Abstract
The mineralocorticoid receptor (MR), a member of the steroid receptor family, regulates blood pressure by mediating the effects of the hormone aldosterone (Aldo) on renal sodium handling. Over the past decade, it has become clear that MR is expressed in the cardiovascular system and interest has grown in understanding the direct role of the MR in regulating vascular function and contributing to cardiovascular disease. This interest stems from multiple clinical studies in which drugs that decrease MR activation also reduce the incidence of heart attacks, strokes, and mortality out of proportion to modest changes in systemic blood pressure. The presence of functional mineralocorticoid receptors in vascular smooth muscle and endothelial cells is now well established and, while still controversial, data supports the vasculature as an Aldo-responsive tissue. This review summarizes recent advances in our understanding of the role of vascular MR in regulating normal vascular function and in promoting vascular disease. In vitro data, in vivo animal studies, and human data are reviewed suggesting a role for MR-activation in promoting vascular oxidative stress, inhibiting vascular relaxation, and contributing to vessel inflammation, fibrosis, and remodeling. These detrimental vascular effects of MR activation appear to be independent of changes in blood pressure and are synergistic with the presence of endothelial dysfunction or damage. Thus, in humans with underlying cardiovascular disease or cardiovascular risk factors, vascular MR activation may promote vascular aging and atherosclerosis thereby contributing to the pathophysiology of heart attack, stroke and possibly even hypertension. Further exploration of the molecular mechanisms for the detrimental vascular effects of MR activation has the potential to identify novel therapeutic targets to prevent or treat common cardiovascular disorders.
Keywords: mineralocorticoid receptor, aldosterone, endothelial function, vascular smooth muscle cells, atherosclerosis, oxidative stress
Introduction: A Role for Mineralocorticoid Receptors in Clinical Vascular Outcomes
The mineralocorticoid receptor (MR), a member of the steroid receptor family, was identified 25 years ago as a critical regulator of blood pressure (BP) by mediating the effects of the hormone aldosterone (Aldo) on renal sodium handling (Rogerson and Fuller, 2000; Arriza et al., 1987). It has since become clear that MR is expressed in non-epithelial cells and interest has grown in understanding the direct role of MR in regulating vascular function and in contributing to cardiovascular diseases. This interest stems from multiple clinical studies demonstrating that inhibition of the renin-angiotensin-aldosterone system (RAAS) prevents vascular ischemic events (heart attacks and strokes) and cardiovascular mortality in diverse patient populations (The SOLVD Investigators., 1991; The SOLVD Investigators., 1992; Dagenais et al., 2001; Dahlof et al., 2002; Pitt et al., 1999; Pitt et al., 2003; Zannad et al., 2011). These benefits were initially attributed to the BP-lowering and potential cardiac remodeling effects of RAAS antagonism with secondary vascular consequences. However, the vascular benefits of MR antagonism in these clinical trials are significantly greater than that expected from the modest decrease in systemic BP with inconsistent effects on cardiac remodeling (Udelson et al., 2010), supporting a direct role for vascular MR-activation in vascular pathology. Additional studies directly support that RAAS antagonists have BP-independent effects on vascular remodeling and cardiovascular events (Dahlof et al., 2002; Lonn et al., 2001). Although the physiologic ligand for vascular MR is still controversial and will be discussed in this review, the importance of understanding the direct vascular effects of Aldo-activated vascular MR has been recently highlighted by clinical data supporting that autonomous Aldo elevation may contribute to a larger fraction of essential hypertension than previously appreciated (Gonzaga and Calhoun, 2008) and by the failure in clinical trials of the HDL-raising CETP-inhibitor torcetrapib, likely due to an off target rise in serum Aldo. In the torcetrapib trials, despite significant improvements in cholesterol profiles (Nissen et al., 2007; Bots et al., 2007; Barter et al., 2007), post hoc analyses support that electrolyte and serum evidence of even mild Aldo excess correlated with increased carotid intimal thickness, coronary atherosclerosis, cardiovascular ischemic events, and mortality in patients with underlying cardiovascular risk factors (Nicholls et al., 2008; Vergeer et al., 2008; Duriez, 2007).
Many studies from the 1970s to the 1990s explored the effects of Aldo on the vasculature and this work has been reviewed previously (Rocha and Funder, 2002). This review specifically focuses on advances in the past decade in our understanding of the role of MR in directly regulating vascular function and in promoting vascular disease. MR activation may alter vascular function via genomic mechanisms, in which MR functions as a ligand-activated transcription factor to modulate vascular gene expression, and by non-genomic, rapid effects of MR that intersect with multiple important vascular signaling pathways including those of epidermal growth factor (EGF), platelet derived growth factor (PDGF), insulin-like growth factor (IGF), and Angiotensin 2 (Ang2). A complete discussion of the non-genomic actions of Aldo and MR is beyond the scope of this review and is summarized elsewhere in this issue. In each section of this review, the most recent data regarding the role of MR will be summarized first in vascular cells in vitro, followed by in vivo studies in animal models, and finally in human subjects.
MR Expression and Function in the Vasculature
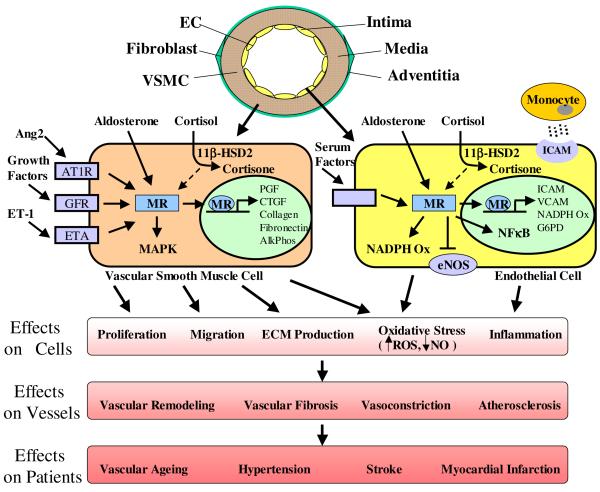
The blood vessel is a layered structure with an inner intima, composed of a single layer of endothelial cells (EC) that line the lumen and contact circulating blood, a medial layer, composed of vascular smooth muscle cells (VSMC), and an outer adventitial layer, containing fibroblasts and extracellular matrix (ECM, see model in Figure 2, top). In the late 1980s and early 1990s, studies demonstrated Aldo binding and MR expression in vascular cells and in whole vessels from animals and humans (reviewed in (Lombes et al., 2000)). More recently, it has been confirmed that endogenous MR in human vascular cells and vessels can directly respond to ligand to regulate vascular-specific gene expression programs that can modulate vascular cell functions involved in cardiovascular pathophysiology (Jaffe and Mendelsohn, 2005; Jaffe et al., 2007; Jaffe et al., 2010; Caprio.M. et al., 2008; Newfell et al., 2011).
Figure 2.
