Abstract
Exact positions of 5-methylcytosine (m5C) on a single strand of DNA can be determined by bisulfite genomic sequencing (BGS). Treatment with bisulfite ion preferentially deaminates unmethylated cytosines, which then convert to uracil upon desulfonation. Amplifying regions of interest from deaminated DNA and sequencing products cloned from amplicons permits determination of methylation at single nucleotide resolution along single DNA molecules, which is not possible with other methylation analysis techniques. This unit describes a BGS technique suitable for most DNA sources, including formaldehyde-fixed tissue. Considerations for experimental design and common sources of error are discussed.
INTRODUCTION
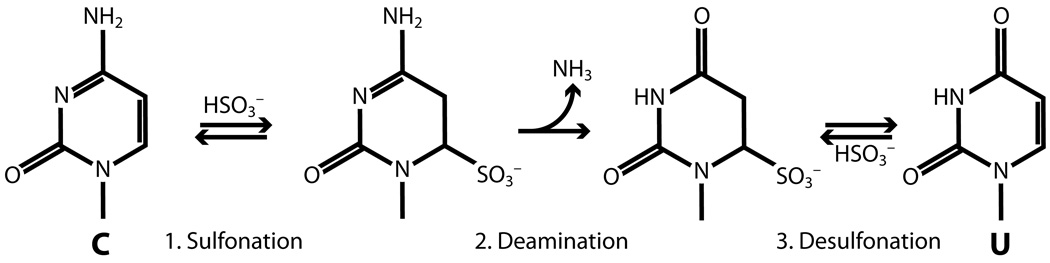
Cytosine methylation is a common post-replicative DNA modification in humans and many other organisms. Methods to identify sites of DNA methylation are useful for the study of gene expression and chromatin structure. Bisulfite deaminates unmethylated cytosine, causing its chemical conversion to uracil upon alkaline desulfonation (Figure 1). Bisulfite converts m5C much more slowly (Hayatsu et al., 1970; Shapiro et al., 1970). By selective conversion of cytosine but not m5C to uracil, followed by PCR and sequencing of cloned amplicon DNA, BGS accurately detects presence of m5C in each region of interest at single-nucleotide resolution (Frommer et al., 1992; Clark et al., 1994). The key advantage of BGS over other techniques is that it provides readout of methylation status of every cytosine along individually cloned molecules. This allows correlations between methylation status of individual cytosines examined within a defined region of the genome and permits identification of differentially methylated DNA species within a population.
Figure 1.
Reaction between cytosine and bisulfite (step 1) leads to deamination (step 2) at acid pH. Afterward, desulfonation at alkaline pH produces uracil (step 3).
This unit describes an improved bisulfite treatment technique, and methods for molecular amplification and cloning of PCR products amplified from bisulfite-converted DNA samples. Optional protocols detail a promising rapid bisulfite treatment technique (Shiraishi and Hayatsu et al., 2004) and genomic DNA preparation from fixed tissue (Ai et al., 2008), greatly extending potential applications of BGS. A supporting protocol covers proper primer design, which remains a significant challenge in BGS. Tips on data analysis are included in the Commentary. Methylation of both CpG and non-CpG sequences are covered with a view toward aiding all DNA methylation research in vertebrates as well as plants and lower organisms. This unit is also applicable for analysis of exogenous methylation used to assess accessibility of chromatin to DNA methyltransferase probes (Kladde et al., 1996; Fatemi et al., 2005; Jessen et al., 2006; Kilgore et al., 2007; UNIT X.X).
(BASIC PROTOCOL 1) BISULFITE CONVERSION OF DNA
The five basic steps in bisulfite conversion are: 1) DNA denaturation; 2) incubation with bisulfite at elevated temperature; 3) removal of bisulfite by desalting; 4) desulfonation of sulfonyl uracil adducts at alkaline pH; and 5) removal of the desulfonation solution. Although there are several commercially available kits for deamination, the “homebrew” approach used herein generally provides more effective deamination.
Up to 2 µg genomic DNA (or 1 ng purified fragment) may be used for BGS. Although 90% or more of DNA is lost during bisulfite treatment, using too much DNA may lead to incomplete deamination, due to re-annealing of complementary sequences. This is especially the case when DNA samples with low sequence complexity (e.g., cloned or PCR-amplified fragments) are deaminated. Multiple unique sequences have been reported from as few as 100 starting molecules (Svoboda et al., 2004).
Cytosines in double-stranded DNA are protected from deamination. Therefore, DNA must be free of protein to allow full denaturation for bisulfite treatment (Warnecke et al., 2002).
Materials
NOTE: Use only molecular biology-grade water (i.e., DNase and nucleic acid free) in all steps and solutions.
DNA of interest: up to 2 µg genomic DNA or 1 ng purified DNA fragment in 20 µl total volume; see Optional Protocol “DNA preparation from fixed tissue” (store indefinitely at −20°C)
Glycogen: molecular biology grade; to be used as carrier if substantially less than 2 µg DNA sample are available (store indefinitely at −20°C)
Degassed distilled H2O (degassed dH2O)
Boil ∼200 ml dH2O for 20 min in a glass beaker containing a stir bar to nucleate boiling and avoid superheating. Carefully pour the boiling water into a 125 ml glass bottle until it is completely full above the lip using surface tension and screw the cap on tightly to ensure an air-tight seal. Cool overnight on the benchtop. Recap the bottle tightly after each use and discard after bisulfite conversion is completed.
3 N NaOH: prepare fresh by weighing ∼0.4 g NaOH pellets and gently dissolving, i.e., avoiding vortexing and hence excessive aeration, in the degassed dH2O using the formula x g NaOH × 8.333 = y ml degassed dH2O diluent (discard after use in desulfonation of bisulfite-converted DNA; see step 8).
WARNING: Causes severe burns. Wear protective eye goggles and gloves.
0.5 M Na2EDTA, pH 8.0 (store indefinitely at room temperature)
100 mM hydroquinone: prepare fresh by gently dissolving ∼0.04 g in a 15 ml conical tube; x g hydroquinone × 90.827 = y ml degassed dH2O diluent (discard after bisulfite conversion of DNA is complete; the solution should remain clear as opposed to oxidizing to brownish)
WARNING: Harmful if swallowed or inhaled; causes irritation to skin and respiratory tract; causes irritation to and risk of serious damage eyes; and possible carcinogen. Possible risk of irreversible effects. Wear protective gear, including gloves, and dust and face masks. Dispose of properly because is very toxic to aquatic organisms.
