Abstract
The effect of K+ ion interaction with monolayers of phosphatidylcholine (lecithin, PC) or cholesterol (Ch) was investigated at the air/water interface. We present surface tension measurements of lipid monolayers obtained using a Langmuir method as a function of K+ ion concentration. Measurements were carried out at 22°C using a Teflon trough and a Nima 9000 tensiometer. Interactions between lecithin and K+ ions or Ch and K+ ions result in significant deviations from the additivity rule. An equilibrium theory to describe the behavior of monolayer components at the air/water interface was developed in order to obtain the stability constants and area occupied by one molecule of lipid–K+ ion complex (LK+). The stability constants for lecithin–K+ ion (PCK+) complex, 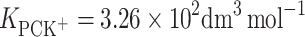 , and for cholesterol–K+ ion (ChK+) complex,
, and for cholesterol–K+ ion (ChK+) complex, 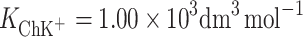 , were calculated by inserting the experimental data. The value of area occupied by one PCK+ complex is 60 Å2 molecule−1, while the area occupied by one ChK+ complex is 40.9 Å2 molecule−1. The complex formation energy (Gibbs free energy) values for the PCK+ and ChK+ complexes are −14.18 ± 0.71 and −16.92 ± 0.85 kJ mol−1, respectively.
, were calculated by inserting the experimental data. The value of area occupied by one PCK+ complex is 60 Å2 molecule−1, while the area occupied by one ChK+ complex is 40.9 Å2 molecule−1. The complex formation energy (Gibbs free energy) values for the PCK+ and ChK+ complexes are −14.18 ± 0.71 and −16.92 ± 0.85 kJ mol−1, respectively.
Keywords: Phosphatidylcholine, Cholesterol, K+ ion, Complex formation equilibria, Monolayer, Langmuir trough
Introduction
The cell’s plasma membrane consists of many different phospholipids that are symmetrically distributed over the two bilayer leaflets. The cytosolic leaflet is enriched in phosphatidylserine (PS), whereas the outer cellular leaflet contains mostly neutral phosphatidylcholine (PC). The properties of membrane lipids depend strongly on the environmental conditions, such as pH and ionic strength. The interactions between membrane lipids and ions, the subject of these studies, plays an important role in many biological processes, such as membrane fusion, enzyme regulation and signal transduction (Chung Peng et al. 1994; Niles et al. 1996; Ravoo et al. 1999; Sovago et al. 2007).
The simplicity of a Langmuir monolayer at the air/water interface offers a clear theoretical advantage for the evaluation and comparison of interfacial models of ion binding in order to study the specific salt effects. In addition, the development of many new experimental techniques that provide details on the structure, ordering and morphology at the mesoscopic and molecular levels, such as X-ray scattering, Brewster angle microscopy, infrared reflection absorption spectroscopy and second harmonic generation spectroscopy, have made DPPC monolayers especially attractive for our purpose (Aroti et al. 2007).
Several investigations of the effects of cations on monolayers (Kmetko et al. 2001; Sovago et al. 2007) and bilayers (Roux and Bloom 1990; Scarpa et al. 2002) exist in the literature, given the importance of H+, Na+, K+, Ca2+ and Mg2+ in altering biomembrane behavior. Relatively few studies have concentrated on anions because the effects of anions, e.g., F−, Cl−, Br− and I−, on lipid monolayers are mostly concentrated on positively charged lipids for which the coulomb interactions are overwhelmingly important (Knock and Bain 2000).
Many ideas about the nature of the specific salt effects have been put forward over the years. Current theories explain specific ion action either through the effect of ions on the structure of water (from here arise the names “kosmotropic” and “chaotropic” ions) (Chaplin 2000) or through dispersive interactions between molecule interfaces and ions (Ninham and Yaminsky 1997) or by the balance between ion–water and water–water interactions (Karlström and Hagberg 2002). In order to better understand the mechanism of action of cations or anions, we have used simple lipid model systems, which allow the examination of several possibilities.
