Abstract
Sphingosine-1-phosphate (S1P) plays important roles regulating functions of diverse biological systems, including the immune system. S1P affects immune cell function mostly by acting through its receptors at the cell membrane but it can also induce S1P receptor-independent responses in the cells where it is generated. S1P produced in allergically stimulated mast cells mediates degranulation, cytokine and lipid mediator production, and migration of mast cells towards antigen by mechanisms that are both S1P receptor-dependent and independent. Even in the absence of an antigen challenge, the differentiation and responsiveness of mast cells can be affected by chronic exposure to elevated S1P from a non-mast cell source, which may occur under pathophysiological conditions, potentially leading to the hyper-responsiveness of mast cells. The role of S1P extends beyond the regulation of the function of mast cells to the regulation of the surrounding or distal environment. S1P is exported out of antigen-stimulated mast cells and into the extracellular space and the resulting S1P gradient within the tissue may influence diverse surrounding tissue cells and several aspects of the allergic disease, such as inflammation or tissue remodeling. Furthermore, recent findings indicate that vasoactive mediators released systemically by mast cells induce the production of S1P in non-hematopoietic compartments, where it plays a role in regulating the vascular tone and reducing the hypotension characteristic of the anaphylactic shock and thus helping the recovery. The dual actions of S1P, promoting the immediate response of mast cells, while controlling the systemic consequences of mast cell activity will be discussed in detail.
INTRODUCTION
Sphingosine-1-phosphate (S1P) is a lipid mediator, formed by phosphorylation of sphingosine (SPH), that is involved in multiple physiological and pathological processes. Of the vast numbers of processes known to be regulated by S1P, vascular development, vascular permeability, angiogenesis, tumorigenesis and lymphocyte trafficking have been extensively explored in the last decade.1–4 The involvement of S1P in these physiological and pathophysiological processes results from its ability to modulate important cellular events such as chemotaxis, cytoskeletal changes, survival and proliferation.5,6 In the last few years, it has become clear that the involvement of S1P in immunological responses is not restricted to its regulation of lymphocyte trafficking but extends to the regulation of immune cell function.7 In mast cells, S1P regulates chemotaxis and mast cell effector responses such as degranulation, cytokine and lipid mediator production.8 A novel picture of S1P as functioning beyond its role as a regulator of mast cell function in allergic disease is emerging, with the new discovery of its role in termination of some allergic reactions.9
S1P AND ITS TARGETS
The generation of S1P is mediated by the enzymatic activity of two cytosolic sphingosine kinase isoforms (SphK1 and SphK2) and occurs mostly at the membrane where the substrate resides and to where these enzymes are translocated after activation.6,10,11 S1P has enough aqueous solubility to partition in the soluble fraction and to diffuse between intracellular membranes.5 However, the site of action is not merely intracellular.6 S1P can be exported out of cells via specific transporters and specifically bind to any of five G-protein coupled receptors (S1PR1-5) present in the cells of origin or in neighboring cells, engaging distinct signaling pathways as mediated by their coupling to specific isoforms of heterotrimeric GTP-binding proteins.5,6,12,13 Thus, activation of any receptor that promotes activation of SphKs and generation of S1P can potentially cause trans-activation of S1P receptors, enhancing the signaling repertoire and creating another wave of signals. Beyond its effect on S1PRs, the acute generation of S1P can also affect certain cell functions independently of its receptors6,10 by either binding and modifying putative intracellular targets or by affecting the relative levels of other lipid products, particularly SPH and ceramide whose effects generally oppose those of S1P1,5,6 (Figure 1). Recently, histone deacytylase was described as the first known intracellular target of S1P. S1P generated in the nucleus, via the translocation and activation of SphK2, was found to bind histone deacetylase, inhibiting its activity and thus enhancing gene transcription.14
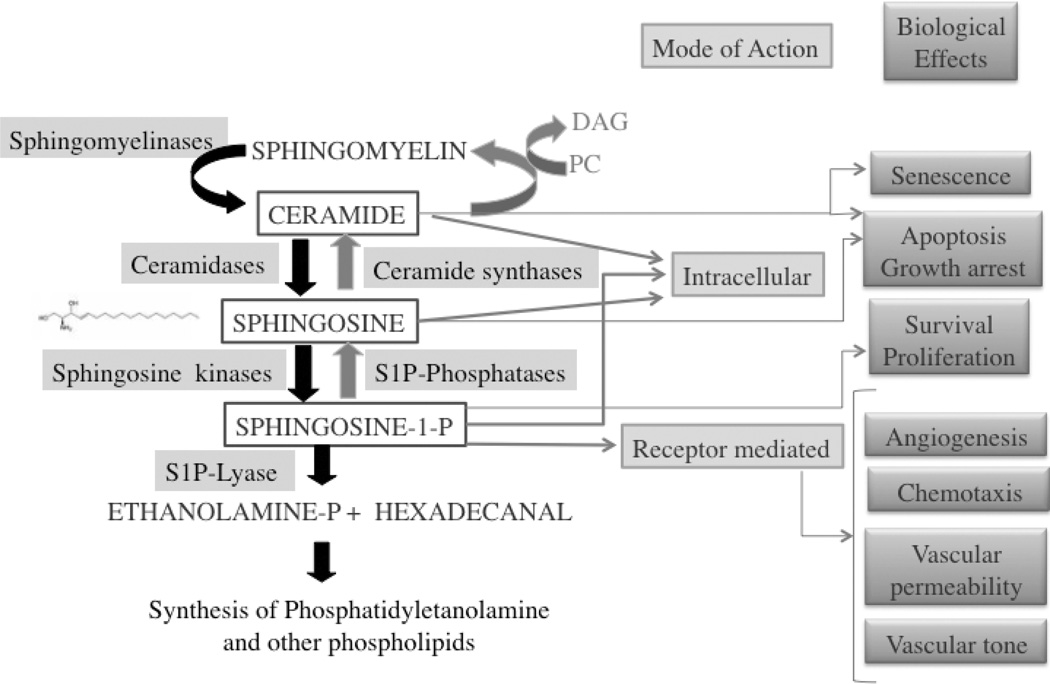
Figure 1. Sphingolipid metabolism and biological functions of sphingolipid derived molecules.
Ceramide, sphingosine and sphingosine-1-phosphate are lipid mediators derived from complex sphingolipids by the action of several enzymes acting on cellular membranes. Sphingomyelinases cleave the phosphocholine group of sphingomyelin to yield ceramide; ceramidases then cleave ceramide to form sphingosine, and sphingosine kinases phosphorylate sphingosine to form sphingosine-1-phosphate (S1P). These enzymes can be selectively or sequentially activated by various stimuli. The sphingolipid metabolites are interconvertible: S1P can be dephosphorylated to form sphingosine by S1P phosphatases and sphingosine acetylated by ceramide synthase. S1P can also be irreversibly degraded by a S1P lyase and the products of its activity used for the resynthesis of lipids. While ceramide and sphingosine were shown to induce apoptosis and cell cycle arrest, S1P prevents apoptosis and promotes cell growth. S1P mediates numerous cellular responses, not only by acting inside cells, but by binding a family of five plasma-membrane G-protein-coupled receptors (known as S1PR1–S1PR5) that are differentially expressed by most cells and mediate multiple functions. S1P produced intracellularly can be exported out of cells by specific transporters, or to a lesser extent, it may be generated in the extracellular space.
