Abstract
Background
Methamphetamine can be neurotoxic to the adult brain; however, many individuals first use methamphetamine during adolescence, and the drug’s impact on this period of brain development is unknown. Therefore, we evaluated young methamphetamine users for possible abnormalities in brain metabolite concentrations.
Methods
Anterior cingulate cortex (ACC), frontal white matter (FWM), basal ganglia, and thalamus were studied with localized proton magnetic resonance spectroscopy in 54 periadolescent (ages 13–23 years) methamphetamine users and 53 comparison subjects. The concentrations of major brain metabolites and their associations with age, sex and cognition were assessed.
Results
FWM total-creatine correlated with age in methamphetamine-using males and comparison females, but not comparison males or methamphetamine-using females, leading to a drug by sex by age interaction (p=0.003) and ACC choline-containing compounds (CHO) correlated with age only in comparison males leading to a drug by sex by age interaction (p=0.03). Higher ACC CHO was associated with faster performance on the Stroop Interference task in the control males. Male methamphetamine users had slowest performance on the Stroop Interference task and did not show showed age-appropriate levels of ACC CHO.
Conclusions
The altered age-appropriate levels of ACC CHO and poorer executive function in male methamphetamine users suggest methamphetamine abuse may interfere with brain maturation. These periadolescents did not have the abnormal neuronal markers previously reported in adult methamphetamine users, suggesting that neuronal abnormalities may be the result of long-term use or interference in normal cortical maturation, emphasizing the need for early intervention for young methamphetamine users.
Keywords: Adolescent, Methamphetamine, Development, Spectroscopy
1. Introduction
Methamphetamine use has reached epidemic proportions in many regions of the United States and elsewhere (Rawson et al., 2007). The drug’s effects on energy and alertness, as well as appetite suppression for weight loss, are particularly attractive to adolescents and young adults (Iritani et al., 2007). Adolescents who abuse methamphetamine often present with symptoms of psychosis (McKetin et al., 2006; King et al., 2010b) and show cognitive impairment on tests of executive function (King et al., 2010a); however, the effects of methamphetamine on adolescent brain chemistry are relatively unknown.
A variety of neuroimaging modalities evaluated adult methamphetamine users (Chang et al., 2007). Several proton magnetic resonance spectroscopy (1H-MRS) studies of adult methamphetamine users reported lower neuronal marker N-acetyl-aspartate (NA) or NA/creatine ratio, and higher glial markers (myo-inositol), in the frontal lobes or basal ganglia (Ernst et al., 2000; Chang et al., 2005b; Nordahl et al., 2005; Salo et al., 2007; Sung et al., 2007). Structural MRI in adult methamphetamine users showed smaller gray matter volumes in the cingulate and adjacent cortices, but larger white matter volumes (Thompson et al., 2004), and lower gray matter density in the right middle frontal cortex that correlated with lower accuracy on a test of executive function and shorter duration of abstinence (Kim et al., 2006). Taken together, these prior findings suggest methamphetamine may be neurotoxic and may cause inflammation or gliosis in the frontal lobes and basal ganglia, leading to poorer cognitive functioning. However, subcortical gray matter, particularly putamen and globus pallidus, were larger in adult methamphetamine users with better cognitive performance and those with lesser cumulative methamphetamine usage, which may reflect neuroinflammation and suggests a compensatory response (Chang et al., 2005a). Additionally, PET studies in adult methamphetamine users found lower radiotracer binding of dopamine transporters (Volkow et al., 2001c) and receptors (Volkow et al., 2001a), and lower glucose metabolism in subcortical structures, orbitofrontal regions (Volkow et al., 2001a; Volkow et al., 2001b; Wang et al., 2004) frontal white matter (FWM)(Volkow et al., 2001a; Kim et al., 2005) and anterior cingulate cortex (ACC) (London et al., 2004). Abnormalities in glucose metabolism also correlated with poorer cognitive performance (Wang et al., 2004; Kim et al., 2005), as well as depressive symptoms or anxiety ratings (London et al., 2004) in the adult methamphetamine users. Taken together, these findings suggest that the frontal lobe and basal ganglia regions of adult methamphetamine users may be particularly vulnerable to the toxic effects of methamphetamine (Chang et al., 2007). Although many methamphetamine users began using the drug during adolescence, little is known about how the drug might impact the developing periadolescent brain.
Therefore, the aim of this study was to investigate the effects of methamphetamine on brain chemistry using 1H-MRS in a group of adolescent and young adult methamphetamine users. Based on prior MRS studies in adult methamphetamine users (Ernst et al., 2000; Chang et al., 2005b; Sung et al., 2007), we hypothesized that brain metabolites indicative of increased cellular metabolism and inflammatory responses to insult, such as total creatine (tCr), myo-Inositol, and choline-containing compounds (CHO), would be elevated in these young methamphetamine users compared to the control subjects, whereas the neuronal marker, NA, would be in the normal range due to the relatively short duration (only 2 years on average compared to 8–10 years in some adult studies) of methamphetamine use in these subjects.
