Cocolonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) is a precursor to vancomycin-resistant S. aureus emergence. MRSA/VRE cocolonization incidence is higher among skilled nursing facility residents with functional disability and indwelling devices and occurs more frequently in wounds than other anatomical sites.
Abstract
Background. Methicillin-resistant Staphylococcus aureus (MRSA) remains sensitive to vancomycin; when vancomycin-resistant S. aureus (VRSA) emerges, treatment becomes more complex. VRSA emergence is attributed to conjugative transfer of the vancomycin-resistance gene cluster from vancomycin-resistant enterococci (VRE) to MRSA. Because cocolonization with MRSA and VRE precedes VRSA development, this study investigates the epidemiology of cocolonization in skilled nursing facility (SNF) residents at high risk for MRSA or VRE colonization.
Methods. A prospective observational study conducted at 15 SNFs in southeast Michigan. Overall, 178 residents (90 with indwelling urinary catheters and/or feeding tubes and 88 device-free) were cultured monthly for MRSA and VRE, and clinical data were recorded.
Results. The incidence of MRSA/VRE cocolonization among residents with indwelling devices was 6.5 per 100 resident-months; 5.2 (95% confidence interval [CI]: 1.49–18.1) times that among those without devices. MRSA/VRE cocolonization in the device group occurred most frequently in wounds (4.1 per 100 resident-months). In a logistic regression analysis limited to residents with devices, functional disability (rate ratio [RR], 1.3; 95% CI: 1.1–1.4) and wound presence (RR, 3.4; 95% CI: 1.4–8.6) were independent risk factors of cocolonization.
Conclusions. In a population of SNF residents, individuals with indwelling devices who also had functional disability or wounds were at greatest risk of MRSA/VRE cocolonization. These individuals should be routinely monitored for the presence of VRSA colonization.
Since the first vancomycin-resistant Staphylococcus aureus (VRSA) isolate was identified in 2002, there have been 10 additional cases reported, the most recent occurring in April 2010 [1]. Interestingly, 8 of the 11 cases occurred in Michigan. Because vancomycin is commonly used to treat methicillin-resistant S. aureus (MRSA) infections and all VRSA isolated to date have been resistant to vancomycin in addition to methicillin, the emergence of VRSA is a major concern [2].
High-level vancomycin resistance in S. aureus (MIC ≥ 16 μg/mL) develops when the vancomycin-resistance gene (vanA) is acquired from a vancomycin-resistant enterococcus (VRE), a bacterium that commonly colonizes the human gastrointestinal tract [3, 4]. Acquisition of vanA occurs by direct conjugal transfer of DNA from VRE to MRSA (frequently mediated by the Inc18-family of plasmids) [3], making proximity requisite for acquisition. Thus, cocolonization with MRSA and VRE is necessary for vanA transfer and subsequent emergence of VRSA. Indeed, several of the VRSA cases had documented coinfection with VRE at the time of VRSA isolation [1, 5]. Therefore, because MRSA/VRE cocolonization precedes VRSA emergence, we chose to investigate the incidence of MRSA/VRE cocolonization in skilled nursing facilities (SNFs), a long-stay population. Using this population, we can observe the incidence rates of MRSA/VRE cocolonization, as well as colonization patterns over time.
Previous studies have investigated the prevalence rates of MRSA/VRE cocolonization but have been limited to clinical cultures or surveillance cultures from specific body sites such as the nares (MRSA surveillance) or rectum (VRE surveillance) [6, 7]. Yet for nearly all documented VRSA cases (10 of 11), the VRSA isolates were recovered from wounds [1]. Therefore, we were interested in using a prospective study design to estimate the overall incidence rate of MRSA/VRE cocolonization among SNF residents, as well as how incidence rates vary at different body sites, especially wounds.
The current study examines risk factors for MRSA/VRE cocolonization and specifically focuses on indwelling device use. Previous analyses from our group demonstrated that SNF residents with indwelling devices are at greater risk for multianatomic site colonization with antibiotic-resistant organisms [8]. Because many of the risk factors for independent colonization with MRSA or with VRE are known, our goal was to determine whether these risk factors were also predictive of MRSA/VRE cocolonization [9, 10]. We used a prospective surveillance approach to identify the risk factors for and incidence rates of cocolonization with MRSA and VRE in SNF residents in southeast Michigan.