Sodium bisulfite/ sodium metabisulfite: unopened, single-use vial containing 5 g (Sigma catalog no. 243973, a mixture of sodium bisulfite and sodium metabisulfite).
WARNING: Harmful if swallowed or inhaled; causes irritation to skin, respiratory tract, and eyes; risk of serious damage to eyes, may cause allergic respiratory reaction; and reacts with acids and water releasing toxic sulfur dioxide gas. Wear protective gear, including gloves, and dust and face masks.
NOTE: Instead of using these expensive 5 g quantities, we prefer to purchase 500 g sodium metabisulfite (≥ 97%; sodium bisulfite, ≥ 90% can also be used) and use a funnel to aliquot the crystalline reagent in a chemical safety hood (oxygen- and water-free environment under argon) into 5 g vials with Teflon® PTFE-lined screw caps. Weighing the amount of reagent to be dispensed into each vial is unnecessary as filling 5 g vials to their rim is sufficient to make a saturated solution. While under argon, the vials are capped tightly and can be stored for at least 2 years in the dark in a sealed vessel containing Drierite without detectable loss in cytosine conversion efficiency. If a chemical safety hood is unavailable, the reagent can be dispensed rapidly into vials by one person and another person can quickly cap them. The reagent should be disposed of properly according to institutional guidelines if the cytosine conversion efficiency begins to decline.
Minicolumn-based DNA purification kit: e.g., Zymo Research catalog no. D5026, which includes desulfonation buffer (store at room temperature)
TE buffer (see recipe; store indefinitely at room temperature)
Deamination or bisulfite conversion of DNA
-
1.
Set a water bath to 50°C.
-
2.
Prepare fresh sample denaturation buffer by gently mixing the following components in ratio of: 0.5 µl 0.5 M EDTA, pH 8.0: 3 µl 3 N NaOH (use the solution made from freshly dissolved pellets): 0.7 µl 3 mg/ml glycogen (if low amount of DNA sample): x µl degassed dH2O, and bring volume to 10 µl.
NOTE: Prepare enough solution for two extra reactions to allow for pipetting error.
-
3.
Add 10 µl sample denaturation buffer to each DNA sample in 20 µl total volume.
-
4.
Prepare fresh saturated sodium metabisulfite (or bisulfite) solution. First, place a 20 ml glass scintillation vial containing a small stir bar onto a stir plate positioned next to a calibrated pH meter. Also, place the degassed dH2O, freshly prepared 100 mM hydroquinone and 3 N NaOH, unopened 5 g vial of sodium metabisulfite, P100 and P1000 micropipettes and tips, 5 ml serological pipette, and pipetting device in the work area. Pipette 7 ml degassed dH2O, and 100 µl 100 mM hydroquinone into the 20 ml vial and stir gently on the stir plate. Next, while stirring gently, open and dump one 5 g vial of sodium metabisulfite into the 20 ml vial, and quickly add 1 ml 3 N NaOH. Adjust the pH to 5.0 while stirring by adding more 3 N NaOH (usually requires 200–300 µl). Cap the vial and preheat the saturated sodium metabisulfite solution to 50°C in a beaker containing water in a water bath. Save the 3 N NaOH solution for desulfonation (step 9).
CAUTION: See above warnings (BASIC PROTOCOL 1; Materials; NaOH, hydroquinone, and bisulfite) and wear protective eye goggles, dust mask, and gloves. The presence of undissolved sodium bisulfite or metabisulfite crystals in the solution is fine, if not preferable, as it ensures preparation of a saturated solution. This recipe provides enough bisulfite solution for at least 30 deamination reactions; however, it is good practice to incubate some unused solution in the capped vial at 50°C in parallel to the samples undergoing bisulfite conversion. The pH of this unused solution is measured in step 7 to make sure that it did not increase significantly, which indicates oxidation of the reactive bisulfite ion to the inert bisulfate ion.
-
5.
Place DNA samples in a thermocycler with a heated lid and denature 5 min at 98˚C. Place a stir plate, P200 micropipette and tips, and saturated sodium metabisulfite (or bisulfite) solution preheated to 50°C next to the thermocycler. Near the end of the denaturation cycle, maintain the thermocycler at 98˚C for step 6.
-
6.
While samples are in the thermocycler at 98˚C, add 200 µl of preheated saturated sodium metabisulfite (or bisulfite) solution from step 4 to the first sample, rapidly remove the tube from the thermocycler, cap it, vortex immediately, and place in a tube float in a water bath preheated to 50°C. It is not necessary to cover samples with mineral oil. Incubate the samples 4–6 hr at 50°C in the dark.
As many as 10–15 samples can be processed sequentially in this manner. The samples can be denatured with the tubes uncapped, but it is then advisable to minimize evaporation by closing the heated lid while each sample is being vortexed and placed at 50°C. Visible, but not complete, evaporation of sample volume does not adversely affect conversion efficiencies of unmethylated cytosines, which are routinely ≥ 99% with this protocol. Longer incubation times lead to increased conversion of m5C to T as well as increased DNA degradation and thus should be avoided.
-
7.
Near the end of the deamination incubation, measure and record the pH of the unused saturated metabisulfite (bisulfite) solution.
NOTE: An increase in pH of more than 0.2 units indicates potential decreased conversion efficiency.
Desalting and desulfonation
A commercially available kit such as the EZ bisulfite DNA Clean-Up Kit (Zymo Research catalog no. D5026) is recommended for steps 8–10.
-
8.
Immobilize DNA by binding to a minicolumn and remove bisulfite solution by centrifugation. Wash with 80% ethanol (v/v) to remove residual bisulfite.
CAUTION: Bisulfite solutions and washes should be disposed of properly according to institutional guidelines.
-
9.
To desulfonate DNA, incubate with 0.3 N NaOH (preheated to 30°C; use the fresh 3 N NaOH prepared from pellets or solution included in the kit) for 15–25 min at 30°C. This can be done on-column by placing the columns in a 30°C incubator.
-
10.
Wash DNA twice with 80% ethanol to desalt before eluting in 20 µl TE preheated to 90°C.