Mineralocorticoid Receptors in Vascular Dysfunction and Disease
Two endogenous ligands, Aldo and cortisol, bind to human MR with equal affinity (Arriza et al., 1987). Although plasma glucocorticoid concentrations are higher than those of Aldo, Aldo-responsive tissues, such as the kidney, maintain Aldo responsiveness by expressing the cortisol-inactivating enzyme 11-beta-hydroxysteroid dehydrogenase type 2 (11ßHSD2, (Funder et al., 1988)). Although still debated, the expression and function of 11ßHSD2 in VSMC and EC has now been demonstrated in multiple studies from many groups supporting the idea that the vasculature is an Aldo-responsive tissue (Kornel, 1994; Brem et al., 1998; Alzamora et al., 2000; Hatakeyama et al., 2000; Kayes-Wandover and White, 2000; Christy et al., 2003; Jaffe and Mendelsohn, 2005; Caprio.M. et al., 2008). Indeed clinical studies demonstrate that even modest increases in serum Aldo levels, as in patients with heart failure, hypertension, or those treated with torcetrapib, produce BP-independent alterations in vascular function lending support for a role for Aldo in directly regulating vascular function in vivo in humans(Farquharson and Struthers, 2000; Gonzaga and Calhoun, 2008; Nissen et al., 2007; Bots et al., 2007; Barter et al., 2007; Nicholls et al., 2008; Vergeer et al., 2008; Duriez, 2007). However, the possibility still exists that under specific conditions or in specific subsets of vascular cells, cortisol could activate vascular MR. For example, in a subset of cultured aortic SMC prone to calcification, 11ßHSD2 expression and function are decreased compared to unselected aortic SMC(Jaffe et al., 2007). Whether subsets of vascular cells respond to cortisol in vivo is not known, and if cortisol does act as a vascular MR-ligand under specific conditions, the differential vascular effects of Aldo- versus cortisol-bound MR remain to be explored. In addition, as with other steroid receptors, MR can be activated by ligand-independent mechanisms. MR-mediated gene transcription in human vascular cells has been shown to be activated by direct action of Ang2 (and other factors in serum) via hormone-independent mechanisms that remain to be clarified (Caprio.M. et al., 2008; Jaffe and Mendelsohn, 2005).
Aldo is produced in the zona glomerulosa of the adrenal gland. Extra-adrenal synthesis of Aldo in tissues including the heart has also been reported (Silvestre et al., 1998; Slight et al., 1999). Expression of Aldo sythase (CYP11B2) and production of Aldo, was originally reported in vascular cells and vessels (Hatakeyama et al., 1994; Takeda et al., 1995a; Takeda et al., 1995b; Takeda et al., 1996; Kayes-Wandover and White, 2000), however, subsequent studies have failed to demonstrate Aldo biosynthesis in the vasculature (Ahmad et al., 2004; Gomez-Sanchez et al., 2004; Jaffe and Mendelsohn, 2005) and the physiological relevance of vascular Aldo production remains controversial. Regardless of the Aldo source, it is now generally accepted that vascular cells contain functional MR capable of responding directly to Aldo through genomic and non-genomic mechanisms to regulate normal vascular function and contribute to cardiovascular disease.
MR, Aldosterone, and Vascular Oxidative Stress
The production of reactive oxygen species (ROS) by the vasculature, termed vascular oxidative stress, is a critical determinant of vascular function and a significant contributor to vascular pathology. Vascular oxidative stress is determined by the balance between vascular damaging ROS and vascular protective nitric oxide (NO). ROS are produced by vascular oxidases including NADPH oxidase, xanthine oxidase, mitochondrial oxidases, and by uncoupling of nitric oxide synthases (NOS) to produce ROS instead of NO. The interaction of ROS with NO also decreases the bioavailability of NO thus further increasing oxidative stress and resulting in impaired EC-dependent vasorelaxation, a marker of endothelial dysfunction. In addition, peroxinitrite, formed by the interaction between ROS and NO, can directly alter many vascular cell functions. Recent reviews address the mechanisms of oxidative stress generation in the vasculature and the role of oxidative stress in cardiovascular disease(Touyz and Briones, 2011; Forstermann, 2010). This section will focus on the relationship between MR signaling and oxidative stress and how this interplay contributes to vascular damage and disease.
Studies exploring the role of MR in promoting vascular oxidative stress are summarized in Table 1. MR activation in VSMC and EC, increases ROS production by increasing the expression and activity of NADPH oxidases (Caprio.M. et al., 2008; Nagata et al., 2006a; Iwashima et al., 2008a; Fiebeler and Luft, 2005; Callera et al., 2005a; Callera et al., 2005b). In EC, Aldo also reduces expression of glucose-6-phosphate dehydrogenase (G6PD), a critical regulator of the intracellular redox state, thereby increasing oxidative stress (Leopold et al., 2007). Although there is some conflicting data in this area, most studies support that MR activation in EC decreases eNOS activity and NO production, further contributing to oxidative stress ((Liu et al., 2003) (Mutoh et al., 2008; Nagata et al., 2006a)and reviewed in (Leopold, 2009)). MR-enhanced vascular oxidative stress has important consequences for vascular cell function. In addition to inhibiting endothelial vasodilatory function, oxidative stress stimulates pro-inflammatory and pro-thrombotic pathways in endothelial cells, important contributors to vascular disease progression and morbidity. In SMC, oxidative stress promotes cell proliferation and a recent study supports a role for oxidative stress in MR-stimulated VSMC migration and proliferation in vitro (Montezano et al., 2008). Oxidative stress may also modulate MR-mediated vascular gene expression with important implications for vascular disease. We recently characterized the Aldo-regulated vascular transcriptome in mouse vessels and identified a subset of pro-atherogenic genes with enhanced Aldo-stimulated, oxidative stress-dependent expression in the setting of vascular injury and in areas predisposed to atherosclerosis (Newfell et al., 2011).
Table 1.
Studies of the Role of MR in Vascular Oxidative Stress
| Model | Treatment (dose; duration) |
Findings | Overall effect of MR activation |
References |
|---|---|---|---|---|
| Endothelial cells | Aldo 0.01-100nM; 10 min |
increased NO production/eNOS activity | ↑ NO/eNOS | Mutoh et al, 2008; Liu et al, 2003 |
| Endothelial cells | Aldo 100nM; 16 hrs | decreased NO production/eNOS activity | ↓ NO/eNOS ↑ROS | Nagata et al, 2006a |
| Endothelial cells | Aldo 10nM; 3 hrs | increased expression of NADPH oxidase subinit Nox4 |
↑ ROS | Caprio et al., 2008 |
| Endothelial cells | Aldo 1-100nM; 24 hrs |
decreased G6PD expression/activity with increased ROS and reduced bioavailable NO; inhibited by NADPH oxidase or Rac1 inhibitor |
↓ NO ↑ROS |
Leopold et al, 2007; Iwashima et al., 2008 |
| Vascular smooth muscle cells |
Aldo 0.1-100nM; 30-60min |
increased ROS production via c-Src, MAPK pathways |
↑ ROS | Callera et al, 2005a&b |
| Vascular smooth muscle cells |
Aldo 1-100nM; 24 hrs |
increased ROS production | ↑ ROS | Maron et al., 2009 |
| Vascular smooth muscle cells |
AngII and Aldo (0.1nM), 5-30min |
Co-stimulation induced c-Src, ERK1/2, JNK & NADPH oxidase activation, superoxide formation & cell migration |
↑ ROS |
Fiebeler & Luft, 2005; Montezano et al, 2008 |
| Aldo-salt hypertensive rat model |
Aldo 0.75 μg/hr ± 1% NaCl; 3-6 wks |
Aldo treatment increased BP and vascular & systemic ROS; abrogated by eplerenone, losartan or tempol (ROS scavenger) |
↑ ROS |
Nishiyama et al, 2004; Iglarz et al., 2004; Hirono et al 2007 |
| DOCA-salt hypertensive rat model |
Apocynin 0.1mM & 1.5mM; acute or 28 days |
NADPH-oxidase inhibitor blocked superoxide formation in aortic rings and in whole body and reduced systolic BP |
↑ ROS | Beswick et al, 2001 |
| APOE-deficient mouse model |
Aldo 2ug/mouse/day; 4 weeks; Eplerenone 200 mg/kg/day; 12 wks |
Aldo increased plaque area and oxidative stress; MR inhibition reduced systolic BP, lipid peroxidation, and plaque area |
↑ ROS |
Keidar et al, 2004; Keidar et al, 2003 |
| APOE-deficient mouse model |
Eplerenone 1.67 g/kg/day or Valsartan 0.5 mg/kg/day; 6 wks |
