Abstract
Background:
Clonidine has emerged as an attractive premedication desirable in laparoscopic surgery wherein significant hemodynamic stress response is seen. The minimum safe and effective dose of intravenous clonidine to attenuate the hemodynamic stress response during laparoscopic surgery has however not yet been determined.
Materials and Methods:
This prospective, randomized, double-blind controlled study was conducted on 90 adults of ASA physical status I and II, scheduled for laparoscopic cholecystectomy under general anesthesia. Patients were randomized to one of the three groups (n= 30). Group I received 100 ml of normal saline, while groups II and III received 1 μg/ kg and 2 μg/ kg of clonidine respectively, intravenous, in 100 ml of normal saline along. All patients received glycopyrrolate 0.004 mg/kg and tramadol 1.5 mg/kg intravenously, 30 min before induction. Hemodynamic variables (heart rate, systolic, diastolic, mean arterial pressure), SpO2, and sedation score were recorded at specific timings. MAP above 20% from baseline was considered significant and treated with nitroglycerine.
Results:
In group I, there was a significant increase in hemodynamic variables during intubation pneumoperitoneum and extubation (P<0.001). Clonidine given 1 μg/kg intravenous attenuated hemodynamic stress response to pneumoperitoneum (P<0.05), but not that associated with intubation and extubation. Clonidine 2 μg/kg intravenous prevented hemodynamic stress response to pneumoperitoneum and that associated with intubation and extubation (P<0.05). As against 14 and 2 patients in groups I and II respectively, no patient required nitroglycerine infusion in group III.
Conclusions:
Clonidine, 2 μg/ kg intravenously, 30 min before induction is safe and effective in preventing the hemodynamic stress response during laparoscopic cholecystectomy.
Keywords: Clonidine, laparoscopic cholecystectomy, pneumoperitoneum, stress response
Introduction
Laparoscopic cholecystectomy has become gold standard surgery for cholelithiasis.[1] Advantages of laparoscopic cholecystectomy are shorter hospital stay, early ambulation, smaller scar, and less compromised postoperative respiratory and gastro-intestinal functions. However, the procedure is not risk free as it is associated with significant hemodynamic changes due to creation of pneumoperitoneum, potential for systemic absorption of carbon dioxide, and reverse Trendelenberg position.[2] Postoperative nausea and vomiting is a major drawback of laparoscopic surgery.[3]
Various pharmacological agents like nitroglycerine, β blocker, and opioids are used to provide hemodynamic stability during pneumoperitoneum,[4] but they have their own disadvantages. Clonidine, a α-2 adrenergic receptor agonist, has shown promising results for attenuation of hemodynamic response associated with laparoscopic surgery.[5–9] However, there is a wide difference in the dose of clonidine used by various authors and there is need for further studies to determine the minimum effective and safe dose of clonidine in laparoscopic surgery. The present study was undertaken with the objective of evaluating the type and extent of hemodynamic changes during laparoscopic cholecystectomy and their modification by two different doses of intravenous clonidine administered as premedication.
Materials and Methods
After institutional review board approval and informed written consent from the patients, this prospective, randomized, double-blind controlled clinical study was carried out in 90 patients of either sex, aged 20–60 years, of ASA physical status I and II, scheduled for laparoscopic cholecystectomy under general anesthesia from October 2009 to September 2010. Exclusion criteria were patients with anticipated difficult airway; body mass index (BMI) >25, history of cardiopulmonary diseases; psychiatric illness; and therapy with α-2 adrenergic agonists, β blocker, methyldopa, MAO inhibitors, tricyclic antidepressant, and benzodiazepines.
In the pre-anesthetic preparation room, monitoring for heart rate (HR), non-invasive systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP), peripheral oxygen saturation (SpO2), and end-tidal CO2 (EtCO2) was instituted. Sedation was rated as per score shown in Table 1.
Table 1.
Sedation score

Patients were randomly divided, by picking up sealed envelopes, into one of the three groups of 30 each. Group I received 100 ml of normal saline, group II, 1 μg/kg of clonidine in 100 ml of normal saline and group III, 2 μg/kg of clonidine in 100 ml of normal saline. The drug was given over 15 min intravenously along with glycopyrrolate 0.004 mg/kg and tramadol 1.5 mg/kg, 30 min before induction of anesthesia.