REGULATION AND FUNCTION OF S1P IN TISSUES AND CIRCULATORY FLUIDS
The levels of S1P inside cells and the interstitium of tissues are usually low in homeostatic conditions, due to irreversible degradation of S1P by a S1P lyase15,16 and to its dephosphorylation to SPH by two specific S1P phosphatases (SPP1 and SPP2)17 (Figure 1) present in intracellular membranes and/or at the plasma membrane facing the extracellular environment. However, local increases in S1P levels may occur under certain conditions, inducing responses in tissue resident or circulating immune cells (which express various combinations of S1PRs) affecting physiological processes.7 For example, during the early stages of inflammation, increases in S1P at the site of inflammation can activate the S1PR1 in T cells18 and hematopoietic stem and progenitor cells,19 preventing their exit into the afferent lymphatics and causing T cell retention in secondary lymphoid tissues and proliferation of tissue-resident myeloid cells, respectively. Furthermore, elevation of local S1P can be involved in modulating the type of immune response by skewing the phenotype of lymphocytes, dendritic cells and macrophages.7 How S1P is elevated in tissues is still unclear; nonetheless, it is known that blood cells,20 and as we will discuss later, mast cells, are likely candidates for increasing S1P in the tissue environment since they can produce and release large quantities of S1P when stimulated.
In contrast to the low levels of S1P in tissues, S1P concentrations in plasma and lymph are well above the binding affinity of S1P for its receptors (although it is unclear what is the “effective” concentration of S1P in circulation). Free S1P may be at least 40 times lower than the total S1P concentration because it is mostly bound to albumin or lipoproteins, but the bound form can be also physiologically active.21,22 The S1P-carrier proteins may provide a sink for excess S1P and/or a regulatory mechanism for protecting S1P from degradation and for delivering S1P to specific cells.21,22 Recent data has uncovered that the high levels of S1P in circulatory fluids play a role in two important physiological processes: the maintenance of endothelial barrier integrity, and lymphocyte recirculation between lymph nodes and periphery. Deletion of both SphK1 and SphK2 in an inducible mouse model renders these “S1P-less mice” with enhanced vascular permeability and induces lethality in response to mediators that induce vessel permeability.23 Resistance to lethality can be restored by transfusion of wild type erythrocytes,23 the cells responsible for maintaining high levels of S1P in blood.24,25 The proposed mechanism for the protective effect by S1P in the vascular barrier is its regulation of S1PR1 activity in endothelial cells.23 S1PR1 is normally abundant in these cells and S1P engagement of this receptor tightens endothelial adherens junctions26 protecting endothelium integrity. On the other hand, targeted deletion of SphK1 and 2 in the lymphatic endothelium, which results in ablation of the high concentrations of S1P in lymph but not in blood, causes impaired egress of lymphocytes from the lymph nodes.27 Previous findings also showed that ablation of the tissue to blood/lymph S1P gradient by inhibition or genetic deletion of S1P lyase prevents the exit of lymphocytes from the lymph nodes,15,28 and that the presence of S1PR1 in lymphocytes is required for their egress from lymph nodes.29,30 Collectively, the findings argue that a major determinant driving the movement of lymphocytes out of the lymph nodes is the S1PR1-dependent sensing by lymphocytes of an S1P gradient that exists between the lymph node (low levels of S1P) and the lymph (high levels of S1P).
THE SPHK/S1P/S1PR AXIS AND MAST CELLS
From the brief discussion above, it is apparent that production and degradation of S1P is tightly and dynamically regulated. This suggests that its export to the extracellular space, its levels in the extracellular space and circulatory fluids, and the regulation of cell surface expression of the multiple S1PRs must be critical in the shaping of a given physiological response. In this review we will summarize the recent knowledge on the regulation of S1P production and the function of S1PRs in mast cell biology and in the allergic responses. We now know that engagement of the high affinity receptor for IgE (FcεRI) induces the activation of SphK1 and SphK2 and the generation of S1P.8 We also know that the activities of SphKs are key to the activation and expression of S1PRs on mast cells, however, it should be recognized that SphKs are also essential to FcεRI-mediated mast cell activation independently of S1PR8,31 When activated, mast cells secrete S1P into the extracellular space, and produce gradients of S1P within resident tissues that may contribute to the inflammatory response and tissue remodeling.32–34 Moreover, dysregulation of S1P can affect the differentiation and responsiveness of mast cells, and thus it may increase mast cell responses in health and (allergic) disease.31,35 S1P also seems to play a vital role in overcoming the severe (patho)physiological responses seen during anaphylaxis.9 Interestingly, its production in a non-hematopoietic compartment was found to be essential for the recovery from anaphylaxis. This indicates that the site of action for S1P may determine its role as a pro- or anti-allergic molecule.
S1P IS GENERATED BY ACTIVATED MAST CELLS
FcεRI ACTIVATES SPHINGOSINE KINASES
It is well recognized that antigen-induced aggregation of IgE bound to FcεRI on mast cells elicits multiple biochemical events culminating in mast cell degranulation and the de novo synthesis and secretion of cytokines and lipid mediators.36 One of the biochemical events that occurs downstream of FcεRI engagement is the activation of SphKs with a consequent increase in intracellular S1P levels, as first shown in RBL-2H3 cells, 37 This finding was later confirmed in non-transformed cultured mast cells, such as bone-marrow derived mast cells (BMMCs), liver-derived mast cells and human mast cells.31,33,38,39 Engagement of FcεRI elicits the partitioning of this receptor to lipid rafts where sphingolipids, such as SPH (the substrate for SphK), are enriched. The Src kinases Lyn and Fyn, which initiate early FcεRI signaling, are needed for the translocation of SphKs to lipid rafts and for their activation.33,40 SphKs interact with Lyn and Fyn, but not with Src or other tyrosine kinases such as Syk, and the activation of SphK1 and 2 depends on the presence of these tyrosine kinases and their downstream signals. Fyn-deficiency caused a complete ablation of SphK activation. 33,40 Further support for the importance of Fyn in SphK activation was provided when Fyn expression was restored, which rescued the activation of SphK1 and SphK2.33 The adaptor protein Gab2 and the lipid kinase PI3K, as well as other undefined signals, were also found to be important for the activation of SphKs downstream of Fyn kinase.33 In comparison, Lyn-deficient mast cells showed a delay in the activation of SphK by IgE/Ag,40 yet these cells recovered to achieve an almost normal SphK activation with time suggesting that Lyn may not be essential for the activity of SphKs but may play a role in facilitating their activation. It has been suggested that PLD 1 activity is also involved in both FcεRI-39 and FcγRI-mediated SphK1 activation41 in mast cells and human myeloid cells, respectively. This view is consistent with previous findings42 as well as our own (AO and JR unpublished observation), that PLD activation is Fyn-dependent in mast cells. This suggests that PLD is also likely to contribute to the activation of SphKs. However, the specific mechanistic detail of how PLD may contribute to SphK activation and the relationship to the translocation/activation of SphK1 and 2 in mast cells is not entirely clear.