Reduced plaque area & expression of oxidative stress-related markers |
↑ ROS | Suzuki et al, 2006 |
| Spontaneously hypertensive rat model |
Eplerenone 30-100 mg/kg/day; 10 weeks |
Reduced systolic BP, aortic media/lumen ratio, NO-dependent vascular relaxation, and systemic oxidative stress |
↑ ROS | Sanz-Rosa et al, 2005b |
| Rat model of heart failure |
Spironolactone 7mg/kg/day |
Prevented decrease in eNOS in the LV & aorta and improved NO-dependent vasorelaxation |
↓ eNOS | Thai et al, 2006 |
| Patients with metabolic syndrome |
Quinapril 20mg/day; 4 weeks |
ACE inhibition reduced isoprostanes & inreased erythrocyte superoxide dismutase activity |
↑ ROS | Khan et al, 2004 |
| Patients with chronic kidney disease |
Spironolactone 25 mg/day; 8 weeks |
Levels of urinary isoprostanes reduced | ↑ ROS | Renke et al, 2008 |
Multiple in vivo studies in diverse animal models support a role for MR-induced vascular oxidative stress in the mechanisms of hypertension and of Aldo-mediated vascular damage (Table 1). In animal models of mineralocorticoid-induced hypertension, the rise in BP was accompanied by increased oxidative stress and in addition, vascular ROS production and hypertension were inhibited by NADPH-oxidase inhibitors(Nishiyama et al., 2004; Iglarz et al., 2004; Beswick et al., 2001; Hirono et al., 2007). MR antagonist treatment decreased NADPH-oxidase activity, increased eNOS activity, and decreased BP in models of both hypertension and atherosclerosis (Keidar et al., 2003; Keidar et al., 2004; Sanz-Rosa et al., 2005b; Thai et al., 2006; Suzuki et al., 2006). Together, these studies support that MR activation in vivo enhances vascular oxidative stress via NADPH oxidase thereby contributing to increased BP and atherosclerosis.
There have been few studies addressing the interaction between MR signaling and oxidative stress in patients with cardiovascular disease. In patients with metabolic syndrome, a population with increased vascular oxidative stress and at high risk for cardiovascular disease, treatment with the ACE inhibitor quinapril reduced markers of vascular oxidative stress(Khan et al., 2004). A recent clinical trial in patients with chronic kidney disease demonstrated that treatment with an MR antagonist decreased oxidative stress, as measured by urinary isoprostanes, supporting a potential role for MR antagonists as clinically effective antioxidants(Renke et al., 2008).
The Role of MR in Vascular Constriction and Relaxation
While still controversial, it has recently been postulated that hypertension could arise from changes in vascular tone, independent of alterations in renal function (Mendelsohn, 2005). The presence of functional MR in blood vessels supports the possibility that MR activation could directly modulate vascular reactivity, and potentially BP, via vascular mechanisms in addition to regulation of renal sodium homeostasis. Vascular relaxation is mediated by dephosphorylation of myosin light-chain in SMC, a process that is regulated by NO activation of guanylyl cyclase (GC) and by calcium signaling (reviewed in (Hofmann, 2005)). As summarized in Table 1, MR activation in vascular SMC and EC increases ROS and decreases bioavailable NO and thus would be expected to promote VSMC contraction by decreasing GC activity. In addition, MR activation in cultured VSMC may directly promote SMC contraction by post-translational modification of soluble GC making it unresponsive to NO or by directly promoting calcium mobilization ((Maron et al., 2009; Leopold, 2009; Michea et al., 2005), see Table 2).
Table 2.
Studies of the Role of MR in Vascular Contraction and Relaxation
| Model | Treatment (dose; duration) |
Findings | Overall Effect of MR Activation |
References |
|---|---|---|---|---|
| Cultured vascular smooth muscle cells |
Aldo 1-100nM; 24 hrs |
guanylyl cyclase modified to become NO insensitive |
Contraction | Maron et al., 2009 |
| Ex vivo rat aorta | Aldo 100nM; 10 min |
enhanced NO-dependent vasorelaxation | Relaxation | Mutoh et al, 2008 |
| Ex vivo rat aorta | Aldo 0.01-100nM; 10 min |
EC-dependent attenuation of PE-mediated vasocontriction, blocked by spiro; higher aldo concentrations (100nM) had no effect |
Relaxation | Liu et al., 2003 |
| Ex vivo rat mesenteric arterioles |
Aldo 0.001-100nM; 30min |
NO-dependent increased vasodilation; denuding endothelium elicited a vasoconstrictor response |
Relaxation/ Contraction with Injury |
Heylen et al, 2009 |
| Ex vivo rabbit aterioles | Aldo 1nM-10μM; 5 min |
counteracted KCl-mediated vasoconstriction, blocked by spiro |
Relaxation | Uhrenholt et al, 2003 |
| Ex vivo rabbit aterioles | Aldo 1-10nM; 10- 60min |
MR-independent vasocontriction | Contraction | Arima et al, 2003 |
| Ex vivo rat arterioles | Aldo 10nM; 10-60min |
vasocontriction in resistance vessels; blocked by eplereone |
Contraction | Michea et al, 2005 |
| Mouse with EC-specific MR overexpression |
no treatment | moderate hypertension and increased vasocontriction with PE, AngII and ET1 |
Contraction | Nguyen Dinh et al, 2010 |
| healthy human brachial artery |
Aldo 500ng/min; 8 min |
increased forearm blood flow; greater vasoconstriction with L-NMMA & PE |
Contraction | Schmidt et al., 2003 |
| healthy human brachial artery |
Aldo 12 pM/kg/min; 4 h |
attenuated endothelium-dependent vasodilatation; no effect on blood flow |
Contraction | Farquharson & Struthers, 2002 |
| healthy human forearm vasculature |
Aldo 0.05 or 0.5 mg; 8 hrs |
increased systemic vascular resistance | Contraction | Schmidt et al, 1999 |
| healthy human forearm vasculature |
Aldo 2.5 pmol/min; 60min |
reduced forearm blood flow | Contraction | Romagni et al, 2003 |
| healthy human forearm vasculature |
Aldo 3.3 to 55 pM/min; 15min or Fludocortisone 0.3mg/d; 14 d |
acute increase in EC-independent vasodilation; chronic increase in EC-dependent vasodilation |
Relaxation | Nietlispach et al, 2007 |
| forearm vasculature in men with coronary heart diease |
Aldo 1mg; 10min |
increased systemic vascular resistance | Contraction | Wehling et al., 1998 |
| brachial artery in men with congestive heart failure |
Aldo 10 ng/min; 10 min |
reduced forearm blood flow | Contraction | Gunaruwan et al, 2005 |
| forearm vasculature in men with congestive heart failure |
Spironolactone 12.5 or 50 mg/d; 1 or 3 months |
increased forearm blood flow; greater vasoconstriction with L-NMMA |
Contraction |
Farquharson & Struthers 2000; Macdonald et al, 2004 |
| brachial artery in subjects with hyperaldosteronism |
Spironolactone 12.5 or 25 mg/d; 3 months |
increased flow mediated dilation | Contraction | Nishizaka et al., 2004 |
| systemic vasculature of subjects with essential hypertension |
Eplerenone 50- 200mg/week; 4- 12 weeks |
80% of patients saw a reduction is systemic blood pressure |
Contraction | Levy et al 2004 |
Our understanding of the immediate response of the vessel to acute MR activation had been limited by seemingly contradictory results of ex vivo vessel studies (reviewed in (Leopold, 2009)). Although the different outcomes may be due to differences in study design (vascular bed, species, duration and dose of Aldo exposure (Table 2, columns 1 and 2)), in virtually all studies, the effects of Aldo are MR-dependent, implicating vascular MR in direct regulation of vascular tone (Liu et al., 2003; Mutoh et al., 2008; Uhrenholt et al., 2003). Interestingly, when Aldo was infused into vessels intraluminally to target the endothelium and compared to Aldo added to the water bath to target VSMC, a vasodilator response was found with intraluminal administration that required the presence of the endothelium, MR, and NO generation via NOS (Heylen et al., 2009). Denudation of the endothelium or co-incubation with NOS inhibitors resulted in a loss of vasodilation and/or enhanced contraction, again implicating endothelial MR in vasodilation and SMC MR in vasoconstriction (Heylen et al., 2009; Schmidt et al., 2003). Studies in the renal vasculature also vary with one study demonstrating Aldo-induced vasoconstriction(Arima et al., 2003) and another supporting relaxation(Uhrenholt et al., 2003). Importantly, the demonstration of MR expression and responsivness of the renal vasculature to Aldo supports the possibility that Aldo and MR could also modulate renal function via a vascular mechanism.