In the operation theatre, after pre-oxygenation, anesthesia was induced with sleep dose of 2.5% thiopentone sodium followed by succinyl choline, 2 mg/kg to facilitate tracheal intubation. When there was no response to Train-of-Four on peripheral nerve stimulation, trachea was intubated with an appropriate sized cuffed, disposable endotracheal tube. Lungs were mechanically ventilated with O2 - N2O (50-50), sevoflurane (1-2%), and vecuronium bromide 0.1 mg/kg bolus followed by 1 mg intermittently for neuromuscular blockade. Tidal volume and ventilator frequency were adjusted to maintain normocapnia (EtCO2 40 ± 4 mmHg). Pneumoperitoneum (PP) was created by insufflations of CO2 and operation table was tilted to about 15° reversed trendelenberg. Intra-abdominal pressure was not allowed to exceed 15 mmHg. Throughout the study period, all the parameters selected (HR, SBP, DBP, MAP, and SpO2) were recorded at specified timings. Any change in hemodynamic variables more than 20% on either side of baseline was considered significant. Any increase in MAP up to 20% from baseline was treated by increasing the concentration of sevoflurane to a maximum 2%. Any increase in MAP more than 20% from baseline was treated with nitroglycerine infusion. Nitroglycerine infusion was adjusted to maintain the MAP within 20% of baseline. Time duration from creation of pneumoperitoneum to the release of pneumoperitoneum was taken as duration of pneumoperitoneum. At the end of surgery, neuromuscular blockade was reversed with neostigmine 50 μg/kg and glycopyrrolate 10 μg/kg intravenously. After satisfying the extubation criteria, trachea was extubated and patients were transferred to post-anesthesia care unit (PACU). In PACU, HR, SBP, DBP, MAP, SpO2, sedation score, and any incidence of complications/adverse event were monitored for next 1 h. Maintenance of MAP and SpO2 within 20% of baseline and sedation score ≤ 2 was considered criteria for recovery.
Sample size of minimum 29 per group was derived using Cohen's formula based on assumption of α error 0.05 and power of study 80% after permitting β error of 0.2 to detect a difference of at least 4 in the quantitative variables between the groups. Mean and standard deviation were calculated for all the quantitative variables using graph-pad prism statistical software. An intra-group comparison was made using paired Student's t-test and comparison between two groups at a time (inter-group comparison) was done using the unpaired t-test. P <0.05 was considered statistically significant.
Results
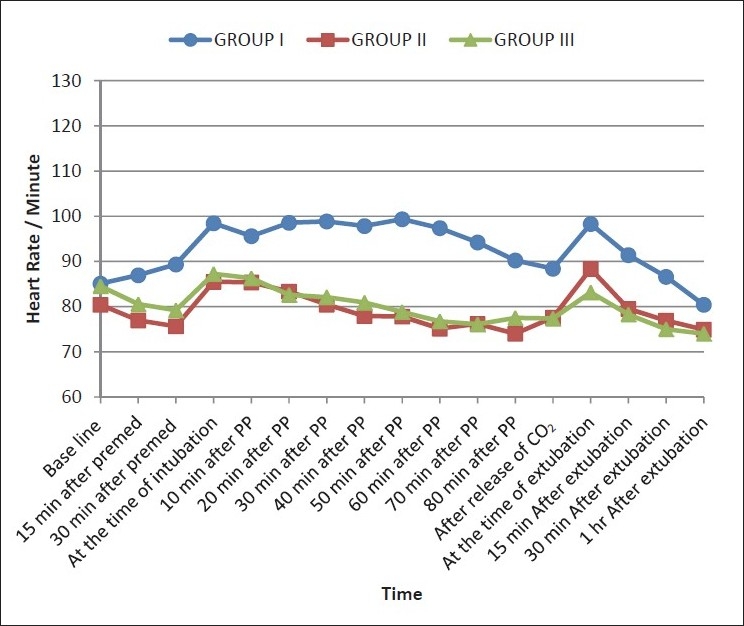
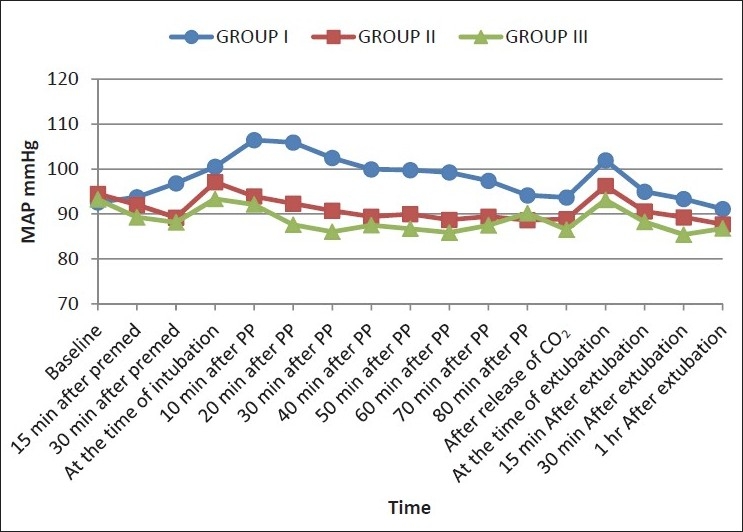
All patients (n=90) completed the study. Demographic parameters were comparable among the three groups (P >0.05) [Table 2]. Duration of pneumoperitoneum in all the patients was 80 min or less except one patient in group I in whom the pneumoperitoneum lasted for 90 min. As the monitored hemodynamic variables at 90-min time point were not available in other groups, this time point was excluded. Hemodynamic variables recorded in three groups at specified timings are shown in Figures 1–3. There was an increase in HR, SBP, DBP, and MAP at tracheal intubation in group I (P <0.001) which continued throughout the study period. All the patients in group I required maximum allowable concentration of 2% sevoflurane to maintain MAP within 20% of baseline. Fourteen patients out of 30 (46.67%) in group I required nitroglycerine infusion for more than 20% rise in MAP above baseline.