(OPTIONAL PROTOCOL 1) RAPID BISULFITE CONVERSION
The basic bisulfite conversion protocol above and in kits offered by commercial suppliers relies on sodium salts of metabisulfite or bisulfite. This promising optional protocol not yet available in commercial kits is based on ammonium bisulfite instead. Increased solubility of ammonium over sodium allows higher bisulfite ion concentration to be achieved and hence leads to a faster deamination reaction (Shiraishi and Hayatsu, 2004). Shorter incubation times reduce conversion of m5C to thymine (Genereux et al., 2008), an adverse side reaction. While faster conversion of unmethylated C residues to U is possible, this protocol has only been used in a few publications (e.g., Bouazoune et al., 2009), and we have obtained lower deamination efficiencies than with sodium bisulfite treatment (BASIC PROTOCOL 1). Thus, widespread application of ammonium bisulfite-based deamination is likely to require further optimization.
Materials
Ammonium bisulfite 45% solution: Spectrum catalog no. A1145; aliquot into 5 g glass vials (with Teflon® PTFE-lined caps) to the rim so there is as little excess volume as possible and cap tightly (store at room temperature in vessel containing Drierite)
Sodium bisulfite (≥90%)
CAUTION: Both bisulfite-based reagents are harmful if swallowed or inhaled. They cause irritation to skin, respiratory tract, and eyes. Risk of serious damage eyes and may cause allergic respiratory reaction. React with acids and water releasing toxic sulfur dioxide gas. Wear protective gear, including gloves, and dust and face masks.
Ammonium sulfite monohydrate (≥ 92%)
CAUTION: Very hazardous in case of ingestion. Hazardous in case of skin contact (irritant), eye contact (irritant), and inhalation. Contact with acids liberates toxic gas. Wear protective gear, including gloves, and dust and face masks.
Rapid deamination or bisulfite conversion of DNA
-
1.
Set water bath to 70°C.
-
2.
Freshly prepare the ammonium bisulfite solution. Add 0.603 g ammonium sulfite monohydrate, 1.872 g sodium bisulfite, and 4.5 ml 45% ammonium bisulfite (freshly opened) to a small beaker or 20 ml glass scintillation vial, which is then loosely capped.
CAUTION: See above warnings (OPTIONAL PROTOCOL 1, Materials; ammonium sulfite, ammonium bisulfite, and sodium bisulfite) and wear protective eye goggles, dust mask, and gloves.
NOTE: The combination of ammonium sulfite, ammonium bisulfite, and sodium bisulfite produces a 9 M bisulfite solution at an acidic pH (∼5.3), thus it is not necessary to titrate the pH further. Recipe provides enough bisulfite solution for 25 deamination reactions.
-
3.
Dissolve bisulfite mixture by heating to 70°C–90°C with gently stirring.
CAUTION: Avoid overheating. If a scintillation vial is used, it must be loosely capped to avoid excessive buildup of pressure potentially causing the vial to explode. It may be most convenient to place the bisulfite-containing vial or beaker inside a heating block or dish partly filled with 70°C water atop a magnetic stir plate.
NOTE: The solution should turn yellow on heating.
-
4.
Denature DNA as above (steps 2, 3, and 5 of BASIC PROTOCOL 1).
-
5.
Add 180 µl of hot ammonium bisulfite solution to DNA, vortex immediately, and incubate 1 hr at 70°C in the dark.
An ultra rapid ammonium bisulfite conversion protocol was recently reported that achieved high-efficiency conversion of a purified DNA fragment (i.e., low complexity) in 10 min at a higher temperature of 90°C (Bouazoune et al., 2009). However, in our hands, poor conversion efficiencies of complex mammalian samples were observed, indicating further optimization is required.
-
6.
Desalting and desulfonation as above (steps 8–10 of BASIC PROTOCOL 1).
(OPTIONAL PROTOCOL 2) DNA PREPARATION FROM FIXED TISSUE
BGS can be performed on formalin-fixed, paraffin-embedded (FFPE) tissue. DNA isolated from FFPE tissue is fragmented and, because further fragmentation occurs during deamination, amplicons should be designed to be of a maximal size of 300 bp (see Support Protocol “Primer design”).
Either tissue blocks, manually dissected, or microdissected tissue can be used. Steps 1–5 are for tissue blocks only. For dissected tissue, capture 5000–10,000 cells of interest from stained tissue sections either manually or with a laser capture microdissector, then go directly to step 6.
Equipment
Microtome
Materials
Xylene
CAUTION: Flammable. Harmful if inhaled and upon contact with skin (irritant).
Ethanol
CAUTION: Flammable.
Lysis buffer (see recipe)
De-paraffinization of tissue blocks
-
1.
Cut tissue blocks into 5 µm thick sections with microtome.
5–20 sections are needed, depending on tissue type and size of sections.
-
2.
Cut away excess paraffin and place sections in a 1.5 ml centrifuge tube. Centrifuge briefly at 5,000 × g to pellet tissue. Store sections at 4°C or below until use.
-
3.
Add 1 ml xylene to each tube, gently vortex for 15 sec, and incubate at room temperature for 10 min. Centrifuge 5 min at 16,100 × g and discard the supernatant. Repeat xylene wash twice.
Make sure the xylene does not remove tube labels.
-
4.
Add 1 ml absolute ethanol to each tube. Gently vortex for 15 sec, centrifuge 5 min at 16,100 × g, and discard the supernatant. Repeat ethanol wash twice.
Make sure the ethanol does not remove tube labels.
-
5.
Remove as much ethanol as possible and dry tissue pellet for 10–20 min at 60°C with the cap open.
Lysis and elution
-
6.
Add 200 µl lysis buffer (50 µl for microdissected tissue) and incubate overnight at 50°C in a water bath. Tissues should be completely digested.
-
7.
Centrifuge 5 min at 9,000 × g to pellet debris. Move the supernatant to a new tube and discard pellet.
-
8.
Determine DNA concentration and store at –20°C until use.
OPTIONAL: DNA can be purified further by phenol: chloroform extraction and ethanol precipitation, although loss of nucleic acid will occur (see Sambrook and Russell 2001).
CAUTION: Phenol causes burns in skin contact, and is toxic if inhaled and if swallowed. Possible risk of irreversible effects. Chloroform is harmful if swallowed, irritating to skin, and a possible carcinogen. Wear protective gloves and a face mask.
(BASIC PROTOCOL 2) MOLECULAR AMPLIFICATION AND CLONING
The procedures below are optimized for deaminated DNA, which presents a special challenge in part due to reduced complexity (i.e., enrichment for adenine-thymine base pairs) of the cloned sequence. After deamination, DNA is single-stranded and prone to form secondary structure; to prevent amplification of non-specific fragments, use a heat-activated Taq polymerase preparation. Excellent results are obtained with HotStar Taq (Qiagen catalog no. 203203).
