Abstract
A diverse group of the pathologic process can produce the enlargement of soft tissues in the oral cavity and often present a diagnostic challenge. This soft tissue enlargement may represent a variation of the normal anatomic structure, inflammatory reaction, cyst, neoplasm, and developmental anomalies. A group of reactive hyperplasias, which develop in response to chronic recurring tissue injury that stimulates an excessive tissue repair response. The pyogenic granuloma (PG) is a reactive enlargement that is an inflammatory response to local irritation such as calculus, a fractured tooth, rough dental restoration, and foreign materials or hormonal (pregnancy tumor) and rarely associated with bone loss. This paper presents a rare case of PG associated with periodontal abscess and bone loss in a 30-year-old male.
Keywords: Bone loss, periodontal abscess, pyogenic granuloma
Introduction
The gingiva is often the site of localized growths that are considered to be reactive rather than neoplastic in nature. Many of these lesions are difficult to be identified clinically and can be identified as specific entity only on the basis of typical and consistent histomorphology. Pyogenic granuloma (PG) or granuloma pyogenicum is a well-known oral lesion. It is a localized granulation tissue overgrowth in reaction to mild irritation.[1] The name pyogenic granuloma (PG) is a misnomer since the condition is not associated with pus and does not represent a granuloma histologically.[2] The majority remains small and lesions more than 1 cm in diameter are rare on the cheeks, tongue, and floor of the mouth possibly because masticatory trauma restricts their size through necrosis and ulceration.[3] PG of the oral cavity is known to involve the gingiva most commonly. PG is a benign lesion; therefore, surgical excision is the treatment of choice. To avoid the possibility of recurrence the lesion must be excised down to the underlying periosteum and predisposing irritant must be removed.
Case Report
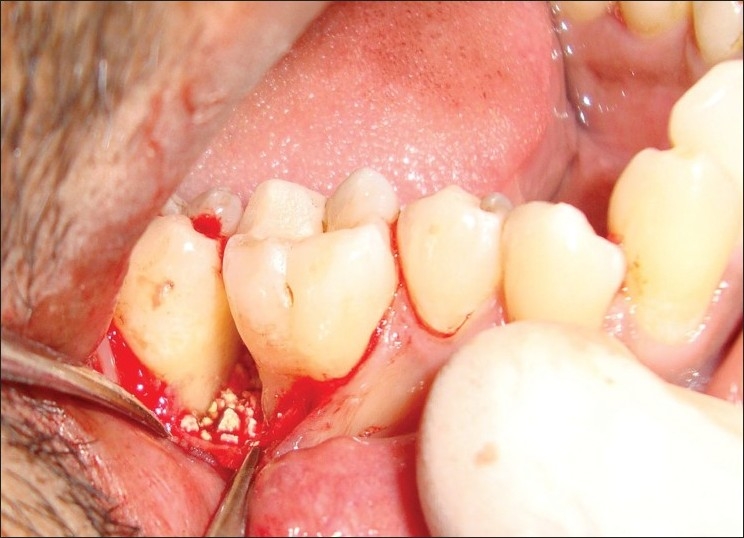
A 30-year-old systemically healthy male patient presented with a chief complaint of growth in the mouth involving lower-left back teeth region of the jaw. The patient had noticed a small painless growth about few years back. There was a very gradual increase in size, which led to discomfort while eating as the extent of growth had reached the occlusal plane [Figure 1] since 3 months. Patient also complained of interference of growth while chewing and food lodgement between molars. Patient complained purulent discharge and constant dull pain in the same region. There was no history of intake of any hormonal supplements, but the patient was given antibiotics by some dentist for the same.
Figure 1.
