotz, M. Hering, S. Huber-Wagner, U. Krehmeier, G. Kanz, M. Kleiner, C. Krettek, C. Kühne, L. Kumpf, K. Ledendecker, I. Marzi, H. Meyer, S. Müller, W. Mutschler, C. Lackner, R. Lefering, D. Nast-Kolb, E. Neugebauer, U. Obertacke, H.J. Oestern, H.-C. Pape, T. Paffrath, Ch. Probst, M. Qvick, M. Raum, D. Rixen, S. Ruchholtz, S. Sauerland, U. Schweigkofler, A. Seekamp, R. Simon, O. Steitz, B. Strohecker, T. Tjardes, F. Walcher, C. Waydhas, M. Wittke, J. Westhoff.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Raum M, Waydhas C. Prehospital fluid resuscitation in trauma. Notfall Rettungsmed. 2006;9:485–500. [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB. Methods for improved hemorrhage control. Crit Care. 2004;8(Suppl 2):S57–60. doi: 10.1186/cc2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nast-Kolb D, Waydhas C, Kastl S, Duswald KH, Schweiberer L. The role of an abdominal injury in follow-up of polytrauma patients. Chirurg. 1993;64:552–9. [PubMed] [Google Scholar]
- 6.White CE, Hsu JR, Holcomb JB. Haemodynamically unstable pelvic fractures. Injury. 2009;40:1023–30. doi: 10.1016/j.injury.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 8.Ziegenfuss T. Emergency management of polytrauma patients. Zentralbl Chir. 1996;121:924–42. [PubMed] [Google Scholar]
- 9.Kreimeier U, Lackner CK, Prückner S, Ruppert M, Peter K. New strategies of volume substitution therapy in polytraumatized patients. Notfall Rettungsmed. 2003;6:77–88. [Google Scholar]
- 10.Williams JG, Riley TR, Moody RA. Resuscitation experience in the Falkland Islands campaign. J R Army Med Corps. 2007;153(Suppl 1):70–2. doi: 10.1136/jramc-153-03s-26. [DOI] [PubMed] [Google Scholar]
- 11.Bickell WH, Stern S. Fluid replacement for hypotensive injury victims: How, when and what risks? Curr Opin Anaesthesiol. 1998;11:177–80. doi: 10.1097/00001503-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bickell WH, Barrett SM, Romine-Jenkins M, Hull SS, Jr, Kinasewitz GT. Resuscitation of canine hemorrhagic hypotension with large-volume isotonic crystalloid: Impact on lung water, venous admixture, and systemic arterial oxygen saturation. Am J Emerg Med. 1994;12:36–42. doi: 10.1016/0735-6757(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 13.Bickell WH. Are victims of injury sometimes victimized by attempts at fluid resuscitation? Ann Emerg Med. 1993;22:155–63. doi: 10.1016/s0196-0644(05)80208-2. [DOI] [PubMed] [Google Scholar]
- 14.Yaghoubian A, Lewis RJ, Putnam B, de Virgilio C. Reanalysis of prehospital intravenous fluid administration in patients with penetrating truncal injury and field hypotension. Am Surg. 2007;73:1027–30. doi: 10.1177/000313480707301023. [DOI] [PubMed] [Google Scholar]
- 15.Kreimeier U, Messmer K. Small-volume resuscitation: From experimental evidence to clinical routine.Advantages and disadvantages of hypertonic solutions. Acta Anaesthesiol Scand. 2002;46:625–38. doi: 10.1034/j.1399-6576.2002.460601.x. [DOI] [PubMed] [Google Scholar]
- 16.Kreimeier U, Christ F, Frey L, Habler O, Thiel M, Welte M, et al. Small-volume resuscitation for hypovolemic shock.Concept, experimental and clinical results. Anaesthesist. 1997;46:309–28. doi: 10.1007/s001010050406. [DOI] [PubMed] [Google Scholar]
- 17.Sapsford W. Should the ‘C’ in ‘ABCDE’ be altered to reflect the trend towards hypotensive resuscitation? Scand J Surg. 2008;1:4–11. doi: 10.1177/145749690809700102. [DOI] [PubMed] [Google Scholar]
- 18.Geeraedts LM, Jr, Kaasjager HA, van Vugt AB, Frölke JP. Exsanguination in trauma: A review of diagnostics and treatment options. Injury. 2009;40:11–20. doi: 10.1016/j.injury.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Marzi I. Hemorrhagic shock. Anaesthesist. 1996;45:976–92. doi: 10.1007/s001010050332. [DOI] [PubMed] [Google Scholar]
- 20.Adams HA, Piepenbrock S, Hempelmann G. Volume replacement solutions--pharmacology and clinical use. Anasthesiol Intensivmed Notfallmed Schmerzther. 1998;33:2–17. doi: 10.1055/s-2007-994204. [DOI] [PubMed] [Google Scholar]
- 21.Bernhard M, Helm M, Aul A, Gries A. Preclinical management of multiple trauma. (quiz 903-4).Anaesthesist. 2004;53:887–902. doi: 10.1007/s00101-004-0732-y. [DOI] [PubMed] [Google Scholar]
- 22.Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess. 2000;4:1–57. [PubMed] [Google Scholar]
- 23.Bone RC. Let's agree on terminology: Definition of sepsis. Crit Care Med. 1991;19:973–6. doi: 10.1097/00003246-199107000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure.On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 25.Lefering R. Development and validation of the revised injury severity classification (RISC) score for severely injured patients. Eur J Trauma Emerg Surg. 2009;35:437–47. doi: 10.1007/s00068-009-9122-0. [DOI] [PubMed] [Google Scholar]
- 26.Trunkey DD. Prehospital fluid resuscitation of the trauma patient.An analysis and review. Emerg Med Serv. 2001;30:93–5. 96,98 passim. [PubMed] [Google Scholar]
- 27.Oestern HJ. Management of polytrauma patients in an international comparison. Unfallchirurg. 1999;102:80–91. doi: 10.1007/s001130050378. [DOI] [PubMed] [Google Scholar]
- 28.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: Impact on in-hospital mortality. J Trauma. 2002;52:1141–6. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]