MATERIALS AND METHODS
Study Design and Population
This prospective observational study involving 15 community-based SNFs in southeast Michigan was conducted between October 2005 and January 2010. Facilities accepted residents from local hospitals but were not hospital-based. Bed size ranged from 71 to 161, and staff and residents were not shared or transferred between facilities. All residents in a facility, regardless of time of admission, were screened for eligibility.
All residents with an indwelling device (enteral feeding tube and/or urinary catheter) were identified and asked to enroll in the study. Upon enrollment of a resident with an indwelling device, a device-free resident was randomly selected using a random number generator and asked to enroll. All residents were followed for a maximum of 12 months or until death, culture refusal, transfer, or device removal. Demographic data were recorded at enrollment, and clinical and microbiologic data were obtained at monthly study visits. This study was approved by the University of Michigan and Veterans Affairs Ann Arbor Health Care System Institutional Review Boards. Written informed consent was obtained from all enrolled residents or their durable power of attorney.
Data Collection and Variables
Participant’s age, sex, and prior length of stay at the SNF were recorded at enrollment. Residents who had been at the facility for >90 days are considered “long stayers.” Clinical data, including antibiotic use, presence of wounds (including pressure ulcers), and hospitalization, all in the previous 30 days, were obtained from all enrolled residents at each monthly study visit by chart review. The Charlson Comorbidity Index was used to assign a comorbidity score ranging from 0 to 11 (where 11 is most comorbidity) [11]. The Lawton and Brody Physical Self-Maintenance Scale was used to assess functional status at each study visit and ranges from 6 to 30, with 30 describing the most physically dependent residents [12].
Microbiologic and Molecular Methods
At each monthly study visit, culture samples were obtained from the anterior nares, oropharynx, groin, and perianal area using Culturette rayon-tipped swabs (Becton Dickenson, Inc). In addition, enteral feeding tubes as well as wounds or pressure ulcers were swabbed and cultured when present. All specimens were tested for MRSA and VRE (see below). One participant refused to provide specimens, so 1 resident-month in the device group is missing for the nares and oropharynx.
For isolation and identification of S. aureus, all swabs were streaked on mannitol salt agar and incubated at 35°C for 24 hours. Colonies presumptive for S. aureus based on phenotype were further confirmed by positive catalase test and agglutination with a rapid test for protein A (Fisher Healthcare). Growth on Mueller-Hinton agar (BD) containing oxacillin (6 μg/mL) identified MRSA. All MRSA colonies were subsequently tested for vancomycin resistance on Mueller-Hinton agar containing vancomycin (6 μg/mL) [13].
Additionally, all culture swabs were streaked on Bile Esculin Azide agar (BD) containing vancomycin (6 μg/mL) for identification of VRE colonies. VRE was confirmed by plating on tryptic soy agar containing 5% sheep’s blood and positive pyroglutamate aminopeptidase tests (pyrrolidonyl-beta-naphthylamide).
Polymerase chain reaction (PCR) using primer pairs: E1: atcaagtacagttagtct and E2: acgattcaaagctaactg and F1: tagagacattgaatatgcc and F2: tcgaatgtgctacaatc identified E. faecalis and E. faecium, respectively [14]. For identification of Inc18-like plasmids, PCR was performed with primer pairs: vanA F: catgaatagaataaaagttgctgcaata and vanA R: cccctttaacgctaatacgatcaa; traA F: taatcgcaatggcttcttatc and traA R: tctgcccaatctttacgaat; and repR F: gcttcatgacggcttgtta and repR R: ttggctgctttgacagattta [15]. PCR was performed in 50-μL reactions with 1-μL colony resuspended in sterile dH2O as template. PCR conditions were as follows: (1) 10 minutes at 94 °C; (2) 30 cycles of 30 seconds at 94°C, 30 seconds at 56°C and 1 minute at 72°C; and (3) 7 minutes at 72°C.
Outcome Measures
Our primary outcome was resident-level incidence rates of cocolonization with MRSA and VRE defined as colonization with MRSA and VRE on the same visit from any combination of sites. A resident could be colonized with both VRE and MRSA at the same anatomic site (eg, wounds) or at different anatomic sites (eg, wound and groin). Colonization with MRSA only or VRE only at the resident level was defined similarly, as colonization with MRSA or VRE at any anatomic site. To determine anatomic site-specific incidence rates, simultaneous isolation of MRSA and VRE from the same culture swab was considered MRSA/VRE cocolonization.