Although 1H-MRS studies of adult methamphetmine users have not evaluated sex-differences or reported no differences, studies of children with prenatal methamphetamine exposure showed more pronounced frontal white matter metabolite abnormalites in the exposed females. Other imaging studies have reported greater methamphetamine effects on subcortical brain volumes in adult females as assessed with MRI (Chang et al., 2005a) and frontal lobe glucose hypometabolism in adult males as assessed with positron emission tomography (Kim et al., 2005). In addition, the brain is still maturing in the adolescent age range (Andersen, 2003; Sowell et al., 2003; Gogtay et al., 2004; Giedd, 2008; Perrin et al., 2008), and studies of children with prenatal methamphetamine exposure have shown age-associated differences in regional brain volumes (Chang et al., 2004). Therefore, we also evaluated for possible sex and age-associated differences in the current study. We hypothesized that methamphetamine abuse would affect males more than females, but the effects would vary with age since female brains mature sooner (Giedd, 2008) and males in this age range are undergoing more rapid changes in brain structure (Perrin et al., 2008), possibly making the males more vulnerable but in an age dependent manner.
2. Methods
2.1. Participants and clinical evaluations
Adolescents and young adults (ages 12–23 years) were recruited from local drug treatment centers, community programs, colleges, and by word-of-mouth. IRB-approved (The Queens’s Medical Center Research and Institutional Review Committee and the University of Hawaii Committee on Human Studies) written informed consents were obtained from the participants 18 years or older, and both parent/guardian consent and participant assent were obtained from those younger than 18 years. Each participant then underwent a detailed medical and drug use history, as well as a physical and neurological examination. We compared 54 (34 female) individuals, who used methamphetamine daily or met Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for methamphetamine dependence, with 53 (30 female) comparison subjects from the same age range but with no history of methamphetamine usage. None of the participants met DSM-IV dependence criteria for any other drugs of abuse, except for nicotine. Individuals were excluded if they had any neurological illness, history of head trauma with loss of consciousness for more than 30 minutes, contraindications for MRI, or were pregnant (verified by urine pregnancy tests). Individuals with a psychiatric illness not resulting from their drug use (e.g., major depression, bipolar disorder, schizophrenia) also were excluded. Each participant also was assessed with a neuropsychological test battery. In a population that included some participants from the current study, we previously showed that adolescent methamphetamine users had poorer performances on the grooved pegboard (fine motor), Wechsler Adult Intelligence Scale Matrices subtest (nonverbal reasoning), and Stroop interference (executive functioning) tasks (King et al., 2010a); therefore, we evaluated the relationship between these test scores and the brain chemistry in the current study participants.
2.2. MRI and MRS protocol
MRI and MRS were performed on a Siemens 3 Tesla Trio scanner. The sequences included: 1) 3-plane localizer, 2) sagittal high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE, repetition time (TR)/time to echo (TE)/inversion time (TI)=2200/4.91/1000ms; image matrix 256×256×160, FOV 256mm, 1mm slice thickness, 0.5mm gap) with near-isotropic resolution (1×1×1.2mm3), 3) transversal fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI=9750/106/2500ms; 3mm slice thickness, no gap, image matrix 154×192×44, FOV 220×176mm2), and 4) localized 1H-MRS using a Point RESolved Spectroscopy (PRESS) acquisition sequence (Bottomley, 1987; Ernst and Chang, 1996) (echo time/relaxation time=3000/30msec, 64 averages) in four brain regions. MRS voxels were prescribed on reconstructed MP-RAGE images in all three planes for the ACC (20×20×20mm3), right frontal white matter (20×17×17mm3), right basal ganglia (including portions of the caudate, putamen, and globus pallidus 20×17×15mm3), and medial thalamus (20×30×15mm3); see Figure 1. Concentrations of metabolites were determined using the fully-relaxed water signal interpolated to TE=0, and included a correction for the partial volume of cerebrospinal fluid (CSF) (Ernst et al., 1993; Kreis et al., 1993). MR spectra were analyzed with LC Model (Provencher, 1993), and metabolite concentrations were calculated. Proportions of gray matter, white matter, and CSF within the ACC and frontal white matter voxels were determined from the high-resolution MP-RAGE data using a customized segmentation program (Andrews-Shigaki et al., 2007) based on the FSL segmentation package (FAST, version 4.1) (Smith, 2002; Smith et al., 2004).
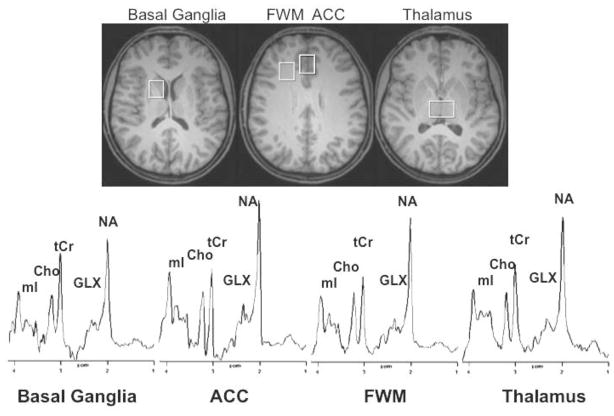
Figure 1.
Representative brain MRI (top) and 1H-MR spectra (bottom) of a healthy 13-year-old male methamphetamine user (transverse reconstruction of MP-RAGE image). Regions of interest and representative spectra are shown in anterior cingulate cortex (ACC), frontal white matter (FWM), thalamus, and basal ganglia. Metabolites of interest are labeled myo-Inositol (mI), choline-containing compounds (CHO), total creatine (tCr), glutamate+glutamine (GLX), and N-acetyl compounds (NA).