The aim of the present work was to examine the possible effect of K+ ions on the PC or Ch monolayer and the molecular interaction between lecithin or Ch and K+ ions. Here, we present evidence for the formation of PCK+ or ChK+ complexes at the air/water interface and calculate their stability constants. Knowledge of the stability constants of the PC–K+ or Ch–K+ system let us understand the processes that take place both in the monolayer itself and on its surface. The results can be used in quantitative descriptions of physical and chemical properties of biological membranes.
Theory
During the formation of a mixed two-component monolayer (lipid–ion K+) on a free electrolyte surface, the individual components (denoted by L, K+ and LK+) can form complexes. The equilibria of such a system are described by the complexation reaction. Let us assume that 1:1 complex is formed in a mixed monolayer at the air/water interface. The reactions
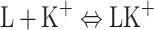 |
1 |
may be described by the system of equations
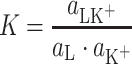 |
2 |
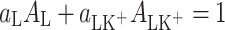 |
3 |
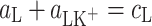 |
4 |
where 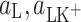 (mol m−2) are the surface concentrations of components L and LK+;
(mol m−2) are the surface concentrations of components L and LK+; 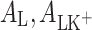 (m2 mol−1) are the surface areas occupied by 1 mole of components L and LK+;
(m2 mol−1) are the surface areas occupied by 1 mole of components L and LK+; 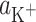 (mol dm−3) is the concentration of K+ ions;
(mol dm−3) is the concentration of K+ ions; 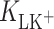 (dm3 mol−1) is the stability constant of the LK+ complex; and
(dm3 mol−1) is the stability constant of the LK+ complex; and 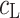 (mol m−2) is the total surface concentration of lipid.
(mol m−2) is the total surface concentration of lipid.
Elimination of the 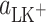 and
and 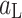 parameters from the set of Eqs. 2–4 yields the basic equation
parameters from the set of Eqs. 2–4 yields the basic equation
 |
5 |
where
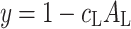 |
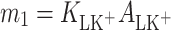 |
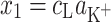 |
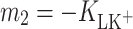 |
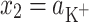 |
The 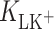 and
and 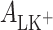 parameters were calculated from the equations presented below:
parameters were calculated from the equations presented below:
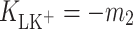 |
6 |
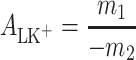 |
7 |
The parameters describing the complexes may be used to calculate theoretical points using the equation presented below (agreement between the theoretical and experimental values implies that the system is well described by the above equations):
 |
8 |
Materials and Methods
Materials
PC (99%; fatty acid composition: 16:0, 33%; 18:0, 4%; 18:1, 30%; 18:2, 14%; 20:4, 4%) and Ch (99%) were purchased from Sigma–Aldrich (St. Louis, MO) and used as received. The 1-chloropropane solvent (>98% pure) was supplied by Sigma–Aldrich. Solutions were prepared by dissolving appropriate amounts of each material in 1-chloropropane at a concentration of 1 mg cm−3 and stored at 4°C.
Electrolyte solutions were prepared from triple-distilled water and potassium chloride (KCl) from POCh (Gliwice, Poland). Solutions of 1, 0.1, 0.01, 0.001 and 0.0001 M were used for experiments.
The water used in the experiments was prepared by triple distillation (the second distillation was performed over KMnO4 and KOH [both from POCh] to remove organic impurities).
Methods
The homemade computer-controlled apparatus used for surface tension measurements was presented previously (Petelska and Figaszewski 2009).
Surface tension measurements were carried out at the water/air interface at 22°C and expressed as surface pressure area per molecule (π-A) isotherms. The apparatus and measurement method were described previously (Petelska and Figaszewski 2009).
For all experiments, the trough was filled with K+ in triple-distilled water as the subphase. Monolayers were prepared by spreading a defined volume of a lecithin solution in 1-chloropropane on the aqueous subphase using a Hamilton (Reno, NV) microsyringe. Ten minutes were allowed for solvent evaporation and monolayer equilibration before an experiment was begun. The monolayer was continuously compressed to obtain the π-A isotherms using the barrier.
The Nima ST9002 computer program was used to calculate the surface pressure of the monolayer (π) as a function of surface area per molecule (A): π = γ − γ 0 = f(A), where γ 0 is the surface tension of the lipid-covered surface and γ is the surface tension of the bare air/water interface.