Statistical Analyses
We identified differences in clinical and demographic characteristics between the device and nondevice groups at baseline. A χ2 test was used to detect differences in categorical variables and a Student t test for differences in continuous variables, both with a 2-sided significance level of α = .05.
We conducted a risk-factor analysis where cocolonization with MRSA and VRE at the resident level was the outcome of interest. Using logistic regression, we estimated rate ratios for cocolonization among residents with devices using 3 different base (comparison) populations: (1) all residents with devices in the study, (2) all residents with devices colonized with MRSA, and (3) all residents with devices colonized with VRE. This analysis highlights differences in outcomes conditional on the base populations used for these analyses. We used generalized estimating equations to describe the associations between MRSA/VRE cocolonization and clinical and demographic characteristics and to adjust for the repeated measures design of the study [16]. A log link function with a Poisson distribution was used to calculate the MRSA/VRE cocolonization rate ratios with robust error variances [17]. Data were analyzed using SAS, version 9.2.
RESULTS
Study Population Characteristics
A total of 178 SNF residents from 15 community-based SNFs were enrolled: 90 with indwelling devices and 88 device-free. The average number of residents enrolled per SNF was 4 (range, 2–83). Residents were followed for a total of 907 resident-months (mean, 5.1); 263 in the device group and 644 in the nondevice group. Residents with devices differed significantly in all characteristics from nondevice residents, except for the proportion of residents with diabetes (26% vs 30%, respectively) (Table 1). Because residents in the device and nondevice groups differed significantly on most characteristics, and indwelling devices are a substantial risk factor for colonization with antibiotic-resistant organisms (Table 2 and [8]), we stratified by device group for all subsequent analyses.
Table 1.
Baseline Characteristics of 178 Skilled Nursing Facility Residents
| Characteristic | Device | Nondevice | P value |
| No. (%) of residents overall | 90 (51) | 88 (49) | |
| Mean ± SD | |||
| Age, years | 78.9 (11.2) | 82.2 (10.7) | .049 |
| Charlson comorbidity score | 3.1 (2.0) | 2.2 (1.6) | .0012 |
| Follow-up, months | 2.9 (3.3) | 7.3 (4.5) | <.0001 |
| PSMS | 21.1 (5.4) | 18.2 (5.7) | .0006 |
| No. (%) of residents | |||
| Male | 41 (46) | 21 (24) | .0024 |
| Diabetes | 23 (26) | 26 (30) | .58 |
| Wound presence | 29 (32) | 9 (10) | .0003 |
| Antibiotic use | 61 (68) | 28 (32) | <.0001 |
| Hospitalization | 66 (73) | 13 (15) | <.0001 |
| Long stayers (>90 days) | 24 (27) | 70 (80) | <.0001 |
| Device type | |||
| Urinary catheter | 48 (53) | … | |
| Feeding tube | 30 (33) | … | |
| Both | 12 (13) | … |
Prospective surveillance study of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci cocolonization conducted in skilled nursing facilities in Michigan, October 2005 to January 2010. Abbreviation: PSMS, Physical Self-Maintenance Scale score (functional status).
Table 2.
Incidence Rates/100 Resident Months of Methicillin-Resistant Staphylococcus aureus/Vancomycin-Resistant Enterococci (MRSA/VRE) Cocolonization, MRSA Colonization Alone, and VRE Colonization Alone, Stratified by Device Group
| Overall |
Device |
Nondevice |
|||||
| EV | IR | EV | IR | EV | IR | IRRa | |
| MRSA/VRE cocolonization | 22 | 2.4 (1.6–3.6) | 17 | 6.5 (3.9–10) | 5 | 0.8 (0.2–1.7) | 5.2 (1.49–18.1)* |
| MRSA only | 189 | 21 (18–24) | 77 | 29 (23–36) | 112 | 18 (14–21) | 1.72 (1.16–2.56)* |
| VRE only | 22 | 2.4 (1.6–3.6) | 10 | 3.8 (1.9–6.8) | 12 | 1.9 (1.0–3.2) | 2.49 (0.96–6.24) |
Prospective surveillance study of methicillin-resistant Staphylococcus aureus/vancomycin-resistant enterococci cocolonization conducted in skilled nursing facilities in Michigan, October 2005 to January 2010 (n = 178). Abbreviations: EV, number of resident months colonized; IR, incidence rate/100 resident-months; IRR, incidence rate ratio; MRSA/VRE, methicillin-resistant Staphylococcus aureus/vancomycin-resistant enterococci.