2.3. Statistical Analysis
Statistical analyses were performed using SAS (SAS Institute, Cary, NC). The effects of methamphetamine use, sex differences and age-dependent levels were evaluated. Duration of methamphetamine use, length of abstinence, and cumulative lifetime amount of methamphetamine were log transformed to yield normally distributed data for further analyses. A two-way ANCOVA was performed, with methamphetamine-use status, age, and sex as independent variables for all brain regions. For the ACC and FWM voxels, the percent gray matter in each voxel was also included as a covariate in the two-way ANCOVA. Post hoc one-way ANCOVAs and correlations between metabolite levels and drug usage or cognitive scores were performed on those measures that showed significant (p<0.0025 Bonferroni corrected for multiple comparisons) or trends for significant (p≤0.05 uncorrected) findings on the initial ANCOVA.
3. Results
3.1. Participant demographics (Table 1)
Table 1.
Participant characteristics (Mean±SEM) for young methamphetamine users and controls. Additionally, log-transformed values are given for those measures that are not normally distributed.
| Sex | Control | Meth | Log | |
|---|---|---|---|---|
| Age | Female | 18.3±0.6 | 18.3±0.4 | na |
| Male | 18.4±0.6 | 18.8±0.5 | ||
|
| ||||
| Sex (# subjects) | Female | 30 | 34 | na |
| Male | 23 | 20 | ||
|
| ||||
| 1st Meth Use (age) | Female | na | 14.8±0.3 | na |
| Male | 15.2±0.4 | |||
|
| ||||
| Last Meth Use (days) | Female | na | 321.7±76.1 | 1.95±0.15 |
| Male | 149.6±73.2 | 1.32±0.21 | ||
|
| ||||
| Duration Meth Use (months) | Female | na | 25.4±3.3 | 1.26±0.07 |
| Male | 32.2±8.2 | 1.30±0.10 | ||
|
| ||||
| Frequency Meth Use (days/week) | Female | na | 5.9±0.3 | na |
| Male | 5.5±0.4 | |||
|
| ||||
| Amount Meth Used (grams/day) | Female | na | 1.1±0.2 | na |
| Male | 1.8±0.8 | |||
|
| ||||
| Cumulative Lifetime Meth Used (grams) | Female | na | 802.4±306.9 | 2.50±0.11* |
| Male | 1427.7±690.0 | 2.52±0.16 | ||
|
| ||||
| Other Drugs Abused | p-value | |||
|
| ||||
| Frequency Nicotine (packs/week) | Female | 0.79±0.35 | 3.82±0.61 | 0.0001 |
| Male | 0.03±0.03 | 4.99±0.60 | <0.0001 | |
|
| ||||
| Lifetime Nicotine (pack years) | Female | 0.38±0.21 | 2.19±0.41* | 0.0003 |
| Male | 0.04±0.03 | 3.77±0.69 | <0.0001 | |
|
| ||||
| Frequency Marijuana (joints/week) | Female | 1.93±0.63* | 7.16±1.28 | 0.0008 |
| Male | 0.16±0.13 | 9.73±1.81 | <0.0001 | |
|
| ||||
| Lifetime Marijuana (cumulative joints) | Female | 317.3±139.7 | 1386.7±293.0* | 0.003 |
| Male | 47.2±45.6 | 2627.0±613.8 | <0.0001 | |
|
| ||||
| Frequency Alcohol (drinks/week) | Female | 3.49±0.88* | 11.28±3.14 | 0.03 |
| Male | 0.79±0.21 | 7.94±1.62 | <0.0001 | |
|
| ||||
| Lifetime Alcohol (cumulative drinks) | Female | 559.5±175.04* | 2727.8±658.5 | 0.004 |
| Male | 131.6±45.3 | 1549.2±412.8 | 0.0006 | |
P-values for differences between methamphetamine users and controls are noted and sex differences (covaried for age) observed for cumulative methamphetamine and other drugs of abuse are indicated (
p<0.05).
All methamphetamine users primarily or exclusively smoked methamphetamine in the crystalline form. Methamphetamine-users and non-users had similar ages (Table 1). Only five of the methamphetamine users were active users with positive urine toxicology for methamphetamine; the other methamphetamine subjects were abstinent, enrolled in or recently completed drug treatment programs, and had negative urine toxicology. Within the methamphetamine-using participants, age at the time of study correlated positively with methamphetamine usage variables (age of first use, duration of use, length of abstinence, and cumulative lifetime amount of methamphetamine). In models that included age and sex as independent variables, only the log cumulative lifetime methamphetamine used showed a significant effect of sex and a sex-by-age interaction. Post hoc correlations revealed a correlation between age and log cumulative lifetime methamphetamine in males (r=0.75; p<0.001) but not females (r=0.2; p=0.3). No other methamphetamine usage measure had significant sex differences. Compared to the controls, more methamphetamine users also smoked nicotine cigarettes (>1 pack lifetime; 87% vs. 23%; χ2=83, p<0.0001), and had greater nicotine pack years (Table 1), smoked >10 joints marijuana lifetime (96% vs. 23%; χ2=120, p<0.0001), with greater estimated lifetime joints (p<0.0001), as well as more regular alcohol use of >7 drinks/week (30% vs. 8%; χ2=14, p<0.0001). Methamphetamine users also experimented with more other drugs, but indicated that methamphetamine was their drug of choice and were not dependent on any other drugs, except nicotine.