The system was enclosed in an acrylic box to minimize water evaporation, to ensure high humidity and to avoid contamination of the system.
All of the reported values are highly reproducible and represent the average of at least five experiments. The standard deviation for surface area measurements was <1%.
Results and Discussion
We present surface tension measurements of lecithin monolayers obtained using a Langmuir method as a function of K+ ion concentration. In this section we present evidence for the formation of PC–K+ ion and Ch–K+ ion complexes at the air/water interface and develop a system of equations to describe the complex formation. Using these equations, the stability constants of the PCK+ and ChK+ complexes were calculated.
PC–K+ Ion System
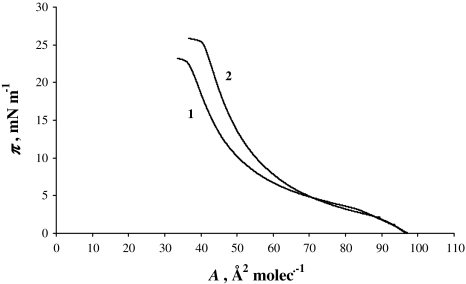
Figure 1 presents two π-A isotherms of PC with absence of K+ ions (denoted by 1) and PC with presence of 0.1 M K+ ions (denoted by 2). This PC isotherm is in satisfactory agreement with that previously reported (Brzozowska and Figaszewski 2002; Gzyl and Paluch 2004). The lecithin monolayer is an example of a liquid-expanded membrane, with the hydrophilic head groups located in the aqueous subphase and the hydrophobic fatty acid tails oriented toward the air. The surface area per lipid molecule assumes various values depending on the length, conformation and degree of unsaturation of the hydrocarbon chains. The surface area for the lecithin molecule in pure water is 56 Å2. This value is in agreement with values reported in the literature (Brzozowska and Figaszewski 2002; Gzyl and Paluch 2004). The surface area for the lecithin molecule in the presence of 0.1 M K+ ion concentrations is 60 Å2, which is presented in Fig. 1 (line denoted by 2).
Fig. 1.
π-A isotherm of phosphatidylcholine monolayers in pure water (1) and 0.1 M K+ ion concentration (2) as a subphase
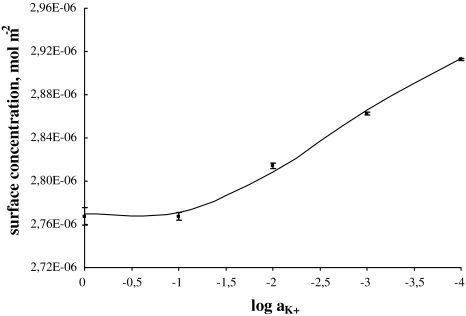
The total surface concentrations of PC versus the logarithm of K+ ion concentration is depicted in Fig. 2. In Fig. 2, the experimental points are compared with the values calculated using Eq. 8 (depicted as a line). Figure 2 refers to the above-presented description (see Theory) where the distribution of the monolayer components on the air/water interface of the lipid layer has been assumed to be uniform. As seen in Eq. 4, the total surface concentration of the lecithin membrane is a sum of the surface concentration of its components, i.e., PC and PCK+.
Fig. 2.
The dependence of total surface concentration of phosphatidylcholine, 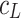 , on the logarithm of K+ ion concentration,
, on the logarithm of K+ ion concentration, 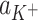 (squares, experimental values; lines, theoretical values) at surface pressure ~25 mN m−1
(squares, experimental values; lines, theoretical values) at surface pressure ~25 mN m−1
According to Eqs 6 and 7, it is possible to determine the area occupied by one molecule of PCK+ complex and the stability constant of this complex, which were valued to be 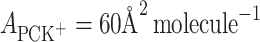 and
and 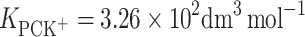 , respectively. The
, respectively. The 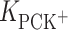 calculated by inserting the experimental data into Eq. 6 is relatively high, which provides additional evidence for the prevalence of a 1:1 complex in PC monolayer with the presence K+ ions.
calculated by inserting the experimental data into Eq. 6 is relatively high, which provides additional evidence for the prevalence of a 1:1 complex in PC monolayer with the presence K+ ions.