The effects of MR activation on vascular reactivity in healthy humans also remains somewhat controversial due to conflicting results from clinical studies with many demonstrating a constrictive response (Farquharson and Struthers, 2002; Schmidt et al., 1999; Romagni et al., 2003) and some showing vascular relaxation(Nietlispach et al., 2007). The discrepancies may be due to differences in the vascular health of the study participants in addition to differences in dose and duration of Aldo infusion (Table 2). However, when patients with underlying cardiovascular diseases are studied, including patients with atherosclerosis, heart failure, and hypertension, the data are quite consistent with MR-activation promoting increased systemic vascular resistance and reduced forearm blood flow(Wehling et al., 1998; Gunaruwan et al., 2005) and MR antagonism producing improved endothelium-dependent vasodilatation, independent of changes in BP(Farquharson and Struthers, 2000; Macdonald et al., 2004; Nishizaka et al., 2004b). The aggregate of the data supports that in healthy vessels, acute MR activation may evoke endothelium-dependent, NO-mediated vasodilatation while, in the presence of endothelial dysfunction, vascular injury, or high vascular oxidative stress (as in patients with cardiovascular risk factors), MR activation promotes vasoconstriction (reviewed in(Skott et al., 2006)).
Whether the direct effects of MR activation on vascular reactivity translate into alterations in systemic BP has yet to be clearly established. A meta-analysis of two trials studying eplerenone treatment in uncomplicated essential hypertension demonstrated that some of the effects of MR antagonism on BP can be distinguished from those on urinary electrolyte excretion supporting a vascular contribution of MR to BP regulation(Funder and Mihailidou, 2009; Levy et al., 2004). Additional support comes from a recent study of a mouse model with inducible over-expression of human MR in only endothelial cells resulting in increased BP, independent of changes in renal sodium transport (Nguyen Dinh et al., 2010). Further studies in vascular-specific MR deficient mice will help to clarify the potential role of endogenous vascular MR in the regulation of blood pressure.
MR and Vascular Inflammation
Vascular inflammation plays a critical role in the pathogenesis of cardiovascular diseases including atherosclerosis and hypertensive vasculopathy. Direct activation of MR in human SMC and EC in vitro has been shown to promote inflammatory gene expression. Specifically, MR activation in human EC promotes expression of intracellular and vascular cell adhesion molecules (ICAM1 and VCAM1) resulting in enhanced leukocyte adhesion to human coronary EC (Caprio.M. et al., 2008; Deuchar et al., 2011). Exposure of human coronary artery SMC to Aldo stimulates expression of interleukin-16 and cytotoxic T-lymphocyte–associated protein 4(Jaffe and Mendelsohn, 2005) and aged rat aortic SMC demonstrate enhanced MR activation associated with increased inflammatory marker expression(Krug et al., 2010).
A pro-inflammatory role for MR in animal models of atherosclerosis has been demonstrated in mice, rabbits, and non-human primates(Keidar et al., 2004; Rajagopalan et al., 2002; Suzuki et al., 2006; Takai et al., 2005; Keidar et al., 2003). In these models, Aldo administration increased atherosclerotic burden and MR antagonists decreased atherosclerotic lesion size, oxidative stress and inflammation marker expression. More recently, the role of MR in atherosclerosis was explored in an apolipoprotein E deficient mouse model with total body genetic deletion of 11βHSD2, allowing for MR activation by glucocorticoids. In this model, MR activation was associated with atherosclerotic plaques enriched in macrophages and lipids with a relative decrease in collagen content (Deuchar et al., 2011). This pro-inflammatory plaque phenotype is associated with plaque rupture in humans, the cause of most heart attacks and strokes. Since this mouse model also had significant hypertension, likely due to renal MR activation by glucocorticoids, further investigation in alternative models is warranted to determine the direct role of vascular MR activation, in the absence of hypertension, in modulating atherosclerotic plaque phenotype with potentially important clinical implications.
MR signaling also contributes to vascular inflammation in animal models of hypertension. In mineralocorticoid-induced hypertension models, MR activation has been associated with perivascular inflammatory cell infiltration, increased expression of pro-inflammatory factors including ICAM1, monocyte chemoattractant protein (MCP-1), cytokines, and COX-2 in cardiac tissue(Rocha et al., 2002b),(Sun et al., 2002) and increased expression of osteopontin, MCP-1, IL-6, and IL-1β in the kidney(Blasi et al., 2003). In these and other studies, MR inhibition reduced the vascular inflammation and ameliorated cardiac and renal injury even without changes in BP, supporting that MR activation participates in vascular inflammation and damage through a BP-independent, direct vascular process(Joffe and Adler, 2005).
The molecular mechanisms for the pro-inflammatory effects of MR are incompletely understood. MR may directly activate inflammatory gene expression in vascular cells. In mouse and human vessels, vascular MR has recently been shown to upregulate expression of the placental growth factor (PGF, a VEGF family member known to promote monocyte chemotaxis), via an MR-responsive element in the upstream PGF gene region(Jaffe et al., 2010) and ICAM1 transcription is regulated by Aldo in EC via the ICAM1 proximal promoter(Caprio.M. et al., 2008). The inflammatory effects of Aldo and MR in hypertensive rat models have been shown to involve cross talk with the pro-inflammatory transcription factor NF-κB(Kobayashi et al., 2005; Sanz-Rosa et al., 2005a; Rocha et al., 2002a; Sun et al., 2002). MR and NF-κB signaling also both interact with other inflammatory signaling pathways such as protein kinase Cε, ERK and Rho kinase (Kobayashi et al., 2005). Our understanding of how these multiple inflammatory pathways interact with MR signaling to contribute to vascular inflammation and disease is incomplete and requires further investigation. Finally, the recent demonstration of functional MR expression in cells of the immune system supports that immune cell MR may also contribute directly to vascular inflammation although the mechanisms and resulting outcomes may depend on the specific immune cells involved. For example, deletion of MR from macrophages in a mouse reduces cerebral infarct size and inflammation as well as mineralocorticoid-induced cardiovascular inflammation and fibrosis (Rickard et al., 2009; Frieler et al., 2011; Usher et al., 2010) while a recent study of Aldo-activated neutrophils support an anti-inflammatory role for MR in these cells(Bergmann et al., 2010). Although complete discussion of the role of MR in immune cell function is beyond the scope of this review, it is clear that activation of MR alters inflammatory cell function and this may be mediated by MR in vascular cells, via direct or paracrine mechanisms, as well as by direct activation of MR in the inflammatory cells.