Incidence rate ratio of colonization for device group compared with nondevice group adjusted for repeated measures using generalized estimating equations.
*P < .01.
Fourteen Residents Were Cocolonized With MRSA and VRE
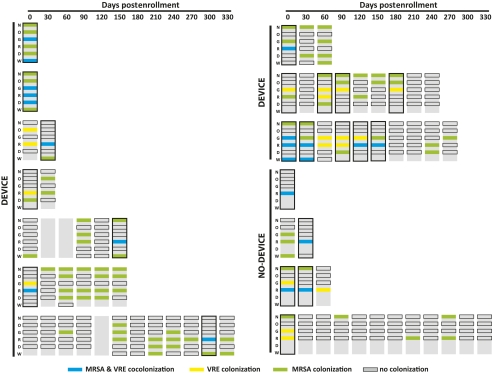
At the resident level, 14 residents were cocolonized with MRSA and VRE on at least 1 study visit, at any combination of body sites (outlined in black in Figure 1). The cocolonized residents were from 8 different SNFs. There was no evidence of clustering of cocolonized residents within facilities or over time. The greatest number of cocolonized residents at a single facility was 4; this facility had the largest number of study participants. Cocolonization with MRSA and VRE at the resident level was dynamic within each resident over time and occurred in 10 residents with devices and in 4 residents without a device (Figure 1). MRSA and VRE colonization were both predictive of one another, and cocolonization occurred more frequently among residents colonized with VRE than among those colonized with MRSA (data not shown).
Figure 1.
Colonization patterns of 14 residents cocolonized a minimum of 1 visit during a prospective surveillance study of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) cocolonization conducted in skilled nursing facilities in Michigan, October 2005 to January 2010 (n = 178). Each row represents the follow-up time for a single study participant. The top horizontal axis denotes the number of days postenrollment. Each vertical rectangle represents a study visit for a particular resident; the 6 anatomical sites eligible for culture: N = nares, O = oropharynx, G = groin, R = rectum, D = device, and W = wound or pressure ulcer. Colored boxes indicate MRSA (green) was cultured at that site, VRE (yellow), and both MRSA and VRE (blue), no organism present (black outline) or not cultured (gray). Residents were defined as cocolonized for the patient-level analysis if they were colonized with MRSA and VRE on the same study visit at any combination of sites (black outlined vertical rectangles). Residents were considered cocolonized for the site-level analysis if MRSA and VRE were both isolated from the same anatomical site at a study visit. Whether residents had indwelling devices is indicated on the y-axis.
The incidence rate of cocolonization was 5.2 (95% confidence interval [CI]: 1.5–18.1) times greater in residents with devices compared to those without, after adjusting for repeated measures. Among residents with devices, the incidence rate of MRSA/VRE cocolonization was less than MRSA colonization alone but greater than VRE colonization alone (Table 2). Also, residents with devices were more likely to be MRSA/VRE cocolonized or singly colonized with MRSA or VRE than residents without devices (Table 2): the incidence rate ratio for cocolonization compared with colonization with MRSA alone was 0.21 (95% CI: .12–.36) and colonization with VRE alone was 1.6 (95% CI: 0.73–3.53).
Associations of Clinical and Demographic Characteristics With MRSA/VRE Cocolonization
We used resident-level cocolonization status for the risk-factor analysis to be comparable to previous studies. Further, we reasoned that within a patient, self-inoculation and cross-contamination of sites by health-care workers might occur. Using logistic regression, functional status and wounds were significant predictors of MRSA/VRE cocolonization among those with devices (Table 3). For every unit increase in physical and self-maintenance scale score (increasing dependency) there is a 30% increase in the rate of MRSA/VRE cocolonization (95% CI: 1.1–1.4). The rate of MRSA/VRE cocolonization in device residents with wounds was 3.4 times that in residents without wounds (95% CI: 1.4–8.6) (Table 3).