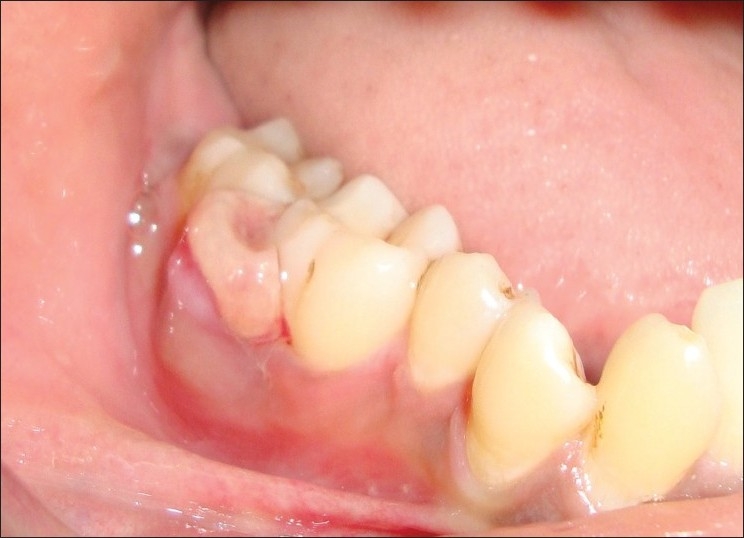
Pre-operative lesion
The extraoral examination did not reveal any facial asymmetry. Lymph nodes were not palpable. Intraoral examination revealed a solitary diffused growth, pale bluish red in color, measuring around 2×1.5 cm seen in the interdental region in relation to the left first molar and second molar region that did not extend lingually [Figure 1]. The superior surface of lesion showed indentation of the upper teeth as a result of surface ulceration. The growth was pedunculated. There was no mobility or pathological migration of any of the molars was not present. Pus discharge while probing [Figure 2]. Oral hygiene of the patient was fair.
Figure 2.
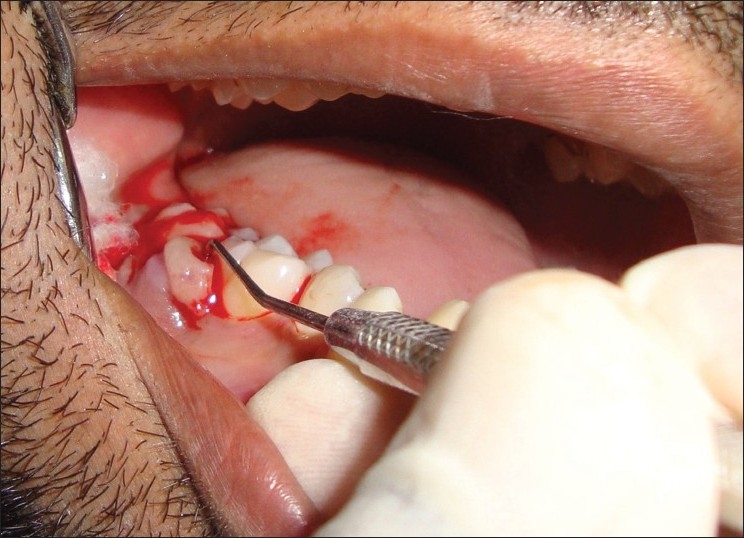
Abscess drainage with help of a periodontal probe
The intraoral periapical radiograph of teeth 46 and 47 region revealed widening of the periodontal ligament space, marked interdental bone loss with change in the trabecular pattern of bone. Roots of the involved teeth did not show any signs of resorption [Figure 3]. The occlusal radiograph did not show the expansion of bony plates.
Figure 3.