Studies of the pro-inflammatory effects of MR activation in humans have relied predominantly on the measurement of circulating biomarkers of inflammation, many of which have been associated with the development of hypertension and cardiovascular ischemic events(Pickering, 2007). Infusion of Aldo or Ang2 into healthy subjects increased circulating IL-6 concentrations and the Ang2 effect was blocked by spironolactone, suggesting an MR-dependent mechanism(Luther et al., 2006). Treatment with the MR antagonist spironolactone has been shown to reduce MCP-1 and plasminogen activator inhibitor (PAI-1) levels in subjects with type II diabetes and hypertension, respectively(Takebayashi et al., 2006; Ma et al., 2005). In human aortas from patients with atherosclerosis undergoing coronary artery bypass grafting, spironolactone inhibits vascular expression of the pro-inflammatory growth factors PGF and connective tissue growth factor (CTGF) supporting that direct effects of vascular MR-activation may contribute to vascular inflammation in humans(Jaffe et al., 2010; Newfell et al., 2011). Overall, cellular, animal, and human studies support that vascular MR activation participates in the inflammatory response by up-regulating adhesion molecules, chemokines, cytokines, and growth factors that promote the recruitment and activation of inflammatory cells and the proliferation of vascular cells and may contribute to the progression of and complications associated with atherosclerotic vascular disease.
MR and Vascular Remodeling
Vascular remodeling is the pathologic response of the vessel to vascular damage and contributes to human ischemic vascular disease. Remodeling occurs when the endothelium is damaged by insults from cardiac risk factors such as cigarette smoke, diabetes, and hypertension or by mechanical injury such as balloon angioplasty and stent implantation during percutaneous revascularization. This damage initiates a cascade of events that constitute the vascular injury response, resulting in the stimulation of VSMC to migrate, proliferate, and produce ECM. This section will review recent studies exploring the mechanisms by which MR promotes vascular remodeling in animals and in humans.
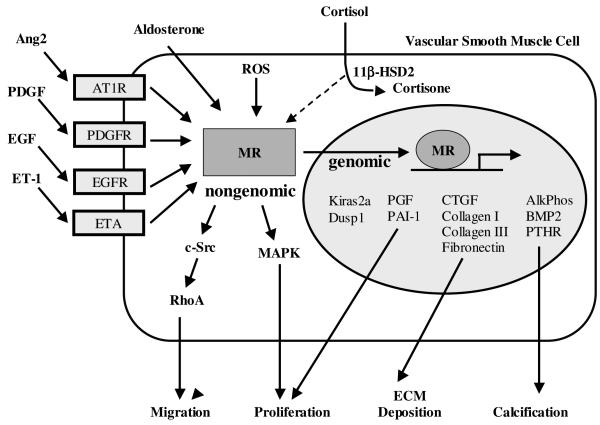
Studies in cultured VSMC demonstrate a mitogenic effect of Aldo that is mediated by SMC MR and is synergistic with Ang2, PDGF, and EGF signaling (see Model in Figure 1). The mechanism involves activation of multiple signaling pathways including a rapid, non-genomic phosphorylation of MAPK followed by a slower, genomic effect involving Kiras2a and Dusp1(Min et al., 2005; Xiao et al., 2000; Montezano et al., 2008). Crosstalk between Aldo and Ang2 also plays a role in VSMC migration via a c-Src-regulated, redox-sensitive, RhoA pathway(Miyata et al., 2005; Montezano et al., 2008). Ang2, acting via the AT1R, has been shown to directly activate MR-mediated gene transcription in human VSMC supporting a new mechanism for the synergy between Aldo and Ang2 in vascular remodeling(Jaffe and Mendelsohn, 2005). MR activation in vascular cells also directly promotes expression of genes that influence vascular remodeling. In human VSMC, Aldo potentiated oxidative stress-induced collagen and fibronectin synthesis in an EGF-dependent manner(Grossmann and Gekle, 2007; Grossmann et al., 2007; Gekle et al., 2007) and gene expression profiling studies in human vascular cells and mouse vessels implicate MR activation in enhancing expression of genes involved in vascular proliferation, fibrosis and calcification (Jaffe and Mendelsohn, 2005; Jaffe et al., 2007; Jaffe et al., 2010; Newfell et al., 2011).
Figure 1.
MR in Vascular Remodeling
Multiple animal models support that Aldo exacerbates vascular remodeling in association with endothelial damage in vivo and that these effects are reversed by Aldo antagonists, implicating MR in the mechanism(Wakabayashi et al., 2006; Pu et al., 2003; Sanz-Rosa et al., 2005b; Virdis et al., 2002; Nagata et al., 2006b). Specifically, eplerenone has been shown to attenuate constrictive remodeling and collagen accumulation in pig coronary arteries after angioplasty(Ward et al., 2001; Van Belle et al., 1995). An increase in vascular fibronectin content has been reported after Aldo administration and eplerenone treatment has been shown to decrease aortic arterial stiffness, a significant cardiovascular risk factor(Pu et al., 2003; Lacolley et al., 2002). In vivo studies have implicated diverse mediators of MR-induced remodeling including endothelin, Ang2, PAI-1, growth factors, and oxidative stress signaling pathways (Figure 1 and reviewed in (Epstein, 2001; Stowasser, 2001)). Several studies have reported that blockade of the endothelin system prevented vascular remodeling in Aldo- and Ang2-induced hypertension(Pu et al., 2003; Virdis et al., 2002; Rajagopalan et al., 1997). More recently, our lab has identified the vascular endothelial growth factor family member, placental growth factor (PGF), as a mediator of Aldo-induced vascular remodeling. Aldo infusion, at doses that did not elevate blood pressure, enhanced vascular SMC proliferation and fibrosis in mouse carotid arteries following wire injury, an effect that was lost in mice deficient in PGF. We further demonstrated that PGF gene expression was directly and transcriptionally regulated by MR in mouse and in human vessels suggesting the PGF/VEGF pathway as a potential renal-independent mechanism for the vascular protective effects of MR antagonists in animal models and in humans(Jaffe et al., 2010).
Evidence for a direct effect of Aldo on vascular structure in humans is supported by studies demonstrating that patients with primary aldosteronism have significantly increased vascular medial thickness and narrowed vessel lumens compared to patients with similar degrees of essential hypertension and other forms of secondary hypertension(Rizzoni et al., 1996; Holaj et al., 2007; Bernini et al., 2008). Excess Aldo is also responsible for arterial stiffness and carotid artery fibrosis in these patients. In a randomized trial of spironolactone in non-diabetic hemodialysis patients, MR antagonism reduced carotid intima-media thickness in treated patients (Vukusich et al., 2010). Thus, MR activation contributes to vascular remodeling by acting synergistically with endothelial damage, Ang2, and growth factor signaling to promote VSMC proliferation, migration, and extracellular matrix deposition. Blockade of the mineralocorticoid receptors may exert beneficial effects by abrogating the MR-induced pathophysiological remodeling. As the details of the molecular mechanisms by which MR promotes vascular remodeling are clarified, novel therapeutic targets may be elucidated to prevent negative vascular remodeling in humans.