ROLE OF SPHKS IN MAST CELL BIOLOGY AND FUNCTION
To directly address the importance of each of these isoforms in mast cell function, we derived mast cells from embryonic liver progenitors of mice deficient in SphK1, SphK2 or both,31 since the double deficiency in SphK1 and 2 is embryonic lethal.43 Although our experiments with BMMC from SphK1 and SphK2 -deficient adult mice showed clearly that SphK2 was the major producer of S1P in these cells,31 mast cell degranulation was not consistently diminished in all tested cultures. This led us to believe that SphK1 and 2 might have some redundant functions. Unlike BMMCs derived from adult mice, mast cells derived from liver progenitors of SphK2-deficient embryos consistently showed a reduction in the extent of the degranulation response and in the production of various cytokines and eicosanoids. In contrast, mast cells derived from liver progenitors of SphK1-deficient embryos showed no impairment in degranulation, whereas cells deficient in both SphK1 and SphK2 had the same impairment as SphK2-deficient mast cells.31 The robust and consistent defect seen in the degranulation response of mast cells derived from the SphK2-null embryos clearly differed from the more mild and variable result seen in SphK2-null BMMCs derived from adult mice. We interpreted these results to reflect a possible compensatory or epigenetic change present in the mast cells of SphK-deficient adult mice (as discussed in the next paragraph), although another possibility is that the two mast cells populations are not phenotypically comparable. This latter possibility is not unexpected as the mast cell phenotype is known to vary depending on the environment they populate in vivo, or the growth factors used in vitro.44 Thus, certain populations of mast cells might differ in their use of SphK1 or SphK2 for compartmentalized or general S1P production. In agreement with the view that the microenvironment may be a determinant in how SphKs are used, we now have evidence (unpublished observation) that peritoneal mast cells from either SphK1 or SphK2-deficient adult mice have impaired degranulation responses (although again, this result was more variable in the SphK2-deficient peritoneal mast cells). Thus, it is plausible that the relative importance of SphK1 and SphK2 depends on the type of mast cell (Table 1). This is also consistent with the findings on the role of SphK1 and SphK2 in human mast cells. In human bone marrow-derived mast cells only SphK1 mRNA was found, and silencing of this isoform by antisense oligonucleotide caused a reduction in degranulation.39 In CD34+-derived human mast cells 33 and in cord blood derived human mast cells (CBMC)38 both SphK1 and 2 were present and could be activated by FcεRI engagement. Silencing of SphK1 and SphK2 by RNAi in the latter cells and in the human mast cell line LAD238 showed that SphK1 was involved in degranulation, migration toward antigen and secretion of the chemokine CCL2/MCP1, whereas silencing of SphK2 reduced cytokine production (Table 1).
TABLE 1.
Reported role of SphK1 and SphK2 isoforms in mast cell responses in vitro and in vivo, using different experimental approaches
| SphK isoform |
Type of SphK alteration |
Type of MC |
Effect in vitro |
Effect on PCA (in vivo) |
Effect on PSA (in vivo) |
|---|---|---|---|---|---|
| SphK1 | siRNA, Antisense oligo |
BMMC* | ↓Degranulation45 ↓ Ca response45 ↓ Cytokine45 |
↓45 | ↓45 |
| Hu MC* | ↓Degranulation38,41 ↓ Ca response41 ↓ Cytokine38 |
||||
| Knockout | BMMC | Normal31 | Normal (unpublished) |
↓Histamine31 ↓ Recovery9 (Strong response) |
|
| LDMC* | Normal31 | ||||
| PMC* | ↓Degranulation (unpublished) |
||||
| SphK2 | siRNA | BMMC | Normal45 | Normal45 | |
| Hu MC | ↓ Cytokine38 | ||||
| Knockout | BMMC | ↓Degranulation31 Normal45 |
↓ (unpublished) |
Normal histamine31 ↑Recovery9 (Poor response) |
|
| LDMC | ↓Degranulation31 ↓ Ca response31 ↓ Cytokine31 |
||||
| PMC | ↓Degranulation (unpublished) |
BMMC, murine bone marrow-derived cultured mast cells; Hu MC, human cultured mast cells (cord blood -or CD4+-derived); LDMC, mouse fetal liver derived mast cells; PMC, mouse peritoneal mast cells.
INCONSISTENCIES IN UNCOVERING THE ROLE OF SPHKS IN MAST CELLS USING SILENCING RNA AND GENETIC DELETION APPROACHES
Apart from the possible heterogeneity of the mast cells in those studies, there are other possible technical considerations when studying the relative involvement of the SphK isoforms. Study of BMMCs derived from SphK1 and SphK2-deficient mouse models, which were generated independently of those we study, showed normal degranulation responses to FcεRI stimulation.45 Yet, the same report shows a reduction in the degranulation of SphK1-deficient BMMCs where SphK1 expression was silenced in vitro or in vivo by siRNA (Table 1). Thus, it is important to consider the possible limitations of employing RNAi silencing or genetic deletion strategies in interpreting the results. The difference observed between the SphK-deficient BMMCs generated by genetic deletion versus RNAi silencing might be most easily ascribed to off-target effects of siRNA or compensatory mechanisms from genetic deletion. The in vivo use of siRNA is also inherently non-specific and would be expected to affect multiple populations of cells, not just mast cells. Alternatively, the genetic deletion could cause changes in the environment that may impose epigenetic changes or compensatory mechanisms in bone marrow progenitors. On this latter point, the SphK2-deficient mice used in our studies had elevated levels of S1P in plasma whereas the SphK1-deficient mice had low levels of circulating S1P.43,46,47 As we demonstrated31 this had a marked effect on mast cell responsiveness when mice were systemically challenged, since SphK2-deficient mice showed normal histamine release. This was unexpected given that fetal-liver derived SphK2-deficient mast cells showed defective degranulation. However, this is consistent with the recent report that S1P can regulate the development of human mast cells derived from cord-blood progenitors into a mast cell type more akin with a skin-type mast cell.35 Thus, it is possible that environmental cues, such as high S1P levels in the blood and lymph, may cause epigenetic alterations that compensate for the SphK deficiency. In fact, we recently found that a passive cutaneous anaphylactic (PCA) challenge of SphK2-deficient mice results in a defective response (unpublished observation). Thus, this skin-localized challenge showed the expected impaired mast cell degranulation of the fetal liver-derived SphK2-deficient mast cells. This is consistent with low levels of S1P in the skin and the findings clearly demonstrate that the high levels of circulating S1P in these mice is a key factor in shifting the poor-responding phenotype of SphK2-deficient mast cells to one of normal response. Obviously, the function of the SphK isoforms is more likely to be unambiguously elucidated by tissue-specific gene targeting in adult mice.
REGULATION OF MAST CELL CALCIUM RESPONSES BY SPHKS
Despite these unresolved issues and dissimilarities, overall the findings using pharmacological inhibitors, silencing RNAi approaches or genetic deletion models, all point to a critical role for SphKs in mast cell function and suggest that some potential overlap for the two isoforms might exist. What has not been studied in detail is the mechanism by which SphK1 or SphK2 affect mast cell function. Our studies on the fetal liver-derived mast cells from SphK2-deficient mice indicated that the underlying cause for the overall impaired mast cell responsiveness was due to an impairment of calcium influx and PKC activation.31 In human mast cells, silencing RNAi approaches have implicated SphK1 in calcium release from intracellular stores.39 However, we uncovered no evidence of a role for SphKs or S1P in mobilizing calcium from endoplasmic reticular (ER) stores. As described in another chapter in this book, calcium influx is well known to be an essential process for FcεRI-induced mast cell degranulation and cytokine production48,49 and thus how SphK2 regulates calcium mobilization is of considerable interest. An essential characteristic of some calcium channels is their modulation by lipids50 and S1P has been shown to regulate calcium mobilization.6,51–53 SphK2-deficient mast cells fail to produce S1P and instead accumulate SPH, which has been reported to inhibit calcium influx.54–57 In contrast, S1P added exogenously to BMMCs had no effect on either FcεRI-induced calcium mobilization31 or store-operated calcium release-activated calcium current (iCRAC) in RBL-2H3,55 suggesting that the effect of S1P on calcium is likely to be intracellular and not through binding of its cell surface receptors on mast cells. As alluded to above, work in RBL-2H3 cells has shown that SPH is an inhibitor of store-operated calcium release-activated calcium current (iCRAC)55 and of the coupling of Orai1/CRACM1 to STIM1, the calcium sensor protein in the ER.58 Since SphK2 controls the intracellular levels of S1P and SPH in mast cells in the resting and activated states, it is possible that an elevation in S1P directly or indirectly leads to the opening of a calcium channel, or that a reduction of SPH releases the inhibitory effect on calcium channel function.31,55 In support of this view, Prieschl and colleagues32 proposed a rheostat model based on the observations that addition of exogenous SPH inhibited mast cell degranulation, and leukotriene and TNF production, effects that could be effectively reversed by S1P. The model stipulates that the balance between SPH and S1P is decisive for the excitability of mast cells. Apart from the possibility that S1P or SPH may directly modify the function of an ion channel in mast cells, a more indirect mechanism is also possible. Recent studies in our lab suggest that reduced levels of non-selective cation channel Transient Receptor Potential Channel 1 (TRPC1) mRNA and protein, in Fyn-deficient BMMCs, is a contributory factor for the defective calcium influx and degranulation of these cells (RS and JR, unpublished results). Moreover, silencing of TRPC1 expression in WT BMMCs also resulted in impaired calcium influx and defective degranulation. As previously mentioned, Fyn-deficient BMMCs failed to activate SphKs after FcεRI stimulation and we found that they also have lower baseline levels of S1P.33 Since S1P was recently shown to affect nuclear transcription by acting directly on histone deacetylases14 one possible scenario is that homeostatic S1P production may be involved in the regulation of certain genes critical for mast cell calcium responses, like TRPC1, and function. Thus, studies on S1P regulation of gene expression may shed further insights on the role of this lipid in regulating mast cell signals and function.