Materials
Molecular amplification:
2.5 mM dNTP mix (see recipe)
25 mM MgCl2
20 µM a1 and a2, or b1 and b2 primers (see Support Protocol “Primer design”)
Hot-start Taq DNA polymerase formulation and buffer supplied by manufacturer
Generic Taq polymerase and buffer supplied by manufacturer
Gel electrophoresis:
Electrophoresis-grade agarose
Tris-acetate EDTA (TAE) buffer (see recipe)
Ethidium bromide
CAUTION: Suspected mutagen; harmful if swallowed; very toxic if inhaled; and irritating to eyes, respiratory system and skin. Possible risk of irreversible effects. Wear protective gear, including gloves, dust and face masks.
Molecular cloning:
Commercial kit for DNA purification
OPTIONAL: Restriction endonucleases and manufacturer’s supplied buffer, depending on primer design or if genomic DNA needs to be digested (see below)
Vector plasmid
T4 DNA ligase and buffer supplied by manufacturer
20 µM primer that anneals upstream of clone insertion site in vector plasmid of choice
20 µM primer that anneals downstream of clone insertion site in vector plasmid of choice
Bacterial growth:
Competent DH5α, DH10B, or Top10 E. coli (see Sambrook and Russell, 2001)
LB-agar + antibiotic (see recipe)
SOC (see recipe)
1 M MgCl2
100 mM IPTG (isopropyl-β-D-1-thiogalactopyranoside, see recipe)
50 g/l X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside, see recipe)
OPTIONAL: Air pore tape sheets (Qiagen, catalog no. 19571)
Molecular amplification
NOTE: As for many PCR applications that amplify rare templates, reagent purity and avoiding contamination are critical. To maintain purity, store multiple small volumes of each reagent. It may also be desirable to work in a PCR workstation, using dedicated pipettes, tubes racks, etc.
-
1.Use 0.2–4 µl deaminated DNA as template in 25 µl polymerase chain reactions (PCR) containing 1× PCR buffer, 1.5–3.5 mM MgCl2, 200 µM dNTPs, 1.2 µM a1 and a2 (or b1 and b2) primer, and 1.25 units hot-start Taq DNA polymerase. Program the thermal cycler according to the manufacturer’s instructions for:
Cycles Time Temperature 1 5 min 94°C 40 45 sec 94°C 45 sec 3°C below the calculated primer Tm 1.5 min 72°C 1 10 min 72°C Extension times for amplifying deaminated DNA are longer because of the presence of uracil, which decreases the rate of DNA polymerization.
-
2.
Check for successful amplification by electrophoresis on a 1% (w/v) agarose TAE gel with 0.1% (w/v) ethidium bromide.
NOTE: If low yield of product was obtained, try to optimize PCR by varying parameters with the following priority:- Amount of deaminated DNA. The amount of DNA needed for successful amplification must be determined empirically. Non-specific DNA sequences interfere with amplification by non-specific incorporation of primers.
- Annealing temperature. If the PCR primers are specific and a single, low-yield amplification product is obtained, decreasing the annealing temperature may increase product yield. In contrast, if yield of specific PCR product is low as more often is the case, higher annealing temperature may increase yield by reducing incorporation of primers into non-specific fragments.
- Increasing extension time or number of cycles.
-
Addition of PCR enhancers, such as trimethylammonium chloride (TMAC; 5 mM stock solution added to 750 µM final concentration), can help increase yield of specific PCR product.CAUTION: TMAC is an irritant to the eyes, skin, and respiratory system. Wear protective gloves, eye goggles, and a dust mask.
-
3.
Once amplification conditions are optimized, perform three separate PCR reactions and, after pooling reactions, purify amplicon DNA with a kit according to the manufacturer’s directions. Electrophorese 2 µl on a 1% agarose TAE gel, versus known amounts of standards, and photograph to quantify DNA yield digitally. Store DNA at –20°C.
Pooling multiple PCR reactions lessens the influence of stochastic events in early-stage amplification of individual template molecules.
Molecular cloning
-
4.
(Optional) Digest approximately 50 ng PCR product and 350 ng vector per sample (plus 100 ng for controls) with appropriate restriction endonucleases (see Support Protocol “Primer design”), according to the directions supplied by the manufacturer. Prompt heat inactivation improves ligation efficiency, so it may be convenient to perform the digest in a thermal cycler.
Directional cloning facilitates analysis of the sequencing end product. However, other cloning strategies such as TA cloning are also effective. We and others typically use the topoisomerase TA cloning kit (TOPO/TA; Invitrogen catalog no. K450040).
-
5.
Ligate digested insert and vector at a 1:1 molar ratio using 200 units T4 DNA ligase, in the buffer supplied by the manufacturer, for either 1–3 hr at room temperature (20–25°C) or 6 hr at 16°C. Prepare three controls for the transformation: 1) a mock ligation with no DNA; 2) a mock ligation with digested vector but no ligase; and 3) a reaction with vector and ligase, but no insert.
-
6.
Transform competent E. coli cells with the ligations: incubate cells with DNA for 10 min on ice, heat shock 45 sec at 42°C, incubate 2 min at 4°C, add 250 µl SOC + 10 mM MgCl2, incubate 1.5 hr at 37°C, and plate on LB-agar containing appropriate concentration of selective antibiotic, after sterilely spreading 40 µl 100 mM IPTG and 20 µl 50 g/l X-gal for blue-white screening. Grow 12–16 hr at 37°C.
Colony PCR
-
7.Screen five or more transformants per plate by colony PCR: suspend each colony in 100 µl LB + antibiotic. Then, add 1 µl of each cell suspension to a 20 µl PCR reaction containing 1× PCR buffer, 2.5 mM MgCl2, 250 µM dNTPs, 1 µM each primer that anneals to sites flanking the insertion/ligation site, and 1.25 units generic thermophile DNA polymerase. Program the thermal cycler according to the manufacturer’s instructions for:
Cycles Time Temperature 1 8 min 94°C 30 30 sec 94°C 30 sec 54°C 1 min 72°C 1 5 min 72°C Bacterial cells remain viable during storage for approximately 10 days at 4°C when suspended in LB + antibiotic instead of water. This allows multiple rounds of colony PCR screening from 96-well plates in cases where cloning efficiency is low. Cells bearing insert-positive plasmids can also be grown for DNA sequencing by re-inoculating into fresh LB + antibiotic (see step 9).