Knowing the area occupied by the PCK+ complex and the stability constant value of this complex, the theoretical values of the surface concentration of the lecithin monolayer in the presence of K+ ions were calculated using Eq. 8. The theoretical values obtained are presented in Fig. 2 and marked by lines; points on the same figure show the experimental values. It can be seen that the agreement between experimental and theoretical points is very good, which verifies the assumption of the formation of the PCK+ complex in the lipid monolayer. Good agreement between experimental and theoretical points verifies the assumption of a 1:1 complex in the PC monolayer. The lack of variation between theoretical and experimental points indicates that the theoretical model (presented under Theory) is sufficient to describe the interaction in the lecithin–K+ ion system. The agreement between the experimental results and the model predictions for the lecithin–K+ ion system justifies the statement that other complexes do not represent a significant component of this system.
Ch–K+ Ion System
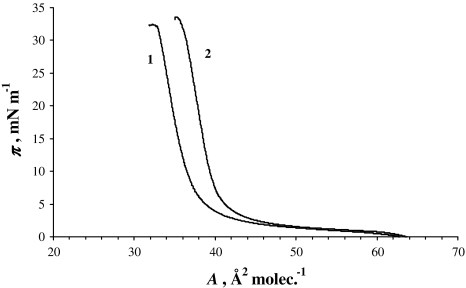
Figure 3 presents two π-A isotherms of Ch in the absence of K+ ions (denoted by 1) and Ch in the presence of K+ ions at a concentration of 0.1 M (denoted by 2). The π–A dependence of Ch is shaped differently from the lecithin isotherm. The slope of the Ch isotherm (denoted by 1 in Fig. 3) is very high, indicating a perpendicular orientation of the molecules at the interface, with the hydrophilic OH group pointing at the aqueous subphase.
Fig. 3.
π-A isotherm of cholesterol monolayer in pure water (1) and 0.1 M K+ ion concentration (2) as a subphase
The surface area per Ch molecule, 38 Å2, was obtained experimentally by extrapolating the isotherm to π = 0. This value is within the range reported in the literature (Joos and Demel 1969; Wege et al. 1999), which varies from 36 to 45 Å2. The surface area for the Ch molecule in the presence 0.1 M K+ ion concentration is 40.6 Å2.
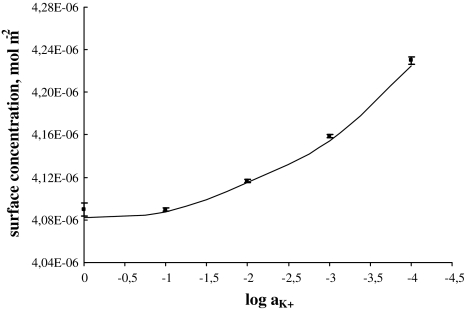
The total surface concentration of Ch versus the logarithm of K+ ion concentration is depicted in Fig. 4. In Fig. 4, the experimental points are compared with the values calculated using Eq. 8 (depicted as line). Figure 4 refers to the above-presented description (see Theory) where the distribution of the monolayer components on the air/water interface of the lipid layer has been assumed to be uniform. As seen in Eq. 4, the total surface concentration of the lecithin membrane is a sum of the surface concentration of its components, i.e., Ch and ChK+.
Fig. 4.
The dependence of total surface concentration of cholesterol, 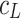 , on the logarithm of K+ ions concentration,
, on the logarithm of K+ ions concentration, 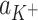 (squares, experimental values; lines, theoretical values) at surface pressure ~35 mN m−1
(squares, experimental values; lines, theoretical values) at surface pressure ~35 mN m−1
According to Eqs 6 and 7, it is possible to determine the area occupied by one molecule of ChK+ complex and the stability constant of this complex, which were valued to be 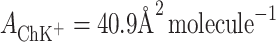 and
and 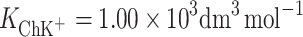 , respectively. The
, respectively. The 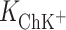 calculated by inserting the experimental data into Eq. 6 is relatively high, which provides additional evidence for the prevalence of a 1:1 complex in Ch monolayer in the presence of K+ ions.
calculated by inserting the experimental data into Eq. 6 is relatively high, which provides additional evidence for the prevalence of a 1:1 complex in Ch monolayer in the presence of K+ ions.