Good quality spectra (based on visual inspection, full-width-half-maximum, and signal-to-noise ratio) were obtained from most voxels. ACC voxels contained 84±4% (range 69–92%) gray matter and frontal white matter voxels contained 86±5% (range 71–96%) white matter.
3.2. Main effects of methamphetamine, age or sex on brain metabolites (Table 2, Figure 2)
Table 2.
Metabolite concentrations (mean±SEM mmoles/kg) and 2-way ANCOVA results (methamphetamine status and sex, covaried for age).
| Control | Meth | ANCOVA: F & p-values for main effects and interactions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SEM | Mean±SEM | F-test | Meth | Sex | Age | MxS | MxA | SxA | MxSxA | |||
| Anterior Cingulate Cortex | NA | Female | 8.44±0.12 | 8.12±0.15 | ns | - | - | - | - | - | - | - |
| Male | 8.48±0.16 | 8.28±0.16 | ||||||||||
|
| ||||||||||||
| GLX | Female | 10.51±0.31 | 9.95±0.27 | ns | - | - | - | - | - | - | - | |
| Male | 10.32±0.39 | 10.26±0.33 | ||||||||||
|
| ||||||||||||
| tCr | Female | 6.37±0.13 | 6.22±0.14 | ns | - | - | - | - | - | - | - | |
| Male | 6.64±0.14 | 6.38±0.12 | ||||||||||
|
| ||||||||||||
| CHO | Female | 1.42±0.04 | 1.46±0.05 | 3.87 | 2.99 | ns | 10.04 | 4.38 | ns | ns | 4.97 | |
| Male | 1.48±0.06 | 1.50±0.06 | 0.001 | 0.09 | 0.002 | 0.04 | 0.03 | |||||
|
| ||||||||||||
| mI | Female | 4.79±0.12 | 4.58±0.11 | ns | - | - | - | - | - | - | - | |
| Male | 4.96±0.13 | 4.72±0.15 | ||||||||||
|
| ||||||||||||
| Frontal White Matter | NA | Female | 8.89±0.14 | 9.14±0.15 | ns | - | - | - | - | - | - | - |
| Male | 8.94±0.14 | 9.01±0.13 | ||||||||||
|
| ||||||||||||
| GLX | Female | 7.18±0.22 | 7.15±0.18 | 2.07 | ns | ns | ns | ns | ns | ns | ns | |
| Male | 7.19±0.29 | 7.08±0.21 | 0.05 | |||||||||
|
| ||||||||||||
| tCr | Female | 5.77±0.10 | 5.76±0.09 | 2.22 | ns | ns | 2.97 | 11.06 | ns | ns | 9.54 | |
| Male | 5.79±0.14 | 5.53±0.12 | 0.03 | 0.09 | 0.001 | 0.003 | ||||||
|
| ||||||||||||
| CHO | Female | 2.00±0.04 | 1.95±0.04 | 2.40 | ns | ns | 6.60 | ns | ns | ns | ns | |
| Male | 2.01±0.06 | 2.00±0.05 | 0.02 | 0.01 | ||||||||
|
| ||||||||||||
| mI | Female | 3.96±0.08 | 4.06±0.11 | ns | - | - | - | - | - | - | - | |
| Male | 4.00±0.11 | 3.95±0.11 | ||||||||||
|
| ||||||||||||
| Basal Ganglia | NA | Female | 7.79±0.17 | 7.91±0.16 | ns | - | - | - | - | - | - | - |
| Male | 7.83±0.16 | 7.48±0.17 | ||||||||||
|
| ||||||||||||
| GLX | Female | 10.21±0.27 | 10.35±0.34 | 2.49 | ns | ns | 6.99 | 3.40 | ns | ns | 3.01 | |
| Male | 11.11±0.31 | 10.55±0.35 | 0.02 | 0.01 | 0.07 | 0.09 | ||||||
|
| ||||||||||||
| tCr | Female | 6.79±0.14 | 6.98±0.13 | ns | - | - | - | - | - | - | - | |
| Male | 7.30±0.18 | 6.91±0.17 | ||||||||||
|
| ||||||||||||
| CHO | Female | 1.53±0.05 | 1.53±0.05 | ns | - | - | - | - | - | - | - | |
| Male | 1.59±0.05 | 1.59±0.05 | ||||||||||
|
| ||||||||||||
| mI | Female | 2.96±0.14 | 3.07±0.15 | ns | - | - | - | - | - | - | - | |
| Male | 3.21±0.15 | 2.93±0.20 | ||||||||||
|
| ||||||||||||
| Thalamus | NA | Female | 9.52±0.15 | 9.60±0.15 | ns | - | - | - | - | - | - | - |
| Male | 9.48±0.15 | 9.23±0.22 | ||||||||||
|
| ||||||||||||
| GLX | Female | 10.00±0.27 | 9.92±0.27 | ns | - | - | - | - | - | - | - | |
| Male | 10.59±0.29 | 9.80±0.26 | ||||||||||