-
8.
Check for successful amplification by electrophoresis on a 1% agarose TAE gel.
Genetic stability of different AT-rich deaminated DNA sequences in various bacteria strains varies unpredictably. If no product was obtained for half or more of the samples tested, repeat step 6 using a different bacterial strain, such as DH5α, DH10B, or Top10.
Sequencing
-
9.
Sequencing is commonly performed by automated techniques such as BigDye (Applied Biosystems) at a core facility or off site. Samples should be prepared according to guidelines supplied by the sequencing facility.
Sequencing results vary at different facilities due to the AT-richness of bisulfite-converted sequences. We obtain excellent, high-quality, and long reads at our core facility (University of Florida, Interdisciplinary Center for Biotechnology Research, ICBR; http://www.biotech.ufl.edu) by amplifying plasmid DNA with the TempliPhi™ DNA amplification kit (GE Healthcare) followed by BigDye sequencing (http://www.biotech.ufl.edu/servicefees.html#sangersequencing). Sequencing by this method conveys significant cost savings as it is not necessary to isolate plasmid DNA. Instead, inoculate 100 µl LB + antibiotic either directly with individual E. coli transformants from selective plates (if insert cloning efficiency is known to be high) or from cell suspensions (see step 7) screened positive for insert by colony PCR. If cells are to be sequenced in 96 well format, which usually confers further cost savings, cover the plate with air pore tape, incubate overnight at 37°C without shaking, bring to 8% (v/v) glycerol, and freeze/store at −70°C.
Number of clones submitted for sequencing depends on the application. Commonly 10–20 samples are sufficient, but for some applications as many as 50 may be necessary.
(SUPPORT PROTOCOL) PRIMER DESIGN
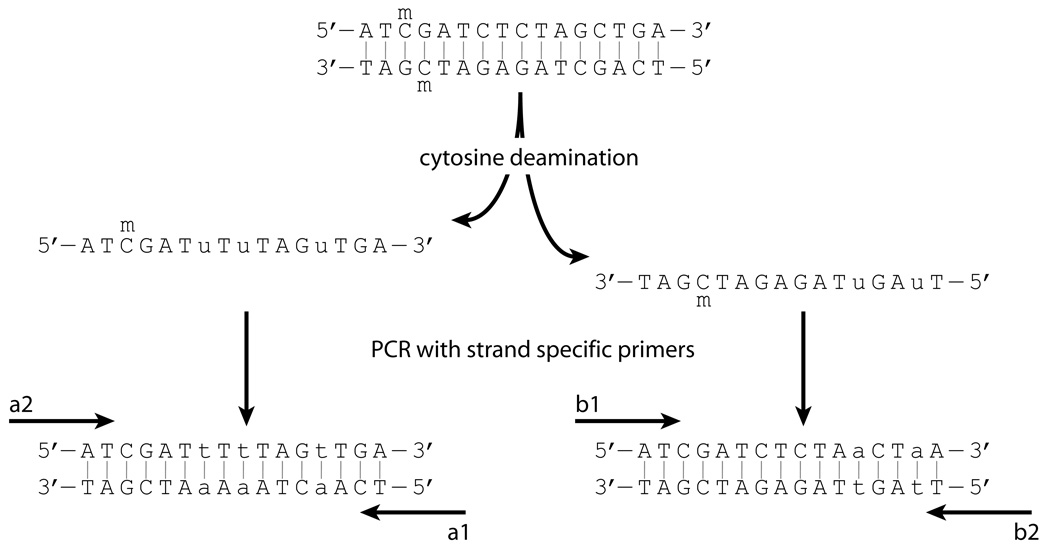
Because deaminated DNA strands are no longer complementary, primer choice dictates which strand is analyzed (Figure 2). Primers are designated a1 and a2, or b1 and b2, based on whether they amplify the top or bottom strand, respectively, by the convention of Frommer et al. (1992). Replacement of G with A in the a1 or b1 primer, and C with T in the a2 or b2 primer, is required for amplification of deaminated DNA. Cytosines within DNA methyltransferase target sites (e.g., CpG in mammalian DNA) should be avoided; if this is not possible, depending on the strand being analyzed, appropriate degenerate bases, R (G and A for a1 and b1 primers) or Y (C and T for a2 and b2 primers), must be used to prevent biased amplification of methylated or unmethylated DNA. Methylation status of sites in primers cannot be scored in the final analysis.
Figure 2.
Substitutions in primers dictate which deaminated strand is amplified. G to A substitutions in a1 and b1 primers complement deaminated sequence. C to T substitutions in a2 and b2 primers complement the complement.
There is an inverse correlation between amplicon size and yield. It is likely this is due to degradation of DNA during and bisulfite conversion and, afterward, presence of uracil in DNA that interferes with processivity of DNA polymerases during the amplification step. While short amplicons maximize yield, it is often desirable to sacrifice yield in favor of determining the methylation status or chromatin structure of longer regions (at least 700 bp of high-quality DNA sequence is typically be obtained by the method we describe). Amplicons should be ≤300 bp for DNA isolated from FFPE tissue or other low abundance sources and subsequently converted with bisulfite. Amplicons from high amounts of deaminated genomic DNA may be as long as 1 kb.
Long stretches of adenine or thymine should be avoided in both the primers and the amplicon. It may be helpful to preview the sequence of the deaminated upper and lower strands of the locus of interest, by substituting T for all C residues, excluding all potential methylation sites, in a text file. Primer melting temperatures should be at least 55°C. Primers ought to be unique within the deaminated genome; for most genomes, this must be determined empirically by testing two or more candidate primer pairs for amplification of a single, specific product. Optional addition of HindIII, XhoI, or other restriction sites at 5′ ends of primers permits directional cloning. Several tools are freely available online to aid in primer design for analysis of CpG methylation in select organisms:
BiSearch (http://bisearch.enzim.hu; Tusnady et al., 2005)
CpGViewer (http://dna.leeds.ac.uk/cpgviewer; Carr et al., 2007)
MethPrimer (http://www.urogene.org/methprimer/index1.html; Li and Dahiya, 2002)
Sequenom (http://www.epidesigner.com/start3.html)
To analyze non-CpG methylation, primers must be designed manually.