FcεRI INDUCES S1P FORMATION AND EXPORT
FcεRI-mediated activation of SphKs not only leads to the formation of S1P inside the cells,37,39 but also results in its export into the extracellular space.32,33,59 This suggests that the site(s) of action for S1P may be both intra- and extra- cellular. As previously mentioned, mast cells lacking SphK2, which do not produce intracellular S1P or export it, have a defect in calcium mobilization and degranulation and these defects are either not restored or only partially restored, respectively, when S1P is added exogenously. This suggests that the some actions of S1P (such as the effect on calcium influx) are independent of its cell surface receptors on the mast cell.31 However, the function of S1P as a second messenger is not well understood and, other than the recent finding of its binding to and regulating histone deacetylase activity,14 its intracellular targets are not defined. In contrast, it has been shown that the receptors for S1P participate in mast cell responses.59 In many cell types, the export of newly synthesized S1P may occur as a localized process with coordinated S1P production on the inner membrane leaflet resulting in receptor activation on the outer leaflet, although the amounts of S1P generated are usually not readily detectable.6,60 Notably, mast cells and platelets can release copious amounts of S1P uponagonist stimulation8,20 suggesting that the S1P released plays a role beyond the autocrine modulation of cell function and may be an important paracrine component of mast cell and platelet regulation of immune responses.
MECHANISMS OF EXPORT
Both platelets and mast cells secrete their granular contents by exocytosis. However, in mast cells, S1P is not pre-accumulated in granules, and degranulation appears to be dissociated from S1P export.61,62 Since S1P is a charged molecule, spontaneous flip-flop of S1P across the bilayer is not likely to occur and thus a facilitating transport mechanism must be in place. The ATP binding cassette (ABC) superfamily of transporters, which catalyze the transport of chemically diverse compounds across cellular membranes, have been involved in the transport of S1P, both from the outside to inside cells63 or from the inside to the outside of the cell.61,62,64 The ABCC7 ion channel CFTR was implicated in the take up of S1P from the mileu.63 However, little is know about the role of this transporter in the uptake of S1P for mast cells. In contrast, several types of ABC transporters have been implicated in the efflux of S1P including ABCB1 in T-cells,64 an ABCA-like transporter in platelets,62 ABCC1 in the RBL-2H3 and human LAD2 mast cell lines,61 and ABCC1 and ABCG2 transporters in breast cancer cells.65 Inhibition of ABCC1 or RNAi silencing of its expression efficiently reduced the FcεRI-stimulated export of S1P from mast cells, but it only partially reduced the constitutive export of S1P observed when SPH was added to the cells, suggesting other transporters or additional mechanisms of export might be present.61 Interestingly, mast cells derived from S1P Lyase-deficient mice, which have markedly elevated levels of S1P and enhanced constitutive as well as FcεRI-induced efflux of S1P into the media, showed an upregulation of an ABCA1 transporter (L. Wright, Y. Kitamura, AO and JR, unpublished observations), although its relevance to S1P export in these cells is still unknown. Recently, it was shown66 that in zebrafish, the protein Spns2, with structural homology to the bacterial glycerol-3-phosphate transporter, can specifically export S1P. A mutation in the Spns2 gene resulted in the inability of yolk syncytial cells to release S1P during heart development, a critical event for the chemoattraction of myocardial precursors bearing the S1P receptor S1PR2 to form the heart tube. A mutation in either Spns2 or in the S1PR2 genes led to the presence of a split heart; a phenotype that was rescued by injecting S1P, Spn2 mRNA or human Spns2 mRNA, but not the homolog Spns1 mRNA. Whether this type of transporter, or its homolog, may also function in mast cells has not been investigated. Mice carrying genetic deletions in ABC transporters or Spns2 may be pivotal to unequivocally define the type of transporter involved in the export of S1P and the contributory role of secreted S1P in allergy and inflammation.
FUNCTIONS OF S1P RECEPTORS IN MAST CELL RESPONSES
Mast cells have been described to express two types of receptors for S1P, S1PR1 and S1PR2,59 although recent observations in our lab identified the presence of mRNA for S1P4 as well, but not S1PR3 or S1PR5. FcεRI activation of SphKs and the production of S1P was shown to induce a ligand-dependent transactivation of S1PR1 and 2 enhancing mast cell functions.59 The transactivation of S1PR1 and S1PR2 can be readily recognized, as these receptors are endocytosed with β-arrestin, a common phenomena observed when a GPCR is activated.67 Antisense oligonucleotide-mediated silencing of S1PR1 or S1PR2 indicated that while S1PR1 was involved in the migration of mast cells toward low concentrations of antigen, S1PR2 participated in FcεRI-induced degranulation. Silencing of S1PR1 reduced, and ectopic expression of S1P1 enhanced, respectively, chemotaxis of mast cells towards antigen without affecting degranulation. On the other hand, overexpression of S1P2 inhibited the chemotactic motility of RBL cells towards antigen.59 Moreover, S1PR2 mRNA expression, but not S1PR1 mRNA, was increased as a late consequence of FcεRI stimulation. These findings argue for a model whereby S1PR1 participates in the recruitment of mast cells to their site of action, while S1PR2 deters migration and contributes to degranulation once mast cells reached their site of action. Additional findings reinforce the conclusion that SphK activation, S1P generation, and export and S1PR1 engagement is necessary for mast cell chemotaxis. Fyn-deficient mast cells, which fail to activate SphKs, also have defective chemotaxis in response to antigen and SCF, but exogenous S1P in combination with FcεRI-generated signals can partly restore the motility defect.33 Similarly, pharmacological inhibition or genetic deletion of SphK in mast cells resulted in reduced chemotactic motility towards antigen or SCF, but these cells were able to migrate normally towards S1P33,59 (AO and JR, unpublished results). Interestingly, inhibition of ABCC1-mediated S1P export blocked migration of mast cells to antigen in vitro, and this was rescued by addition of S1P,61 suggesting that mast cell chemotaxis to antigen or SCF is, at least in part, dependent on mast cell derived S1P.