Table 3.
Associations Between Methicillin-Resistant Staphylococcus aureus/Vancomycin-Resistant Enterococci Cocolonization and Clinical and Demographic characteristicsa
| MRSA/VRE cocolonizationb | No cocolonizationc |
MRSA onlyd |
VRE onlye |
||||
| Characteristic | (n = 17) | (n = 246) | rate ratio (95% CI) | (n = 77) | rate ratio (95% CI) | (n = 10) | rate ratio (95% CI) |
| Mean | |||||||
| Age, years | 75.9 | 77.9 | 1.0 (1.0–1.1) | 78. | 1.0 (1.0–1.0) | 79.4 | 1.0 (1.0–1.0) |
| CCS | 3.2 | 3.2 | .9 (.8–1.1) | 3.1 | 0.9 (.7–1.2) | 3.5 | 0.9 (.7–1.2) |
| Follow-up, months | 6.8 | 6.6 | 1.0 (.9–1.2) | 7.3 | 1.0 (.8–1.1) | 2.9 | 1.1 (1.0–1.2)* |
| PSMS | 26.3 | 22 | 1.3 (1.1–1.4)*** | 23.5 | 1.2 (1.0–1.4)* | 21.7 | 1.1 (1.0–1.3)* |
| No. (%) of residents | |||||||
| Male | 13 (76) | 103 (42) | 3.1 (.9–10.4) | 30 (39) | 3.3 (1.1–10.2)* | 7 (70) | 1.0 (.5–2.0) |
| Diabetes | 7 (41) | 63 (27) | 1.3 (.3–4.8) | 19 (25) | 1.1 (.3–3.9) | 4 (40) | 0.9 (.4–2.1) |
| Wound | 8 (53) | 62 (27) | 3.4 (1.4–8.6)** | 24 (35) | 2.6 (1.3–5.3)** | 4 (40) | 1.2 (.48–2.84) |
| Antibiotics | 9 (53) | 82 (33) | 3.0 (1.0–9.1) | 18 (23) | 3.7 (1.3–10.3)* | 9 (90) | 0.5 (.3–0.9)* |
| Hospitalization | 6 (38) | 69 (30) | 1.6 (.3–9.9) | 14 (21) | 10.6 (.7–151.4) | 8 (80) | 0.5 (.3–1.1) |
| Long stayf | 7 (41) | 97 (39) | 1.8 (.5–6.3) | 29 (38) | 1.3 (.4–4.3) | 1 (10) | 1.9 (1.0–3.7) |
Prospective surveillance study of methicillin-resistant Staphylococcus aureus/vancomycin-resistant enterococci (MRSA/VRE) cocolonization conducted in skilled nursing facilities in Michigan, October 2006 to January 2010 (n = 178). Cocolonized participants are compared to all other participants, to those colonized only with MRSA, and with those colonized only with VRE.
Abbreviations: n, number of resident-months colonized; CCS, Charlson comorbidity score (range, 0–11 with increasing comorbidity); PSMS, Physical Self-Maintenance Scale score (range, 6–30, with increasing functional disability).
Adjusted for repeated measures using generalized estimating equations.
MRSA/VRE cocolonization is the outcome in each analysis. Subpopulations that the logistic regression analyses were conducted in are listed in notes c, d, and e.
All device residents (included residents singly colonized with VRE alone or MRSA alone).
MRSA only colonization.
VRE only colonization.
Admission at facility >90 days.
*P < .05.
**P < .01.
***P < .001.
Among residents with devices, predictors of cocolonization compared with residents with MRSA alone or VRE alone differed. Among residents colonized with MRSA, residents who received antibiotics in the previous 30 days had a near 4-fold increase in MRSA/VRE cocolonization rates compared with residents who did not receive antibiotics (rate ratio [RR], 3.7; 95% CI: 1.3–10.3). Functional status (RR, 1.2; 95% CI: 1.04–1.38), male sex (RR, 3.4, 95% CI: 1.1–10.2), and wounds (RR, 2.6; 95% CI: 1.3–5.3) were also significantly associated with cocolonization among MRSA colonized residents (Table 3).