REAGENTS AND SOLUTIONS
NOTE: Use distilled deionized water for all reagents and steps. For common stock solutions, see Appendix X, for suppliers see Appendix X.
dNTP mix
2.5 mM dATP
2.5 mM dCTP
2.5 mM dGTP
2.5 mM dTTP
Dissolve in 100 µl water and store as aliquots at –20°C.
IPTG
100 mM IPTG (isopropyl β-D-1-thiogalactopyranoside)
Dissolve in 1 ml water and filter sterilize. Store at –20°C.
LB-agar
15 g/l agar
10 g/l tryptone
10 g/l NaCl
5 g/l yeast extract
Dissolve in 1 l water and autoclave. Add appropriate antibiotic (e.g., 100 µg/l ampicillin) after autoclaving and cooling to about 45°C, then pour ∼20 ml plates. Wrap plates in aluminum foil and store at 4°C. Shortly before use, pipette 40 µl 100 mM IPTG and 20 µl 50 g/l X-gal on plates and spread sterilely. Alternatively, plates can be made to contain 200 µM IPTG and 50 mg/l X-gal.
Lysis buffer
10 mM Tris-HCl, pH 8.0
1 mM Na2EDTA, pH 8.0
1% SDS (w/v)
Dissolve in 50 ml water and store indefinitely at room temperature. Immediately before use, add proteinase K and ribonuclease A to 200 µl per sample.
SOC
20 g/l tryptone peptone
5 g/l yeast extract
0.186 g/l KCl
0.5 g/l NaCl
3.6 g/l dextrose
Autoclave and store at room temperature for up to 3 months. Add sterile MgCl2 to 10 mM immediately before use.
50× TAE buffer
2 M Tris base
1 M acetic acid
50 mM Na2EDTA, pH 8.0
Dissolve in 1 l water and store indefinitely at room temperature.
TE buffer
10 mM Tris-Cl, pH 8.0
1 mM Na2EDTA, pH 8.0
Dissolve in 200 ml water. Filter sterilize and store indefinitely at room temperature.
X-Gal
50 g/l X-Gal stock solution
Dissolve in 1 ml dimethylformamide and filter sterilize. Store at –20°C.
COMMENTARY
Background Information
Although bisulfite conversion of nucleosides was first reported in 1970 (Hayatsu et al., 1970; Shapiro et al. 1970), it took 22 years for the reaction to be adapted to sequence genomic cytosine methylation (Frommer et al., 1992). Cytosine methylation is the primary genetically programmed DNA modification in mammals, and is found in many other organisms as well. It is generally associated with transcriptionally-repressed chromatin structure, possibly originating as a mechanism to suppress invasive DNA (reviewed in Zilberman, 2008). Historically, BGS has been used largely for analysis of single CpG islands, but recent advances in high-throughput sequencing and computational analysis have made it possible to perform whole-genome BGS (Cokus et al., 2008; Lister et al., 2008; Meissner et al., 2008; Hodges et al., 2009; Zhang et al., 2009; Zeschnigk et al., 2009).
BGS remains the gold standard for DNA methylation analysis. Although other techniques such as pyrosequencing (Colella et al., 2003; Dupont et al., 2004) or COBRA (combined bisulfite restriction analysis, Xiong and Laird, 1997) give more quantitative data on degree of methylation at particular sites in a population of cells, BGS is the best way to uncover variability that exists within a population. The key strength of BGS is that it reports methylation status along single DNA strands. For instance, at an imprinted locus, half the copies of the DNA are methylated and half are not; with BGS, such bimodal patterns are readily apparent. BGS is also ideal for methyltransferase accessibility experiments used to characterize diversity of chromatin structure on individual templates (MAPit, UNIT X.X; reviewed in Pondugula et al., 2008). Furthermore, BGS requires little technical expertise, no special equipment, and can be done using commercially available kits.
A technical problem in BGS to date is fragmentation of DNA during deamination, which may preclude some potential applications. In particular, DNA fragmentation during deamination, together with DNA fragmentation in FFPE tissue, greatly increases the difficulty of performing BGS on fixed tissue. FFPE tissue is one of the most widely used methods for clinical sample preservation and archiving. It is estimated that over a billion tissue samples, most of them FFPE, are being stored in numerous hospitals, tissue banks, and research laboratories around the world. The protocol reported here, adapted from previous work (Ai et al., 2008), allows researchers to apply this vast resource toward research in epigenetics.
Critical Parameters and Troubleshooting
In vitro methylated DNA
Unmethylated and fully-methylated samples of DNA can serve as controls for deamination, cloning, and sequencing. An equimolar mixture of these DNA samples is also useful to test for potential biases in amplification of bisulfite-converted DNA and cloning (see below). DNA from placenta, which is unmethylated at most sequences, and PCR product or plasmid containing region of interest can serve as sources of unmethylated DNA. They can be methylated to completion in vitro with commercially available enzymes such as M.SssI (New England Biolabs, catalog no. M0226), using the buffers and instructions supplied by the manufacturer. Note that the S-adenosylmethionine cofactor is labile. If Dcm methylation (Cm5CA/TGG) of the studied region is of concern, plasmid DNA can be isolated from a dcm− strain of E. coli, e.g. GM2163 (supplied free by New England Biolabs with any order).
DNA preparation
DNA degradation occurs during deamination, most likely due to the depurination induced by the alkaline conditions during desulfonation. This degradation reduces the ability to amplify long PCR products considerably (Munson et al., 2007). Thus, preparation of sufficient DNA is critical for BGS. Non-specific fragmentation of DNA during preparation, as occurs when extracting DNA from fixed tissue, further reduces yield of long amplicons. However, fragmentation of DNA by a restriction endonuclease that cuts outside the primer binding sites and severs the region of interest from repetitive sequences may be necessary to achieve and maintain complete DNA denaturation.
DNA preparations must be free of proteins, as these interfere with deamination efficiency. While removal of RNA has also been reported to be necessary (Warnecke et al., 2002), we have found that its presence does not adversely affect deamination efficiency. DNA denaturation under alkaline conditions at 98°C achieves complete hydrolysis of RNA.
Bisulfite treatment
Errors in bisulfite conversion are not usually apparent until the sequencing results are obtained. Presence of cytosines outside known methylation sequences (CpG for mammals) is evidence of incomplete deamination; for most experiments, deamination efficiency ≥98% should be sufficient. Low deamination efficiency can be caused by oxidation of bisulfite to bisulfate, incomplete strand separation at the initial denaturation step and during deamination, leftover protein residues after DNA extraction, or an excess of DNA during bisulfite conversion, especially with sequences of low complexity.