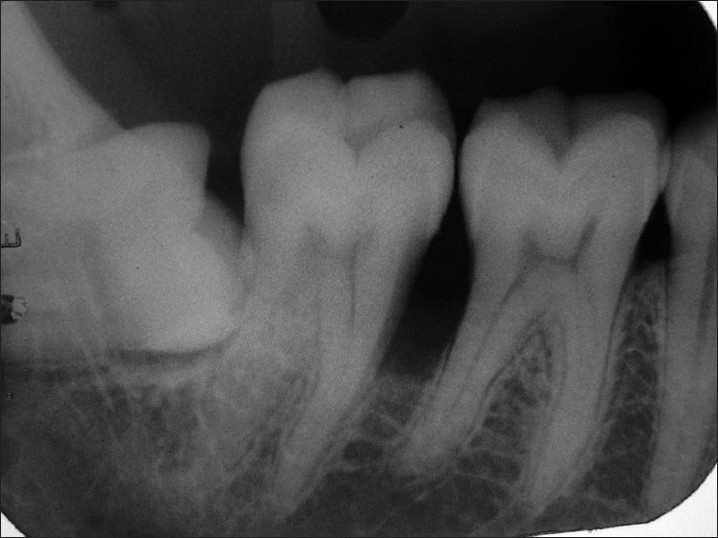
Intraoral periapical radiograph showing interdental bone loss and change in the trabecular pattern in between first and second molars
Blood examination revealed normal values. The treatment comprised of oral prophylaxis and surgical excision of the growth by gingivectomy procedure under local anesthesia.
Treatment
Although many treatment techniques have been described for PG, when it is large or occurs in a surgically difficult surgically area, choosing an appropriate treatment modality can be difficult. Excisional biopsy is indicated for the treatment of PG. Except when the procedure would produce marked deformity: in such a case, incisional biopsy is mandatory. Conservative surgical excision and removal of causative irritants (plaque, calculus, foreign materials, and source of trauma) are the usual treatments[4,5] for gingival lesions.
Here, local anesthesia 1 : 80,000 given to the patient and abscess was drained with the help of the periodontal probe. In this case, PG was associated with periodontal abscess and bone loss, so interval bevel gingivectomy was performed. The flap is designed in the manner that removed lesion form interdental papilla. Periosteum reflected and exposed the underlying bone [Figure 4].
Figure 4.
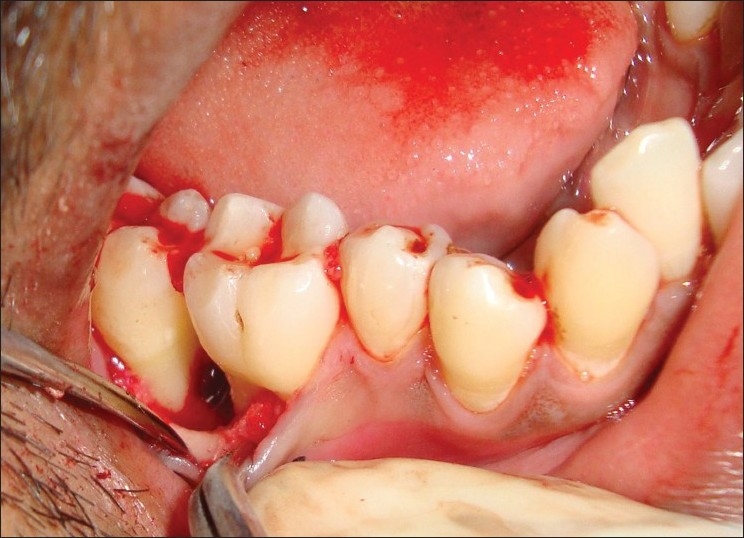
Exposing the underlying bone after excision of growth
After complete degranulation and scaling, root planning angular defect was filled with G-BONE® bone alloplast (hydoxyapatite + tricalcium sulphate) [Figure 5] and flap was sutured. Periodontal pack was given for facilitate healing. The biopsy specimen was sent for the microscopic examination.
Figure 5.
Defect filled with G-BONE® bone alloplast
Ten days later, periodontal pack and suture were removed and satisfactory healing of the gingiva was seen.
Patient revisited after 3 months. Complete healing of the operated area was observed at 3 month follow-up visit [Figure 6]. Radiograph also shows sign of bone fill [Figure 7].
Figure 6.