Knowing the area occupied by the ChK+ complex and the stability constant value of this complex, the theoretical values of the surface concentration of the Ch monolayer in the presence of K+ ions were calculated using Eq. 8. The theoretical values are presented in Fig. 4 and marked by lines; points on the same figure show the experimental values. It can be seen that the agreement between experimental and theoretical points is very good, which verifies the assumption of the formation of the ChK+ complex in the lipid monolayer. Good agreement between experimental and theoretical points verifies the assumption of a 1:1 complex in the Ch monolayer. The lack of variation between theoretical and experimental points indicates that the theoretical model (presented under Theory) is sufficient to describe the interaction in the Ch–K+ ion system. The agreement between the experimental results and the model predictions for the Ch–K+ ion system justifies the statement that other complexes do not represent a significant component of this system.
We find that a K+ ion affects the PC and Ch monolayers, like calcium ions, which perturb the lipid organization significantly (Sovago et al. 2007; Petelska et al. 2010). The effect of calcium on lipid organization suggests that the Ca2+ ion interacts with the head group, most likely the phosphate group. Calcium and phosphate are known to form a strong ion pair in water (Zhang et al. 1991), and the strength of this interaction is likely to be increased in the lipid head group region, where the surrounding dielectric permittivity is reduced.
With potassium ions present in the subphase, the compression isotherm for PC shifts to slightly higher surface pressures in the LE and LE + LC regions. The same effect was observed previously (Sovago et al. 2007; Aroti et al. 2004) and explained by disorder of the lipid chains induced by ions binding to the LE phase. At higher surface pressure, potassium has no effect on the compression isotherm, indicating no significant interaction of K+ with the LC phase: The ions are probably being “squeezed out” from the head group region.
Conclusions
In conclusion, the stability constants for PCK+ and ChK+ complexes in monolayers are reported here for the first time. The stability constants 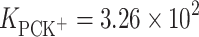 and
and 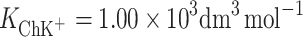 were calculated by inserting the experimental data into Eq. 6.
were calculated by inserting the experimental data into Eq. 6.
The experimental area occupied by one PCK+ complex is 60 Å2 molecule−1, while the area occupied by a ChK+ complex is 40.9 Å2 molecule−1. The excellent agreement between the experimental and theoretical points validates the assumption of lipid–K+ ion complex formation between the lecithin or Ch monolayer and K+ ions.
The complex formation energy (Gibbs free energy) values for the PCK+ and ChK+ complexes are −14.18 ± 0.71 and −16.92 ± 0.85 kJ mol−1, respectively.
The data presented in this work are of great importance for the interpretation of phenomena occurring in lipid monolayers and bilayers, especially the effect of K+ ions. The interactions between membrane lipids and monovalent ions are studied by several techniques; however, there we are still lacking a quantitative description of the systems, which would be required for a better understanding of the processes that take place in biological membranes to use the artificial membrane that would resemble very closely the properties of natural membranes.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aroti A, Leontidis E, Maltseva E, Brezesinski G. Effects of Hofmeister anions on DPPC Langmuir monolayers at the air–water interface. J Phys Chem B. 2004;108:15238–15245. doi: 10.1021/jp0481512. [DOI] [Google Scholar]
- Aroti A, Leontidis E, Dubois M, Zembb T, Brezesinski G. Monolayers, bilayers and micelles of zwitterionic lipids as model systems for the study of specific anion effects. Colloids Surf A. 2007;303:144–158. doi: 10.1016/j.colsurfa.2007.03.011. [DOI] [Google Scholar]