On the other hand, in the VRE colonized group, residents were half as likely to be cocolonized with MRSA if they received antibiotics in the previous 30 days (RR, 0.5; 95% CI: .3–.9) (Table 3). Among the residents colonized with VRE, again increasing physical and self-maintenance score was significantly associated with cocolonization (RR, 1.1; 95% CI: 1.04–1.25).
Incidence Rate of Cocolonization by Anatomic Site
Incidence rates for cocolonization with MRSA and VRE were determined for 6 different anatomical sites and compared with the rate of colonization with MRSA alone and VRE alone. For the site-level analysis, cocolonization was defined as simultaneous colonization with MRSA and VRE at the same site on the same study visit (blue boxes in Figure 1). Among the device group, the incidence rate for cocolonization with MRSA and VRE was highest in wounds and the rectum (4.1/100 and 3.8/100 resident-months, respectively) (Table 4). Of note, MRSA/VRE cocolonization was never observed in the nares or oropharynx. Additionally, in the device group, when devices and wounds were colonized with VRE, there was always cocolonization with MRSA (Table 4). Importantly, MRSA/VRE cocolonization only occurred in the rectum in nondevice residents, at a rate of 0.6/100 resident-months (Table 4).
Table 4.
Incidence Rates Per 100 Resident-Months of Cocolonization With Methicillin-Resistant Staphylococcus aureus (MRSA) and Vancomycin-Resistant Enterococcus (VRE), Colonization With VRE Only and Colonization With MRSA Only at 6 Different Anatomical Sites Stratified by Device Groupa
| Incidence rateb (95% CI) |
||||||
| Device |
Nondevice |
|||||
| MRSA/VRE | VRE only | MRSA on | MRSA/VRE | VRE only | MRSA only | |
| Nares | 0 | 0 | 15.3 (11–21) | 0 | 0 | 11.0 (8.7–14) |
| Oropharynx | 0 | 3.8 (.2–19) | 8.0 (5.1–12) | 0 | 0 | 0.9 (.4–1.9) |
| Groin | 1.1 (.3–3.1) | 5.3 (3.0–8.7) | 7.6 (4.8–12) | 0 | 1.7 (.9–3.0) | 3.1 (2.0–4.7) |
| Rectum | 3.8 (1.9–6.8) | 3.8 (1.9–6.8) | 12.9 (10–18) | 0.6 (.2–1.5) | 1.4 (.7–2.6) | 5.3 (3.7–7.3) |
| Device | 0.4 (0.02–1.9) | 0 | 14.5 (10–20) | … | … | … |
| Wound | 4.1 (1.0–11) | 0 | 14.9 (7.8–26) | 0 | 0. | 13.1 (6.1–15) |
Prospective Surveillance Study of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci cocolonization conducted in skilled nursing facilities in Michigan, October 2005 to January 2010 (n = 178).
Abbreviations: CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
There were 263 and 644 resident-months exposed in the device and nondevice groups, respectively. There were 74 and 61 resident-months exposed to wounds in the device and nondevice groups, respectively.
Colonization/100 resident-months.
Molecular Analysis of VRE Isolates
We used PCR of enterococci-specific genes (ddl) to identify all VRE isolates to the species level [1, 14]. E. faecalis was isolated more commonly in both the device and nondevice groups and accounted for over half (52%) of the total VRE isolates. E. faecium was isolated more commonly from cocolonized residents (41%) compared with residents who were not cocolonized (19%), although this difference was not statistically significant (P = .063).
Plasmids of the Inc18 family have been associated with several of the VRSA cases because of successful conjugation with MRSA [1]. Therefore, we determined the prevalence of Inc18-like plasmids in our study population. PCR for the repR, traA, and vanA genes was used to identify Inc18-like plasmids carried by the enterococcal isolates. Only 2 (3%) of 66 of the VRE isolates were positive for the presence of Inc18-like plasmids. This is similar to the prevalence of Inc18-like plasmids reported by Zhu et al [1] for ICUs in Michigan.