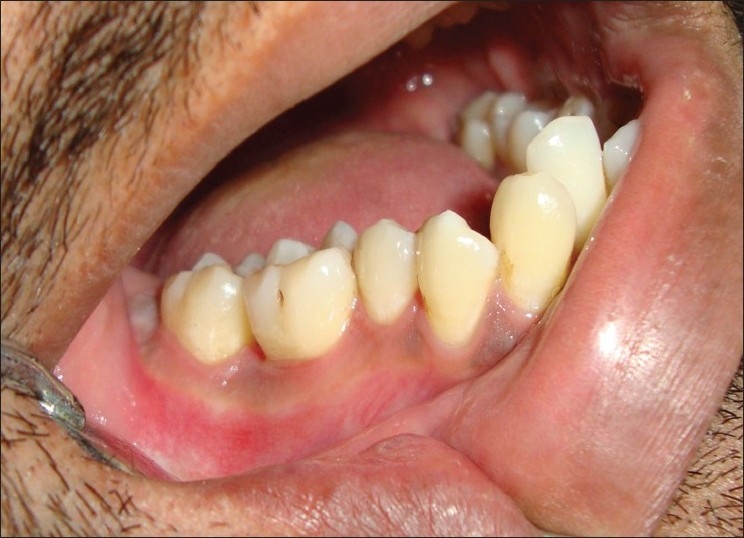
Complete healing after 3 months of operation
Figure 7.
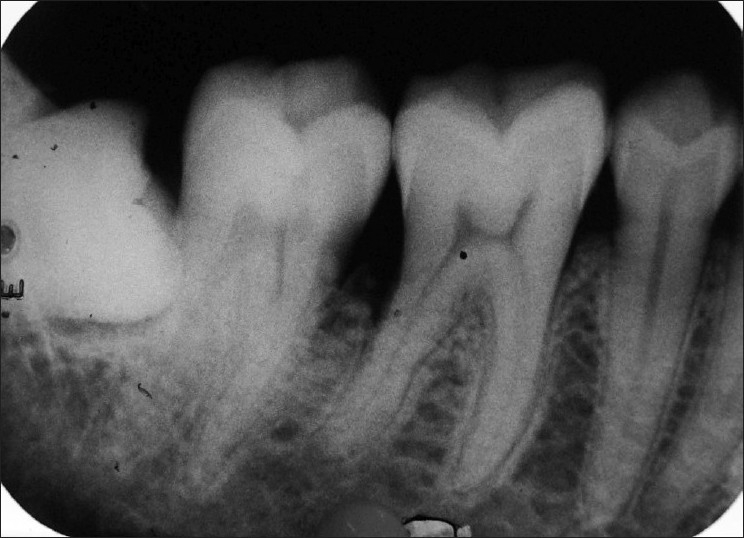
Radiograph also shows sign of bone fill after 3 months
Histopathological examination
Histopathological findings in the photomicrograph shows hematoxilin–eosin-stained section showing the overlying epithelium had hyperplastic parakeratinized stratified squamous epithelium exhibiting areas of pseudoepitheliomatous hyperplasia [Figure 8]. Highly vascular connective tissue exhibiting numerous small and large endothelium-lined channels engorged with red blood cells. Thickened wall of blood vessels, proliferating endothelial cells and few lymphatic vessels were evident. Mixed inflammatory cell infiltrate consisting predominantly of neutrophils and lymphocytes were also seen [Figure 9]. There was no evidence of atypia or malignancy. The clinical and histopathological features were suggestive of PG.
Figure 8.
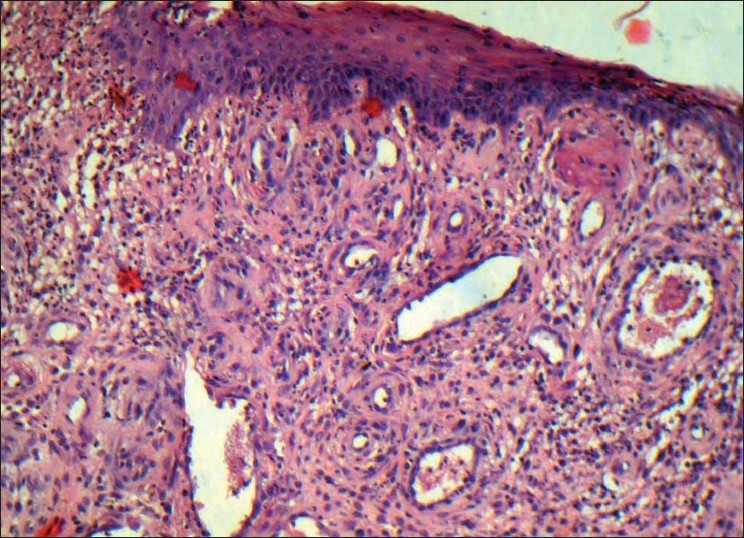
Photomicrograph shows hyperplastic parakeratinized stratified squamous epithelium exhibiting areas of pseudoepitheliomatous hyperplasia
Figure 9.