Summary and Conclusions
The presence of functional mineralocorticoid receptors in the vasculature is now well established and, while still controversial, data supports the vasculature as an Aldo-responsive tissue. In vitro, animal, and human data support a role for MR-activation in promoting vascular cell oxidative stress, inflammation, proliferation, migration and ECM production thereby promoting vasoconstriction, atherosclerosis, vascular remodeling and fibrosis (see Model in Figure 2). The detrimental vascular effects of MR activation appear to be independent of changes in blood pressure and are synergistic with the presence of endothelial dysfunction and/or vascular oxidative stress. Thus, vascular MR may contribute to the progression of vascular dysfunction in humans with cardiovascular risk factors that promote endothelial dysfunction. MR activation in these patients may contribute to atherosclerosis and plaque instability, resulting in heart attacks and strokes, to vascular aging with associated vascular stiffness, and possibly even to hypertension. Further investigation of the molecular mechanisms for the detrimental vascular effects of MR activation has the potential to identify novel therapeutic targets to prevent or treat common cardiovascular disorders.
MCE 7898 Research Highlights.
Mineralocorticoid receptors are expressed in vascular smooth muscle and endothelial cells.
MR-activation promotes vascular oxidative stress vascular contraction.
MR-activation contributes to vessel inflammation, fibrosis, and remodeling.
In humans with cardiovascular risk factors, vascular MR-activation may promote vascular aging and atherosclerosis.
Abbreviations
- 11ßHSD2
11-beta-hydroxysteroid dehydrogenase type 2
- Aldo
Aldosterone
- Ang2
Angiotensin 2
- AT1R
Angiotensin type1 receptor
- BP
blood pressure
- CETP
cholesterol-ester-transfer-protein
- CTGF
connective tissue growth factor
- EC
endothelial cells
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- GC
guanylyl cyclase
- HDL
high density lipoprotein
- ICAM1
intercellular adhesion molecule 1
- IGF
insulin-like growth factor
- MCP-1
monocyte chemoattractant protein
- MR
Mineralocorticoid Receptor
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAI-1
plasminogen activator inhibitor 1
- PDGF
platelet derived growth factor
- PGF
placental growth factor
- RAAS
renin-angiotensin-aldosterone system
- ROS
reactive oxygen species
- VCAM1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahmad N, Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Do human vascular endothelial cells produce aldosterone? Endocrinology. 2004;145(8):3626–9. doi: 10.1210/en.2004-0081. [DOI] [PubMed] [Google Scholar]
- Alzamora R, Michea L, Marusic ET. Role of 11beta-hydroxysteroid dehydrogenase in nongenomic aldosterone effects in human arteries. Hypertension. 2000;35(5):1099–104. doi: 10.1161/01.hyp.35.5.1099. [DOI] [PubMed] [Google Scholar]
- Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. Journal of the American Society of Nephrology. 2003;14(9):2255–63. doi: 10.1097/01.asn.0000083982.74108.54. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE,I. Effects of torcetrapib in patients at high risk for coronary events. New England Journal of Medicine. 2007;357(21):2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Eulenberg C, Wellner M, Rolle S, Luft F, Kettritz R. Aldosterone abrogates nuclear factor kappaB-mediated tumor necrosis factor alpha production in human neutrophils via the mineralocorticoid receptor. Hypertension. 2010;55(2):370–9. doi: 10.1161/HYPERTENSIONAHA.109.141309. [DOI] [PubMed] [Google Scholar]
- Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. Journal of Hypertension. 2008;26(12):2399–405. doi: 10.1097/HJH.0b013e32831286fd. [DOI] [PubMed] [Google Scholar]
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38(5):1107–11. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney International. 2003;63(5):1791–800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ, RADIANCE 2 Investigators Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- Brem AS, Bina RB, King TC, Morris DJ. Localization of 2 11beta-OH steroid dehydrogenase isoforms in aortic endothelial cells. Hypertension. 1998;31:459–462. doi: 10.1161/01.hyp.31.1.459. [DOI] [PubMed] [Google Scholar]
- Callera GE, Montezano AC, Yogi A, Tostes RC, He Y, Schiffrin EL, Touyz RM. c-Src-dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension. 2005a;46(4):1032–8. doi: 10.1161/01.HYP.0000176588.51027.35. [DOI] [PubMed] [Google Scholar]
- Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005b;45(4):773–9. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- Caprio M, Newfell BG, LaSala A, Baur WE, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional Mineralocorticoid Receptors in Human Vascular Endothelial Cells Regulate ICAM-1 Expression and Promote Leukocyte Adhesion. Circulation Research. 2008;102(11):1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy C, Hadoke PW, Paterson JM, Mullins JJ, Seckl JR, Walker BR. 11beta-hydroxysti dehydrogenase type 2 in mouse aorta: localization and influence on response to glucocorticoids. Hypertension. 2003;42:580–587. doi: 10.1161/01.HYP.0000088855.06598.5B. [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Yusuf S, Bourassa MG, Yi Q, Bosch J, Lonn EM, Kouz S, Grover J, Investigators HOPE. Effects of ramipril on coronary events in high-risk persons: results of the Heart Outcomes Prevention Evaluation Study. Circulation. 2001;104:522–526. doi: 10.1161/hc3001.093502. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen 0, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, The LIFE,S.G. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Deuchar GA, Mclean D, Hadoke PWF, Brownstein DG, Webb DJ, Mullins JJ, hapman K, eckl JR, otelevtsev YV. 11Beta-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe-/- mice. Endocrinology. 2011;1(152):236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez P. CETP inhibition. Lancet. 2007;370(9603):1882–3. doi: 10.1016/S0140-6736(07)61788-7. [DOI] [PubMed] [Google Scholar]
- Epstein M. Aldosterone as a determinant of cardiovascular and renal dysfunction. Journal of the Royal Society of Medicine. 2001;94(8):378–83. doi: 10.1177/014107680109400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clinical Science. 2002;103(4):425–31. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- Fiebeler A, Luft FC. The mineralocorticoid receptor and oxidative stress. Heart Failure Reviews. 2005;10(1):47–52. doi: 10.1007/s10741-005-2348-y. [DOI] [PubMed] [Google Scholar]
- Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv. 2010;6(459):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42(1):179–85. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Mihailidou AS. Aldosterone and mineralocorticoid receptors: Clinical studies and basic biology. Molecular & Cellular Endocrinology. 2009;301(1-2):2–6. doi: 10.1016/j.mce.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Grossmann C. Altered collagen homeostasis in human aortic smooth muscle cells (HAoSMCs) induced by aldosterone. Pflugers Archiv - European Journal of Physiology. 2007;454(3):403–13. doi: 10.1007/s00424-007-0211-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of Aldosterone in the Rat Heart. Endocrinology. 2004;145(11):4796–4802. doi: 10.1210/en.2004-0295. [DOI] [PubMed] [Google Scholar]
- Gonzaga CC, Calhoun DA. Resistant hypertension and hyperaldosteronism. Current Hypertension Reports. 2008;10(6):496–503. doi: 10.1007/s11906-008-0092-0. [DOI] [PubMed] [Google Scholar]
- Grossmann C, Gekle M. Non-classical actions of the mineralocorticoid receptor: misuse of EGF receptors? Molecular & Cellular Endocrinology. 2007;277(1-2):6–12. doi: 10.1016/j.mce.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Grossmann C, Krug AW, Freudinger R, Mildenberger S, Voelker K, Gekle M. Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. American Journal of Physiology - Endocrinology & Metabolism. 2007;292(6):E1790–800. doi: 10.1152/ajpendo.00708.2006. [DOI] [PubMed] [Google Scholar]