The involvement of S1PR2 in degranulation is not as clear. Exogenous addition of S1P alone or in combination with FcεRI stimulation does not induce significant degranulation in the RBL-2H3 cell line or in murine mast cells.33,59 However, RNAi silencing of S1PR2 or its genetic deletion in BMMCs resulted in a 40 to 50% reduction in FcεRI-induced degranulation. Of note, the degranulation response to ATP59 and to phorbol esters and ionomycin (AO and JR, unpublished results) were also reduced in S1PR2-deficient BMMCs, suggesting a potential defect in the exocytotic machinery in these cells. Interestingly, inhibition of ABCC1-mediated S1P export did not affect FcεRI-induced degranulation, indicating that export of S1P by this transporter is needed for migration but not for degranulation.61 Although it is possible that a yet unidentified transporter brings a distinct pool of S1P to the proximity of S1PR2, other possibilities include that the S1P/S1PR2 axis functions independently of a transporter for the degranulation response, or perhaps in a model similar to that proposed for other S1PRs68 in which a constitutively active S1PR2 facilitates a signaling platform that promotes FcεRI signals. In this later model, the S1PR2 could act as a sensor for S1P in the environment and thus as a modifier of FcεRI responses. For example, in normal tissues where the levels of S1P are low S1PR2 activity on mast cells could also be low. Thus, in tissues mast cells might require higher amounts of antigen to trigger a response, whereas in mast cells that are in closer vicinity to the circulatory system, where the S1P concentration is high or under conditions where S1P is elevated, the activity of the S1PR2 would be higher, lowering the threshold of activation via the FcεRI. Nevertheless, this is hypothetical and more conclusive evidence for the role of this receptor in mast cells responses in vivo is needed. Our recent studies of systemic anaphylaxis in the S1PR2-null mice suggest that these mice have a normal immediate hypersensitivity response upon systemic antigen challenge. However, we uncovered a mast-cell independent contribution of this receptor in the recovery from anaphylaxis, and thus a mast-cell specific genetic deletion of S1PR2 is needed to more directly assess its involvement in the mast cell responses during anaphylaxis or other allergic disorders.
PARACRINE FUNCTION OF MAST CELL-RELEASED S1P
As tissue-resident cells, mast cells are exposed to a variety of stimuli whose profile can change given a particular circumstance. Antigen-stimulation of FcεRI, for instance, has been demonstrated to induce the production and release of S1P into the interstitium, and thus S1P may participate in promoting allergic inflammation. It is known that the levels of S1P also increase locally during acute inflammation in the footpad,18 in the airways of asthmatic individuals hours after a challenge,69 and in the joints of rheumatic patients.70,71 The origin of the elevated S1P in tissues under these conditions is not known, but one reasonable candidate is the mast cell. Changes in S1P levels can have drastic effects in the surrounding tissue, not only by affecting angiogenesis and vascular permeability,1,72 but also by causing fundamental changes to the immune system.7,70,73 However, the specific role of S1P in inflammation or in allergic inflammation is still unclear, but it is likely that the particular challenge and the type of immune cells that respond to this challenge may be a determinant of S1P function in inflammation. Increases in the levels of S1P and/or increased expression of S1PRs can result in the accumulation of immune cells in the local site. Several mechanisms may account for this accumulation: induction of chemotaxis,29,59,74–78 retention of hematopoietic stem cell progenitors,19 and retention of mature T cells in the tissues; the latter two through increased LFA-1–ICAM-1 and VLA-4–VCAM-1-mediated adhesion to the afferent lymphatic endothelium.18 In vitro and in vivo experiments in murine systems suggest that S1P can also cause a shift in T-cell responses promoting TH2- and TH17-cell responses while disfavoring TH1-cell responses, thus potentially skewing responses toward allergic phenotypes. This, in part, can be mediated through the reported effects of S1P on dendritic cell maturation. S1P was shown to impair the ability of lipopolysaccharide (LPS)-stimulated human monocyte-derived DCs to cause naïve CD4+ T cells differentiation to T helper 1 (TH1)-cells and instead promoted TH2-cell differentiation.7,76 In this regard, it should also be kept in mind that S1P also seems to be important in the effector phase of allergic disease through control mast-cell responsiveness. Thus, in certain circumstances, it appears that S1P can play a major role in promoting allergic inflammation.
An anti-inflammatory role for S1P has also been described. For example, S1P can induce a switch from proinflammatory M1 to the anti-inflammatory M2 macrophage subtype,7 which explains the observed protective effect of S1P against atherosclerotic lesions.79 Furthermore, topical application in the skin80 or inhalation of S1P or the S1P mimetic FTY72081 suppressed ear swelling in a model of allergic contact dermatitis and airway inflammation, and bronchial hyperresponsiveness in an asthma model, respectively. The underlying mechanism for suppression appears to be the inhibition of dendritic or Langerhans cell migration to the lymph nodes and thus an impaired generation of allergen-specific T cells.80,81 Furthermore, FTY720 also induced a decrease in the induction of TH1- and TH2-cell effector cytokines and the establishment of a stable synapse between DCs and T cells.81 Intriguingly, lesional skin biopsies of individuals with atopic dermatitis82 and psoriasis83 show increased expression of two enzymes involved in the degradation of S1P, S1P Lyase and S1P-phosphatase type 2 (SPP2), respectively. The elevated presence of these enzymes could result in reduced levels of S1P and the perpetuation of the disease. Consistent with this view, an anti-inflammatory role for S1P was also suggested by the reduction in IL-1β and IL8 production by stimulated HUVEC or neutrophils when its degradation was blocked by RNAi silencing of SPP2.83 Alternatively, the SPP2-mediated conversion of S1P to SPH, which can be used to generate ceramide,17 or the breakdown of S1P by S1P-Lyase into metabolites that can be used for the synthesis of other lipids, particularly phosphatidylethanolamine,16 may also play a role in the course of these distinct diseases by affecting the lipid composition of the skin. Interestingly, application of an emulsion of ceramide to the skin appears to reduce the severity of atopic dermatitis.84–86 However, at the moment it is difficult to distinguish if the increase in expression of enzymes involved in the catabolism of S1P contributes to the development of the disease by reducing the presence of an anti-inflammatory S1P, or if this is a mechanism to suppress disease by eliminating a pro-inflammatory S1P and producing other lipid products that improve the symptoms.
S1P-MEDIATED REGULATION OF THE MAST CELL PHENOTYPE AND RESPONSIVENESS
Increased levels of S1P in the circulation were found to correlate with the ability to release granule-stored mast-cell allergic mediators (degranulation), such as histamine. This was unexpectedly uncovered when we compared the responses of mast cells derived from SphK-deficient mice in vitro to the responses of mast cells during IgE-dependent systemic anaphylaxis. SphK-deficient mice have altered levels of circulating S1P and these changes in S1P levels are independent of the mast cells. 31 Genetic deletion of SphK1 resulted in the reduction of circulating S1P,31,46 because this isozyme is the major activity present in RBC, the cells most responsible for maintaining S1P levels in the blood. In contrast, genetic deletion of SphK2 caused an elevation in the circulating levels of S1P,31,47,87 perhaps due to a compensatory elevation of SphK1 activity in RBC. As previously mentioned, FcεRI-dependent stimulation of SphK2-deficient mast cells in vitro resulted in a defective degranulation response. However, when SphK2-deficient mice were challenged systemically, in an IgE/Ag-dependent manner, the mast cell response (as measured by histamine release) was similar to WT mice suggesting that the higher levels of S1P in these mice could cause SphK2-deficient mast cells to function normally. In contrast, SphK1-deficient mice, whose mast cells showed normal degranulation in vitro, had decreased histamine release upon a systemic challenge. This apparent disconnect between the in vitro and in vivo results appeared to indicate that the exposure to high or low levels of S1P caused an alteration of the mast-cell phenotype31 (Figure 2 and Table 1). As discussed earlier, it was also possible that the phenotype of the mast cells differentiated in vitro did not reflect the in vivo phenotype of mast cells participating in the anaphylactic response. However, additional experiments (AO and JR, unpublished observations) showed that a local (cutaneous) IgE/Ag-challenge of SphK1- or SphK2-deficient mice showed a normal or defective mast cell response, respectively, that mirrored the results observed in vitro (Table 1). This was not due to a difference in the number of mast cells in the skin or differences in granule content since the local (cutaneous) response to compound 48/80, a secretagogue, was comparable in all the mutant and WT mice. This suggests that, in systemic anaphylaxis, changes in the levels of circulating S1P may influence the responsiveness of the mast cell and thus the sensitivity and/or severity of this response. In the skin, where levels of S1P are inherently low, the role of mast-cell extrinsic S1P on mast cell responsiveness may not be so influential.