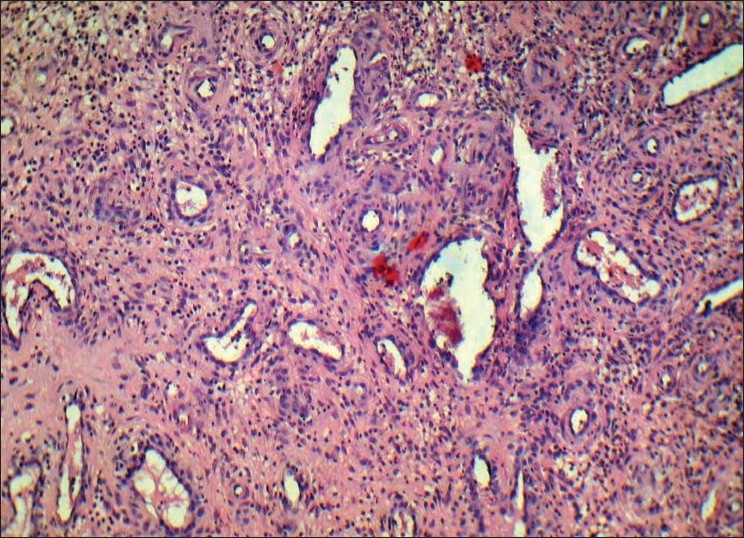
Photomicrograph showing endothelium lined channels, mixed inflammatory cell infiltrate consisting predominantly of neutrophils and lymphocytes
Discussion
The incidence of PG has been describe between 28.8% to 32% of all reactive lesions.[6] As reported by Buchner et al. in study of 302 consecutive localized gingival outgrowths it was found PG occurred in all ages but mainly in youngsters. More in the third decade[7] PG was thought to be a mitotic infection contracted from horses.[8,9] It was claimed by Inagi K (1991) without any scientific evidence that PG results from purulent change within benign oral tumors.[10] Recently, angiogenesis-associated factors Tie2, angiopoietin-1, angiopoietin-2, ephrin B2, and Eph B4 have been detected in PG in immunohistochemistry.[11] It is now generally accepted that the lesion is exaggerated localized connective tissue reaction to minor trauma or irritation.
The principal oral site affected by PG is the gingiva. Other oral sites are the lower lip, tongue, buccal mucosa, upper lip, and palate. These findings are consistent with those of others.[8,12] With regard to site, gingival PG is more common in the maxilla than in the mandible and in the anterior region than in the posterior regions of both jaws.[12]
Gingival irritation as a result of calculus, overhanging edges or rough restorations might be the predisposing factor for the development of gingival PG. It is possible that microulceration from these irritants in an already inflamed gingiva allows the ingress into the gingival connective tissue of low virulent oral microflora. This evokes an exaggerated vascular hyperplastic response in the connective tissue resulting in the formation of PG.[13]
PG is a common reactive lesion that generally develops rapidly, bleeds easily, and ulcerates causing the erroneous clinical impression of the malignant tumor.[14] It is however a well-circumscribed benign soft tissue tumor of innammatory rather than the neoplastic nature arising from the connective tissue of the skin or mucous membrane.[15]
Clinically, PG is a smooth or lobulated exophytic lesion manifesting as small, red erythematous papules on a pedunculated or sometimes sessile base, which is usually hemorrhagic and compressible. The size varies in diameter from a few millimeters to several centimeters.[4,5] Rarely PG exceeds 2.5 cm in size and it usually reaches its full size within weeks or months, remaining indefinitely thereafter. Clinical development of the lesion is slow, asymptomatic, and painless,[4] but it may also grow rapidly. The surface is characteristically ulcerated and friable which may be covered by a yellow, fibrinous membrane and its color ranges from pink to red to purple, depending on the age of the lesion. Young PGs are highly vascular in appearance because they are composed predominantly of hyperplastic granulation tissue in which capillaries are prominent. Thus, minor trauma to the lesion may cause considerable bleeding, due to its pronounced vascularity,[4] whereas older lesions tend to become more collagenized and pink. Rarely, PG may cause significant bone loss, as reported by Goodman–Topper and Bimstein.[16] In our case also, bone loss was associated with PG.