Table 2.
Patient characteristics given as mean± SD
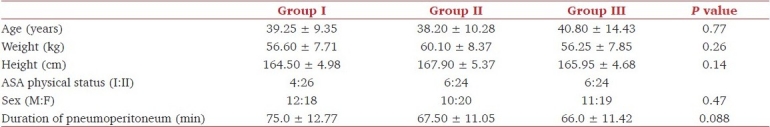
Figure 1.
Changes in heart rate at various specified timings in three groups
Figure 3.
Changes in MAP at various specified timings in three groups
Figure 2.
Changes in SBP and DBP at various specified timings in three groups
In group II, HR, SBP, DBP, and MAP decreased from baseline within 30 min of clonidine premedication (P <0.05), but the decrease was never more than 20%. HR and SBP increased at the time of intubation (P <0.05), but the increase was less than that observed in group I at the same time (P <0.05). An increase in DBP and MAP at tracheal intubation was not significant as compared to baseline (P >0.05). An increase in hemodynamic variables at the time of intubation approached baseline within 20 min of pneumoperitoneum with a statistically significant decrease observed within 40 min which continued throughout the duration of pneumoperitoneum. At tracheal extubation, HR increased (P <0.05) but a rise in SBP, DBP, and MAP was not statistically significant. The MAP of 20 patients could be maintained with 1% sevoflurane, while 10 patients required an increase up to 2% to maintain MAP within 20% of baseline. Two patients in group II (6.66 %) required nitroglycerine infusion.
In group III, a decrease in HR, SBP, DBP, and MAP from baseline was observed within 15 min of clonidine premedication (P <0.05), but at no time, this decrease was more than 20% from baseline. At tracheal intubation, HR and DBP increased (P >0.05), while SBP decreased (P >0.05) and MAP remained comparable to baseline. Within 40 min of pneumoperitoneum HR and within 20 min SBP, DBP, and MAP decreased (P <0.05) and remained so throughout the study period Hemodynamic variables at the time of extubation remained comparable to baseline. All the patients maintained MAP comparable to baseline with 1% sevoflurane. No patient in group III required nitroglycerine infusion. SpO2 remained stable and comparable to baseline in all the three groups.
Higher sedation score was observed in group III as compared to group II at specified timings (P <0.05) [Figure 4], but it never approached 2 at any time and no sign of respiratory depression observed. No patient in any group demanded supplemental analgesic up to 1 h postoperatively. 30%, 20%, and 10% patients in group I had nausea, vomiting, and shivering respectively in the postoperative period while none had any complication in other two groups.
Figure 4.