|
| ||||||||||||
| tCr | Female | 6.99±0.12 | 7.04±0.13 | ns | - | - | - | - | - | - | - | |
| Male | 7.12±0.14 | 6.66±0.18 | ||||||||||
|
| ||||||||||||
| CHO | Female | 1.94±0.05 | 1.98±0.04 | ns | - | - | - | - | - | - | - | |
| Male | 1.99±0.05 | 1.97±0.06 | ||||||||||
|
| ||||||||||||
| mI | Female | 4.22±0.11 | 4.47±0.12 | ns | - | - | - | - | - | - | - | |
| Male | 4.34±0.12 | 4.41±0.14 | ||||||||||
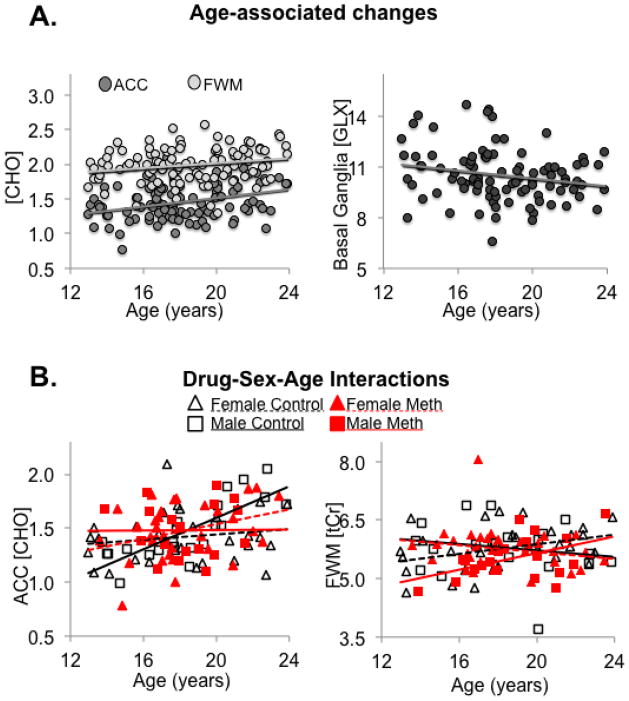
Figure 2.
Age-associated brain metabolite concentrations in young methamphetamine users and controls. A) Concentrations (mmoles/kg) of choline-containing compounds (CHO) were higher in older participants in both the anterior cingulate cortex (ACC: r=0.34, p=0.002) and frontal white matter (FWM: r=0.20, p=0.007), whereas basal ganglia glutamate+glutamine (GLX) concentrations were lower in older participants (r=−0.21, p=0.007). B) Interactions between methamphetamine-use, sex, and age indicate that only male controls show higher CHO in the ACC with older age (ANCOVA-interaction-p=0.03; male controls r=0.79 p<0.0001, others have no correlations with age), and only male methamphetamine users and female controls showed higher total creatine (tCr) in the FWM with older age (ANCOVA-interaction p=0.003; female controls r=0.36 p=0.05, male methamphetamine-users r=0.47, p=0.04, others have no correlations with age).
After Bonferroni correction, only ACC CHO remained significant on ANCOVA F-test (p=0.0007), with trends for significance in the FWM glutamate+glutamine (GLX) (p=0.05), tCr (p=0.03) and CHO (p=0.02), as well as basal ganglia GLX (p=0.02). Significance was driven by age-related changes; for instance, age showed positive correlations with CHO in the ACC (p=0.002) and FWM (p=0.01), and negative correlations with GLX in the basal ganglia (p=0.01). However, we observed no main effects of methamphetamine or sex on brain metabolites.
3.3. Interaction effects of methamphetamine, age and sex, on brain metabolites (Table 2, Figure 2)
Interaction effects were observed for ACC CHO between methamphetamine-use and sex (p=0.04), as well as age, sex, and methamphetamine-use (p=0.03). Post hoc analyses demonstrated a positive correlation between ACC CHO and age across all subjects (r=0.34, p=0.0005) but this was primarily due to control males (r=0.86; p<0.0001), whereas no significant correlations with age were observed in male methamphetamine users or either group of female participants. FWM tCr also showed interactions between methamphetamine-use and sex (p=0.001) as well as age, sex, and methamphetamine-use (p=0.003). Specifically, in the FWM, tCr correlated with age in male methamphetamine users (r=0.55; p=0.02) but not in male controls, whereas tCr correlated with age in female controls (r=0.54; p=0.03) but not in female methamphetamine users. Furthermore, in the basal ganglia, GLX showed trends for interactions between methamphetamine-use and sex (p=0.07) as well as age, sex, and methamphetamine-use (p=0.09). In particular, although basal ganglia GLX correlated negatively with age across all participants (r=−0.21, p=0.04), significant correlations were found only in male controls (r=−0.39; p=0.05) and female methamphetamine users (r=−0.42; p=0.02).