Differential diagnosis of PG includes parulis, peripheral giant cell granuloma, peripheral ossifying fibroma, hemangioma, peripheral fibroma, leiomyoma, hemangioendothelioma, hemangiopericytoma, bacillary angiomatosis, kaposis sarcoma, metastatic tumor, pregnancy tumor and post extraction granuloma.[9]
Definitive diagnosis of PG can only be made by histopathologic examination of biopsied tissue. PG histologically shows a highly vascular proliferation that resembles granulation tissue. Numerous small and larger endothelium- lined channels are formed that are engorged with red blood cells. These vessels sometimes are organized in lobular aggregates and some pathologists require this lobular arrangement for the diagnosis (lobular capillary hemangioma). The surface that is usually ulcerated replaced the thick fibrinopurulent membrane. A mixed inflammatory cell infiltrate of neutrophils, plasma cells, and lymphocytes is evident. Neutrophils are more prevalent near the ulcerated surface; chronic inflammatory cells are found deeper in the specimen. Older lesions may have areas with a more fibrous appearance. Many gingival fibromas probably represent PG that have undergone fibrous maturation.[17]
PG is a benign lesion; therefore, surgical excision is the treatment of choice. Other conventional surgical modalities for the treatment of PG reported is cryosurgery in form of either liquid nitrogen spray or a cryoprobe, which has been used for eradication of the lesion. It is a safe, easy, and inexpensive technique suited for out patient's clinic setting.[18,19] Nd: YAG and CO2, and flash lamp pulsed dye lasers have also been used for the PG.[20,21] Lasers have shown to be a successful option for the excision of PG with advantages of minimal pain and invasiveness and the lack of need for suturing or packing. The recurrence rate of 16% however has been reported for PG.[6]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Laskaris G. 4th ed. New York: Thieme publishers; 1997. Colour atlas of oral diseases; pp. 400–1. [Google Scholar]
- 2.Shafer WG, Hine MK, Levy BM. 4th ed. Philadelphia: WB Saunders; 1983. A textbook of oral pathology; pp. 359–60. [Google Scholar]
- 3.Daley TD, Wysocki GP, Wysocki PD, Wysocki DM. The major epulides: clinicopathological correlations. J Can Dent Assoc. 1990;56:627–30. [PubMed] [Google Scholar]
- 4.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia: WB Saunders; 2002. Oral and Maxillofacial Pathology; pp. 473–95. [Google Scholar]
- 5.Regezi JA, Sciubba JJ, Jordan RC. 4th ed. Philadelphia: WB Saunders; 2003. Oral pathology: clinical pathologic considerations; pp. 115–6. [Google Scholar]
- 6.Kfir Y, Buchner A, Hansen LS. Reactive lesion of the gingiva: A clinicopathologic study of 471 cases. J Periodontol. 1980;51:655–61. doi: 10.1902/jop.1980.51.11.655. [DOI] [PubMed] [Google Scholar]
- 7.Buchner A, Calderon S, Raman Y. Localized hyperplastic lesions of the gingiva: A clinicopathologic study of 302 lesions. J Periodontol. 1977;48:101–4. doi: 10.1902/jop.1977.48.2.101. [DOI] [PubMed] [Google Scholar]