Bisulfite reacts with m5C to produce two diastereomers, one of which converts to thymine upon desulfonation (Shiragami et al., 1975). Conversion of m5C to T presents a problem, as it cannot be discerned from an unmethylated cytosine that has been converted. Reaction of bisulfite with m5C occurs much more slowly than cytosine deamination. Inappropriate conversion is expected to affect only ∼1% of m5C under the conditions given in this protocol (Genereux et al., 2008).
Molecular amplification
The choice of locus to be analyzed depends on the availability of suitable primers. Although algorithms for BGS primer design are publicly available, a thorough understanding of the requirements of BGS is still necessary. In addition to these requirements (see Support Protocol “Primer Design” for details, above), the usual pitfalls of PCR still apply: secondary structure in the primers or amplicon can preclude successful fragment amplification.
PCR is a potential source of artifacts. If secondary structure in the amplicon is affected by the presence of guanines or cytosines on either strand, PCR could be biased toward amplification of differentially methylated templates (Warnecke et al., 1997). A possible simple solution is to substitute a1 and a2 for b1 and b2 primers, or vice versa. BGS on the opposite strand will confirm whether results are affected by strand-specific secondary structure. If this does not solve the problem, in practice it is usually easiest to design one or two new primers; shifted by a few bases upstream or downstream, that anneals to entirely new sequences, or split the original amplicon into two shorter, overlapping amplicons.
When designing new primers is not possible, the extent of suspected amplification and/or cloning bias can be tested by performing BGS on a 50:50 mixture of unmethylated to fully-methylated DNA. Equal representation of unmethylated and methylated patterns among a set of sequenced molecules rules out biases in both PCR amplification and cloning of the product (see below). Significant skewing in the number of either unmethylated or methylated sequences indicates bias in PCR, cloning, or both. Testing for suspected bias due to PCR amplification as opposed to cloning must involve direct quantification of methylation in the PCR product, without cloning.
A second type of PCR artifact occurs when an incompletely extended strand derived from one template DNA molecule primes extension on a template strand derived from another template molecule (Warnecke et al., 2002). This can be detected when the template DNA contains sequence polymorphisms. Long templates and short extension times increase the likelihood of such events. BGS of an equimolar mixture of unmethylated DNA and highly-methylated DNA provides “epigenetic polymorphisms” that can also serve as a test for template priming by incompletely extended molecules. Presence of contiguous spans of C (m5C in original DNA mixture) followed by thymine (C in original sample), or vice versa, among sequenced clones suggests a template priming artifact.
A third source of PCR error is the use of Taq polymerase rather than higher fidelity enzymes. The presence of uracil in the deaminated template precludes the use of higher fidelity enzymes, at least for the initial amplification cycle, since they do not tolerate uracil-rich templates. Less than 1% of cytosines are expected to be affected by misincorporation during PCR (Genereux et al. 2008). A mixture of HotStar Taq (Qiagen) and a DNA polymerase with proofreading activity, e.g. Pfu (Stratagene; 16 U HotStar Taq: 1 U Pfu), can be used to further minimize base substitutions.
Cloning
Cloning bias occurs when AT-rich sequence derived from unmethylated DNA is more or less stable in bacteria than sequence derived from highly-methylated DNA. Either low transformation efficiency or a high proportion of transformants that do not contain the BGS insert can be evidence of cloning bias. Skewed representation of either methylated or unmethylated molecules among sequenced molecules after BGS of a 50:50 mixture of unmethylated to methylated DNA may also indicate a cloning bias. To confirm suspected cloning bias, pyrosequencing can be performed on amplified bisulfite-treated DNA. Choice of vector and host strain affects whether cloning bias occurs with a particular BGS product.
Sequence analysis
Several tools for analysis of CpG methylation are available online: BiQ analyzer (http://biq-analyzer.bioinf.mpi-inf.mpg.de/; Bock et al., 2005), its companion program BDPC (http://biochem.jacobs-university.de/BDPC/BDPC_version_2/; Zhang et al., 2009b), and CpGViewer (http://dna.leeds.ac.uk/cpgviewer/; Carr et al., 2007). Analysis of non-CpG methylation can be performed by sequencing alignment programs such as Sequencher™ and generating images of methylation patterns with an improved version of CpGViewer (Pardo, C. E., Carr, I., Markham, A. F., Bonthron, and Kladde, M. P., manuscript in preparation).
BGS is not precisely quantitative when a small number of sequences are obtained, and the rarer a species is within the population, the more sequences are needed to quantify its abundance accurately.
Anticipated Results
The low (4–14%) DNA yield after bisulfite treatment is overcome by PCR amplification. Thus, this protocol can be used with as little as 10 ng of mammalian genomic DNA (but 2 µg is recommended if obtainable), or as little as 1 ng of a purified DNA fragment. Transformation of PCR products into bacteria typically produces several fold more clones than are needed for sequencing.
Time Considerations
Minimally, this protocol requires 2–3 days to complete. However, most of this time is due to incubations that need not be supervised. Generally it takes longer to obtain sequencing results than it does to prepare samples.
DNA preparation can take as little as 1 hr or may require an overnight incubation with proteinase K and RNase A. Deamination and desulfonation together take about 2–7 hr, depending on the protocol used. Molecular amplification needs 3–4 hr including setup. The optional restriction digest takes 2 hr. Ligation may be performed in 1 hr or overnight. Bacterial transformation takes less than 1 hr, with overnight growth of colonies on a selective plate. Preparation of samples for sequencing may require another overnight incubation.
Acknowledgements
We would like to thank Nancy Nabilsi for helpful comments on the chapter and support from the National Institutes of Health (CA102289 to KDB and CA95525 to MPK) as well as the Department of Defense, Breast Cancer Research Program (BC062914 and BC087311 to MPK).
Footnotes
Internet Resources
http://www.urogene.org/methprimer/index1.html
http://www.epidesigner.com/start3.html
Free primer design suitable for CpG analysis only.
http://biq-analyzer.bioinf.mpi-inf.mpg.de/
http://biochem.jacobs-university.de/BDPC/BDPC_version_2/
Aligns sequencing reads and visualizes CpG methylation.
http://dna.leeds.ac.uk/cpgviewer/
Useful for primer design as well as alignment and visualization of CpG methylation.