- Brzozowska I, Figaszewski ZA. The equilibrium of phosphatidylcholine-cholesterol in monolayers at the air/water interface. Colloids Surf B. 2002;23:51–58. doi: 10.1016/S0927-7765(01)00209-0. [DOI] [Google Scholar]
- Chaplin MF. A proposal for the structuring of water. Biophys Chem. 2000;83:211–221. doi: 10.1016/S0301-4622(99)00142-8. [DOI] [PubMed] [Google Scholar]
- Chung Peng C, Laudederkind SJF, Ballou LR. Sphingosine-mediated phosphatidylinositol metabolism and calcium mobilization. J Biol Chem. 1994;269:5849–5856. [PubMed] [Google Scholar]
- Gzyl B, Paluch M. Monolayers of lipids at the water–air interface. Prog Colloid Polym Sci. 2004;126:60–63. [Google Scholar]
- Joos P, Demel RA. The interaction energies of cholesterol and lecithin in spread mixed monolayers at the air–water interface. Biochim Biophys Acta. 1969;183:447–457. doi: 10.1016/0005-2736(69)90159-X. [DOI] [PubMed] [Google Scholar]
- Karlström G, Hagberg D. Toward an understanding of the Hofmeister effect: a computer game with dipoles and an ion. J Phys Chem B. 2002;106:11585–11592. doi: 10.1021/jp021252k. [DOI] [Google Scholar]
- Kmetko J, Datta A, Evmenenko G, Dutta P. The effects of divalent ions on Langmuir monolayer and subphase structure: a grazing-incidence diffraction and bragg rod study. J Phys Chem B. 2001;105:10818–10825. doi: 10.1021/jp0122169. [DOI] [Google Scholar]
- Knock MM, Bain CD. Effect of counterion on monolayers of hexadecyltrimethylammonium halides at the air–water interface. Langmuir. 2000;16:2857–2865. doi: 10.1021/la991031e. [DOI] [Google Scholar]
- Niles WD, Silvius JR, Cohen FS. Resonance energy transfer imaging of phospholipid vesicle interaction with a planar phospholipids membrane. J Gen Physiol. 1996;107:329–351. doi: 10.1085/jgp.107.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninham BW, Yaminsky V. Ion binding and ion specificity: the Hofmeister effect and Onsager and Lifshitz theories. Langmuir. 1997;13:2097–2108. doi: 10.1021/la960974y. [DOI] [Google Scholar]
- Petelska AD, Figaszewski ZA. The equilibria of phosphatidylethanolamine-cholesterol and phosphatidylcholine-phosphatidylethanolamine in monolayers at the air/water interface. J Macromol Sci A. 2009;46:607–614. doi: 10.1080/10601320902851884. [DOI] [Google Scholar]
- Petelska AD, Niemcunowicz-Janica A, Szeremeta M, Figaszewski ZA. Equilibria of phosphatidylcholine-Ca2+ ions in monolayer at the air/water interface. Langmuir. 2010;26:13359–13363. doi: 10.1021/la102118n. [DOI] [PubMed] [Google Scholar]
- Ravoo BJ, Weringa WD, Engberts JB. Membrane fusion in vesicles of oligomerizable lipids. Biophys J. 1999;76:374–386. doi: 10.1016/S0006-3495(99)77204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Bloom M. Ca2+, Mg2+, Li+, Na+, and K+ distributions in the headgroup region of binary membranes of phosphatidylcholine and phosphatidylserine as seen by deuterium NMR. Biochemistry. 1990;29:7077–7089. doi: 10.1021/bi00482a019. [DOI] [PubMed] [Google Scholar]
- Scarpa MV, Maximiano FA, Chaimovich H, Cuccovia IM. Interfacial concentrations of chloride and bromide and selectivity for ion exchange in vesicles prepared with dioctadecyldimethylammonium halides, lipids, and their mixtures. Langmuir. 2002;18:8817–8823. doi: 10.1021/la025652a. [DOI] [Google Scholar]
- Sovago M, George WH, Wurpel MS, Müller M, Bonn M. Calcium-induced phospholipid ordering depends on surface pressure. J Am Chem Soc. 2007;129:11079–11084. doi: 10.1021/ja071189i. [DOI] [PubMed] [Google Scholar]
- Wege HA, Holgado-Terriza JA, Galvez-Ruiz MJ, Cabrerizo-Vilchez MA. Development of a new Langmuir-type pendant-drop film balance. Colloids Surf B. 1999;12:339–349. doi: 10.1016/S0927-7765(98)00088-5. [DOI] [Google Scholar]
- Zhang JW, Ebrahimpour A, Nancollas GH. Ion association in calcium phosphate solutions at 37°C. J Sol Chem. 1991;20:455–465. doi: 10.1007/BF00650802. [DOI] [Google Scholar]