- 8.Al-Khateeb T, Ababneh K. Oral pyogenic granuloma in Jordanians: A retrospective analysis of 108 cases. J Oral Maxillofac Surg. 2003;61:1285–8. doi: 10.1016/s0278-2391(03)00729-8. [DOI] [PubMed] [Google Scholar]
- 9.Kerr DA. Granuloma pyogenicum. Oral Surg Oral Med Oral Pathol. 1951;4:158–76. doi: 10.1016/0030-4220(51)90432-x. [DOI] [PubMed] [Google Scholar]
- 10.Inagi K, Takahashi HO, Yao K, Kamata T. Study of pyogenic granuloma of oral cavity. Nippon Jibinkoka Gakkai Kaiho. 1991;94:1857–64. doi: 10.3950/jibiinkoka.94.12_1857. [DOI] [PubMed] [Google Scholar]
- 11.Yaun K, Jin YT, Lin MT. Expression of Tie 2, angiopoietin-1. angiopoietin-2. ephrin 62 and EphB4 in pyogenic granuloma of the human gingiva implicates their role in inflammatory angiogenesis. J Periodontal Res. 2000;35:165–71. doi: 10.1034/j.1600-0765.2000.035003165.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee L, Miller PA, Maxymiw WG, Messner HA, Rotstein LE. Intraoral pyogenic granuloma after allogeneic bone marrow transplant. Report of three cases. Oral Surg Oral Med Oral Pathol. 1994;78:607–10. doi: 10.1016/0030-4220(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 13.Bragado R, Bello E, Requena L, Renedo G, Texeiro E, Alvarez MV, Castilla MA, et al. Increased expression of vascular endothelial growth factor in pyogenic granulomas. Acta Derm Venereol. 1999;79:422–5. doi: 10.1080/000155599750009834. [DOI] [PubMed] [Google Scholar]
- 14.Correll RW, Wescott WB, Siegel WM. Rapidly growing non painful, ulcerated swelling in postrolateral palate. J Am Dent Assoc. 1983;106:494–5. doi: 10.14219/jada.archive.1983.0085. [DOI] [PubMed] [Google Scholar]
- 15.Angelopolous AP. Pyogenic granuloma of oral cavity: Statistical analysis of its clinical features. J Oral Surg. 1971;29:840–7. [PubMed] [Google Scholar]
- 16.Goodman-Topper ED, Bimstein E. Pyogenic granuloma as a cause of bone loss in a twelve year old child: report of case. ASDC J Dent Child. 1994;61:65–7. [PubMed] [Google Scholar]
- 17.Fowler EB, Cuenin MF, Thompson SH, Kudryk VL, Billman MA. Pyogenic granuloma associated with guided tissue regeneration: A case report. J Periodontol. 1996;67:1011–5. doi: 10.1902/jop.1996.67.10.1011. [DOI] [PubMed] [Google Scholar]
- 18.Pogrel MA. Application of laser and cryosurgery in oral and maxillofacial surgery. Curr Opin Dent. 1991;1:263–70. [PubMed] [Google Scholar]
- 19.Meffert JJ, Cagna DR, Meffert RM. Treatment of oral granulation tissue with the flashlamp pulsed dye laser. Dermatol Surg. 1998;24:845–8. doi: 10.1111/j.1524-4725.1998.tb04261.x. [DOI] [PubMed] [Google Scholar]
- 20.White JM, Chaudhry SI, Kudler JJ, Sekandari N, Schoelch ML, Silverman S., Jr Nd:YAG and CO2 laser therapy of oral mucosal lesions. J Clin Laser Med Surg. 1998;16:299–304. doi: 10.1089/clm.1998.16.299. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Nakanishi H, Seike T, Koizumi Y, Mihara K, Kubo Y. Treatment of pyogenic granuloma with sclerosing agent. Dermato Surge. 2001;27:521–3. doi: 10.1046/j.1524-4725.2001.01039.x. [DOI] [PubMed] [Google Scholar]