DISCUSSION
In this prospective study of 178 residents of SNFs in southeast Michigan, the incidence of MRSA/VRE cocolonization was 2.4/100 resident-months overall and 5.2 (95% CI: 1.49–18.1) times greater in residents with indwelling devices compared with residents without. Ours is the first study to actively culture multiple anatomic sites, including wounds, over time within a patient to identify MRSA/VRE cocolonization. This protocol allowed us to identify wounds as the most common site for cocolonization compared with other sites cultured in patients with indwelling devices. Consistent with our findings is that 10 of 11 previously reported cases of VRSA were identified from wounds [18]. Wounds may be an important reservoir for VRSA emergence because of the propensity for biofilm formation within wounds; biofilms can enhance bacterial growth and lateral gene transfer [19].
Indwelling devices are a well-known risk factor for colonization with antibiotic-resistant organisms [20]. We show that the risk of MRSA/VRE cocolonization is higher in individuals with indwelling devices, as is cocolonization at more anatomic sites than individuals without an indwelling device. In residents without devices, MRSA/VRE cocolonization occurred only at a single site, the rectum. Interestingly, we observed no cocolonization among the wounds present in residents without indwelling devices. Therefore, among residents with wounds, an indwelling device may pose a specific risk for MRSA/VRE cocolonization of the wound.
MRSA and VRE colonization were not independent of one another; this is not surprising given the similarity of risk factors, such as presence of indwelling devices, antibiotic use, and duration of hospital stay, for both MRSA colonization alone and VRE colonization alone [21, 22]. However, different predictors important for cocolonization were identified between the MRSA-colonized and VRE-colonized groups. Among residents colonized with MRSA in the device group, 17 (18%) of 94 resident-months were also colonized with VRE (thus cocolonized) compared with those colonized with VRE, where 17 (63%) of 27 resident-months were also colonized with MRSA. The frequency of cocolonization among residents with devices was also greater than VRE colonization alone in these individuals. Furthermore, VRE colonization alone never occurred at device or wound sites in the residents with indwelling devices; that is, if residents were colonized with VRE at these sites they were always also colonized with MRSA (or cocolonized). Thus, our findings support the suggestion that preventing VRE colonization among residents with indwelling devices would limit MRSA/VRE cocolonization [1]. Additional studies are needed to understand the significance of predictive ability of VRE colonization for MRSA/VRE cocolonization.
Our study is limited by its sample size and selection procedure. Because we restricted our investigation of risk factors for MRSA/VRE cocolonization to residents with indwelling devices, it is difficult to determine the true role diabetes and functional status may play in MRSA/VRE cocolonization independent of device use. Additionally, it would be interesting to know risk factors for cocolonization specific to each anatomic site. Nevertheless, we present important risk factors for cocolonization among SNF residents with indwelling devices, a high-risk cohort common in these facilities. Increasing awareness for the role functional status plays in colonization with multiple drug-resistant pathogens has led to clinical studies that specifically investigate this association [20, 23]. Additional studies are needed to confirm these findings, as well as identify additional risk factors responsible for cocolonization.
Prevalence estimates of MRSA/VRE cocolonization in the literature vary dramatically, from 0.27% to 34% [24–26]. The wide range of previous prevalence estimates can be explained by variability in study populations, the use of clinical and/or surveillance cultures, and whether screening was conditional on the prior positive identification of MRSA or VRE colonization within the subject [6, 7, 25–27]. In our prospective study, the cross-sectional prevalence of cocolonization at baseline was 7.9% when active surveillance was performed on 5 anatomical sites regardless of prior MRSA or VRE colonization status. This may be a better representation of true prevalence. Because we found wounds to be the most common site of MRSA/VRE cocolonization among residents with devices, additional studies should be conducted to determine risk factors specific for wound cocolonization and their interaction with device status. Because cocolonization is an important precursor for VRSA emergence, it is crucial to identify high-risk groups most amenable to infection prevention interventions. The greater the prevalence of MRSA/VRE cocolonization, the greater the possibility of gene transfer leading to new VRSA strains—some that may transmit well between persons, an event that has yet to be documented.
Notes
Acknowledgments.
The authors would like to thank Bonnie Lansing, Jay Fisch, and Tisha Moore for enrollment of participants and sample collection, Kathleen Symons and Kay Cherian for sample processing, and Heidi Reichert for help with the statistical analyses.
Financial support.