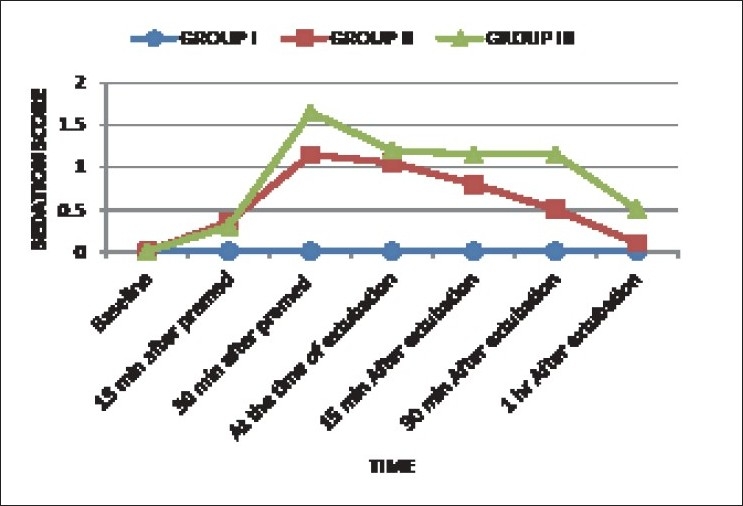
Sedation score in three groups at various specified timings
Discussion
An appraisal of the potential problems in laparoscopic surgery is essential for optimal anesthetic care of patients. The anesthetic technique for upper abdominal laparoscopic surgery is generally limited to general anesthesia with neuromuscular blockade, tracheal intubation, and mechanical ventilation. Pneumoperitoneum during laparoscopic surgery leads to significant hemodynamic changes such as an increase in MAP and systemic vascular resistance (SVR) and a decrease in cardiac output. The decline in cardiac output and venous return can be attenuated by volume infusion before pneumoperitoneum. However, an increase in MAP and SVR requires therapeutic intervention. Techniques like reduction in intra-abdominal pressure during pneumoperitoneum and gasless laparoscopy using abdominal elevators have been tried to counteract these detrimental effects of pneumoperitoneum.[10–12] Pharmacological agents like β-blocker, opioids, increasing concentration of inhalational anaesthetic agents, nitroglycerine, and α-2 adrenergic agonist have been used to minimize these hemodynamic derangements during laparoscopy with varied results.
Clonidine, a selective α-2 adrenergic agonist, has desirable actions like anxiolysis, sedation, analgesia, antiemesis, and prevention of shivering. It is a potent hypotensive agent. Clonidine inhibits catecholamine and vasopressin-mediated increase in SVR caused by pneumoperitoneum.[13] Studies using oral clonidine (150 μg) administered 60 to 90 min before induction of anesthesia have shown promising results in minimizing the hemodynamic changes during laparoscopic surgery.[6,14–16] Although the bioavailability of clonidine after oral administration is over 90%, it requires 2 to 4 h to develop its peak effect necessitating its ingestion at least 2 h prior to induction to have clinically desirable actions. As intravenous clonidine has an onset of action within 15 min (with a peak at 30 min), its administration half an hour before induction of anesthesia is a better option. Numerous studies using intravenous clonidine found effective prevention of hemodynamic derangements during laparoscopic cholecystectomy. 3 μg/kg[5] and 8 μg/kg[9] of intravenous clonidine used as premedication effectively attenuated hemodynamic changes during laparoscopic cholecystectomy without any side effects. A study using 4.5 μg/kg of clonidine intramuscularly[7] found a significant decrease in MAP and HR during pneumoperitoneum. These studies demonstrated significantly less analgesic requirement when clonidine was used as premedication. Clonidine thus is a desirable premedication for laparoscopic surgery.
Though, various studies found intravenous clonidine effective in attenuating the hemodynamic changes during laparoscopic surgery, there is wide difference in the dose of clonidine used. Joris et al[9] used 8 μg/kg of clonidine intravenously and surprising reported stable intraoperative hemodynamic. They did not find any adverse effect in perioperative period, though they felt the need of further research to determine the ideal dose of clonidine required. This study was planned with the objective of determining the minimum safe and effective dose of intravenous clonidine as premedication in laparoscopic surgery by comparing effects of 1 μg/kg and 2 μg/kg doses.
This was essential to exclude hemodynamic variation due to hypercapnia. Diamante et al[17] reported a 35% decrease in cardiac output in dogs with a raised intra-abdominal pressure of 40 mmHg. Ishizaki et al[18] observed a significant fall in cardiac output at 16 mmHg intra-abdominal pressure, but hemodynamic alteration was not observed at 12 mmHg intra-abdominal pressure. In two studies, ejection fraction of left ventricle was assessed using trans-esophageal echocardiography during pneumoperitoneum and no significant change in ejection fraction was reported up to 15 mmHg intra-abdominal pressure.[19,20] Based on these observations, the current recommendation is to monitor intra-abdominal pressure and to keep it as low as possible. Intra-abdominal pressure was kept below 15 mmHg in this study.