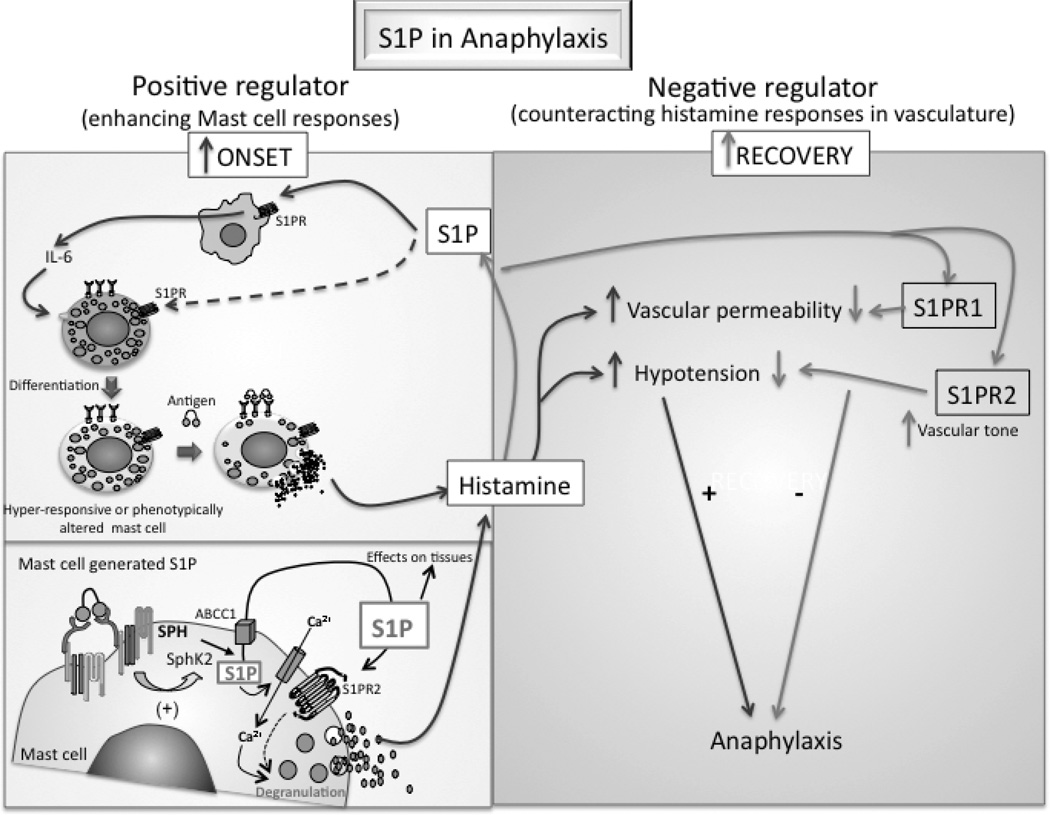
Figure 2. Dual role of sphingosine -1-phosphate in the regulation of systemic anaphylaxis.
While S1P can enhance the signaling and function of mast cells (left panels), which initiate the allergic response, it alleviates the symptoms of the anaphylactic shock acting in a mast cell-independent manner (right panel). Exposure of mast cell progenitors to higher levels of S1P in the cellular environment can change the differentiated phenotype and/or enhance the responsiveness of mast cells. The effect of S1P on mast cells can be indirect, involving monocytes/macrophages, or direct (left, upper panel). Thus, under circumstances where S1P is increased, mast cells can become more sensitive to a stimulus and thus influence the initiation of an allergic response. Mast cells can produce S1P via the activation of SphKs (left lower panel). The activation of SphK2 by FcεRI is necessary for the activation of calcium channels at the plasma membrane and for the degranulation of mast cells. The S1P generated is exported to the outside of the cell, where its concentration increases locally and can thus engage the S1PRs on the mast cells or on other neighboring cells, affecting the inflammatory response. The release of histamine by mast cells triggers SphK1 activity and the production of S1P from non-mast cell sources (right panel). This S1P activates S1PR2 receptors in the vasculature, regulating the vascular tone and thus blood pressure, which in turn can controls glomerular filtration rate and the clearance of histamine resulting in the recovery from anaphylaxis. SphK1 and S1P production can also activate S1PR1 receptors, which prevent vascular leakage and this also may contribute to recovery.
Additional data in models other than SphK-null mice also support the idea that in vivo regulation of S1P homeostasis (extrinsic to the mast cell) can regulate mast cell responsiveness. Mice from an 129Sv genetic background, which undergo strong TH2-cell responses and anaphylaxis,88 have higher levels of circulating S1P as compared to C57BL/6 mice, which have modest TH2-cell responses.89 Increasing the levels of circulating S1P in C57BL/6 mice, by using an inhibitor of S1P lyase,15 results in mice with enhanced mast cell responses as determined by increased histamine in the plasma following a systemic challenge. The level of the response resembles that observed in 129Sv mice, suggesting a link between S1P levels and the enhanced mast cell phenotype (AO and JR, unpublished observation). Recent findings also indicate that exposure of human cord blood progenitors to both S1P and stem cell factor enhances the rate of differentiation of these progenitors to mast cells and causes them to become more like a connective tissue type mast cell (containing chymase and tryptase in their granules) rather than a mucosal type (containing tryptase) mast cells, which is the normal when cells are cultured in the presence of stem cell factor alone.35 The mechanism for how S1P causes the developmental shift in the phenotype of human mast cells appears to be partially indirect, involving the production of IL-6 by cells of monocyte/macrophage lineage.35 Our unpublished observations on the effect of S1P during murine BMMCs differentiation indicate that chronic treatment with S1P induces genetic changes that give rise to a hyper-responsive mast cell. This may be a direct action of S1P on the mast cells, but whether this is mediated by a S1PR on the mast cell remains to be determined.
Thus, S1P homeostasis in circulation or the tissue environment, which can be dysregulated by genetic causes or by dietary or environmental factors, may result in alterations in the phenotype of mast cells and the susceptibility to particular allergic reactions. Although the discussed data supports an effect of the extrinsic S1P in vivo as a regulator of mast cell responsiveness, other findings argue otherwise. Adult mice lacking S1P, by conditional deletion of both SphK1 and SphK2, had normal mast cell responses to an anaphylactic challenge when challenged with strong stimuli,23 suggesting normal mast cell function in the absence of S1P. Additional studies are needed to determine if the susceptibility of mast cells to S1P in the environment or within the mast cell depends on the type or the strength of the stimulus.