3.4. Cognitive performance and its relationship with brain metabolites
We previously reported that adolescent methamphetamine users had poorer performance than controls on the Grooved Pegboard test, WAIS matrices, and the Stroop Interference task (King et al., 2010a). However, in the current smaller cohort, the adolescent methamphetamine users showed similar performance to controls on their Pegboard test, and their 12% poorer scores on the WAIS matrices were no longer significantly different when covaried for education or age. The Stroop interference task showed only a drug-by-sex interaction (p=0.01, when covaried for education), with male methamphetamine-users performing worse than male (p=0.003) and female (p=0.04) controls, as well as female methamphetamine-users (p=0.03). Post hoc correlations indicated that faster responses on the Stroop Interference task were associated with higher ACC CHO levels only in the male controls (r=−0.46 p=0.03). In addition, poorer performance on the WAIS matrices was associated with higher FWM tCr (r=−0.5, p=0.0006) in methamphetamine users but not in controls.
3.5. Correlations between brain metabolites and other clinical variables (Table 2, Figure 3)
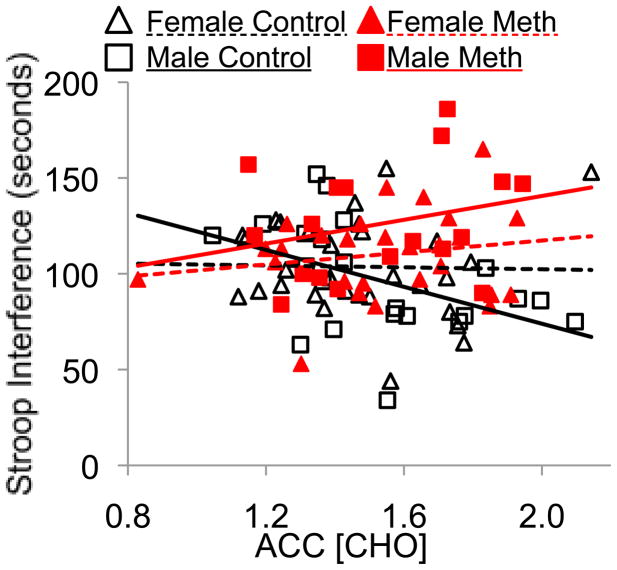
Figure 3.
Brain metabolites correlate with a measure of executive function in male control subjects. Shorter (faster) response time for the Stroop interference task is associated with higher ACC CHO concentrations only in male control participants (black solid line). ANCOVA: methamphetamine effect p=0.01, methamphetamine by sex Interaction p=0.008, Pearson correlation r=−0.46, p=0.03
Only the basal ganglia GLX concentrations in male methamphetamine users correlated with age of first methamphetamine use (r=−0.47; p=0.03); other brain metabolites did not correlate with measures of methamphetamine or other drug usage. Brain metabolite levels did not correlate with body mass index or with sexual maturation (Tanner scale, the majority of participants were at stage 4 or 5).
4. Discussion
This is the first study that evaluated brain metabolite levels in a group of adolescents whose drug of choice was methamphetamine. Compared to the controls, these young primarily methamphetamine users had similar brain metabolite levels as a group, but their brain metabolite levels in relation to their ages and sex were altered, particularly in the frontal lobes and basal ganglia. These are the same brain regions that show abnormal metabolite levels in adult methamphetamine users, and these regions are still undergoing maturational changes in the adolescents. Some of the metabolites that showed age-associated differences, such as GLX in the basal ganglia, also correlated with age of first methamphetamine use. Findings from this study emphasize the need for early intervention to prevent brain injury observed in older methamphetamine users.
4.1. Lack of brain metabolite abnormalities in adolescent methamphetamine users
Adolescent methamphetamine users in the current study did not show abnormal brain metabolite concentrations in the four brain regions measured and had relatively normal cognitive function. Only the male methamphetamine users showed deficits in executive function, and lacked age and sex appropriate-levels of brain metabolites. Several studies of adult methamphetamine users, all with average ages greater than 30 years, reported evidence of neuronal loss or injury, with lower NA, tCr or NA/tCr ratio in the basal ganglia or the frontal lobe (Ernst et al., 2000; Chang et al., 2005b; Nordahl et al., 2005; Salo et al., 2007; Sung et al., 2007). Since lower frontal lobe NA concentrations and NA/tCr correlated with greater lifetime methamphetamine use in adults (Ernst et al., 2000; Sung et al., 2007), continued methamphetamine exposure in these adolescents may eventually lead to lower NA concentrations. Additionally, animal models demonstrate greater neurotoxicity in adults compared to adolescents, due to higher concentrations and greater potential for cytoplasmic release of dopamine, as well as age-dependent differences in monoamine transporter function (Volz et al., 2009).
The typical glial response found in adult methamphetamine users, with elevated ACC myo-inositol and CHO (Ernst et al., 2000; Chang et al., 2005b) also are not observed in these cadolescent methamphetamine users. Conversely, children with prenatal methamphetamine exposure showed opposite findings from those of adult methamphetamine users, having elevated tCr in the striatum (Smith et al., 2001) or elevated neuronal metabolites (tCr, NA and GLX) in the frontal white matter and lower than normal level of glial metabolite myo-Inositol in the thalamus (Chang et al., 2009a). In contrast to both the developed brain in adults and the early developing brain of younger children, the brains of these adolescents showed little or no effect to the relatively short duration of methamphetamine use. Evidence from rodent models suggests maturation of dopaminergic modulation of cortical interneurons occurs at different times for different cell types. For example, only D1 receptors have an excitatory effect on interneurons in prepubescent animals, but the D2 receptors begin to exert excitatory effects after puberty (Tseng and O’Donnell, 2007). This may in part explain the apparently contradictory results from adult vs. prenatal methamphetamine exposure and the observation that this adolescent population is in the middle of this transition resulting in our modest intermediate findings. Similarly, repeated exposure to methamphetamine during adolescence prevented decreased VMAT2 activity from a challenge dose during adulthood, but did not alter VMAT2 on its own (McFadden et al., 2011), indicating that while the adolescent exposure did not have an obvious effect on this synaptic measure, it did have long-term consequences. Like the current study, minimal effects of methamphetamine on the adolescent brain do not preclude long-term consequences and periadolescents in the current study could be more vulnerable to neurotoxic effects of amphetamines as they grow older.