L. M. was supported by NIA K23 AG028943, ASP/AGS T. Franklin Williams Research Scholarship, NIA R01AG032298 and The Claude D. Pepper Older Americans Independence Center and Michigan Institute for Clinical and Health Research, University of Michigan Pilot Grant Program. E. F. was supported by a Rackham Spring/Summer Research Grant.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zhu W, Murray PR, Huskins WC, et al. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:4314–20. doi: 10.1128/AAC.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century–a clinical super-challenge. N Engl J Med. 2009;360:439–43. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 3.Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:4580–7. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–8. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 5.Finks J, Wells E, Dyke TL, et al. Vancomycin-resistant Staphylococcus aureus, Michigan, USA, 2007. Emerg Infect Dis. 2009;15:943–5. doi: 10.3201/eid1506.081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren DK, Nitin A, Hill C, Fraser VJ, Kollef MH. Occurrence of co-colonization or co-infection with vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2004;25:99–104. doi: 10.1086/502357. [DOI] [PubMed] [Google Scholar]
- 7.Furuno JP, Perencevich EN, Johnson JA, et al. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization. Emerg Infect Dis. 2005;11:1539–44. doi: 10.3201/eid1110.050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55:1921–6. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce JM. Methicillin-resistant Staphylococcus aureus: detection, epidemiology, and control measures. Infect Dis Clin North Am. 1989;3:901–13. [PubMed] [Google Scholar]
- 10.Zirakzadeh A, Patel R. Epidemiology and mechanisms of glycopeptide resistance in enterococci. Curr Opin Infect Dis. 2005;18:507–12. doi: 10.1097/01.qco.0000186849.54040.2a. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Laboratory detection of vancomycin-intermediate/resistant Staphylococcus aureus (VISA/VRSA) 2010. ; Available at: http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html. Accessed 24 November 2010. [Google Scholar]
- 14.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–7. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrob Agents Chemother. 2008;52:452–7. doi: 10.1128/AAC.00908-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twisk JW, Smidt N, de Vente W. Applied analysis of recurrent events: a practical overview. J Epidemiol Community Health. 2005;59:706–10. doi: 10.1136/jech.2004.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindquist K. How can I estimate relative risk in SAS using proc genmod for common outcomes in clinical studies? Resources for SAS. . Available at: http://www.ats.ucla.edu/stat/sas/faq/relative_risk.htm. Accessed 3 March 2011.
- 18.Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA) 2010. November 24, 2010 [cited 2011 March 3, 2011]; Available at: http://www.cdc.gov/HAI/settings/lab/vrsa_lab_search_containment.html. Accessed 24 November 2010. [Google Scholar]
- 19.Ehrlich GD, Ahmed A, Earl J, et al. The distributed genome hypothesis as a rubric for understanding evolution in situ during chronic bacterial biofilm infectious processes. FEMS Immunol Med Microbiol. 2010;59:269–79. doi: 10.1111/j.1574-695X.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody L, Bradley SF, Galecki A, et al. Conceptual model for reducing infections and antimicrobial resistance in skilled nursing facilities: focusing on residents with indwelling devices. Clin Infect Dis. 2011;52:654–61. doi: 10.1093/cid/ciq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13:686–707. doi: 10.1128/cmr.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 23.Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc. 2008;56:1485–9. doi: 10.1111/j.1532-5415.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 24.Ray AJ, Pultz NJ, Bhalla A, Aron DC, Donskey CJ. Coexistence of vancomycin-resistant enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin Infect Dis. 2003;37:875–81. doi: 10.1086/377451. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JR, Engemann JJ, Kaye KS, Sexton DJ. Co-infection or co-colonization with vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus in a network of community hospitals. Infect Control Hosp Epidemiol. 2004;25:622. doi: 10.1086/503504. [DOI] [PubMed] [Google Scholar]
- 26.Reyes K, Malik R, Moore C, et al. Evaluation of risk factors for coinfection or cocolonization with vancomycin-resistant enterococcus and methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2010;48:628–30. doi: 10.1128/JCM.02381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurup A, Wong YY, Tan KY, Low JG. Clinical correlates of vancomycin-resistant enterococci/meticillin-resistant Staphylococcus aureus co-colonization and co-infection in Singapore. J Hosp Infect. 2008;70:291–2. doi: 10.1016/j.jhin.2008.08.001. [DOI] [PubMed] [Google Scholar]