RECOVERY FROM ANAPHYLAXIS IS POSITIVELY REGULATED BY S1P FROM A NON-MAST CELL SOURCE
S1P receptors are ubiquitously expressed and affect multiple physiological functions in various tissues.7 Thus, it is likely that, beyond their role in mast cell biology and function, these receptors may influence other aspects of the allergic disease. For instance, S1PR1, S1PR2 and S1PR3, are known to regulate the vascular system affecting vascular tone, vascular permeability and heart rate.90–92 In endothelial cells, S1PR1 preserves vascular integrity and regulates vascular permeability.23,91,93 S1PR3 functions in myocardial contractility and in the regulation of bradycardia and hypertension induced by S1P;94 and S1PR2 increases vascular permeability and regulates renal, mesenteric, and local blood flow in various organs.91,95,96 All these are processes are vastly altered during an allergic reaction like systemic anaphylaxis. Thus, it is not surprising that S1P produced during anaphylaxis by mast cells, or other cells, could influence the severity of the response by acting on the vascular system. Recently, we discovered9 that S1PR2 plays an important role in counteracting the vasodilator actions of histamine and therefore the hypotension associated with systemic anaphylaxis (Figure 2). We found that histamine drives S1P production by activating SphK1 and this is required for the engagement of S1PR2 and the recovery from anaphylaxis. Genetic deletion of SphK1 or S1PR2 disrupts this regulatory loop during histamine or IgE/Ag-induced anaphylaxis, causing a severe hypotension, with a consequent delay in the renal rate of histamine excretion, and thus a delay in the recovery from anaphylaxis.9 Importantly, the delayed recovery from anaphylaxis could be rescued in SphK1-deficient by intravenous injection of S1P during anaphylaxis. However, S1P injection had no effect on the delayed recovery of S1PR2-deficient mice, indicating that the presence of this receptor is required for S1P-induced rescue from anaphylaxis.
Other studies suggest that S1PR1 may also play a role in recovery from anaphylaxis.23 Inducible genetic deletion of both SphK1 and SphK2 results in mice that lack circulating or tissue S1P. When challenged with IgE/Ag or PAF, these mice undergo a severe anaphylactic response with resulting lethality. The IgE (SPE-7 clone) and PAF used in this study are potent inducers of mast cell degranulation. A massive loss of circulatory fluids by vascular extravasation was observed and this was attributed to the loss of S1PR1 function, since lethality was partially prevented by injection of a S1PR1 agonist.23 Similar anaphylactic lethality was also seen when SphK1-deficient mice were challenged with PAF, and these mice also showed enhanced vascular fluid extravasation.23 In contrast, we did not observe differences in vascular fluid extravasation between WT and SphK1-deficient mice in our model of IgE/Ag (using a H1-DNPε clone) or histamine-induced anaphylaxis, although SphK1-deficient mice clearly showed more severe anaphylaxis. Thus, whether S1PR1 or S1PR2 is dominant in recovery from anaphylaxis may well depend on the stimuli. Alternatively, models where lethality is the measureable outcome may not reflect the normal anaphylactic response to a stimulus, since recovery is common.97 Regardless, these two different studies suggest that generation of S1P during anaphylaxis and/or the presence of S1P in the circulation have a protective effect via the activation of either or both S1PR1 and S1PR2. While S1PR1 plays a role in the maintenance of the vascular barrier integrity, S1PR2 regulates the vascular contractility that allows for the recovery from hypotension resulting from a systemic anaphylactic challenge. It is known that, in humans, the two main anaphylactic triggers are IgE- or IgG-mediated and the type and the prevalence of released mediators differs, with PAF release being more abundant in IgG-mediated anaphylaxis. Thus, from a therapeutic perspective, a combination of S1PR1 or S1PR2 agonists used at different ratios may prove useful in the treatment of anaphylaxis. In this regard, we found that treatment of WT mice with S1P during histamine-induced anaphylaxis expedited their recovery from anaphylaxis at a similar rate as treatment with adrenaline, the first choice of treatment for anaphylaxis. However, administration of adrenaline carries some risks, particularly in the elderly population, including cardiac arrhythmias, myocardial infarction and hypertensive intracerebral bleeds.98,99 Since the S1PR2 receptor does not mediate bradycardia, tachycardia or influence myocardial function in vivo and its effects on blood pressure and flow rate appear to be mostly vascular,95 specific receptor agonists may be a safer and useful alternative for anaphylaxis treatment. As a note of caution, it is important to point out that S1PR2 has the opposite role of S1PR1 in vascular permeability, as its activation in the endothelial cells can lead to increased vascular permeability. However, the fact that the S1PR1 is predominantly expressed in endothelial cells and that S1PR2-deficiency in our model of histamine-induced anaphylaxis did not reveal differences in vascular permeability, provides an argument for further exploration of S1PR2 agonists in counteracting anaphylaxis.
Overall, another important conclusion suggested from the studies on anaphylaxis is that SphK/S1P is a “Janus” regulator of the systemic allergic response (Figure 2). On one hand, it is part of the signals generated upon engagement of FcεRI, and functions to enhance the activation of mast cells. Also, the presence of S1P in the microenvironment may render mast cells with a more responsive phenotype. On the other hand, SphK/S1P also seems to regulate other aspects of the allergic response that are independent of the mast cell.9 In the case of systemic anaphylaxis, although it enhances mast cell immediate responses, it also mediates the recovery from shock, thus affecting positively and negatively, respectively, the onset and duration of anaphylaxis. Such dual roles for physiological mediators are not unusual in biology since, in a similar way, a given cytokine may have distinct roles as an initiator or attenuator of the immune responses.100–102
CONCLUDING REMARKS
SphK1, SphK2, their product S1P, and the receptors for S1P are all emerging as important regulators of allergic responses. Their roles extend beyond the regulation of the effector function of mast cells and other immune cells to the regulation of the surrounding environment and the (patho)physiological response. Due to the complexity of their actions and the various types of cells involved, further investigations must be aimed at determining the specific sites of action and the relative importance of these molecules to the resulting outcomes. The models that completely eliminate the expression of these molecules (through genetic deletion) in the entire mouse have been useful so far, but they are limited in allowing a further understanding of the role of S1P in different cell types and compensatory mechanisms may complicate the interpretation of their observed involvement. For example, studies on the development of arthritis in SphK1-deficient mice indicated that SphK1 had no involvement in the severity and incidence of inflammation.103 However, studies where pharmacological inhibitors of SphK or RNAi for SphK1 were administered, showed that the incidence, severity and articular inflammation were reduced.71,104 Although the reasons for these discrepancies are not entirely clear, one of the possibilities is that the pharmacological inhibitors or the silencing RNAi may target a population(s) of cells that is more susceptible to these treatments and are essential in the disease progression. Tissue-specific targeting of the SphKs or S1PRs for deletion in specific cells would be highly beneficial in evaluating these possibilities. This could be particularly valuable when considering SphKs or S1PR2 as targets for therapy, and in furthering our understanding of their role in health and disease. Nevertheless, we conclude from the work cited herein that SphKs, S1P, and S1PRs are key components in the regulation of allergic responses and beyond.