4.2. Lack of sex differences in adolescents
The adolescents in the current study also did not show sex-differences on any of their brain metabolite levels. Prior studies of younger children found higher NA/tCr in the FWM of girls than boys(Ozturk et al., 2009), but studies of older adults showed higher FWM NA (Schweinsburg et al., 2003) and ACC CHO (Chang et al., 1999) in men than women. The age-associated increase in ACC CHO, seen only in the male adolescent controls, may represent the early development of the sex-differences in CHO that occurs later in older adults.
4.3. Effect of age on brain metabolites
In all adolescent subjects, the lower GLX in their basal ganglia of the older subjects is consistent with the age-related decline in glutamate, also in the basal ganglia, of older adults (Chang et al., 2009b). In addition, the higher levels of CHO in older subjects are consistent with that reported in several studies of healthy aging adults (≥18 years) (Chang et al., 1996; Haga et al., 2009). In contrast, brain development of younger (<5 years) children typically shows an age-related decline in CHO in the cortical gray matter (Pouwels et al., 1999). The concentration of CHO in oligodendrocytes and astrocytes is two to three times higher than in neurons (Urenjak et al., 1993), and both myelin formation and breakdown would lead to greater CHO turnover and concentration. In addition, choline phospholipid content is higher in mature white matter axonal cell membranes than in myelin itself (DeVries et al., 1981). Therefore, the higher CHO levels in the older subjects may reflect normal developmental changes in cellularity and axonal maturation (e.g., neuronal branching or myelination, or cell membrane breakdown during pruning). Furthermore, the higher CHO levels in the ACC of the older male, but not female, controls may reflect more pruning, which is consistent with the more rapid decline in gray matter volumes reported in boys 6–17 year old boys (De Bellis et al., 2001).
4.4. Methamphetamine, sex and age interaction effects on brain metabolites
The altered age-appropriate levels of CHO in the ACC (higher in the younger but lower in the older) male methamphetamine users suggests that the drug might have disrupted the maturation of their ACC, and that pruning or myelination in this region might have occurred too early in some subjects or was arrested in the older subjects.
The sex-specific effects of methamphetamine on the frontal white matter of these adolescents may be related to the sex-specific trajectories for increasing FWM volume through adolescence and early adulthood (Giedd, 2008; Perrin et al., 2008), as well as sex-specific D1 and D2 receptor density overproduction and pruning during adolescence (Andersen et al., 1997). The modest correlation between age and FWM tCr of female controls could indicate normal metabolic increases in support of axonal maturation and myelination that were not present in the female methamphetamine users. However, the steeper age associated correlations with tCr levels of male methamphetamine users also could indicate a greater glial metabolic demand (e.g., inflammation or astrocytosis) in response to the as yet undetectable level of neuronal injury.
Dopaminergic modulation of the excitation-inhibition balance in the prefrontal cortex changes during adolescence (O’Donnell, 2010). In addition, both puberty and brain maturation occur at different ages between the sexes (Giedd, 2008) and with hormonal changes (Perrin et al., 2008). Therefore, it is perhaps not surprising that a drug with profound effects on the dopaminergic system, taken during a time when the interaction between the dopaminergic system and the frontal cortex is in transition, would result in age-associated changes. Additionally, sex differences that interact with age and drug-use are also not surprising given the ongoing hormonal changes during later adolescence. Future studies should address the interaction between methamphetamine and underlying mechanisms that influence brain maturation such as hormone levels.
4.5. Effects of methamphetamine use on cognitive performance in relation to brain metabolites
In addition to physiological maturation of the frontal lobes, we also observed age-associated improved performance on the Stroop Interference task, a widely used measure of executive function that is sensitive to methamphetamine-associated deficits in adolescents (King et al., 2010a) and in adults (Salo et al., 2008). The positive correlation between ACC CHO and cognitive performance in the control participants further suggests that this metabolite measure reflects normal maturation of the ACC. Conversely, the group with the poorest executive function, the male methamphetamine users, did not show age-appropriate levels of ACC CHO, and had poorer performance on the non-verbal reasoning task in those with higher FWM tCr. Rat studies show that although methamphetamine dose tolerance decreases with age from p21 (weanling) to P60 (young adult), vulnerability to impaired learning in adulthood is only observed in late-adolescent males and females (P40–50), but not in younger or older animals, illustrating a unique vulnerability at this age (Vorhees et al., 2005). Likewise, most of the participants used methamphetamine during their late adolescence and showed cognitive deficits (King et al., 2010a). Therefore, disruption of normal frontal lobe maturation by methamphetamine use, specifically during late adolescence, might have contributed to poorer cognition. The lack of ACC CHO maturation in the male methamphetamine users could have greater negative impact on cognition as this group ages. The ACC is important for normal executive function and decision making, and was shown to be impaired or less efficient in drug-abusing populations and implicated in several maladaptive behaviors associated with drug-abuse (Goldstein and Volkow, 2002); therefore, lack of maturation of the ACC could impact both cognition and the decision to remain abstinent from drug abuse.