- Gunaruwan P, Schmitt M, Sharman J, Lee L, Struthers A, Frenneaux M. Effects of aldosterone on forearm vasculature in treated chronic heart failure. American Journal of Cardiology. 2005;95(3):412–4. doi: 10.1016/j.amjcard.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Inaba S, Takeda R, Miyamori I. 11beta-hydroxysteroid dehydrogenase in human vascular cells. Kidney International. 2000;57:1352–1357. doi: 10.1046/j.1523-1755.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J. Biol. Chem. 1994;269:24316–24320. [PubMed] [Google Scholar]
- Heylen E, Huang A, Sun D, Kaley G. Nitric oxide-mediated dilation of arterioles to intraluminal administration of aldosterone. Journal of Cardiovascular Pharmacology. 2009;54(6):535–42. doi: 10.1097/FJC.0b013e3181bfb00d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148(4):1688–96. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- Hofmann F. The biology of cyclic GMP-dependent protein kinases. Journal of Biological Chemistry. 2005;280(1):1–4. doi: 10.1074/jbc.R400035200. [DOI] [PubMed] [Google Scholar]
- Holaj R, Zelinka T, Wichterle D, Petrak 0, Strauch B, Widimsky J., Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. Journal of Hypertension. 2007;25(7):1451–7. doi: 10.1097/HJH.0b013e3281268532. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. American Journal of Hypertension. 2004;17(7):597–603. [PubMed] [Google Scholar]
- Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149(3):1009–14. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circulation Research. 2005;96(6):643–50. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Ehsan A, Mendelsohn ME. Placental growth factor mediates aldosterone-dependent vascular injury in mice. Journal of Clinical Investigation. 2010;120(11):3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27(4):799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Failure Reviews. 2005;10(1):31–7. doi: 10.1007/s10741-005-2346-0. [Review] [40 refs] [DOI] [PubMed] [Google Scholar]
- Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. Journal of Clinical Endocrinology & Metabolism. 2000;85:2519–2525. doi: 10.1210/jcem.85.7.6663. [DOI] [PubMed] [Google Scholar]
- Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R, Aviram M. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. Journal of Cardiovascular Pharmacology. 2003;41(6):955–63. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109(18):2213–20. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- Khan BV, Sola S, Lauten WB, Natarajan R, Hooper WC, Menon RG, Lerakis S, Helmy T. Quinapril, an ACE inhibitor, reduces markers of oxidative stress in the metabolic syndrome. Diabetes Care. 2004;27(7):1712–5. doi: 10.2337/diacare.27.7.1712. [DOI] [PubMed] [Google Scholar]
- Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita S, Nakano S, Tsubokou Y, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension. 2005;45(4):538–44. doi: 10.1161/01.HYP.0000157408.43807.5a. [DOI] [PubMed] [Google Scholar]
- Kornel L. Colocalization of 11 beta-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. American Journal of Hypertension. 1994;7(1):100–3. doi: 10.1093/ajh/7.1.100. [DOI] [PubMed] [Google Scholar]
- Krug AW, fer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55(6):1476–83. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation. 2002;106:2848–2853. doi: 10.1161/01.cir.0000039328.33137.6c. [DOI] [PubMed] [Google Scholar]
- Leopold JA. Rapid aldosterone signaling and vascular reactivity: relax or don’t do it. Journal of Cardiovascular Pharmacology. 2009;54(6):465–7. doi: 10.1097/FJC.0b013e3181c37ddc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nature Medicine. 2007;13(2):189–97. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DG, Rocha R, Funder JW. Distinguishing the antihypertensive and electrolyte effects of eplerenone. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2736–40. doi: 10.1210/jc.2003-032149. [DOI] [PubMed] [Google Scholar]
- Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R, Feldman RD. Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Circulation. 2003;108(19):2400–6. doi: 10.1161/01.CIR.0000093188.53554.44. [DOI] [PubMed] [Google Scholar]
- Lombes M, Farman N, Bonvalet JP, Zennaro MC. Identification and role of aldosterone receptors in the cardiovascular system. Annales d Endocrinologie. 2000;61:41–46. [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K, SECURE,I. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE) Circulation. 2001;103:919–925. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48(6):1050–7. doi: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- Ma J, Albornoz F, Yu C, Byrne DW, Vaughan DE, Brown NJ. Differing effects of mineralocorticoid receptor-dependent and -independent potassium-sparing diuretics on fibrinolytic balance. Hypertension. 2005;46(2):313–20. doi: 10.1161/01.HYP.0000174327.53863.86. [DOI] [PubMed] [Google Scholar]
- Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90(7):765–70. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. Journal of Biological Chemistry. 2009;284(12):7665–72. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME. In hypertension, the kidney is not always the heart of the matter. Journal of Clinical Investigation. 2005;115(4):840–4. doi: 10.1172/JCI24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michea L, Delpiano AM, Hitschfeld C, Lobos L, Lavandero S, Marusic ET. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology. 2005;146(3):973–80. doi: 10.1210/en.2004-1130. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circulation Research. 2005;97(5):434–42. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. Journal of the American Society of Nephrology. 2005;16(10):2906–12. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arteriosclerosis, Thrombosis & Vascular Biology. 2008;28(8):1511–8. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- Mutoh A, Isshiki M, Fujita T. Aldosterone enhances ligand-stimulated nitric oxide production in endothelial cells. Hypertension Research - Clinical & Experimental. 2008;31(9):1811–20. doi: 10.1291/hypres.31.1811. [DOI] [PubMed] [Google Scholar]
- Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006a;48(1):165–71. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006b;47(4):656–64. doi: 10.1161/01.HYP.0000203772.78696.67. [DOI] [PubMed] [Google Scholar]
- Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone Regulates Vascular Gene Transcription via Oxidative Stress-Dependent And - Independent Pathways. Arterioscler Thromb Vasc Biol. 2011;31 doi: 10.1161/ATVBAHA.111.229070. (published online May 26, 2011), 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Dinh CA, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB Journal. 2010;24(7):2454–63. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118(24):2506–14. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- Nietlispach F, Julius B, Schindler R, Bernheim A, Binkert C, Kiowski W, Brunner-La Rocca HP. Influence of acute and chronic mineralocorticoid excess on endothelial function in healthy men. Hypertension. 2007;50(1):82–8. doi: 10.1161/HYPERTENSIONAHA.107.088955. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43(4):841–8. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109(23):2857–61. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM, ILLUSTRATE,I. Effect of torcetrapib on the progression of coronary atherosclerosis. New England Journal of Medicine. 2007;356(13):1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- Pickering TG. Stress, inflammation, and hypertension. Journal of Clinical Hypertension. 2007;9(7):567–71. doi: 10.1111/j.1524-6175.2007.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New England Journal of Medicine. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension. 2003;42(1):49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105:2212–2216. doi: 10.1161/01.cir.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- Renke M, Tylicki L, Knap N, Rutkowski P, Neuwelt A, Larczynski W, Wozniak M, Rutkowski B. Spironolactone attenuates oxidative stress in patients with chronic kidney disease. Hypertension. 2008;52(5):e132–3. doi: 10.1161/HYPERTENSIONAHA.108.120568. author reply e134. [DOI] [PubMed] [Google Scholar]
- Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54(3):537–43. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti-Rosei E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension. 1996;28(5):785–90. doi: 10.1161/01.hyp.28.5.785. [DOI] [PubMed] [Google Scholar]
- Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Annals of the New York Academy of Sciences. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [Review] [50 refs] [DOI] [PubMed] [Google Scholar]
- Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002a;143(12):4828–36. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. American Journal of Physiology - Heart & Circulatory Physiology. 2002b;283(5):H1802–10. doi: 10.1152/ajpheart.01096.2001. [see comment] [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- Romagni P, Rossi F, Guerrini L, Quirini C, Santiemma V. Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis. 2003;166(2):345–9. doi: 10.1016/s0021-9150(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Sanz-Rosa D, Cediel E, De las HN, Miana M, Balfagon G, Lahera V, Cachofeiro V. Participation of aldosterone in the vascular inflammatory response of spontaneously hypertensive rats: role of the NFkappaB/IkappaB system. Journal of Hypertension. 2005a;23(6):1167–72. doi: 10.1097/01.hjh.0000170379.08214.5a. [DOI] [PubMed] [Google Scholar]
- Sanz-Rosa D, Oubina MP, Cediel E, De las HN, Aragoncillo P, Balfagon G, Cachofeiro V, Lahera V. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxidants & Redox Signaling. 2005b;7(9-10):1294–1301. doi: 10.1089/ars.2005.7.1294. [DOI] [PubMed] [Google Scholar]
- Schmidt BM, Montealegre A, Janson CP, Martin N, Stein-Kemmesies C, Scherhag A, Feuring M, Christ M, Wehling M. Short term cardiovascular effects of aldosterone in healthy male volunteers. Journal of Clinical Endocrinology & Metabolism. 1999;84(10):3528–33. doi: 10.1210/jcem.84.10.6020. [DOI] [PubMed] [Google Scholar]
- Schmidt BM, Oehmer S, Delles C, Bratke R, Schneider MP, Klingbeil A, Fleischmann EH, Schmieder RE. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42(2):156–60. doi: 10.1161/01.HYP.0000083298.23119.16. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. Journal of Biological Chemistry. 1998;273(9):4883–91. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- Skott O, Uhrenholt TR, Schjerning J, Hansen PB, Rasmussen LE, Jensen BL. Rapid actions of aldosterone in vascular health and disease--friend or foe? Pharmacology & Therapeutics. 2006;111(2):495–507. doi: 10.1016/j.pharmthera.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Slight SH, Joseph J, Ganjam VK, Weber KT. Extra-adrenal mineralocorticoids and cardiovascular tissue. Journal of Molecular & Cellular Cardiology. 1999;31(6):1175–84. doi: 10.1006/jmcc.1999.0963. [DOI] [PubMed] [Google Scholar]
- Stowasser M. New perspectives on the role of aldosterone excess in cardiovascular disease. Clinical & Experimental Pharmacology & Physiology. 2001;28(10):783–91. doi: 10.1046/j.1440-1681.2001.03523.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. American Journal of Pathology. 2002;161(5):1773–81. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii T, Higaki J, Horiuchi M. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arteriosclerosis, Thrombosis & Vascular Biology. 2006;26(4):917–21. doi: 10.1161/01.ATV.0000204635.75748.0f. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H, Miyazaki M. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension. 2005;46(5):1135–9. doi: 10.1161/01.HYP.0000184640.81730.22. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. Journal of Clinical Endocrinology & Metabolism. 2006;91(6):2214–7. doi: 10.1210/jc.2005-1718. [DOI] [PubMed] [Google Scholar]
- Takeda R, Hatakeyama H, Takeda Y, Iki K, Miyamori I, Sheng WP, Yamamoto H, Blair IA. Aldosterone biosynthesis and action in vascular cells. Steroids. 1995a;60(1):120–4. doi: 10.1016/0039-128x(94)00026-9. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Yoneda T, Hatakeyama H, Inaba S, Furukawa K, Mabuchi H, Takeda R. Regulation of aldosterone synthase in human vascular endothelial cells by angiotensin II and adrenocorticotropin. Journal of Clinical Endocrinology & Metabolism. 1996;81:2797–2800. doi: 10.1210/jcem.81.8.8768832. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh FY, Takeda R. Production of aldosterone in isolated rat blood vessels. Hypertension. 1995b;25(2):170–3. doi: 10.1161/01.hyp.25.2.170. [DOI] [PubMed] [Google Scholar]
- Thai HM, Do BQ, Tran TD, Gaballa MA, Goldman S. Aldosterone antagonism improves endothelial-dependent vasorelaxation in heart failure via upregulation of endothelial nitric oxide synthase production. Journal of Cardiac Failure. 2006;12(3):240–5. doi: 10.1016/j.cardfail.2006.01.002. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. New England Journal of Medicine. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertension Research. 2011;1(34):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, Konstam MA. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circulation: Heart Failure. 2010;3(3):347–53. doi: 10.1161/CIRCHEARTFAILURE.109.906909. [DOI] [PubMed] [Google Scholar]
- Uhrenholt TR, Schjerning J, Hansen PB, Norregaard R, Jensen BL, Sorensen GL, Skott O. Rapid inhibition of vasoconstriction in renal afferent arterioles by aldosterone. Circulation Research. 2003;93(12):1258–66. doi: 10.1161/01.RES.0000106135.02935.E1. [DOI] [PubMed] [Google Scholar]
- Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. Journal of Clinical Investigation. 2010;120(9):3350–64. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle E, Bauters C, Wernert N, Hamon M, McFadden EP, Racadot A, Dupuis B, Lablanche JM, Bertrand ME. Neointimal thickening after balloon denudation is enhanced by aldosterone and inhibited by spironolactone, and aldosterone antagonist. Cardiovascular Research. 1995;29(1):27–32. [PubMed] [Google Scholar]
- Vergeer M, Bots ML, van Leuven SI, Basart DC, Sijbrands EJ, Evans GW, Grobbee DE, Visseren FL, Stalenhoef AF, Stroes ES, Kastelein JJ. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 2008;118(24):2515–22. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40(4):504–10. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, Cavada G, Michea L, Marusic ET. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clinical Journal of The American Society of Nephrology: CJASN. 2010;5(8):1380–7. doi: 10.2215/CJN.09421209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Suzuki H, Sato T, Iso Y, Katagiri T, Takeyama Y. Eplerenone suppresses neointimal formation after coronary stent implantation in swine. International Journal of Cardiology. 2006;107(2):260–6. doi: 10.1016/j.ijcard.2005.03.078. [see comment] [DOI] [PubMed] [Google Scholar]
- Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation. 2001;104(4):467–72. doi: 10.1161/hc3001.091458. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spes CH, Win N, Janson CP, Schmidt BM, Theisen K, Christ M. Rapid cardiovascular action of aldosterone in man. Journal of Clinical Endocrinology & Metabolism. 1998;83(10):3517–22. doi: 10.1210/jcem.83.10.5203. [DOI] [PubMed] [Google Scholar]
- Xiao F, Puddefoot JR, Vinson GP. Aldosterone mediates angiotensin II-stimulated rat vascular smooth muscle cell proliferation. Journal of Endocrinology. 2000;165:533–536. doi: 10.1677/joe.0.1650533. [DOI] [PubMed] [Google Scholar]
- Zannad F, Mcmurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group Eplerenone in patients with systolic heart failure and mild symptoms. New England Journal of Medicine. 2011;364(1):11–21. [Google Scholar]