Footnotes
The research of the authors included herein was supported by the intramural research program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
References
- 1.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 2.Schwab SR, Cyster JG. Finding a way out: Lymphocyte egress from lymphoid organs. Nature Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 3.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 4.Kono M, Allende ML, Proia RL. Sphingosine-1-phosphate regulation of mammalian development. Biochim Biophys Acta. 2008;1781:435–441. doi: 10.1016/j.bbalip.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel S, Milstien S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 7.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: Lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 9.Olivera A, Eisner C, Kitamura Y, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock. J Clin Invest. 2010 doi: 10.1172/JCI40659. "in press". [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 11.Olivera A, Spiegel S. Sphingosine kinase: A mediator of vital cellular functions. Prostaglandins. 2001;64:123–134. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 13.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 14.Hait NC, Allegood J, Maceyka M, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through s1p lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 16.Bektas M, Allende ML, Lee BG, et al. S1P lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem. doi: 10.1074/jbc.M109.081489. 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: From modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 18.Ledgerwood LG, Lal G, Zhang N, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 19.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Yatomi Y, Miura Y, et al. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 21.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 23.Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 25.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 26.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 27.Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 207:17–27. S11–S14. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogel P, Donoviel MS, Read R, et al. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 30.Cyster JG. Specifying the patterns of immune cell migration. Novartis Found Symp. 2007;281:54–61. doi: 10.1002/9780470062128.ch6. discussion 61-54, 208-209. [DOI] [PubMed] [Google Scholar]
- 31.Olivera A, Mizugishi K, Tikhonova A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Prieschl EE, Csonga R, Novotny V, et al. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after FcεRI triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivera A, Urtz N, Mizugishi K, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 34.Jolly PS, Rosenfeldt HM, Milstien S, et al. The roles of sphingosine-1-phosphate in asthma. Mol Immunol. 2002;38:1239–1245. doi: 10.1016/s0161-5890(02)00070-6. [DOI] [PubMed] [Google Scholar]
- 35.Price MM, Kapitonov D, Allegood J, et al. Sphingosine-1-phosphate induces development of functionally mature chymase-expressing human mast cells from hematopoietic progenitors. FASEB J. 2009;23:3506–3515. doi: 10.1096/fj.08-128900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 38.Oskeritzian CA, Alvarez SE, Hait NC, et al. Distinct roles of sphingosine kinases 1 and 2 in human mast cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 40.Urtz N, Olivera A, Bofill-Cardona E, et al. Early activation of sphingosine kinase in mast cells and recruitment to FcεRI are mediated by its interaction with lyn kinase. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melendez A, Floto RA, Gillooly DJ, et al. FcγRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J Biol Chem. 1998;273:9393–9402. doi: 10.1074/jbc.273.16.9393. [DOI] [PubMed] [Google Scholar]
- 42.Choi WS, Hiragun T, Lee JH, et al. Activation of RBL-2H3 mast cells is dependent on tyrosine phosphorylation of phospholipase D2 by Fyn and Fgr. Mol Cell Biol. 2004;24:6980–6992. doi: 10.1128/MCB.24.16.6980-6992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as "Tunable" effector and immunoregulatory cells: Recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 45.Pushparaj PN, Manikandan J, Tay HK, et al. Sphingosine kinase 1 is pivotal for Fc epsilon RI-mediated mast cell signaling and functional responses in vitro and in vivo. J Immunol. 2009;183:221–227. doi: 10.4049/jimmunol.0803430. [DOI] [PubMed] [Google Scholar]
- 46.Allende ML, Sasaki T, Kawai H, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 47.Zemann B, Kinzel B, Muller M, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 48.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit Rev Immunol. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vig M, Kinet JP. The long and arduous road to crac. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardie RC. Trp channels and lipids: From drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu SZ, Muraki K, Zeng F, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birchwood CJ, Saba JD, Dickson RC, et al. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J Biol Chem. 2001;276:11712–11718. doi: 10.1074/jbc.M010221200. [DOI] [PubMed] [Google Scholar]
- 54.Titievsky A, Titievskaya I, Pasternack M, et al. Sphingosine inhibits voltage-operated calcium channels in GH4C1 cells. J Biol Chem. 1998;273:242–247. doi: 10.1074/jbc.273.1.242. [DOI] [PubMed] [Google Scholar]
- 55.Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (icrac) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–25030. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 56.Budde K, R LS, Nashan B, et al. Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant. 2003;3:846–854. doi: 10.1034/j.1600-6143.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 57.Blom T, Bergelin N, Slotte JP, et al. Sphingosine kinase regulates voltage operated calcium channels in GH4C1 rat pituitary cells. Cell Signal. 2006;18:1366–1375. doi: 10.1016/j.cellsig.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Calloway N, Vig M, Kinet JP, et al. Molecular clustering of Stim1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jolly PS, Bektas M, Olivera A, et al. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hobson JP, Rosenfeldt HM, Barak LS, et al. Role of the sphingosine-1-phosphate receptor Edg-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 61.Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi N, Nishi T, Hirata T, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Boujaoude LC, Bradshaw-Wilder C, Mao C, et al. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: Modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 64.Honig SM, Fu S, Mao X, et al. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takabe K, Kim RH, Allegood JC, et al. Estradiol induces export of sphingosine-1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawahara A, Nishi T, Hisano Y, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 67.Kovacs JJ, Hara MR, Davenport CL, et al. Arrestin development: Emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waters CM, Long J, Gorshkova I, et al. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. Faseb J. 2006;20:509–511. doi: 10.1096/fj.05-4810fje. [DOI] [PubMed] [Google Scholar]
- 69.Ammit AJ, Hastie AT, Edsall LC, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 70.Kitano M, Hla T, Sekiguchi M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: Regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 71.Lai WQ, Irwan AW, Goh HH, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 72.Lee OH, Kim YM, Lee YM, et al. Sphingosine 1-phosphate induces angiogenesis: Its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- 73.Oskeritzian CA, Milstien S, Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol Ther. 2007;115:390–399. doi: 10.1016/j.pharmthera.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walzer T, Chiossone L, Chaix J, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 75.Roviezzo F, Del Galdo F, Abbate G, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Idzko M, Panther E, Corinti S, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of th2 immune responses. Faseb J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 77.Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168–174. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 78.Czeloth N, Schippers A, Wagner N, et al. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 79.Nofer JR, Bot M, Brodde M, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 80.Reines I, Kietzmann M, Mischke R, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:1954–1962. doi: 10.1038/jid.2008.454. [DOI] [PubMed] [Google Scholar]
- 81.Idzko M, Hammad H, van Nimwegen M, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo EY, Park GT, Lee KM, et al. Identification of the target genes of atopic dermatitis by real-time PCR. J Invest Dermatol. 2006;126:1187–1189. doi: 10.1038/sj.jid.5700234. [DOI] [PubMed] [Google Scholar]
- 83.Mechtcheriakova D, Wlachos A, Sobanov J, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19:748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 84.Kucharekova M, Schalkwijk J, Van De Kerkhof PC, et al. Effect of a lipid-rich emollient containing ceramide 3 in experimentally induced skin barrier dysfunction. Contact Dermatitis. 2002;46:331–338. doi: 10.1034/j.1600-0536.2002.460603.x. [DOI] [PubMed] [Google Scholar]
- 85.Kang JS, Yoon WK, Youm JK, et al. Inhibition of atopic dermatitis-like skin lesions by topical application of a novel ceramide derivative, K6PC-9P, in Nc/Nga mice. Exp Dermatol. 2008;17:958–964. doi: 10.1111/j.1600-0625.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 86.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 87.Mizugishi K, Li C, Olivera A, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamashita Y, Charles N, Furumoto Y, et al. Cutting edge: Genetic variation influences Fc epsilon RI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 89.Rivera J, Tessarollo L. Genetic background and the dilema of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 90.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JF, Gordon S, Estrada R, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296:H33–H42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82:193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singleton PA, Dudek SM, Chiang ET, et al. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 94.Forrest M, Sun SY, Hajdu R, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 95.Lorenz JN, Arend LJ, Robitz R, et al. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–R446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 96.Sanchez T, Skoura A, Wu MT, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors rock and pten. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 97.Lieberman P, Camargo CA, Jr, Bohlke K, et al. Epidemiology of anaphylaxis: Findings of the american college of allergy, asthma and immunology epidemiology of anaphylaxis working group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 98.El-Shanawany T, Williams PE, Jolles S. Clinical immunology review series: An approach to the patient with anaphylaxis. Clin Exp Immunol. 2008;153:1–9. doi: 10.1111/j.1365-2249.2008.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 100.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: A double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 101.Mosser DM, Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 103.Michaud J, Kohno M, Proia RL, et al. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 104.Lai WQ, Irwan AW, Goh HH, et al. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183:2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]