4.6. Other considerations and limitations of the study
Several issues could confound the findings and interpretations of this study. First, concentrations of several metabolites differ between white and gray matter (e.g., CHO is higher in white matter than gray matter); therefore, differences in gray-white composition of the voxels could influence brain metabolite concentrations. However, we found no group differences or age-associated changes in voxel gray-white composition, and we covaried for gray matter content in our analysis. Second, the young methamphetamine users had greater usage of nicotine, marijuana, and alcohol than the controls; however, covarying for these drug usage did not diminish our findings. Nevertheless, future studies should include nicotine, marijuana, and alcohol-using comparison populations to further delineate the potential contribution of these other drugs. In addition, age at the time of study positively correlated with most measures of methamphetamine usage (age of onset, duration of use, length of abstinence, and cumulative lifetime amount of methamphetamine). Although adding any of these drug usage measures as a covariate did not substantially alter the results, their potential to influence our observations, particularly in terms of age-associated effects, needs to be emphasized as they could also introduce sampling biases (Kraemer et al., 2000).
The relatively normal neuronal metabolite levels in these adolescent methamphetamine users suggest that early intervention might be helpful in preventing potential long-term brain injury, such as that seen with lower NA in adult methamphetamine users. Although the findings of abnormal age-associated levels of brain metabolites from this cross-sectional study suggest abnormal brain maturation in adolescent methamphetamine users, a longitudinal study is needed to confirm these findings, and to determine if the maturation processes may normalize with abstinence or worsen with continued methamphetamine use into adulthood.
This periadolescent profile appears to be unique compared to that in adult methamphetamine users, as well as to children with prenatal methamphetamine exposure, and may reflect a window of vulnerability to drugs of abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andrews-Shigaki B, Yakupov R, Chang L, Ernst T. 13th Annual Meeting of the Organization for Human Brain Mapping, Neuroimage. Elsevier Inc; Chicago: 2007. Analysis of Segmentation Methods for Partial Volume Correction in Magnetic Resonance Spectroscopy Voxels. [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009a;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005a;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005b;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009b;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- DeVries GH, Zetusky WJ, Zmachinski C, Calabrese VP. Lipid composition of axolemma-enriched fractions from human brains. J Lipid Res. 1981;22:208–216. [PubMed] [Google Scholar]
- Ernst T, Chang L. Elimination of artifacts in short echo time 1H MR spectroscopy of the frontal lobe. Magn Reson Med. 1996;36:462–468. doi: 10.1002/mrm.1910360320. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I: compartments and water. J Magn Reson. 1993;B102:1–8. [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with (1)H magnetic resonance spectroscopy, in healthy aging. Neurobiol, Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Iritani BJ, Hallfors DD, Bauer DJ. Crystal methamphetamine use among young adults in the USA. Addiction. 2007;102:1102–1113. doi: 10.1111/j.1360-0443.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Sung YH, Lee HY, Lee DS, Jeong DU, Renshaw PF. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30:1383–1391. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 2010a;212:243–249. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. J Neuroimmune Pharmacol. 2010b;5:582–591. doi: 10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II: metabolite concentrations. J Magn Reson. 1993;B102:9–19. [Google Scholar]
- London E, Simon S, Berman S, Mandelkern M, Lichtman A, Bramen J, Shinn A, Miotto K, Learn J, Dong Y, Matochik J, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Hoonakker AJ, Vieira-Brock PL, Stout KA, Sawada NM, Ellis JD, Allen SC, Walters ET, Nielsen SM, Gibb JW, Alburges ME, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine treatment during development attenuates the dopaminergic deficits caused by subsequent high-dose methamphetamine administration. Synapse. 2011;65:771–777. doi: 10.1002/syn.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Degaonkar M, Matson MA, Wells CT, Mahone EM, Horska A. Proton MR spectroscopy correlates of frontal lobe function in healthy children. Am J Neuroradiol. 2009;30:1308–1314. doi: 10.3174/ajnr.A1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, Frahm J. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res. 1999;46:474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, McCann M, Ling W. Use of methamphetamine by young people: is there reason for concern? Addiction. 2007;102:1021–1022. doi: 10.1111/j.1360-0443.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Galloway GP, Leamon MH. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry. 2008;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell E, Peterson B, Thompson P, Welcome S, Henkenius A, Toga A. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Thompson P, Hayashi K, Simon S, Geaga J, Hong M, Sui Y, Lee J, Toga A, Ling W, London E. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine d(2) receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Franceschi D, Sedler M, Gatley S, Hitzemann R, Ding Y, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001b;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, Sedler M, Gatley S, Hitzemann R, Ding Y, Logan J, Wong C, Miller E. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Age-dependent differences in dopamine transporter and vesicular monoamine transporter-2 function and their implications for methamphetamine neurotoxicity. Synapse. 2009;63:147–151. doi: 10.1002/syn.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler J. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]