Following pneumoperitoneum with carbon dioxide, ventilation was adjusted to maintain normocapnia. In spite of maintaining normocapnia and the intra-abdominal pressure below 15 mmHg, a significant rise in HR, SBP, DBP, and MAP was observed in group I. Fourteen patients in group I had a rise in MAP more than 20% from base line and required nitroglycerine infusion. Pneumoperitoneum produces significant hemodynamic derangements which may be detrimental and needs to be prevented, especially in high risk hemodynamically compromised patients. In this study, 1 μg/kg of clonidine could achieve hemodynamic stability during pneumoperitoneum but hemodynamic response to intubation and extubation could not be prevented, though the magnitude of this stress response was less as compared to group I. With 2 μg/kg of intravenous clonidine, hemodynamic stress response of pneumoperitoneum as well as of tracheal intubation and extubation was effectively prevented.
A decrease in sympathetic tone by central action and pre-synaptically mediated inhibition of norepinephrine and vagomimetic action at nucleus tractus solitarius by clonidine is responsible for bradycardia. Bradycardia was however not observed in both the treatments groups in this study. Many studies reported significant reduction in HR, SBP, DBP, and MAP during laparoscopic surgery.[5,6,9,15,16] We have not compared the results of this study with that of these studies because of wide differences in the doses used.
The sedation score was comparatively higher in group III, but it never approached 2 in any patient and no sign of respiratory depression was noted. A comparatively higher sedation score in group III can be viewed as an advantage as all the patients were sleeping comfortably. Monitoring depth of anesthesia using a bispectral index monitor could have been an additional tool to assess the efficacy of clonidine as a supplement to general anesthesia but it was not used in this study due to non-availability. The mean sedation score in both the treatment groups was less than 1 at 1 h post-operative which proved that these doses of clonidine did not prolong recovery.
Ten per cent patients in group I experienced shivering against none in other two groups. This finding corroborates the finding of Nicolaou et al who stated that clonidine could be used as an effective perioperative anti-shivering agent as it inhibits the cold thermoregulatory response due to an effect on central integration control and output from the thermoregulatory center.[21] Clonidine increases gastrointestinal motility by decreasing sympathetic and increasing parasympathetic outflow from the central nervous system, thus reduces incidence of post-operative nausea and vomiting.[22] We found a statistically significant decrease in the incidences of postoperative nausea and vomiting compared to the control group in our study.
In conclusion, significant hemodynamic derangements during pneumoperitoneum of laparoscopic surgery can be effectively attenuated by premedication with 1 μg and 2 μg/kg of intravenous clonidine. We recommend the use of clonidine 2 μg/kg intravenously, 30 min before induction of anesthesia to attenuate the hemodynamic stress response of pneumoperitoneum and tracheal intubation/extubation in otherwise healthy patients.
Acknowledgments
This work was supported by Sir Takhtasinhji Hospital, Government Medical College, Bhavnagar, Gujarat, India. None of the authors has any conflict of interest.
Footnotes
Source of Support: Sir Takhtasinhji Hospital, Government medical college, Bhavnagar, Gujarat, India
Conflict of Interest: None declared.
References
- 1.Cunningham AJ, Brull SJ. Laparoscopic Cholecystectomy: Anesthetic implications. Anaesth Analg. 1993;76:1120–33. doi: 10.1213/00000539-199305000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Jean LJ. Anaesthesia for Laparoscopic surgery. In: Miller RD, editor. Anesthesia. 7th ed. New York: Churchill Livingstone; 2010. pp. 2185–202. [Google Scholar]
- 3.Thune A, Appalgren L, Haglind E. Prevention of post operative nausea and vomiting after laparoscopic cholecystectomy. Eur J Surg. 1995;161:265–8. [PubMed] [Google Scholar]
- 4.Feig BW, Berger DH, Doughtery TB, Dupuis JF, His B, Hickey RC, et al. Pharmacologic intervention can reestablish baseline hemodynamic parameters during laparoscopy. Surgery. 1994;116:733–9. [PubMed] [Google Scholar]