Literature Cited
- Ai L, Kim WJ, Demircan B, Dyer LM, Bray KJ, Skehan RR, Massoll NA, Brown KD. The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis. 2008;29:510–518. doi: 10.1093/carcin/bgm280. [DOI] [PubMed] [Google Scholar]
- Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21:4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Miranda TB, Jones PA, Kingston RE. Analysis of individual remodeled nucleosomes reveals decreased histone-DNA contacts created by hSWI/SNF. Nucleic Acids Res. 2009 Jun 30; doi: 10.1093/nar/gkp524. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr IM, Valleley EM, Cordery SF, Markham AF, Bonthron DT. Sequence analysis and editing for bisulphite genomic sequencing projects. Nucleic Acids Res. 2007;35:e79. doi: 10.1093/nar/gkm330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- Dupont JM, Tost J, Jammes H, Gut IG. De novo quantitative bisulfite sequencing using the pyrosequencing technology. Anal. Biochem. 2004;333(1):119–127. doi: 10.1016/j.ab.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Fatemi M, Pao MM, Jeong S, Gal-Yam EN, Egger G, Weisenberger DJ, Jones PA. Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 2005;33:e176. doi: 10.1093/nar/gni180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. First demonstrated the utility of bisulfite conversion in sequencing of 5-methylcytosine.
- Genereux DP, Johnson WC, Burden AF, Stoger R, Laird CD. Errors in the bisulfite conversion of DNA: modulating inappropriate- and failed-conversion frequencies. Nucleic Acids Res. 2008;36:e150. doi: 10.1093/nar/gkn691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu H, Wataya Y, Kai K, Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970;9:2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Hodges E, Smith AD, Kendall J, Xuan Z, Ravi K, Rooks M, Zhang MQ, Ye K, Bhattacharjee A, Brizuela L, McCombie WR, Wigler M, Hannon GJ, Hicks JB. High definition profiling of mammalian DNA methylation by array capture and single molecule bisulfite sequencing. Genome Res. 2009;19:1593–1605. doi: 10.1101/gr.095190.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen WJ, Hoose SA, Kilgore JA, Kladde MP. Active PHO5 chromatin encompasses variable numbers of nucleosomes at individual promoters. Nat. Struct. Mol. Biol. 2006;13:256–263. doi: 10.1038/nsmb1062. [DOI] [PubMed] [Google Scholar]
- Kilgore JA, Hoose SA, Gustafson TL, Porter W, Kladde MP. Single-molecule and population probing of chromatin structure using DNA methyltransferases. Methods. 2007;41:320–332. doi: 10.1016/j.ymeth.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kladde MP, Xu M, Simpson RT. Direct study of DNA-protein interactions in repressed and active chromatin in living cells. EMBO J. 1996;15:6290–6300. [PMC free article] [PubMed] [Google Scholar]
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson K, Clark J, Lamparska-Kupsik K, Smith SS. Recovery of bisulfite-converted genomic sequences in the methylation-sensitive QPCR. Nucleic Acids Res. 2007;35:2893–2903. doi: 10.1093/nar/gkm055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula S, Kladde MP. Single-molecule analysis of chromatin: changing the view of genomes one molecule at a time. J. Cell. Biochem. 2008;105:330–337. doi: 10.1002/jcb.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd edition. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Shapiro R, Servis RE, Welcher M. Reactions of uracil and cytosine derivatives with sodium bisulfite. A specific deamination method. J. Am. Chem. Soc. 1970;92:422–424. [Google Scholar]
- Shiragami M, Iida S, Kudo I, Hayatsu H. Formation of diastereomers of 5,6-dihydrothymine-6-sulfonate by deamination of 5-methylcytosine with bisulfite. Chem. Pharm. Bull. 1975;23:3027–3029. [Google Scholar]
- Shiraishi M, Hayatsu H. High-speed conversion of cytosine to uracil in bisulfite genomic sequencing analysis of DNA methylation. DNA Res. 2004;11:409–415. doi: 10.1093/dnares/11.6.409. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Filipowicz W, Schultz RM. Lack of homologous sequence-specific DNA methylation in response to stable dsRNA expression in mouse oocytes. Nucleic Acids Res. 2004;32:3601–3606. doi: 10.1093/nar/gkh697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnady GE, Simon I, Varadi A, Aranyi T. BiSearch: primer-design and search tool for PCR on bisulfite-treated genomes. Nucleic Acids Res. 2005;33:e9. doi: 10.1093/nar/gni012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke PM, Stirzaker C, Melki JR, Millar DS, Paul CL, Clark SJ. Detection and measurement of PCR bias in quantitative methylation analysis of bisulphite-treated DNA. Nucleic Acids Res. 1997;25:4422–4426. doi: 10.1093/nar/25.21.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke PM, Stirzaker C, Song J, Grunau C, Melki JR, Clark SJ. Identification and resolution of artifacts in bisulfite sequencing. Methods. 2002;27:101–107. doi: 10.1016/s1046-2023(02)00060-9. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeschnigk M, Martin M, Betzl G, Kalbe A, Sirsch C, Buiting K, Gross S, Fritzilas E, Frey B, Rahmann S, Horsthemke B. Massive parallel bisulfite sequencing of CG-rich DNA fragments reveals that methylation of many X-chromosomal CpG islands in female blood DNA is incomplete. Hum. Mol. Genet. 2009;18:1439–1448. doi: 10.1093/hmg/ddp054. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rohde C, Tierling S, Jurkowski TP, Bock C, Santacruz D, Ragozin S, Reinhardt R, Groth M, Walter J, Jeltsch A. DNA Methylation analysis of chromosome 21 gene promoters at single base pair and single allele resolution. PLoS Genet. 2009;5:e1000438. doi: 10.1371/journal.pgen.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rohde C, Tierling S, Stamerjohanns H, Reinhardt R, Walter J, Jeltsch A. DNA methylation analysis by bisulfite conversion, cloning, and sequencing of individual clones. Methods Mol. Biol. 2009b;507:177–187. doi: 10.1007/978-1-59745-522-0_14. [DOI] [PubMed] [Google Scholar]
- Zilberman D. The evolving functions of DNA methylation. Curr. Opin. Plant. Biol. 2008;11:554–559. doi: 10.1016/j.pbi.2008.07.004. [DOI] [PubMed] [Google Scholar]