- 5.Malek J, Knor J, Kurzova A, Lopourova M. Adverse hemodynamic changes during laparoscopic cholecystectomy and their possible suppression with clonidine premedication: Comparison with intravenous and intramuscular premedication. RozhlChir. 1999;78:286–91. [PubMed] [Google Scholar]
- 6.Sung CS, Lin SH, Chan KH, Chang WK, Chow LH, Lee TY. Effect of oral clonidine premedication on perioperative haemodynamic response and postoperative analgesic requirement for patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Sin. 2000;38:23–9. [PubMed] [Google Scholar]
- 7.Laisalmi M, Koivusalo AM, Valta P, Tikkanen I, Lindgren L. Clonidine provides opioid sparing effect, stable haemodynamics and renal integrity during laparoscopic cholecystectomy. Surg Endosc. 2001;15:1331–5. doi: 10.1007/s004640090126. [DOI] [PubMed] [Google Scholar]
- 8.Raval DL, Mehta MK. Oral clonidine pre medication for attenuation of haemodynamic response to laryngoscopy and intubation. Indian J Anaesth. 2002;46:124–9. [Google Scholar]
- 9.Joris JL, Chiche JD, Canivet JL, Jacquet NJ, Legros JJ, Lamy ML. Hemodynamic changes induced by laparoscopy and their endocrine correlates: Effect of clonidine. J Am CollCardiol. 1998;32:1389–96. doi: 10.1016/s0735-1097(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren L, Koivusalo AM, Kellokumpu I. Conventional pneumoperitoneum compared with abdominal wall lift for laparoscopic cholecystectomy. Br J Anaesth. 1995;75:567–72. doi: 10.1093/bja/75.5.567. [DOI] [PubMed] [Google Scholar]
- 11.Mcdermott JP, Regan MC, Page R, Stokes MA, Kevin B, Moriarty DC, et al. Cardiorespiratory effects of laparoscopy with or without gas insufflation. Arch Surg. 1995;130:984–88. doi: 10.1001/archsurg.1995.01430090070022. [DOI] [PubMed] [Google Scholar]
- 12.Casati A, Valentini G, Ferrari S, Senatove R, Zangrillo A, Torri G. Cardio respiratory changes during gynecological laparoscopy by abdominal wall elevation: Comparison with carbon dioxide pneumoperitoneum. Br J Anaesth. 1997;78:51–4. doi: 10.1093/bja/78.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Zalunardo MP, Zollinger A, Spahn DR, Seifert B, Radjaipour M, Gautschi K, et al. Effects of intravenous and oral clonidine on hemodynamic and plasma-catecholamine response due to endotracheal intubation. J Clin Anesth. 1997;9:143–7. doi: 10.1016/S0952-8180(97)00239-0. [DOI] [PubMed] [Google Scholar]
- 14.Goyaji T, Tanaka M, Nishikawa T. Oral clonidine premedication reduces awakening concentration of Isoflurane. Anesth Analg. 1998;86:410–3. doi: 10.1097/00000539-199802000-00036. [DOI] [PubMed] [Google Scholar]
- 15.Mrinmoy D, Manjushree R, Gauri M. Haemodynamic changes during laparoscopic cholecystectomy: Effect of oral clonidine premedication. Indian J Anaesth. 2007;51:2005–10. [Google Scholar]
- 16.Yu HP, Hseu SS, Yien HW. Oral clonidine premedication preserves heart rate variability for patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2003;47:185–90. doi: 10.1034/j.1399-6576.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 17.Diamant M, Benumof JL, Saidman LJ. Haemodynamics of increased intra-abdominal Pressure: inter action with hypovolemia and halothane anesthesia. Anesthesiology. 1978;48:23–7. doi: 10.1097/00000542-197801000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ishizaki Y, Bandal Y, Shimomura K. Safe intra-abdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery. 1993;114:549–54. [PubMed] [Google Scholar]
- 19.Cunningham AJ, Turner J, Rosenbaum S, Rafferty T. Transesophageal echocardiographic assessment of haemodynamicfunction during laparoscopic cholecystectomy. Br J Anaesth. 1993;70:621–5. doi: 10.1093/bja/70.6.621. [DOI] [PubMed] [Google Scholar]
- 20.Dorsay GA, Greene FL, Baysinger CL. Haemodynamic changes during laparoscopic cholecystectomy monitored with transesophageal echocardiography. Surg Endosc. 1995;9:128–34. doi: 10.1007/BF00191952. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaou G, Chen AA, Johnston CE, Kenny GP, Bristow GK, Giesdbrecht GG. Clonidine decreases vasoconstriction and shivering threshold without affecting the sweating threshold. Can J Anaesth. 1997;44:636–44. doi: 10.1007/BF03015448. [DOI] [PubMed] [Google Scholar]
- 22.Javaherfroosh F, Pipelzadeh MR, Namazi M. Clonidine reduces post operative nausea and vomiting in laparoscopic gynecological surgery. Pak J Med Sci. 2009;25:782–85. [Google Scholar]


